Epigenetic Regulation of Corneal Epithelial Differentiation by TET2
Abstract
:1. Introduction
2. Results
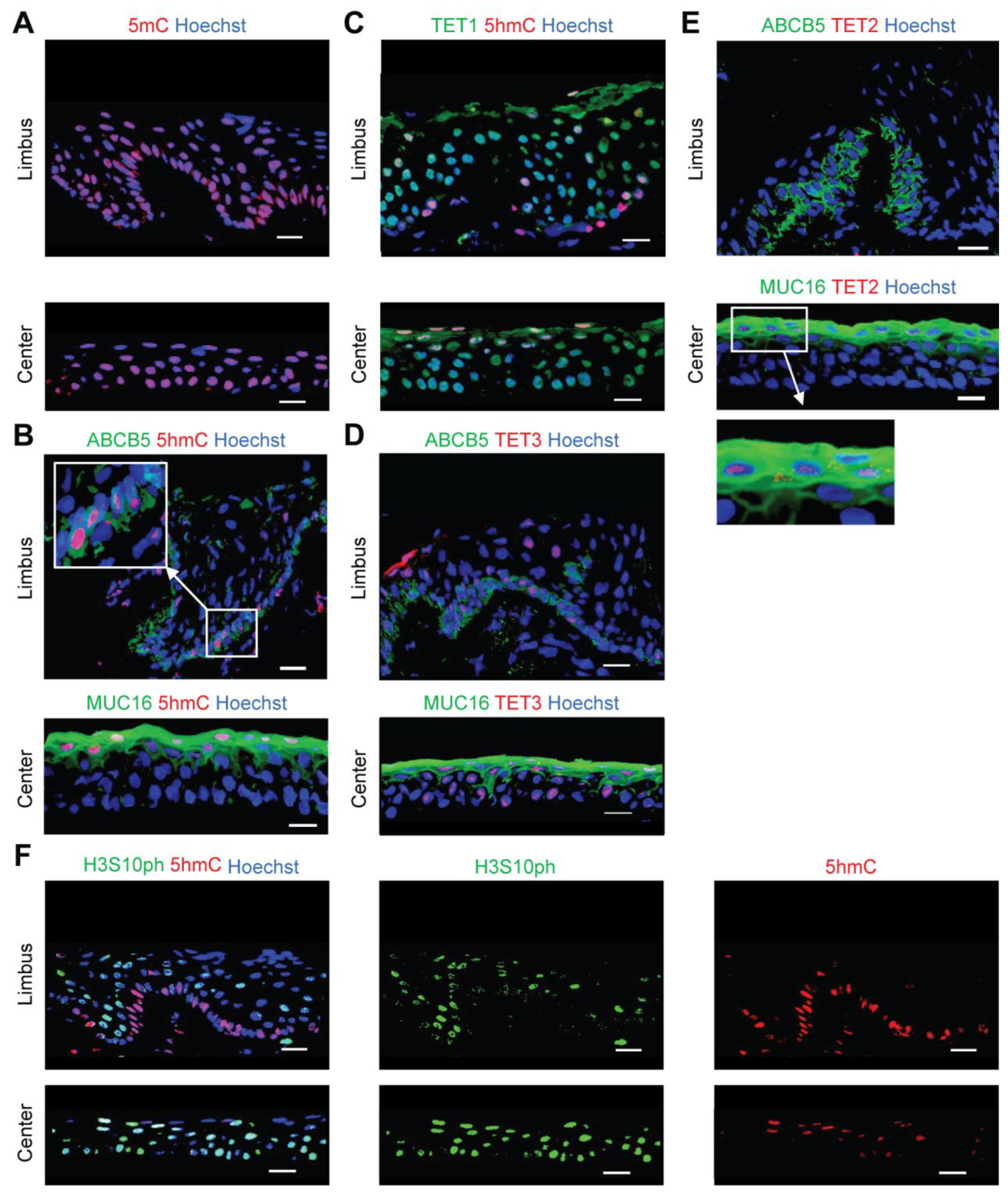
2.1. 5hmC Marks Quiescent Cells in the Limbus
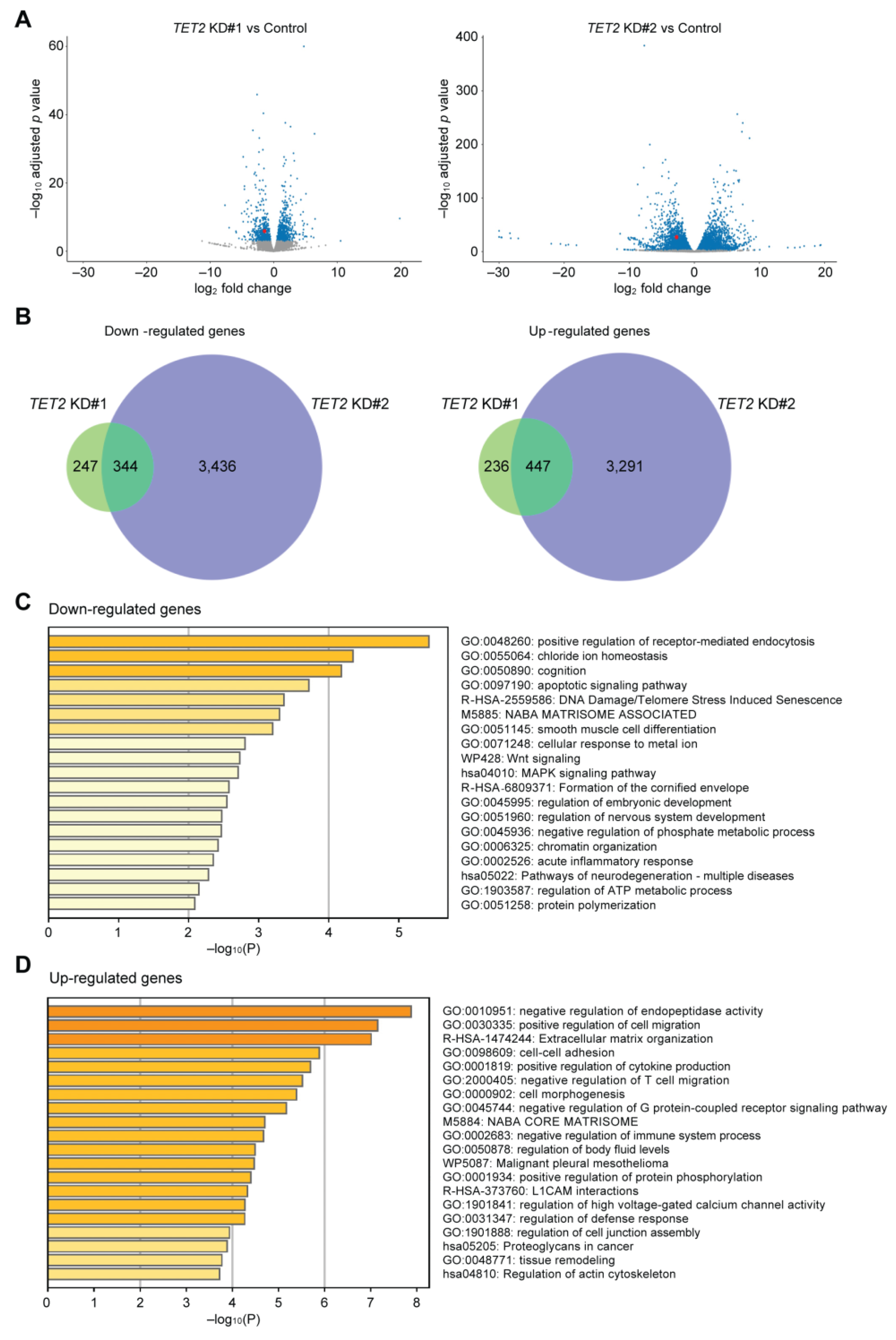
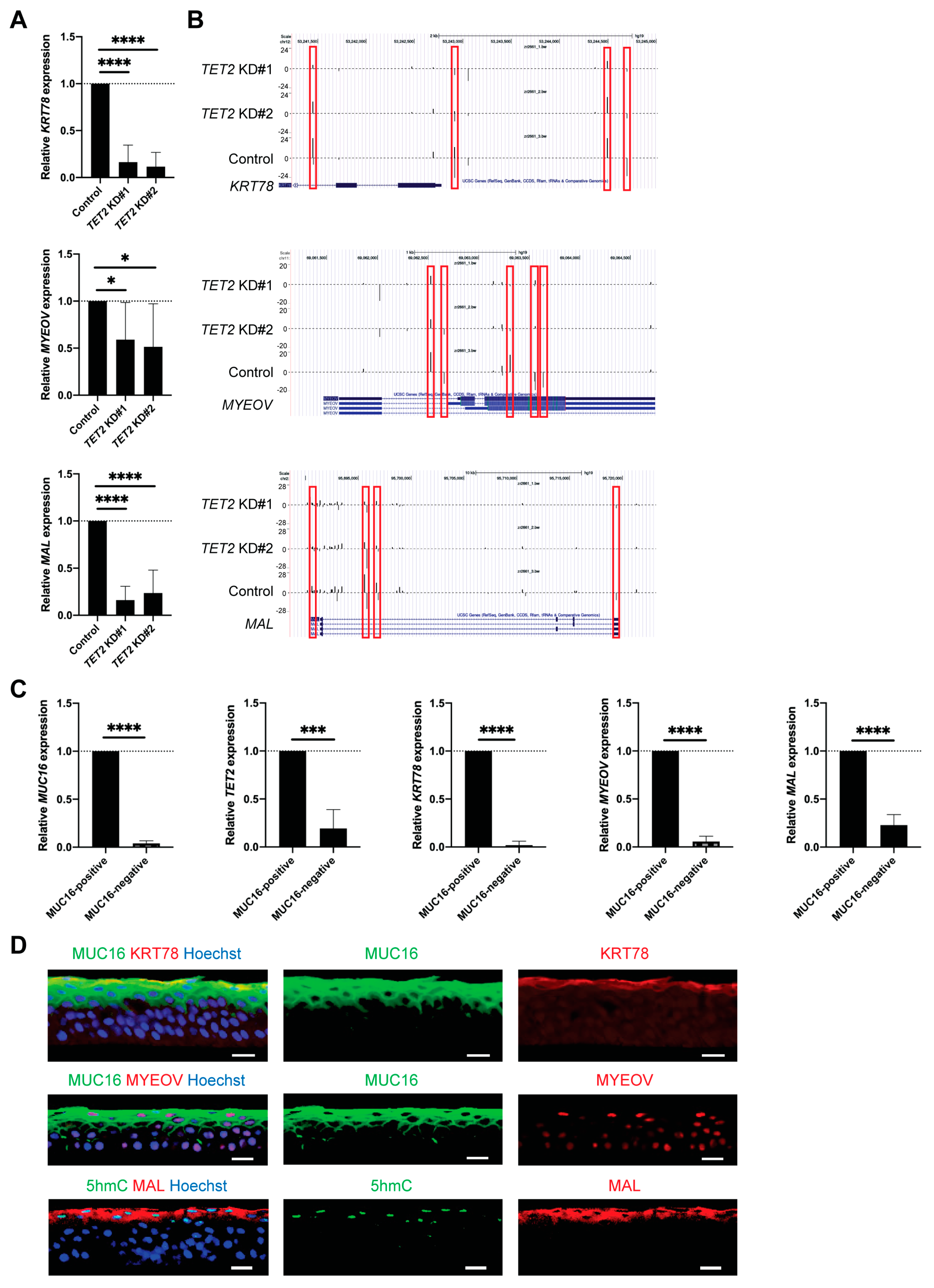
2.2. TET2 Regulates the Expression of Corneal Differentiation Genes
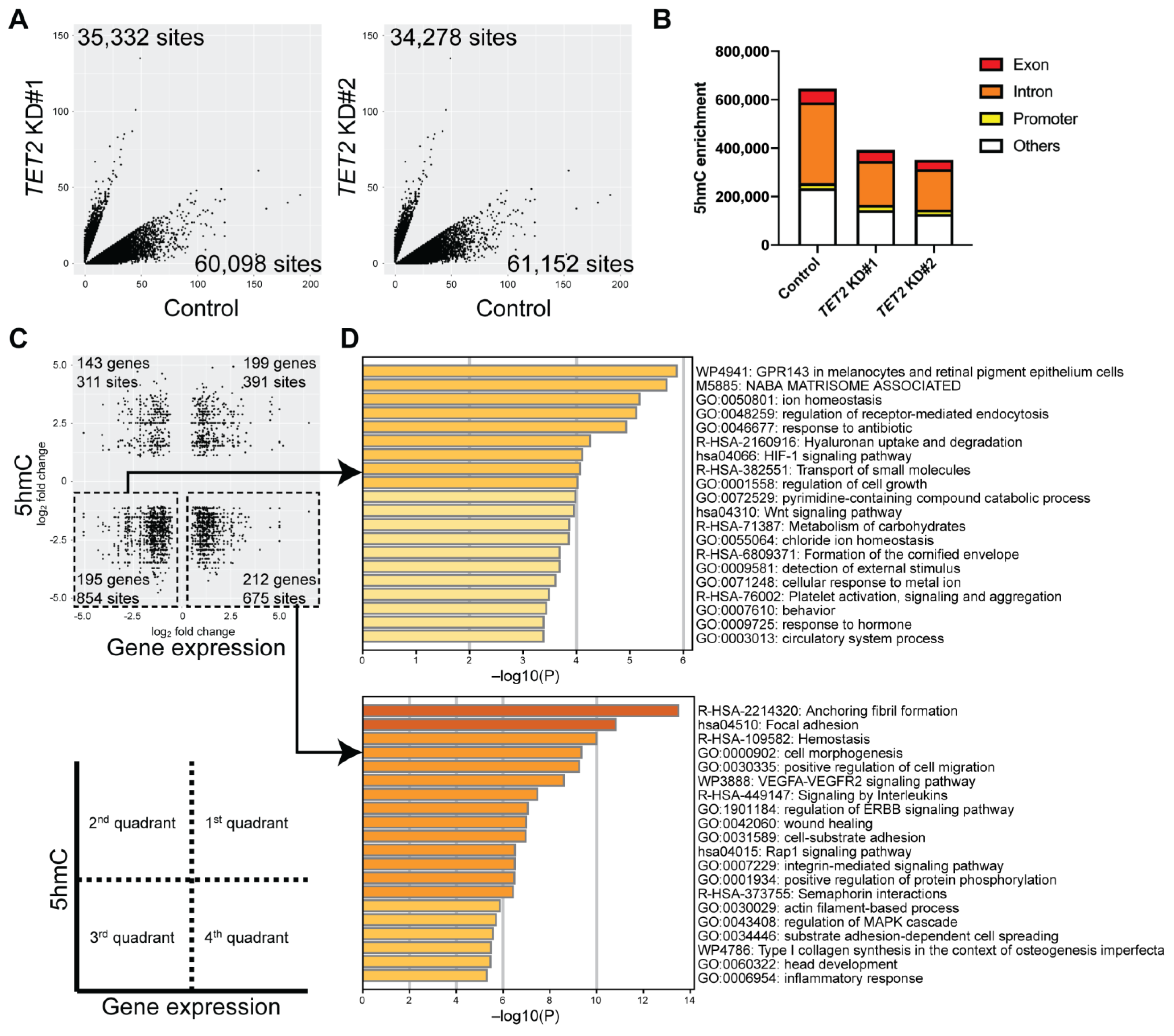
2.3. Identification of Genes Regulated by TET2 through 5hmC Modification
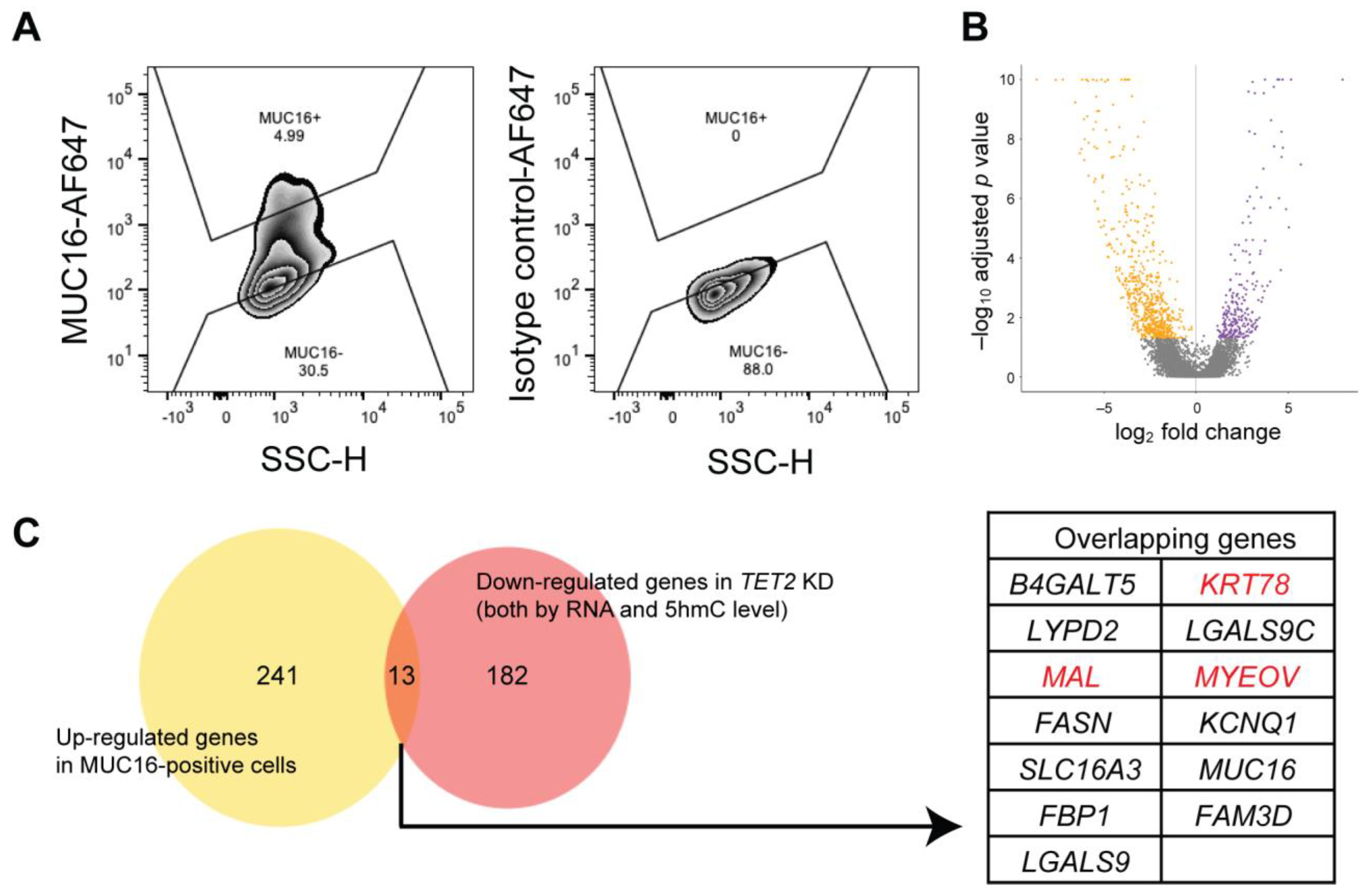
2.4. Identification of Novel Corneal Differentiation Genes Epigenetically Regulated by TET2
3. Discussion
4. Methods
4.1. Human Tissues
4.2. Tissue Processing
4.3. Immunostaining
4.4. RNA Interference
4.5. Western Blot
4.6. RNA-Seq
4.7. Genome-Wide 5hmC Analysis
4.8. Quantitative Reverse Transcription PCR (qRT-PCR)
4.9. Flow Cytometric Analyses
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Gonzalez, G.; Sasamoto, Y.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Limbal stem cells: Identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e303. [Google Scholar] [CrossRef] [PubMed]
- Jongkhajornpong, P.; Nakamura, T.; Sotozono, C.; Nagata, M.; Inatomi, T.; Kinoshita, S. Elevated expression of ABCB5 in ocular surface squamous neoplasia. Sci. Rep. 2016, 6, 20541. [Google Scholar] [CrossRef]
- Kureshi, A.K.; Dziasko, M.; Funderburgh, J.L.; Daniels, J.T. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci. Rep. 2015, 5, 16186. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.J.; Ismail, S.; McGhee, J.J.; McGhee, C.N.; Sherwin, T. Sphere-forming cells from peripheral cornea demonstrate the ability to repopulate the ocular surface. Stem. Cell Res. Ther. 2016, 7, 81. [Google Scholar] [CrossRef]
- Norrick, A.; Esterlechner, J.; Niebergall-Roth, E.; Dehio, U.; Sadeghi, S.; Schröder, H.M.; Ballikaya, S.; Stemler, N.; Ganss, C.; Dieter, K.; et al. Process development and safety evaluation of ABCB5(+) limbal stem cells as advanced-therapy medicinal product to treat limbal stem cell deficiency. Stem. Cell Res. Ther. 2021, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.J.; Kavianpour, B.; Wu, K.L.; Xie, Y.; Brown, D.J.; Jester, J.V. Immunofluorescence Tomography of Mouse Ocular Surface Epithelial Stem Cells and Their Niche Microenvironment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7338–7344. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Ahmad, S.; Md Latar, N.; Ali, S.; Meeson, A. A Human Corneal Epithelial Cell Line Model for Limbal Stem Cell Biology and Limbal Immunobiology. Stem. Cells Transl. Med. 2017, 6, 761–766. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Osei-Bempong, C.; Ahmad, S.; Rooney, P.; Ali, S.; Oldershaw, R.; Meeson, A. Human limbal mesenchymal stem cells express ABCB5 and can grow on amniotic membrane. Regen. Med. 2016, 11, 273–286. [Google Scholar] [CrossRef]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, Y.; Lee, C.A.; Wilson, B.J.; Buerger, F.; Martin, G.; Mishra, A.; Kiritoshi, S.; Tran, J.; Gonzalez, G.; Hildebrandt, F.; et al. Limbal BCAM expression identifies a proliferative progenitor population capable of holoclone formation and corneal differentiation. Cell Rep. 2022, 40, 111166. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K.; Argueso, P. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar]
- Chen, E.; Bohm, K.; Rosenblatt, M.; Kang, K. Epigenetic regulation of anterior segment diseases and potential therapeutics. Ocul. Surf. 2020, 18, 383–395. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, C.; Zhang, X.; Fan, Y.; Zeng, H.; Liu, J.; Meng, H.; Bai, D.; Peng, J.; Zhang, Q.; et al. Tissue-specific 5-hydroxymethylcytosine landscape of the human genome. Nat. Commun. 2021, 12, 4249. [Google Scholar] [CrossRef]
- Luo, G.; Jing, X.; Yang, S.; Peng, D.; Dong, J.; Li, L.; Reinach, P.S.; Yan, D. DNA Methylation Regulates Corneal Epithelial Wound Healing by Targeting miR-200a and CDKN2B. Investig. Ophthalmol. Vis. Sci. 2019, 60, 650–660. [Google Scholar] [CrossRef]
- Khuc, E.; Bainer, R.; Wolf, M.; Clay, S.M.; Weisenberger, D.J.; Kemmer, J.; Weaver, V.M.; Hwang, D.G.; Chan, M.F. Comprehensive characterization of DNA methylation changes in Fuchs endothelial corneal dystrophy. PLoS ONE 2017, 12, e0175112. [Google Scholar] [CrossRef]
- Shen, L.; Wu, H.; Diep, D.; Yamaguchi, S.; D’Alessio, A.C.; Fung, H.L.; Zhang, K.; Zhang, Y. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell 2013, 153, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Bachman, M.; Uribe-Lewis, S.; Yang, X.; Williams, M.; Murrell, A.; Balasubramanian, S. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat. Chem. 2014, 6, 1049–1055. [Google Scholar] [CrossRef]
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef]
- Shi, D.Q.; Ali, I.; Tang, J.; Yang, W.C. New Insights into 5hmC DNA Modification: Generation, Distribution and Function. Front. Genet. 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; An, J.; Bandukwala, H.S.; Chavez, L.; Aijo, T.; Pastor, W.A.; Segal, M.F.; Li, H.; Koh, K.P.; Lähdesmäki, H.; et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 2013, 497, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, S.-I.; Kishida, S.; Michiue, T.; Fukui, A.; Sakamoto, I.; Takada, S.; Asashima, M.; Kikuchi, A. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol. Cell Biol. 2001, 21, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Seritrakul, P.; Gross, J.M. Tet-mediated DNA hydroxymethylation regulates retinal neurogenesis by modulating cell-extrinsic signaling pathways. PLoS Genet. 2017, 13, e1006987. [Google Scholar] [CrossRef]
- Prigent, C.; Dimitrov, S. Phosphorylation of serine 10 in histone H3, what for? J. Cell Sci. 2003, 116, 3677–3685. [Google Scholar] [CrossRef]
- Sun, T.-T.; Tseng, S.C.G.; Huang, A.J.-W.; Cooper, D.; Schermer, A.; Kavanaugh-Lynch, M.; Weiss, R.; Eichner, R. Monoclonal antibody studies of mammalian epithelial keratins: A review. Ann. N. Y. Acad Sci. 1985, 455, 307–329. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Bojic, S.; Dorgau, B.; Moyse, N.; Molina, M.M.; Yang, C.; Dey, S.; Reynolds, G.; et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul. Surf. 2021, 21, 279–298. [Google Scholar] [CrossRef]
- Shimmura-Tomita, M.; Wang, M.; Taniguchi, H.; Akiba, H.; Yagita, H.; Hori, J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS ONE 2013, 8, e63620. [Google Scholar] [CrossRef]
- Kitayama, K.; Hayashida, Y.; Nishida, K.; Akama, T.O. Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J. Biol. Chem. 2007, 282, 30085–30096. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Pastor, W.A.; Pape, U.J.; Huang, Y.; Henderson, H.R.; Lister, R.; Ko, M.; McLoughlin, E.M.; Brudno, Y.; Mahapatra, S.; Kapranov, P.; et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature 2011, 473, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Moran-Crusio, K.; Reavie, L.; Shih, A.; Abdel-Wahab, O.; Ndiaye-Lobry, D.; Lobry, C.; Figueroa, M.E.; Vasanthakumar, A.; Patel, J.; Zhao, X.; et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 2011, 20, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haffner, M.C.; Chaux, A.; Meeker, A.K.; Esopi, D.M.; Gerber, J.; Pellakuru, L.G.; Toubaji, A.; Argani, P.; Iacobuzio-Donahue, C.; Nelson, W.G.; et al. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2011, 2, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Qiu, R.; Wu, X.; Li, A.X.; Zhang, H.; Wang, J.; Jui, J.; Jin, S.-G.; Jiang, Y.; Pfeifer, G.P.; et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013, 3, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Argueso, P.; Spurr-Michaud, S.; Russo, C.L.; Tisdale, A.; Gipson, I.K. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Lin, C.-T.; Ko, P.-S.; Yeh, P.-T.; Kuan, Y.-H.; Hu, F.-R.; Yang, C.-M. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology 2009, 116, 1038–1047. [Google Scholar] [CrossRef]
- Saini, J.S.; Khandalavla, B. Corneal epithelial fragility in diabetes mellitus. Can. J. Ophthalmol. 1995, 30, 142–146. [Google Scholar] [PubMed]
- Hodges, R.R.; Dartt, D.A. Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 2013, 117, 62–78. [Google Scholar] [CrossRef]
- Mantelli, F.; Argueso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef]
- Miyashita, H.; Yokoo, S.; Yoshida, S.; Kawakita, T.; Yamagami, S.; Tsubota, K.; Shimmura, S. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem. Cells Transl. Med. 2013, 2, 758–765. [Google Scholar] [CrossRef]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.B.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Hayashi, R.; Hara, S.; Sasamoto, Y.; Harrington, J.; Tsujikawa, M.; Nishida, K. KLF4 prevents epithelial to mesenchymal transition in human corneal epithelial cells via endogenous TGF-beta2 suppression. Regen. Ther. 2019, 11, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Petterson, A.; Chung, T.H.; Tan, D.; Sun, X.; Jia, X.Y. RRHP: A tag-based approach for 5-hydroxymethylcytosine mapping at single-site resolution. Genome Biol. 2014, 15, 456. [Google Scholar] [CrossRef]

| Downregulated genes with reduced 5hmC peaks in Exon regions in TET2 KD cells | |||||||||
| MYEOV | BEST1 | COLCA1 | CREBBP | KRT34 | FIBCD1 | TNNI2 | PLPP2 | TLCD1 | PLCB3 |
| TMC5 | TIMM44 | SLC4A2 | FAM107A | CARD10 | PDXP | MIB2 | TET3 | SNX8 | TNFSF14 |
| TEAD4 | NFATC4 | KRT23 | FASN | CFD | SEMA3B | LSP1 | ATAD3C | CCDC88B | CAPS |
| COX4I2 | MED24 | ATP2A1 | TNK2 | ENO1 | WNK2 | MIDN | IFI27 | TXNRD2 | EMILIN2 |
| CAPN12 | PLEKHB1 | DOCK6 | MUC4 | UPP1 | P4HTM | SERPINF1 | PTPRH | MSI1 | NDUFA4L2 |
| ZNF768 | ATG16L2 | MAL | B4GALT5 | SPTBN2 | KLF16 | SLC9A1 | LEFTY1 | MB | C15orf39 |
| KLK13 | EPHB6 | WTIP | TMEM205 | DOT1L | QRICH2 | ISYNA1 | CHD1 | HGD | RASGRP2 |
| HES6 | TNFSF13 | MKNK2 | REC8 | MYLPF | FBXL19 | REEP6 | RNF223 | MLPH | CCK |
| TBC1D1 | LTC4S | SIGIRR | ZNF652 | FUT4 | PALM | H19 | ARRB2 | MSI2 | TF |
| SLC7A1 | ABCA7 | ||||||||
| Downregulated genes with reduced 5hmC peaks in Intron regions in TET2 KD cells | |||||||||
| ATP6V1B1 | AGAP1 | INF2 | PALM | B4GALT5 | NCMAP | ADIPOR2 | MLPH | PPARGC1B | WNK2 |
| KCNQ1 | PPP1R9B | CARD10 | DOT1L | SLC6A17 | BFSP2-AS1 | ABCA7 | REEP6 | SSBP3 | SMDT1 |
| CORO2A | SIGIRR | TEAD4 | NTF3 | MSI2 | CCDC57 | TNK2 | SLC4A2 | BGN | PRKCB |
| LYPD5 | MUC4 | SNX8 | FAM20A | LMTK3 | TXNRD2 | TJP3 | CSK | PHLPP1 | JDP2 |
| TMC5 | EMILIN2 | PKN1 | ATAD3C | ASPHD2 | WTIP | LSP1 | SLC29A2 | EMP1 | PRIM1 |
| ZNF652 | TIMP2 | MIDN | SEC1P | TET3 | HYAL1 | LYPD2 | SH2D4A | BICDL1 | SGF29 |
| FAM3D-AS1 | MKNK2 | SERPINF1 | PLPP2 | LEFTY1 | PLCB3 | SLC16A3 | CAPN12 | PTPRH | MAL |
| GMPR | SPTBN2 | ENO1 | SLC9A3R2 | FAM107B | SLC12A5 | HEXB | R3HDM2 | SLC9A1 | SLC25A37 |
| ATG16L2 | NDUFA4L2 | VWF | ECI1 | CREBBP | KRT23 | TNFSF14 | MUC16 | MIB2 | WNT3A |
| EPHA8 | SNHG17 | KRT8 | FASN | S100P | CAPNS1 | SH3GL3 | ATP2A1 | MED24 | MROH6 |
| FBP1 | RAB26 | KRT78 | UPP1 | APOL6 | IFI27 | TLCD1 | NQO2 | PTPRD | BEST1 |
| SIX1 | CYBA | MOCOS | RNF223 | EEF1A2 | DGUOK-AS1 | TBC1D1 | SLC6A8 | FAM120A | ARRB2 |
| MRPL12 | CRMP1 | FIBCD1 | FBXL19 | P4HTM | TMCO4 | TIMM44 | PPP2CB | CRIP1 | VEGFA |
| TMEM205 | MCRIP2 | MT1M | TMEM164 | RASGRP2 | MSI1 | C15orf39 | MAP4K1 | KLK13 | N4BP3 |
| KLF16 | VKORC1 | LGALS9C | LEMD1 | ALMS1 | TF | SLC7A1 | RASA4B | FAM3D | NXPH4 |
| MYEOV | TET2 | LRRC56 | DCTPP1 | LGALS9 | ABCA4 | WNT6 | SGMS1-AS1 | PDXP | DUSP5 |
| H19 | IZUMO4 | ||||||||
| Downregulated genes with reduced 5hmC peaks in Promoter regions in TET2 KD cells | |||||||||
| INF2 | HGD | PRR34-AS1 | B4GALT5 | EIF4EBP3 | CYSRT1 | LSP1 | KRT78 | SERPINF1 | LYPD2 |
| KRT8 | RAB26 | TLCD1 | NQO2 | ARRB2 | FAM107A | SPTBN2 | PTPRH | CSK | NAT14 |
| TJP3 | SLC4A2 | LEFTY1 | PCYT2 | MLPH | TMCO4 | LGALS9C | TMEM205 | CAPS | UPP1 |
| IFI27 | MAL | LRRC56 | MYEOV | CCDC88B | JDP2 | UBXN10 | S100P | SGMS1-AS1 | N4BP3 |
| GMPR | CARD10 | RHBDL1 | IZUMO4 | SMDT1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasamoto, Y.; Wu, S.; Lee, C.A.A.; Jiang, J.Y.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Epigenetic Regulation of Corneal Epithelial Differentiation by TET2. Int. J. Mol. Sci. 2023, 24, 2841. https://doi.org/10.3390/ijms24032841
Sasamoto Y, Wu S, Lee CAA, Jiang JY, Ksander BR, Frank MH, Frank NY. Epigenetic Regulation of Corneal Epithelial Differentiation by TET2. International Journal of Molecular Sciences. 2023; 24(3):2841. https://doi.org/10.3390/ijms24032841
Chicago/Turabian StyleSasamoto, Yuzuru, Siyuan Wu, Catherine A. A. Lee, Jason Y. Jiang, Bruce R. Ksander, Markus H. Frank, and Natasha Y. Frank. 2023. "Epigenetic Regulation of Corneal Epithelial Differentiation by TET2" International Journal of Molecular Sciences 24, no. 3: 2841. https://doi.org/10.3390/ijms24032841
APA StyleSasamoto, Y., Wu, S., Lee, C. A. A., Jiang, J. Y., Ksander, B. R., Frank, M. H., & Frank, N. Y. (2023). Epigenetic Regulation of Corneal Epithelial Differentiation by TET2. International Journal of Molecular Sciences, 24(3), 2841. https://doi.org/10.3390/ijms24032841






