Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms
Abstract
:1. Introduction
2. Quercetin and Breast Cancer (BC)
2.1. Quercetin Free Form in BC Experimental Models
2.2. Effects of Combination of First-Line Treatments with Quercetin in BC Experimental Models
2.3. Delivery Systems for Quercetin in BC Experimental Models
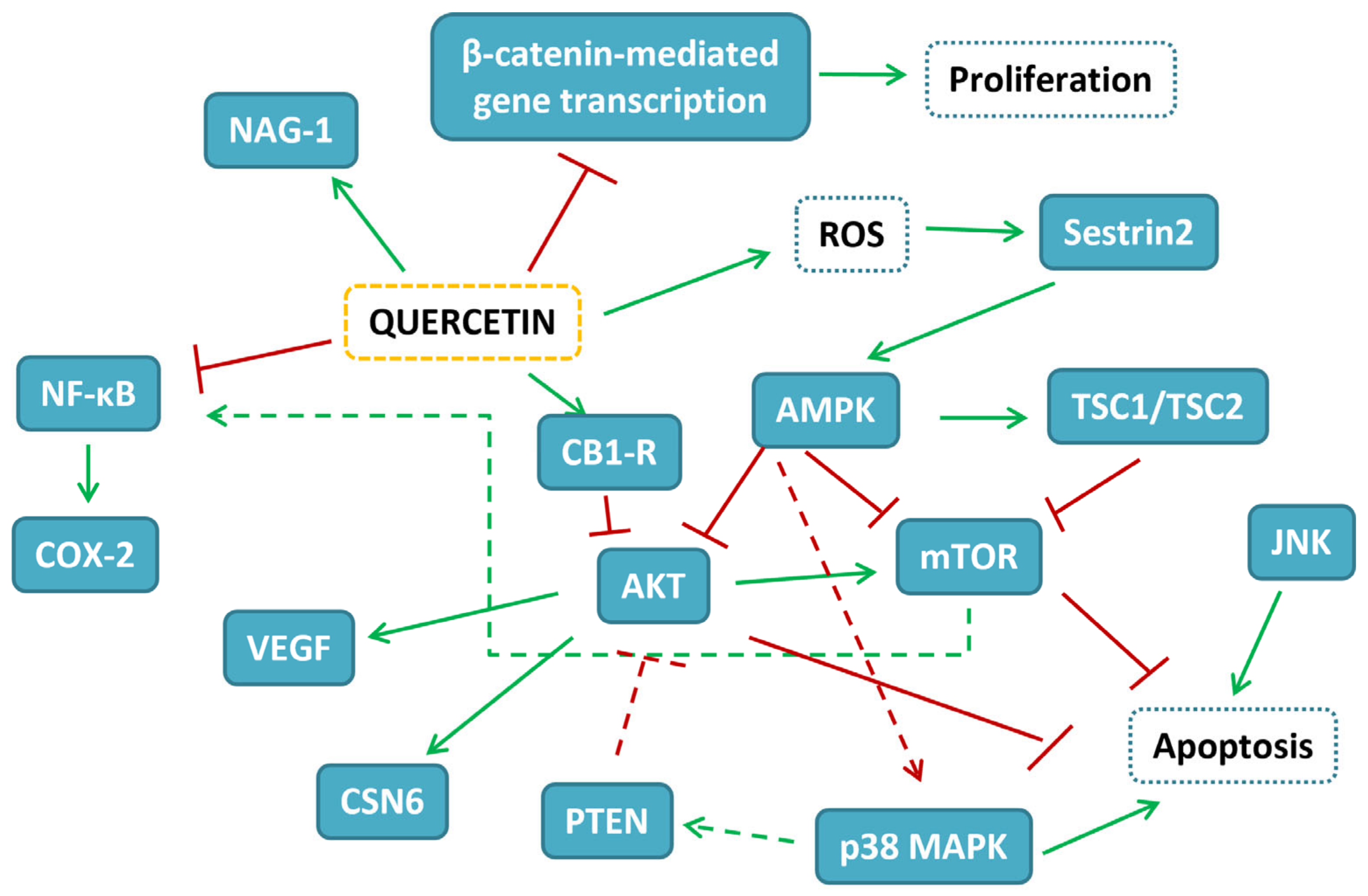
3. Quercetin and Colorectal Cancer (CRC)
3.1. Quercetin Free Form in CRC Experimental Models
3.2. Effects of Combination of First-Line Treatments with Quercetin in CRC Experimental Models
3.3. Delivery Systems for Quercetin in CRC Experimental Models
4. Quercetin and Hepatocellular Cancer (HCC)
4.1. Quercetin Free Form in HCC Experimental Models
4.2. Effects of Combination of First-Line Treatments with Quercetin in HCC Experimental Models
4.3. Delivery Systems for Quercetin in HCC Experimental Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Li, A. Plant Natural Products: Promising Resources for Cancer Chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-Geranyloxy-7-methoxycoumarin Cooperate in the Cytotoxic Effect of Citrus bergamia (Bergamot) Essential Oil in Human Neuroblastoma SH-SY5Y Cell Line. Toxins 2021, 13, 275. [Google Scholar] [CrossRef]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein inhibits STAT3, activates the apoptotic machinery, and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment with a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharmacol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Clementino, M.; Shi, X.; Zhang, Z. Prevention of Polyphenols Against Carcinogenesis Induced by Environmental Carcinogens. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 87–98. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Micali, A.; Marini, H.R.; Freni, J.; Santoro, G.; Puzzolo, D.; Squadrito, F.; Pallio, G.; Navarra, M.; Cirmi, S.; et al. A Flavonoid-Rich Extract from Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, Shows Protective Effects in a Murine Model of Cadmium-Induced Testicular Injury. Pharmaceuticals 2021, 14, 386. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective Effect of Bergamot Juice in 6-OHDA-Induced SH-SY5Y Cell Death, an In Vitro Model of Parkinson’s Disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Lombardo, G.E.; Russo, C.; Musumeci, L.; Gangemi, S.; Calapai, G.; Barreca, D.; Navarra, M. A Flavonoid-Rich Extract of Mandarin Juice Counteracts 6-OHDA-Induced Oxidative Stress in SH-SY5Y Cells and Modulates Parkinson-Related Genes. Antioxidants 2021, 10, 539. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid Fraction of Citrus reticulata Juice Reduces Proliferation and Migration of Anaplastic Thyroid Carcinoma Cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef]
- Seba, V.; Silva, G.; dos Santos, M.B.; Baek, S.J.; De Castro França, S.; Fachin, A.L.; Regasini, L.O.; Marins, M. Chalcone Derivatives 4′-Amino-1-Naphthyl-Chalcone (D14) and 4′-Amino-4-Methyl-1-Naphthyl-Chalcone (D15) Suppress Migration and Invasion of Osteosarcoma Cells Mediated by p53 Regulating EMT-Related Genes. Int. J. Mol. Sci. 2018, 19, 2838. [Google Scholar] [CrossRef] [Green Version]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular Targets of Genistein and Its Related Flavonoids to Exert Anticancer Effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; De Luca, L.; Gitto, R.; Lombardo, G.E.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. The SIRT2 Pathway Is Involved in the Antiproliferative Effect of Flavanones in Human Leukemia Monocytic THP-1 Cells. Biomedicines 2022, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin content in some food and herbal samples. Food Chem. 2007, 100, 699–704. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C. T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of Citrus bergamia juice: A cell-free, in silico, and in vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.A.; Velasco, J.A.; Cansado, J.; Notario, V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994, 54, 2424–2428. [Google Scholar]
- Singhal, R.L.; Yeh, Y.A.; Praja, N.; Olah, E.; Sledge, G.W., Jr.; Weber, G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem. Biophys. Res. Commun. 1995, 208, 425–431. [Google Scholar] [CrossRef]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Miodini, P.; Fioravanti, L.; Di Fronzo, G.; Cappelletti, V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. Cancer 1999, 80, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Kim, J.Y.; Lee, J.Y.; Kang, C.M.; Kwon, H.J.; Yoo, Y.D.; Kim, T.W.; Lee, Y.S.; Lee, S.J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, M.; Yu, L.; Zhao, Y.; He, N.; Yang, X. Antitumor activities of quercetin and quercetin-5′,8-disulfonate in human colon and breast cancer cell lines. Food Chem. Toxicol. 2012, 50, 1589–1599. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Lee, W.S.; Park, O.J. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 2009, 41, 201–207. [Google Scholar] [CrossRef]
- Van der Woude, H.; Gliszczynska-Swiglo, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, H.; Ter Veld, M.G.; Jacobs, N.; van der Saag, P.T.; Murk, A.J.; Rietjens, I.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Vivacqua, A.; Fasanella, G.; Recchia, A.G.; Sisci, D.; Pezzi, V.; Montanaro, D.; Musti, A.M.; Picard, D.; Ando, S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004, 279, 27008–27016. [Google Scholar] [CrossRef]
- Waite, K.A.; Sinden, M.R.; Eng, C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005, 14, 1457–1463. [Google Scholar] [CrossRef]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.; Jhanwar-Uniyal, M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006, 26, 1177–1181. [Google Scholar]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- He, C.L.; Bian, Y.Y.; Xue, Y.; Liu, Z.X.; Zhou, K.Q.; Yao, C.F.; Lin, Y.; Zou, H.F.; Luo, F.X.; Qu, Y.Y.; et al. Pyruvate Kinase M2 Activates mTORC1 by Phosphorylating AKT1S1. Sci. Rep. 2016, 6, 21524. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef] [Green Version]
- Cardaci, S.; Filomeni, G.; Ciriolo, M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012, 125, 2115–2125. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Sultan, A.S.; Khalil, M.I.; Sami, B.M.; Alkhuriji, A.F.; Sadek, O. Quercetin induces apoptosis in triple-negative breast cancer cells via inhibiting fatty acid synthase and β-catenin. Int. J. Clin. Exp. Pathol. 2017, 10, 156–172. [Google Scholar]
- Lenzi, M.; Cocchi, V.; Novakovic, A.; Karaman, M.; Sakac, M.; Mandic, A.; Pojic, M.; Barbalace, M.C.; Angeloni, C.; Hrelia, P.; et al. Meripilus giganteus ethanolic extract exhibits pro-apoptotic and anti-proliferative effects in leukemic cell lines. BMC Complement. Altern. Med. 2018, 18, 300. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, O.J. Regulation of mutual inhibitory activities between AMPK and Akt with quercetin in MCF-7 breast cancer cells. Oncol. Rep. 2010, 24, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.K.; Oesterreich, S.; Lemieux, P.; Sarge, K.D.; Fuqua, S.A. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem. Biophys. Res. Commun. 1997, 239, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Kiyga, E.; Sengelen, A.; Adiguzel, Z.; Ucar, E.O. Investigation of the role of quercetin as a heat shock protein inhibitor on apoptosis in human breast cancer cells. Mol. Biol. Rep. 2020, 47, 4957–4967. [Google Scholar] [CrossRef]
- Caldas-Lopes, E.; Cerchietti, L.; Ahn, J.H.; Clement, C.C.; Robles, A.I.; Rodina, A.; Moulick, K.; Taldone, T.; Gozman, A.; Guo, Y.; et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl. Acad. Sci. USA 2009, 106, 8368–8373. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef] [Green Version]
- Devipriya, S.; Vani, G.; Ramamurthy, N.; Shyamaladevi, C.S. Regulation of intracellular calcium levels and urokinase activity in MDA MB 231 cells by quercetin. Chemotherapy 2006, 52, 60–65. [Google Scholar] [CrossRef]
- Umar, S.M.; Kashyap, A.; Kahol, S.; Mathur, S.R.; Gogia, A.; Deo, S.V.S.; Prasad, C.P. Prognostic and therapeutic relevance of phosphofructokinase platelet-type (PFKP) in breast cancer. Exp. Cell Res. 2020, 396, 112282. [Google Scholar] [CrossRef]
- Lee, J.H.; Liu, R.; Li, J.; Zhang, C.; Wang, Y.; Cai, Q.; Qian, X.; Xia, Y.; Zheng, Y.; Piao, Y.; et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Shimomura, I.; Kogure, A.; Usuba, W.; Takahashi, R.U.; Ochiya, T.; Yamamoto, Y. Quercetin Inhibits Lef1 and Resensitizes Docetaxel-Resistant Breast Cancer Cells. Molecules 2020, 25, 2576. [Google Scholar] [CrossRef]
- Safi, A.; Heidarian, E.; Ahmadi, R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell. Med. 2021, 10, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, J.I.; Ahn, T.G. Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice. Obstet. Gynecol. Sci. 2019, 62, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Roshanazadeh, M.; Rezaei, H.B.; Rashidi, M. Quercetin synergistically potentiates the anti-metastatic effect of 5-fluorouracil on the MDA-MB-231 breast cancer cell line. Iran. J. Basic Med. Sci. 2021, 24, 928–934. [Google Scholar] [CrossRef]
- Mawalizadeh, F.; Mohammadzadeh, G.; Khedri, A.; Rashidi, M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol. Biol. Rep. 2021, 48, 7733–7742. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Turner, K.A.; Kalafatis, M. TRAIL-Induced Apoptosis in TRAIL-Resistant Breast Carcinoma Through Quercetin Cotreatment. Breast Cancer 2018, 12, 1178223417749855. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Wang, B.; Yuan, S.; Wang, X.; Li, K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 2018, 32, 1530–1536. [Google Scholar] [CrossRef]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. Combination Therapy of Doxorubicin and Quercetin on Multidrug-Resistant Breast Cancer and Their Sequential Delivery by Reduction-Sensitive Hyaluronic Acid-Based Conjugate/d-alpha-Tocopheryl Poly(ethylene glycol) 1000 Succinate Mixed Micelles. Mol. Pharm. 2020, 17, 1415–1427. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Song, H.; Canup, B.S.B.; Gou, S.; She, Z.; Dai, F.; Ke, B.; Xiao, B. Inhibition of growth and lung metastasis of breast cancer by tumor-homing triple-bioresponsive nanotherapeutics. J. Control. Release 2020, 328, 454–469. [Google Scholar] [CrossRef]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008, 68, 7210–7218. [Google Scholar] [CrossRef]
- Mansourizadeh, F.; Alberti, D.; Bitonto, V.; Tripepi, M.; Sepehri, H.; Khoee, S.; Crich, S.G. Efficient synergistic combination effect of Quercetin with Curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Colloids Surf. B Biointerfaces 2020, 191, 110982. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, Y.; Liang, X.; Wusiman, Z.; Yin, Y.; Shen, Q. Synergistic inhibition of migration and invasion of breast cancer cells by dual docetaxel/quercetin-loaded nanoparticles via Akt/MMP-9 pathway. Int. J. Pharm. 2017, 523, 300–309. [Google Scholar] [CrossRef]
- Sun, Z.J.; Chen, G.; Hu, X.; Zhang, W.; Liu, Y.; Zhu, L.X.; Zhou, Q.; Zhao, Y.F. Activation of PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the apoptosis-evasion in human salivary adenoid cystic carcinoma: Its inhibition by quercetin. Apoptosis 2010, 15, 850–863. [Google Scholar] [CrossRef]
- Cheng, J.C.; Chou, C.H.; Kuo, M.L.; Hsieh, C.Y. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 2006, 25, 7009–7018. [Google Scholar] [CrossRef] [Green Version]
- Boehm, J.S.; Zhao, J.J.; Yao, J.; Kim, S.Y.; Firestein, R.; Dunn, I.F.; Sjostrom, S.K.; Garraway, L.A.; Weremowicz, S.; Richardson, A.L.; et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 2007, 129, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Bhat, F.A.; Singh, P.R.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Agullo, G.; Gamet-Payrastre, L.; Fernandez, Y.; Anciaux, N.; Demigne, C.; Remesy, C. Comparative effects of flavonoids on the growth, viability and metabolism of a colonic adenocarcinoma cell line (HT29 cells). Cancer Lett. 1996, 105, 61–70. [Google Scholar] [CrossRef]
- Shan, B.E.; Wang, M.X.; Li, R.Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Investig. 2009, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Van Erk, M.J.; Roepman, P.; van der Lende, T.R.; Stierum, R.H.; Aarts, J.M.; van Bladeren, P.J.; van Ommen, B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur. J. Nutr. 2005, 44, 143–156. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shen, S.C.; Chow, J.M.; Ko, C.H.; Tseng, S.W. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int. J. Oncol. 2004, 25, 661–670. [Google Scholar] [CrossRef]
- Piazzi, M.; Bavelloni, A.; Gallo, A.; Faenza, I.; Blalock, W.L. Signal Transduction in Ribosome Biogenesis: A Recipe to Avoid Disaster. Int. J. Mol. Sci. 2019, 20, 2718. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.K.; Kim, B.S.; Lee, S.H.; Park, Y.S.; Park, B.K.; Kim, S.J.; Kim, J.; Choi, C.; Kim, J.S.; et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef]
- Narayansingh, R.; Hurta, R.A. Cranberry extract and quercetin modulate the expression of cyclooxygenase-2 (COX-2) and IκBα in human colon cancer cells. J. Sci. Food Agric. 2009, 89, 542–547. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzatsos, A.; Tsichlis, P.N. Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J. Biol. Chem. 2007, 282, 18069–18082. [Google Scholar] [CrossRef] [PubMed]
- Niero, E.L.; Rocha-Sales, B.; Lauand, C.; Cortez, B.A.; de Souza, M.M.; Rezende-Teixeira, P.; Urabayashi, M.S.; Martens, A.A.; Neves, J.H.; Machado-Santelli, G.M. The multiple facets of drug resistance: One history, different approaches. J. Exp. Clin. Cancer Res. 2014, 33, 37. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, Y.M. Quercetin Regulates Sestrin 2-AMPK-mTOR Signaling Pathway and Induces Apoptosis via Increased Intracellular ROS in HCT116 Colon Cancer Cells. J. Cancer Prev. 2013, 18, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef]
- Luo, H.; Yang, Y.; Duan, J.; Wu, P.; Jiang, Q.; Xu, C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013, 4, e481. [Google Scholar] [CrossRef]
- Ho, K.K.; McGuire, V.A.; Koo, C.Y.; Muir, K.W.; de Olano, N.; Maifoshie, E.; Kelly, D.J.; McGovern, U.B.; Monteiro, L.J.; Gomes, A.R.; et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J. Biol. Chem. 2012, 287, 1545–1555. [Google Scholar] [CrossRef]
- Bulzomi, P.; Galluzzo, P.; Bolli, A.; Leone, S.; Acconcia, F.; Marino, M. The pro-apoptotic effect of quercetin in cancer cell lines requires ERbeta-dependent signals. J. Cell. Physiol. 2012, 227, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Wannatung, T.; Lee, S.; Yang, W.K.; Chung, S.H.; Lim, J.S.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis 2012, 17, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Bang, M.H.; Kim, E.S.; Kang, N.E.; Jung, K.C.; Cho, H.J.; Park, J.H. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 2005, 16, 155–162. [Google Scholar] [CrossRef]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.K.; Yang, C.H. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Tafaro, A.; Bianco, G.; Gigante, I.; Scavo, M.P.; D’Alessandro, R.; Refolo, M.G.; Messa, C.; Caruso, M.G.; et al. Cannabinoid Receptor-1 Up-regulation in Azoxymethane (AOM)-treated Mice After Dietary Treatment with Quercetin. Anticancer. Res. 2018, 38, 4485–4491. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.W.; Min, D.S.; Chang, J.S.; Lee, Y.H.; Park, Y.B.; Choi, K.S.; Kwon, T.K. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis 2007, 12, 411–421. [Google Scholar] [CrossRef]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000, 21, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, Y.; Zhang, X. Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells Through Regulating Toll-like Receptor 4/Nuclear Factor-kappa B Pathway. Pharmacogn. Mag. 2016, 12, S237–S244. [Google Scholar] [CrossRef]
- Feng, J.; Song, D.; Jiang, S.; Yang, X.; Ding, T.; Zhang, H.; Luo, J.; Liao, J.; Yin, Q. Quercetin restrains TGF-beta1-induced epithelial-mesenchymal transition by inhibiting Twist1 and regulating E-cadherin expression. Biochem. Biophys. Res. Commun. 2018, 498, 132–138. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Sun, Q.; Yang, Z.; Liu, M.; Tang, H. DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-kappaB pathway in colorectal cancer. Int. J. Cancer 2018, 142, 2068–2079. [Google Scholar] [CrossRef] [Green Version]
- Xavier, C.P.; Lima, C.F.; Preto, A.; Seruca, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett. 2009, 281, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, T.; Chen, D.; Ma, Q.; Zheng, Y.; Liao, S.; Wang, Y.; Zhang, J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 2019, 43, 117–124. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Psahoulia, F.H.; Moumtzi, S.; Roberts, M.L.; Sasazuki, T.; Shirasawa, S.; Pintzas, A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis 2007, 28, 1021–1031. [Google Scholar] [CrossRef]
- Pang, B.; Xu, X.; Lu, Y.; Jin, H.; Yang, R.; Jiang, C.; Shao, D.; Liu, Y.; Shi, J. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019, 10, 5339–5349. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Xu, P.; Yang, B. Integrated Whole Transcriptome Profiling and Bioinformatics Analysis for Revealing Regulatory Pathways Associated With Quercetin-Induced Apoptosis in HCT-116 Cells. Front. Pharmacol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Erdogan, M.K.; Agca, C.A.; Askin, H. Quercetin and Luteolin Improve the Anticancer Effects of 5-Fluorouracil in Human Colorectal Adenocarcinoma In Vitro Model: A Mechanistic Insight. Nutr. Cancer 2022, 74, 660–676. [Google Scholar] [CrossRef]
- Lin, C.; Yu, Y.; Zhao, H.G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Han, H.; Ma, L.; Zhou, H.; Zhao, C.-X. The chemosensitization effect of quercetin on cisplatin induces the apoptosis of human colon cancer HT-29 cell line. Int. J. Clin. Exp. Med. 2016, 9, 2285–2292. [Google Scholar]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015, 18, 635–643. [Google Scholar] [PubMed]
- Muller, P.A.; Vousden, K.H. p53 mutations in cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, L.; Li, X.; Song, W.; Liu, Y.; Huang, L. Nanoformulated Codelivery of Quercetin and Alantolactone Promotes an Antitumor Response through Synergistic Immunogenic Cell Death for Microsatellite-Stable Colorectal Cancer. ACS Nano 2019, 13, 12511–12524. [Google Scholar] [CrossRef]
- Rashedi, J.; Haghjo, A.G.; Abbasi, M.M.; Tabrizi, A.D.; Yaqoubi, S.; Sanajou, D.; Ashrafi-Jigheh, Z.; Namvaran, A.; Mohammadi, A.; Khoshraj, J.M.; et al. Anti-tumor Effect of Quercetin Loaded Chitosan Nanoparticles on Induced Colon Cancer in Wistar Rats. Adv. Pharm. Bull. 2019, 9, 409–415. [Google Scholar] [CrossRef]
- Shao, M.; Chang, C.; Liu, Z.; Chen, K.; Zhou, Y.; Zheng, G.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Polydopamine coated hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for overcoming multidrug resistance. Colloids Surf. B Biointerfaces 2019, 183, 110427. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Hu, T.G.; Li, L.; Wu, H. Preparation and Characterization of Electrospun Colon-Specific Delivery System for Quercetin and Its Antiproliferative Effect on Cancer Cells. J. Agric. Food Chem. 2018, 66, 11550–11559. [Google Scholar] [CrossRef]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro. Anticancer. Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Qiu, H.; Zhu, L.; Ren, Z.; Zhao, W.; Zhang, T.; Liu, L. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food Funct. 2015, 6, 1518–1525. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kwon, S.; Ban, K.; Hong, Y.K.; Ahn, C.; Sung, J.S.; Choi, I. Regulation of the Intracellular ROS Level Is Critical for the Antiproliferative Effect of Quercetin in the Hepatocellular Carcinoma Cell Line HepG2. Nutr. Cancer 2019, 71, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Gautam, P.K.; Tomar, M.S.; Verma, P.K.; Singh, S.P.; Kumar, S.; Acharya, A. Protein kinase C-alpha and the regulation of diverse cell responses. Biomol. Concepts 2017, 8, 143–153. [Google Scholar] [CrossRef]
- Ogawara, Y.; Kishishita, S.; Obata, T.; Isazawa, Y.; Suzuki, T.; Tanaka, K.; Masuyama, N.; Gotoh, Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 2002, 277, 21843–21850. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates NF-kappa B and AP-1/JNK pathways to induce cell death in human hepatoma cells. Nutr. Cancer 2010, 62, 390–401. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019, 8, 4806–4820. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Ma, Y.X.; Li, W.T.; Li, L.; Zhu, H.Z.; Wu, M.H.; Zhou, J.R. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J. Nutr. Biochem. 2019, 69, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Matsushima-Nishiwaki, R.; Kozawa, O. Quercetin suppresses the migration of hepatocellular carcinoma cells stimulated by hepatocyte growth factor or transforming growth factor-alpha: Attenuation of AKT signaling pathway. Arch. Biochem. Biophys. 2020, 682, 108296. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-mTOR Pathway. Molecules 2019, 24, 1993. [Google Scholar] [CrossRef]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Jin, R.; Wang, W.; Zhang, T.; Sang, J.; Li, N.; Han, Q.; Zhao, W.; Li, C.; Liu, Z. STAT3 regulates glycolysis via targeting hexokinase 2 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 24777–24784. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, Y.M.; Handoussa, H.; Youness, R.A.; Assal, R.A.; El-Khatib, A.H.; Linscheid, M.W.; El Tayebi, H.M.; Abdelaziz, A.I. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018, 32, 2217–2220. [Google Scholar] [CrossRef]
- Lee, R.H.; Cho, J.H.; Jeon, Y.J.; Bang, W.; Cho, J.J.; Choi, N.J.; Seo, K.S.; Shim, J.H.; Chae, J.I. Quercetin Induces Antiproliferative Activity Against Human Hepatocellular Carcinoma (HepG2) Cells by Suppressing Specificity Protein 1 (Sp1). Drug Dev. Res. 2015, 76, 9–16. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Wang, B.; Yu, B.; Ge, J.; Shi, X. Quercetin suppresses the chymotrypsin-like activity of proteasome via inhibition of MEK1/ERK1/2 signaling pathway in hepatocellular carcinoma HepG2 cells. Can. J. Physiol. Pharmacol. 2018, 96, 521–526. [Google Scholar] [CrossRef]
- Pi, J.; Li, B.; Tu, L.; Zhu, H.; Jin, H.; Yang, F.; Bai, H.; Cai, H.; Cai, J. Investigation of quercetin-induced HepG2 cell apoptosis-associated cellular biophysical alterations by atomic force microscopy. Scanning 2016, 38, 100–112. [Google Scholar] [CrossRef]
- Salama, Y.A.; El-Karef, A.; El Gayyar, A.M.; Abdel-Rahman, N. Beyond its antioxidant properties: Quercetin targets multiple signalling pathways in hepatocellular carcinoma in rats. Life Sci. 2019, 236, 116933. [Google Scholar] [CrossRef]
- Bahman, A.A.; Abaza, M.S.I.; Khoushiash, S.I.; Al-Attiyah, R.J. Sequencedependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action. Int. J. Mol. Med. 2018, 42, 1695–1715. [Google Scholar] [CrossRef]
- Yu, C.-P.; Qiu, R.-G.; Shi, L.; Liang, J. Celecoxib and quercetin induce apoptosis in human hepatocarcinoma. Biomed. Res. 2017, 28, 3465–3470. [Google Scholar]
- Dai, W.; Gao, Q.; Qiu, J.; Yuan, J.; Wu, G.; Shen, G. Quercetin induces apoptosis and enhances 5-FU therapeutic efficacy in hepatocellular carcinoma. Tumour Biol. 2016, 37, 6307–6313. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhao, J.; Jiao, H.J. Synergistic growth-suppressive effects of quercetin and cisplatin on HepG2 human hepatocellular carcinoma cells. Appl. Biochem. Biotechnol. 2014, 172, 784–791. [Google Scholar] [CrossRef]
- Sharma, A.; Bhat, M.K. Enhancement of carboplatin- and quercetin-induced cell death by roscovitine is Akt dependent and p53 independent in hepatoma cells. Integr. Cancer Ther. 2011, 10, NP4–NP14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Xu, W.; Han, J.; Liu, Q.Y.; Gao, L.F.; Wang, X.H.; Li, X.L. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer Drugs 2020, 31, 684–692. [Google Scholar] [CrossRef]
- Herrmann, D.; Conway, J.R.; Vennin, C.; Magenau, A.; Hughes, W.E.; Morton, J.P.; Timpson, P. Three-dimensional cancer models mimic cell-matrix interactions in the tumour microenvironment. Carcinogenesis 2014, 35, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Peluso, J.; Chalhoub, S.; Gillet, Y.I.; Benkirane-Jessel, N.; Rochel, N.; Fuhrmann, G.; Ubeaud-Sequier, G. Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1alpha and MDR1. PLoS ONE 2020, 15, e0240676. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zheng, Y.F.; Ge, W.; Wang, S.B.; Mou, X.Z. Synergistic Anti-tumour Effects of Quercetin and Oncolytic Adenovirus expressing TRAIL in Human Hepatocellular Carcinoma. Sci. Rep. 2018, 8, 2182. [Google Scholar] [CrossRef]
- Igbe, I.; Shen, X.F.; Jiao, W.; Qiang, Z.; Deng, T.; Li, S.; Liu, W.L.; Liu, H.W.; Zhang, G.L.; Wang, F. Dietary quercetin potentiates the antiproliferative effect of interferon-alpha in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase. Oncotarget 2017, 8, 113734–113748. [Google Scholar] [CrossRef]
- Srisa-Nga, K.; Mankhetkorn, S.; Okonogi, S.; Khonkarn, R. Delivery of Superparamagnetic Polymeric Micelles Loaded With Quercetin to Hepatocellular Carcinoma Cells. J. Pharm. Sci. 2019, 108, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabou, A.A.; Ahmed, H.H. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv. Med. Sci. 2017, 62, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Jafarian, A.; Salehi, G.; Zolfaghari, B. Comparing different sterol containing solid lipid nanoparticles for targeted delivery of quercetin in hepatocellular carcinoma. J. Liposome Res. 2014, 24, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, L.; Wu, C.; Wu, J.; Zhu, C.; Yuan, G. RGD peptide targeted lipid-coated nanoparticles for combinatorial delivery of sorafenib and quercetin against hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2016, 42, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Li, Y.H.; Wu, G.; Ren, J.Z.; Lu, H.B.; Li, Z.M.; Han, X.W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef] [Green Version]


| Cell [or Animal] Model | Concentration | Effect | Reference |
|---|---|---|---|
| MDA-MB-468 | 23–55 µM | Arrest of cells in G2/M phase and growth inhibition | [36] |
| MCF-7 | 17.2 µM | Reduction in cell growth | [37] |
| MCF-7 | 4.9 µM | Counteractive effects on pro-proliferative effects of E2 and TNF- α | [38] |
| MCF-7 | 1–20 µM | Induction of apoptosis and arrest of cells in G2/M phase (p21 dependent) | [39] |
| MCF-7 | 48 µM | Increased ROS production | [40] |
| MCF-7 | 100 µM | Increased ROS production; induction of apoptosis; AMPK activation and decrease of COX-2 protein levels | [41] |
| MCF-7 | 100 µM | Reduction in proliferation | [42] |
| MCF7, T47D (ER+) MDA-MB-231, HCC-38 (ER−) | 0.1–60 µM | (ER+): pro-proliferative effects at lower concentrations; anti-proliferative effects at higher concentrations (ER−): anti-proliferative effects | [43] |
| MCF-7, MDA-MB-231 | 5–20-100 µM | Lower concentrations: pro-proliferative effects Higher concentrations: anti-proliferative effects | [44] |
| MCF-7, SK-Br-3 | 100 µM | Induction of c-fos; activation of MEKs and ERK1/2; EGFR and MAPK activation | [45] |
| MCF-7 | 0.1–1000 nM | Increase in PTEN protein level; decrease in phosphorylated AKT; increase in p27; arrest of cell cycle | [46] |
| HCC1937 (PTEN−/−), T47D (PTEN+/+) | 25 µM | Reduction in phosphorylated AKT level | [47] |
| MCF-7, MDA-MB-231 | 30 µM [50 mg/kg] | Decrease in cellular invasion and migration capacities; decrease in AKT/mTOR activity; autophagy induction; decrease in GLUT1, PKM2, LDHA; reduction in tumor volume | [48] |
| BCSC (CD44+ from MCF-7) [xenograft] | 50 µM | Reduction in cell viability and metastatic properties; reduction in tumor volume | [50] |
| HCC1806, HCC70, HCC1937, BT-549, BT-20, Hs578T, MDA-MB231, MDA-MB 157 and MDA-MB-468 BT-549 | 200 µM | Migration inhibition and decreased invasion abilities; decreased AKT phosphorylation; decrease in GSK3α/β and WNK-1; reduced phosphorylation of β-catenin, ERK1/2, JNK1/2/3, p38α; increase in CHK2 phosphorylation | [51] |
| MDA-MB-231, MDA-MB-435 | 15 µM | Increase in AMPK phosphorylation; decrease in AKT activity; growth inhibition and arrest of cell cycle in G2/M phase | [52] |
| MDAMB-231, MDA-MB-157 | 230, 415 µM | Reduced lipid synthesis; inhibition of FAS; reduction of cell viability and induction of apoptosis; decrease in FASN, β-catenin, Bcl-2 | [55] |
| MCF-7 | Increased level of phosphorylated AMPK; decreased level of phosphorylated AKT | [57] | |
| MDA-MB-231 | 20 µM | Decreased cell viability; increased apoptosis; cell cycle arrest; JNK and FOXO3a increase | [58] |
| MCF-7, MDA-MB-231 [xenograft] | Induction of apoptosis; negative regulation of EGFR; increase in miR-146a expression; reduction in tumor volume | [59] | |
| MCF-7, MDA-MB-231 | 100 µM | Reduction in HSP70 and HSP27, HSP90 protein levels | [60,61] |
| BCSC ALDH+, AS-B145, AS-B244 | 0–200 µM | Reduction in HSP27; decrease in mammosphere dimension, cell migration and EMT; decrease in nuclear translocation of NF-κB and proteasomal degradation of IκBα HSP27-mediated | [63] |
| MDA-MB-231 | 150 µM | Growth inhibition; decrease in intracellular calcium concentration; decrease in urokinase activity | [64] |
| TNBC MDA-MB-231 | 25 µM | Reduction in PFKP and LDHA protein levels; decrease in cellular invasiveness and migration | [65] |
| Cell [or Animal] Model | First-Line Agent | Effect of Combination | Reference |
|---|---|---|---|
| MCF7-DR | Docetaxel | Synergistic increase in cytotoxicity; decrease in Lef1 | [67] |
| MDA-MB-231 | Docetaxel | Synergistic induction of apoptosis; increase in p53 and BAX; decrease in pAKT, pERK1/2, pSTAT3 | [68] |
| EMT6 [xenograft] | Cisplatin | Greater cytotoxic effects; reduction in tumor volume | [69] |
| MDA-MB-231 | 5-fluorouracil | Greater decrease in cell viability and migration; decrease in MMP-2 and -9 expression | [70] |
| MCF-7 | 5-fluorouracil | Synergistic increase in apoptosis | [71] |
| MCF-7, BT-20 | rhTRAIL | Enhancement of apoptosis; induction of proteasomal degradation; reduction in c-FLIP and increase in DR5 | [72] |
| MCF-7/ADR | Paclitaxel | Downregulation of P-glycoprotein | [73] |
| MCF-7/ADR | Doxorubicin, paclitaxel, vincristine | Enhancement of cytotoxic effects; decrease in P-glycoprotein protein level and YB-1 nuclear translocation | [74] |
| Cell [or Animal] Model | Delivery System | Effect | Reference |
|---|---|---|---|
| MCF-7/ADR | Encapsulated quercetin and paclitaxel in MSNs-ChS@PQ | Augmented cytotoxicity and apoptosis | [75] |
| MDA-MB-231/MDR1 | Encapsulated quercetin and doxorubicin in mixed micelles of HA-based conjugate and d-α-tocopheryl poly-(ethylene glycol) 1000 succinate | More efficient induction of apoptosis | [76] |
| 4T1 [xenograft] | Encapsulated quercetin in nanoparticles of RSF coated with LyP-1-QU-NPs | Greater inhibition of cell viability; stronger apoptosis induction; reduced tumor volume | [77] |
| MCF-7 | Nanoparticles of apoferritin loaded with quercetin and curcumin | Increase in ROS production and apoptosis induction | [79] |
| MCF-7 | Solid lipid nanoparticles loaded with quercetin and curcumin | Increase in ROS production and apoptosis induction | [80] |
| 4T1 | Nanoparticles formed by PLGA, linked to PEI, and bound to HA of quercetin and docetaxel | Decrease in phosphorylated AKT and MMP-9 protein level; decrease in NF-κB activity | [81] |
| MCF-7, MDA-MB-231 | Quercetin-conjugated gold nanoparticles | Decreased EMT, migration and invasion abilities; strong inhibition of PI3K/AKT pathway; reduction in EGFR activity | [85] |
| Cell (or Animal) Model | Concentration | Effect | Reference |
|---|---|---|---|
| SW480/ mouse CRC clone 26 | 60/160 µM | Reduction in cell growth; cell cycle blockage | [87] |
| Caco-2 | 5–50 µM | Reduction in cell growth; downregulation of cell cycle-related factor mRNAs | [88] |
| HT-29 | 15 µM | Cell growth inhibition | [86] |
| HT-29, HCT-116 | 0–70 µM | Pro-proliferative effects | [42] |
| HT-29, COLO205, COLO205-X | 200 µM | Reduction in cell viability | [89] |
|
HT-29 [xenograft] | 100 µM [50–100 mg/kg] | Growth inhibition; chromatin condensation; cell cycle arrest in G1 phase; AMPK activation; induction of apoptosis | [91] |
| HT-29 | 1–100 µM | COX-2 protein level decrease; induction of apoptosis; increase in IκBα expression | [41,92] |
| Caco-2, SW620 | 35–20 µM | Growth inhibition; induction of caspase-dependent apoptosis; reduction in p65 and IκBα phosphorylated protein levels; increase in IκBα expression | [93] |
| HCT116, HT-29 | 25–50 µM | ROS production; apoptosis induction; increase in sestrin2 | [96,98] |
| DLD-1 | 1 µM | Induction of apoptosis by activation of ERβ1; increased p38MAPK phosphorylated and PTEN expression; PI3K/AKT/mTOR pathway activation | [101] |
|
HCT116 [xenograft] | 100 µM [50 mg/kg] | Apoptosis induction; inhibition of AMPK activity; reduction in tumor volume and AMPK phosphorylation | [102] |
| HT-29 | 81.65 µM | Induced strong cellular morphological changes and apoptosis; modulation of CSN6 activity | [103] |
| HT-29, SW480 | 100 µM | Induction of apoptosis; decrease in ErbB2 (HER2) and 3 (HER3); decrease in PI3K activation | [104] |
| Caco-2, DLD-1 | 50 µM | Up-regulation of CB1-R; inhibition of growth; inhibition of PI3K/AKT activity; JNK and c-Jun activation | [105,106] |
| [AOM/DSS-induced CRC] | [30 mg/kg] | Decrease in tumor size and volume | [107,108] |
| HCT-116 | 40 µM | Increase in NAG-1 expression; increase in SP1 and EGR-1 transcription factors | [109] |
| DLD-1 | 20 µM | TNF-alpha-stimulated COX-2 expression | [110] |
| Caco-2 | 5 µM | Inhibition of TLR-4 and NF-κB activity | [111] |
| SW480 | 100 µM | Inhibition of TGF-β1-induced EMT; increase in E-cadherin; decrease in vimentin and Twist1 | [112] |
| HCT-15, CO-115 | 20 µM | Apoptosis induction; inhibition of RAS and PI3K activity | [114] |
| SW480, HCT116, DLD-1KRASG13D | 100 µM | Induction of extrinsic and intrinsic apoptosis; inhibition of AKT and activation of JNK | [115] |
| Cell [or Animal] Model | First-Line Agent | Effect of Combination | Reference |
|---|---|---|---|
| HCT-15 | 5-fluorouracil | Enhancement of caspase-independent apoptosis | [120] |
| HT-29 | 5-fluorouracil | Increase in apoptosis induction | [121,126] |
| HT-29 | 5-fluorouracil | Increase in p53 expression; decrease in AKT and mTOR pathways | [122] |
| DLD-1 [Xenograft] | Ionizing radiation | Reduction in tumor volume | [123] |
| HT-29 | Cisplatin | Reduction in cell viability; induction of apoptosis and cell cycle blockage | [124] |
| CSC | Doxorubicin | Enhancement of cytotoxic activity and cell arrest in G2/M phase | [124,125] |
| Cell [or Animal] Model | Delivery System | Effect | Reference |
|---|---|---|---|
| CT26-FL3 [xenograft] | Encapsulation of quercetin and alantolactone in micelles of DSPEPEG2000 and TPGS | Greater cytotoxicity (IC50 drop from 148 to 8 µM); reduction in tumor volume; decrease in mTOR phosphorylation | [127] |
| [CRC rats] | Nanoparticles of quercetin cross-linked to chitosan | Reduction in tumor angiogenesis and mitosis rate; increase in apoptosis | [128] |
| HCT-8/TAX | Encapsulated doxorubicin and quercetin in hollow mesoporous silica nanoparticles, coated with polydopamine bound to mPEG-NH2 | Better uptake; decrease in P-glycoprotein protein level | [129] |
| Caco-2 | Encapsulated quercetin in chitosan nanoparticles coated with sodium alginate by coaxial electrospinning | Increase in growth inhibition; induction of apoptosis; arrest in G0/G1 phase | [130] |
| Cell (or Animal) Model | Concentration | Effect | Reference |
|---|---|---|---|
| KIM-1, KYN-1, -2, -3, HAK-1A, -1B, -2, -3, -4, -5, and -6 | 50–100 µM | Growth inhibition; apoptosis induction; cell cycle blockage | [131] |
| HepG2 | 40 µM | Apoptosis induction; increase in intracellular ROS dependent on upregulation of PIG3 expression | [132] |
| Huh-7, HepG2 | 80 µM | Decrease in ROS; decrease in PI3K p85α subunit phosphorylation, total PKC activity, PKCα and COX-2 protein level; increase in p53 | [133,134] |
| HepG2 | 50 µM | JNK activation; decreased ERK and AKT phosphorylation level; decreased nuclear translocation of NF-κB; increased nuclear translocation of AP-1 | [139] |
| LM3 (Xenograft) | 90 µM (100 mg/kg) | Growth inhibition; autophagy and apoptosis induction; decrease in invasiveness, EMT and migration; reduced JAK2 and STAT3 phosphorylation levels | [140] |
| SMMC-7721, HepG2 (Xenograft) | 40 µM (100 mg/kg) | Autophagy induction by inhibition of AKT/mTOR pathway and increase in MAPKs activities | [141] |
| Huh-7 | 7–30 µM (+HGF or +TNF-α) | Reduced cell migration, PI3K/AKT pathway; E-cadherin increase | [142] |
| Bel-7402, SMMC-7721 (Xenograft) | <50 µM (50 mg/kg) | Inhibition of cell viability, downregulation of HK2 | [143] |
| Huh-7 | 82.7 µM | Increased expression of miR-1275; degradation of IGF2BPs mRNA | [146] |
| HepG2 | 12.9 µM | Downregulation of Sp1 | [147] |
| HepG2 | 50 µM | Induction of apoptosis; influence of chymotrypsin-like proteasomal activity; ERK1/ERK2 decreased activity; decrease in proteasome β expression | [148] |
| HepG2 | 40 µM | Increase in aggregation of F-actin, cytoskeleton disruption and membrane perturbation | [149] |
| (HCC-induced mice) | (100 or 25 mg/mL, before or after HCC induction, respectively) | Normalization of hepatic enzymes; reduction in liver oxidative stress; decrease in CK2-α and Notch and Hedgehog pathways | [150] |
| Cell [or Animal] Model | First-Line Agent | Effect of Combination | Reference |
|---|---|---|---|
| HepG2, Hep3B | Sorafenib | Combination or pre-treatment with quercetin lowered sorafenib IC50 | [151] |
| HepG2, MDBK, Huh-7 | Celecoxib | Growth inhibition and apoptosis induction | [152] |
| SMCC-7721, HepG2 (Xenograft) | 5-fluorouracil | Decrease in cell growth by apoptosis induction; reduction in tumor volume | [153] |
| HepG2 | Cisplatin | Induction of growth inhibition and apoptosis | [154] |
| HepG2, Hep3B | Roscovitine | Induction of growth inhibition; decrease in AKT phosphorylation; pro-apoptotic protein levels increase | [155] |
| HepG2/GEM | Gemcitabine | Decrease in cell viability and higher apoptosis rate | [156] |
| HepG2 (3D) | Doxorubicin | Induction of apoptosis | [158] |
| SMMC-7721, HepG2, Huh-7 (Xenograft) | ZD55 adenovirus | Reduction in cell growth and tumor volume | [159] |
| HepG2, Huh-7 | Interferon-α | Increase in anti-proliferative effects; inhibition of SHP2 | [160] |
| Cell (or Animal) Model | Delivery System | Effect | Reference |
|---|---|---|---|
| HepG2.2.15 | Encapsulated quercetin and superparamagnetic iron oxide nanoparticles into micelles | Decrease in the concentration needed to arrest cell cycle | [161] |
| HepG2 | Encapsulated quercetin in nanoparticles formed by PLGA decorated with chitosan and PEG | Stronger reduction in cell viability compared to quercetin free form; induction of apoptosis | [162] |
| HepG2 | Encapsulated quercetin in solid-lipid nanoparticles of cholesterol | More efficient reduction in cell growth compared to free-form quercetin | [163] |
| HepG2 (Xenograft) | Encapsulated quercetin and sorafenib in modified lipid nanoparticles coated with RGD | Inhibition of cell growth; reduction in tumor volume | [164] |
| MHCC97H, Hep3B, HCCLM3 and Bel7402 | PLGA encapsulated gold quercetin nanoparticles | Increase in growth inhibition; reduction in both PI3K/AKT and MEK/ERK pathway activities; hTERT reduced level; inhibition of COX-2 expression; reduction in NF-κB nuclear translocation; downregulation of AP-2β signaling; reduced tumor volume | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. https://doi.org/10.3390/ijms24032952
Maugeri A, Calderaro A, Patanè GT, Navarra M, Barreca D, Cirmi S, Felice MR. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. International Journal of Molecular Sciences. 2023; 24(3):2952. https://doi.org/10.3390/ijms24032952
Chicago/Turabian StyleMaugeri, Alessandro, Antonella Calderaro, Giuseppe Tancredi Patanè, Michele Navarra, Davide Barreca, Santa Cirmi, and Maria Rosa Felice. 2023. "Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms" International Journal of Molecular Sciences 24, no. 3: 2952. https://doi.org/10.3390/ijms24032952
APA StyleMaugeri, A., Calderaro, A., Patanè, G. T., Navarra, M., Barreca, D., Cirmi, S., & Felice, M. R. (2023). Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. International Journal of Molecular Sciences, 24(3), 2952. https://doi.org/10.3390/ijms24032952










