Retinal Tissue Shows Glial Changes in a Dravet Syndrome Knock-in Mouse Model
Abstract
1. Introduction
2. Results
2.1. Morphology and Distribution and Quantification of Retinal Microglia
2.1.1. Outer Plexiform Layer (OPL)
2.1.2. Inner Plexiform Layer (IPL)
2.1.3. Nerve Fiber Layer-Ganglion Cell Layer (NFL-GCL)
2.2. Morphology and Distribution and Quantification of Retinal Astrocytes
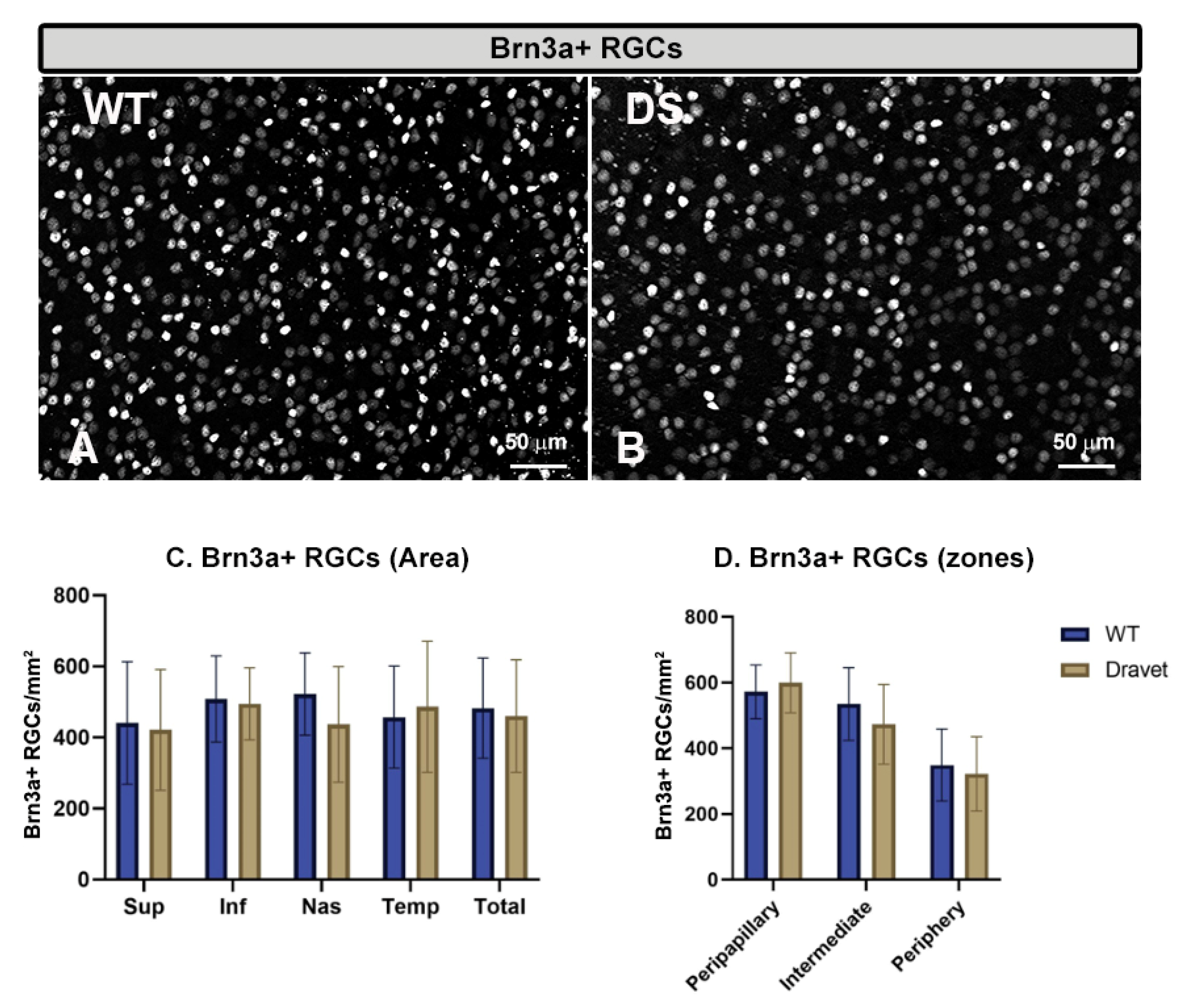
2.3. Quantitative Study of Retinal Ganglion Cells (RGC) and Amacrine GABAergic Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Genotyping
4.2. Immunohistochemistry
4.2.1. Retinal Quantifications
4.2.2. Microglial Quantifications
Number of Microglia Iba-1+
Area Occupied by Each Microglial Cell
Arbor Area
4.3. Astroglial Quantifications
4.4. Brn3a+ RGC and GABAergic GAD65/67 Amacrine Cells Quantifications
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín-Suárez, S.; Abiega, O.; Ricobaraza, A.; Hernandez-Alcoceba, R.; Encinas, J.M. Alterations of the Hippocampal Neurogenic Niche in a Mouse Model of Dravet Syndrome. Front. Cell Dev. Biol. 2020, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Skluzacek, J.v.; Watts, K.P.; Parsy, O.; Wical, B.; Camfield, P. Dravet Syndrome and Parent Associations: The IDEA League Experience with Comorbid Conditions, Mortality, Management, Adaptation, and Grief. Epilepsia 2011, 52, 95–101. [Google Scholar] [CrossRef]
- Guerrini, R. Dravet Syndrome: The Main Issues. Eur. J. Paediatr. Neurol. 2012, 16, S1–S4. [Google Scholar] [CrossRef]
- Dravet, C.; Bureau, M.; Oguni, H.; Fukuyama, Y.; Cokar, O. Severe Myoclonic Epilepsy in Infancy: Dravet Syndrome. Adv. Neurol. 2005, 95, 71–102. [Google Scholar] [PubMed]
- Jansen, F.E.; Sadleir, L.G.; Harkin, L.A.; Vadlamudi, L.; McMahon, J.M.; Mulley, J.C.; Scheffer, I.E.; Berkovic, S.F. Severe Myoclonic Epilepsy of Infancy (Dravet Syndrome): Recognition and Diagnosis in Adults. Neurology 2006, 67, 2224–2226. [Google Scholar] [CrossRef] [PubMed]
- Oguni, H.; Hayashi, K.; Osawa, M.; Awaya, Y.; Fukuyama, Y.; Fukuma, G.; Hirose, S.; Mitsudome, A.; Kaneko, S. Severe Myoclonic Epilepsy in Infancy: Clinical Analysis and Relation to SCN1A Mutations in a Japanese Cohort. Adv. Neurol. 2005, 95, 103–117. [Google Scholar]
- Depienne, C.; Trouillard, O.; Saint-Martin, C.; Gourfinkel-An, I.; Bouteiller, D.; Carpentier, W.; Keren, B.; Abert, B.; Gautier, A.; Baulac, S.; et al. Spectrum of SCN1A Gene Mutations Associated with Dravet Syndrome: Analysis of 333 Patients. J. Med. Genet. 2009, 46, 183–191. [Google Scholar] [CrossRef]
- Tai, C.; Abe, Y.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Impaired Excitability of Somatostatin- and Parvalbumin-Expressing Cortical Interneurons in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, E3139–E3148. [Google Scholar] [CrossRef]
- Cheah, C.S.; Yu, F.H.; Westenbroek, R.E.; Kalume, F.K.; Oakley, J.C.; Potter, G.B.; Rubenstein, J.L.; Catterall, W.A. Specific Deletion of NaV1.1 Sodium Channels in Inhibitory Interneurons Causes Seizures and Premature Death in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 14646–14651. [Google Scholar] [CrossRef]
- Bender, A.C.; Morse, R.P.; Scott, R.C.; Holmes, G.L.; Lenck-Santini, P.P. SCN1A Mutations in Dravet Syndrome: Impact of Interneuron Dysfunction on Neural Networks and Cognitive Outcome. Epilepsy Behav. 2012, 23, 177–186. [Google Scholar] [CrossRef]
- Oakley, J.C.; Kalume, F.; Catterall, W.A. Insights into Pathophysiology and Therapy from a Mouse Model of Dravet Syndrome. Epilepsia 2011, 52, 59–61. [Google Scholar] [CrossRef]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced Sodium Current in GABAergic Interneurons in a Mouse Model of Severe Myoclonic Epilepsy in Infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Nav1.1 Localizes to Axons of Parvalbumin-Positive Inhibitory Interneurons: A Circuit Basis for Epileptic Seizures in Mice Carrying an Scn1a Gene Mutation. J. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Dutt, K.; Papale, L.; Rusconi, R.; Shankar, A.; Hunter, J.; Tufik, S.; Yu, F.H.; Catterall, W.A.; Mantegazza, M.; et al. A BAC Transgenic Mouse Model Reveals Neuron Subtype-Specific Effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) Mutation. Neurobiol. Dis. 2009, 35, 91–102. [Google Scholar] [CrossRef]
- Mancuso, M.; Filosto, M.; Naini, A.; Rocchi, A.; Del Corona, A.; Sartucci, F.; Siciliano, G.; Murri, L. A Screening for Superoxide Dismutase-1 D90A Mutation in Italian Patients with Sporadic Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron. Disord. 2002, 3, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Dutt, K.; Papale, L.A.; Dubé, C.M.; Dutton, S.B.; de Haan, G.; Shankar, A.; Tufik, S.; Meisler, M.H.; Baram, T.Z.; et al. Altered Function of the SCN1A Voltage-Gated Sodium Channel Leads to γ-Aminobutyric Acid-Ergic (GABAergic) Interneuron Abnormalities. J. Biol. Chem. 2010, 285, 9823–9834. [Google Scholar] [CrossRef]
- Dutton, S.B.; Makinson, C.D.; Papale, L.A.; Shankar, A.; Balakrishnan, B.; Nakazawa, K.; Escayg, A. Preferential Inactivation of Scn1a in Parvalbumin Interneurons Increases Seizure Susceptibility. Neurobiol. Dis. 2013, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.; Hawkins, N.A.; Mccollom, C.E.; Kearney, J.A. Mapping Genetic Modifiers of Survival in a Mouse Model of Dravet Syndrome. Genes Brain Behav. 2014, 13, 163–172. [Google Scholar] [CrossRef]
- Rubinstein, M.; Han, S.; Tai, C.; Westenbroek, R.E.; Hunker, A.; Scheuer, T.; Catterall, W.A. Dissecting the Phenotypes of Dravet Syndrome by Gene Deletion. Brain 2015, 138, 2219–2233. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lee, M.L.; Chang, C.Y.; Fan, H.H.; Yu, I.S.; Chen, Y.T.; You, J.Y.; Chen, C.Y.; Chang, F.C.; Hsiao, J.H.; et al. Functional and Structural Deficits of the Dentate Gyrus Network Coincide with Emerging Spontaneous Seizures in an Scn1a Mutant Dravet Syndrome Model during Development. Neurobiol. Dis. 2015, 77, 35–48. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.G.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef]
- Satta, V.; Alonso, C.; Díez, P.; Martín-Suárez, S.; Rubio, M.; Encinas, J.M.; Fernández-Ruiz, J.; Sagredo, O. Neuropathological Characterization of a Dravet Syndrome Knock-In Mouse Model Useful for Investigating Cannabinoid Treatments. Front. Mol. Neurosci. 2021, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Boer, K.; Crino, P.B.; Gorter, J.A.; Nellist, M.; Jansen, F.E.; Spliet, W.G.M.; van Rijen, P.C.; Wittink, F.R.A.; Breit, T.M.; Troost, D.; et al. Gene Expression Analysis of Tuberous Sclerosis Complex Cortical Tubers Reveals Increased Expression of Adhesion and Inflammatory Factors. Brain Pathol. 2010, 20, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Zurolo, E.; Spliet, W.G.M.; van Rijen, P.C.; Baayen, J.C.; Gorter, J.A.; Aronica, E. Evaluation of the Innate and Adaptive Immunity in Type I and Type II Focal Cortical Dysplasias. Epilepsia 2010, 51, 1763–1773. [Google Scholar] [CrossRef]
- Koh, S. Role of Neuroinflammation in Evolution of Childhood Epilepsy. J. Child Neurol. 2017, 33, 64–72. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory Properties of Inflammatory Cytokines and Their Impact on Neuronal Excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Hayatdavoudi, P.; Hosseini, M.; Hajali, V.; Hosseini, A.; Rajabian, A. The Role of Astrocytes in Epileptic Disorders. Physiol. Rep. 2022, 10, e15239. [Google Scholar] [CrossRef]
- Coulter, D.A.; Steinhäuser, C. Role of Astrocytes in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022434. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sone, T.; Higurashi, N.; Sakuma, T.; Suzuki, S.; Ishikawa, M.; Yamamoto, T.; Mitsui, J.; Tsuji, H.; Okano, H.; et al. Generation of D1-1 TALEN Isogenic Control Cell Line from Dravet Syndrome Patient IPSCs Using TALEN-Mediated Editing of the SCN1A Gene. Stem Cell Res. 2018, 28, 100–104. [Google Scholar] [CrossRef]
- Salobrar-García, E.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; Ramírez, J.M. Ophthalmologic Psychophysical Tests Support OCT Findings in Mild Alzheimer’s Disease. J. Ophthalmol. 2015, 2015, 736949. [Google Scholar] [CrossRef]
- Salobrar-García, E.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Rojas, P.; Vazirani, R.; Amarante, C.; Yubero, R.; Gil, P.; Pinazo-Durán, M.D.; et al. Changes in Visual Function and Retinal Structure in the Progression of Alzheimer’s Disease. PLoS ONE 2019, 14, e0220535. [Google Scholar] [CrossRef] [PubMed]
- Salobrar-Garcia, E.; Hoyas, I.; Leal, M.; De Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; et al. Analysis of Retinal Peripapillary Segmentation in Early Alzheimer’s Disease Patients. Biomed Res. Int. 2015, 2015, 636548. [Google Scholar] [CrossRef]
- Salobrar-García, E.; Rodrigues-Neves, A.C.; Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; López-Cuenca, I.; Ramírez, J.M.; Ambrósio, A.F.; Salazar, J.J. Microglial Activation in the Retina of a Triple-Transgenic Alzheimer’s Disease Mouse Model (3xTg-AD). Int. J. Mol. Sci. 2020, 21, 816. [Google Scholar] [CrossRef]
- Salobrar-García, E.; López-Cuenca, I.; Sánchez-Puebla, L.; de Hoz, R.; Fernández-Albarral, J.A.; Ramírez, A.I.; Bravo-Ferrer, I.; Medina, V.; Moro, M.A.; Saido, T.C.; et al. Retinal Thickness Changes Over Time in a Murine AD Model APPNL-F/NL-F. Front. Aging Neurosci. 2021, 12, 625642. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; de Hoz, R.; Ramírez, A.I.; Ferreras, A.; Salobrar-García, E.; Muñoz-Blanco, J.L.; Urcelay-Segura, J.L.; Salazar, J.J.; Ramírez, J.M. Changes in Retinal OCT and Their Correlations with Neurological Disability in Early ALS Patients, a Follow-Up Study. Brain Sci. 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Ramírez, A.I.; Cadena, M.; Fernández-Albarral, J.A.; Salobrar-García, E.; López-Cuenca, I.; Santos-García, I.; de Lago, E.; Urcelay-Segura, J.L.; Ramírez, J.M.; et al. Retinal Ganglion Cell Loss and Microglial Activation in a SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2021, 22, 1663. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Chieffo, D.; Battaglia, D.; Brogna, C.; Contaldo, I.; de Clemente, V.; Losito, E.; Dravet, C.; Mercuri, E.; Guzzetta, F. A Prospective Longitudinal Study on Visuo-Cognitive Development in Dravet Syndrome: Is There a “Dorsal Stream Vulnerability”? Epilepsy Res. 2015, 109, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, D.; Battaglia, D.; Lettori, D.; del Re, M.; Brogna, C.; Dravet, C.; Mercuri, E.; Guzzetta, F. Neuropsychological Development in Children with Dravet Syndrome. Epilepsy Res. 2011, 95, 86–93. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Mora-Jimenez, L.; Puerta, E.; Sanchez-Carpintero, R.; Mingorance, A.; Artieda, J.; Nicolas, M.J.; Besne, G.; Bunuales, M.; Gonzalez-Aparicio, M.; et al. Epilepsy and Neuropsychiatric Comorbidities in Mice Carrying a Recurrent Dravet Syndrome SCN1A Missense Mutation. Sci. Rep. 2019, 9, 14172. [Google Scholar] [CrossRef]
- Valassina, N.; Brusco, S.; Salamone, A.; Serra, L.; Luoni, M.; Giannelli, S.; Bido, S.; Massimino, L.; Ungaro, F.; Mazzara, P.G.; et al. Scn1a Gene Reactivation after Symptom Onset Rescues Pathological Phenotypes in a Mouse Model of Dravet Syndrome. Nat. Commun. 2022, 13, 161. [Google Scholar] [CrossRef]
- Sokolova, T.v.; Zabrodskaya, Y.M.; Litovchenko, A.v.; Paramonova, N.M.; Kasumov, V.R.; Kravtsova, S.v.; Skiteva, E.N.; Sitovskaya, D.A.; Bazhanova, E.D. Relationship between Neuroglial Apoptosis and Neuroinflammation in the Epileptic Focus of the Brain and in the Blood of Patients with Drug-Resistant Epilepsy. Int. J. Mol. Sci. 2022, 23, 12561. [Google Scholar] [CrossRef] [PubMed]
- Terrone, G.; Salamone, A.; Vezzani, A. Inflammation and Epilepsy: Preclinical Findings and Potential Clinical Translation. Curr. Pharm. Des. 2017, 23, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.L.; Löscher, W.; White, H.S.; Galanopoulou, A.S. Neuroinflammation in Epileptogenesis: Insights and Translational Perspectives from New Models of Epilepsy. Epilepsia 2017, 58, 39–47. [Google Scholar] [CrossRef] [PubMed]
- de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Triviño, A.; Ramírez, A.I.; Salazar, J.J.; García-Sánchez, J. Immunohistochemical Study of Human Retinal Astroglia. Vision Res. 1994, 34, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Müller Glial Cells in Retinal Disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Pannicke, T.; Biedermann, B.; Uckermann, O.; Weick, M.; Bringmann, A.; Wolf, S.; Wiedemann, P.; Habermann, G.; Buse, E.; Reichenbach, A. Physiological Properties of Retinal Müller Glial Cells from the Cynomolgus Monkey, Macaca Fascicularis—A Comparison to Human Müller Cells. Vision Res. 2005, 45, 1781–1791. [Google Scholar] [CrossRef]
- Grote, A.; Heiland, D.-H.; Taube, J.; Helmstaedter, C.; Ravi, V.M.; Will, P.; Hattingen, E.; Schüre, J.-R.; Witt, J.-A.; Reimers, A.; et al. ‘Hippocampal Innate Inflammatory Gliosis Only’ in Pharmacoresistant Temporal Lobe Epilepsy. Brain 2022, awac293. [Google Scholar] [CrossRef]
- López-Cuenca, I.; Marcos-Dolado, A.; Yus-Fuertes, M.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Salazar, J.J.; Ramírez, A.I.; Sánchez-Puebla, L.; Fuentes-Ferrer, M.E.; et al. The Relationship between Retinal Layers and Brain Areas in Asymptomatic First-Degree Relatives of Sporadic Forms of Alzheimer’s Disease: An Exploratory Analysis. Alzheimers Res. Ther. 2022, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Oku, Y.; Taneichi, H.; Tanaka, T.; Igarashi, N.; Niida, Y.; Nishida, N. Two Autopsy Cases of Sudden Unexpected Death from Dravet Syndrome with Novel de Novo SCN1A Variants. Brain Dev. 2020, 42, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Harkin, L.A.; McMahon, J.M.; Iona, X.; Dibbens, L.; Pelekanos, J.T.; Zuberi, S.M.; Sadleir, L.G.; Andermann, E.; Gill, D.; Farrell, K.; et al. The Spectrum of SCN1A-Related Infantile Epileptic Encephalopathies. Brain 2007, 130, 843–852. [Google Scholar] [CrossRef] [PubMed]
- van Hook, M.J.; Nawy, S.; Thoreson, W.B. Voltage- and Calcium-Gated Ion Channels of Neurons in the Vertebrate Retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef]
- Zenisek, D.; Henry, D.; Studholme, K.; Yazulla, S.; Matthews, G. Voltage-Dependent Sodium Channels Are Expressed in Nonspiking Retinal Bipolar Neurons. J. Neurosci. 2001, 21, 4543. [Google Scholar] [CrossRef]
- Vahedi, K.; Depienne, C.; le Fort, D.; Riant, F.; Chaine, P.; Trouillard, O.; Gaudric, A.; Morris, M.A.; LeGuern, E.; Tournier-Lasserve, E.; et al. Elicited Repetitive Daily Blindness: A New Phenotype Associated with Hemiplegic Migraine and SCN1A Mutations. Neurology 2009, 72, 1178–1183. [Google Scholar] [CrossRef]
- Choi, C.; Khuddus, N.; Mickler, C.; Tuli, S.; Tuli, S. Occlusive Patch Therapy for Reduction of Seizures in Dravet Syndrome. Clin. Pediatr. 2011, 50, 876–878. [Google Scholar] [CrossRef]
- López-Cuenca, I.; de Hoz, R.; Salobrar-García, E.; Elvira-Hurtado, L.; Rojas, P.; Fernández-Albarral, J.A.; Barabash, A.; Salazar, J.J.; Ramírez, A.I.; Ramírez, J.M. Macular Thickness Decrease in Asymptomatic Subjects at High Genetic Risk of Developing Alzheimer’s Disease: An OCT Study. J. Clin. Med. 2020, 9, 1728. [Google Scholar] [CrossRef]
- Alonso, C.; Satta, V.; Díez-Gutiérrez, P.; Fernández-Ruiz, J.; Sagredo, O. Preclinical Investigation of β-Caryophyllene as a Therapeutic Agent in an Experimental Murine Model of Dravet Syndrome. Neuropharmacology 2022, 205, 108914. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.A.; Perez, Z.D.; Foresti, M.L.; Arisi, G.M.; Ribak, C.E. Morphological and Ultrastructural Features of Iba1-Immunolabeled Microglial Cells in the Hippocampal Dentate Gyrus. Brain Res. 2009, 1266, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP Induces Upregulation of GFAP and MHC-II and Microglia Reactivity in Mice Retina Contralateral to Experimental Glaucoma. J. Neuroinflammation 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Jiménez-López, M.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; de la Villa, P.; Vidal-Sanz, M. Functional and Morphological Effects of Laser-Induced Ocular Hypertension in Retinas of Adult Albino Swiss Mice. Mol. Vis. 2009, 15, 2578–2598. [Google Scholar] [PubMed]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; de Hoz, R.; Matamoros, J.A.; Chen, L.; López-Cuenca, I.; Salobrar-García, E.; Sánchez-Puebla, L.; Ramírez, J.M.; Triviño, A.; Salazar, J.J.; et al. Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study. Biomedicines 2022, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- de Hoz, R.; Ramírez, A.I.; González-Martín, R.; Ajoy, D.; Rojas, B.; Salobrar-García, E.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Bilateral Early Activation of Retinal Microglial Cells in a Mouse Model of Unilateral Laser-Induced Experimental Ocular Hypertension. Exp. Eye Res. 2018, 171, 12–29. [Google Scholar] [CrossRef]
- de Gracia, P.; Gallego, B.I.; Rojas, B.; Ramírez, A.I.; de Hoz, R.; Salazar, J.J.; Triviño, A.; Ramírez, J.M. Automatic Counting of Microglial Cells in Healthy and Glaucomatous Mouse Retinas. PLoS ONE 2015, 10, e0143278. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of Glaucoma. Int. J.Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, J.J.; Satriano, A.; Matamoros, J.A.; Fernández-Albarral, J.A.; Salobrar-García, E.; López-Cuenca, I.; de Hoz, R.; Sánchez-Puebla, L.; Ramírez, J.M.; Alonso, C.; et al. Retinal Tissue Shows Glial Changes in a Dravet Syndrome Knock-in Mouse Model. Int. J. Mol. Sci. 2023, 24, 2727. https://doi.org/10.3390/ijms24032727
Salazar JJ, Satriano A, Matamoros JA, Fernández-Albarral JA, Salobrar-García E, López-Cuenca I, de Hoz R, Sánchez-Puebla L, Ramírez JM, Alonso C, et al. Retinal Tissue Shows Glial Changes in a Dravet Syndrome Knock-in Mouse Model. International Journal of Molecular Sciences. 2023; 24(3):2727. https://doi.org/10.3390/ijms24032727
Chicago/Turabian StyleSalazar, Juan J., Andrea Satriano, José A. Matamoros, José A. Fernández-Albarral, Elena Salobrar-García, Inés López-Cuenca, Rosa de Hoz, Lidia Sánchez-Puebla, José M. Ramírez, Cristina Alonso, and et al. 2023. "Retinal Tissue Shows Glial Changes in a Dravet Syndrome Knock-in Mouse Model" International Journal of Molecular Sciences 24, no. 3: 2727. https://doi.org/10.3390/ijms24032727
APA StyleSalazar, J. J., Satriano, A., Matamoros, J. A., Fernández-Albarral, J. A., Salobrar-García, E., López-Cuenca, I., de Hoz, R., Sánchez-Puebla, L., Ramírez, J. M., Alonso, C., Satta, V., Hernández-Fisac, I., Sagredo, O., & Ramírez, A. I. (2023). Retinal Tissue Shows Glial Changes in a Dravet Syndrome Knock-in Mouse Model. International Journal of Molecular Sciences, 24(3), 2727. https://doi.org/10.3390/ijms24032727












