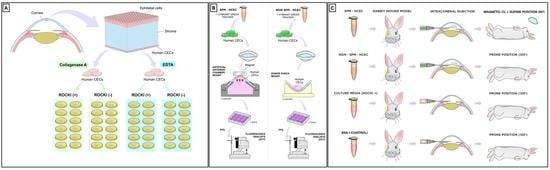
A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach
Abstract
1. Introduction
2. Results
2.1. HCEC Isolation and Expansion
2.2. Effect of Y27632 on Corneal Endothelial Cell Culture System
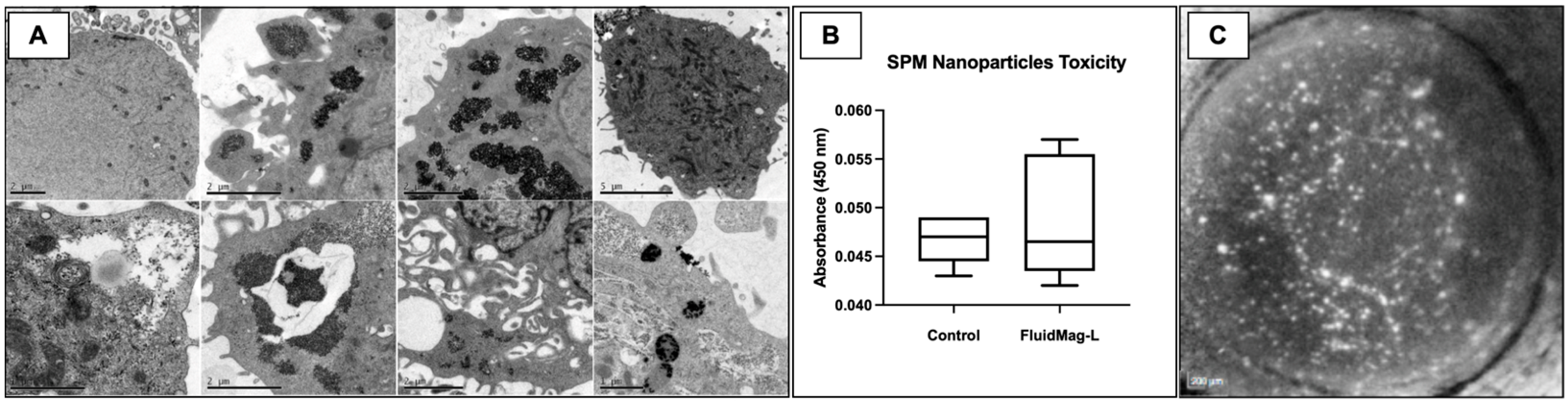
2.3. Cell Viability and Attachment after HCEC SPM Embedding
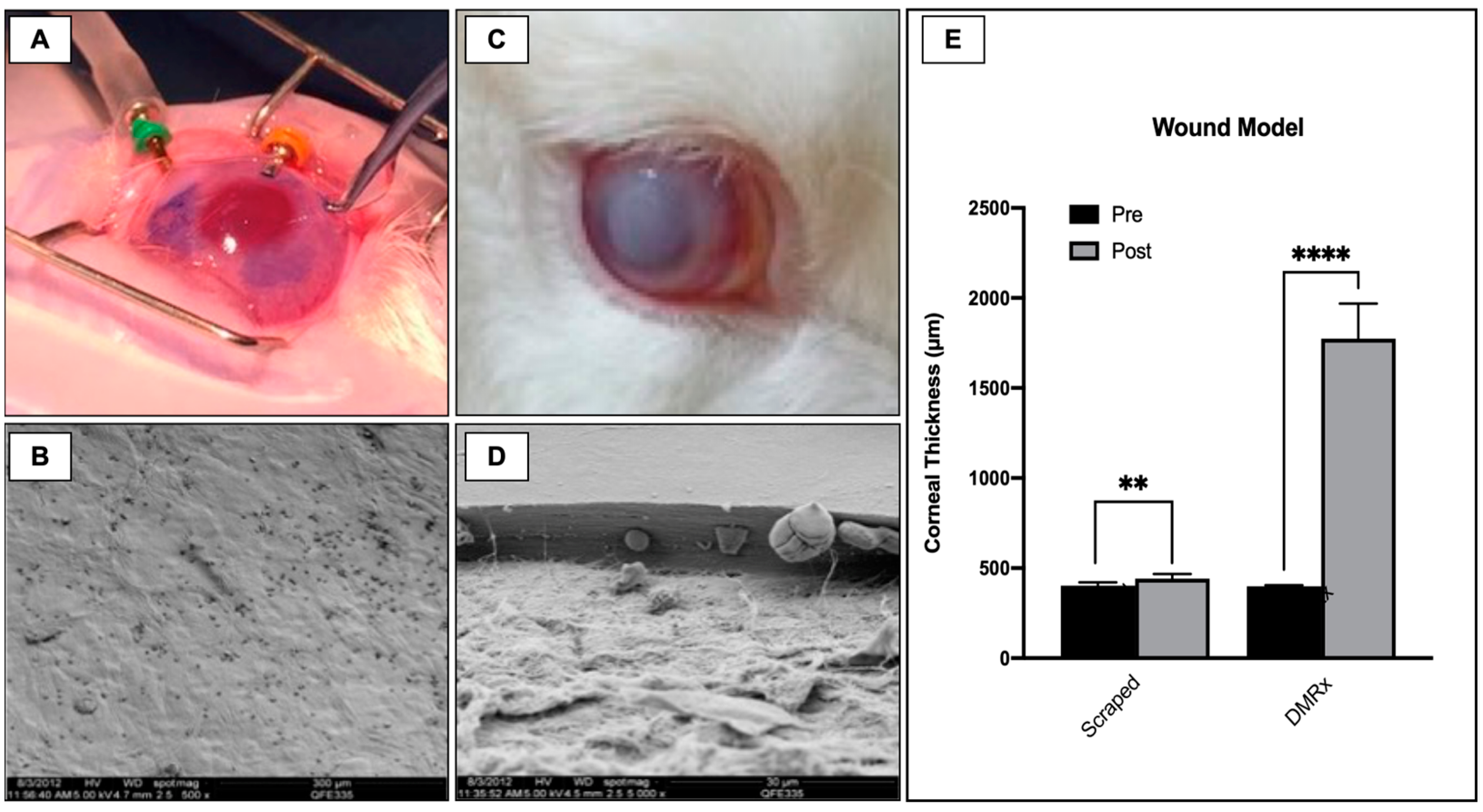
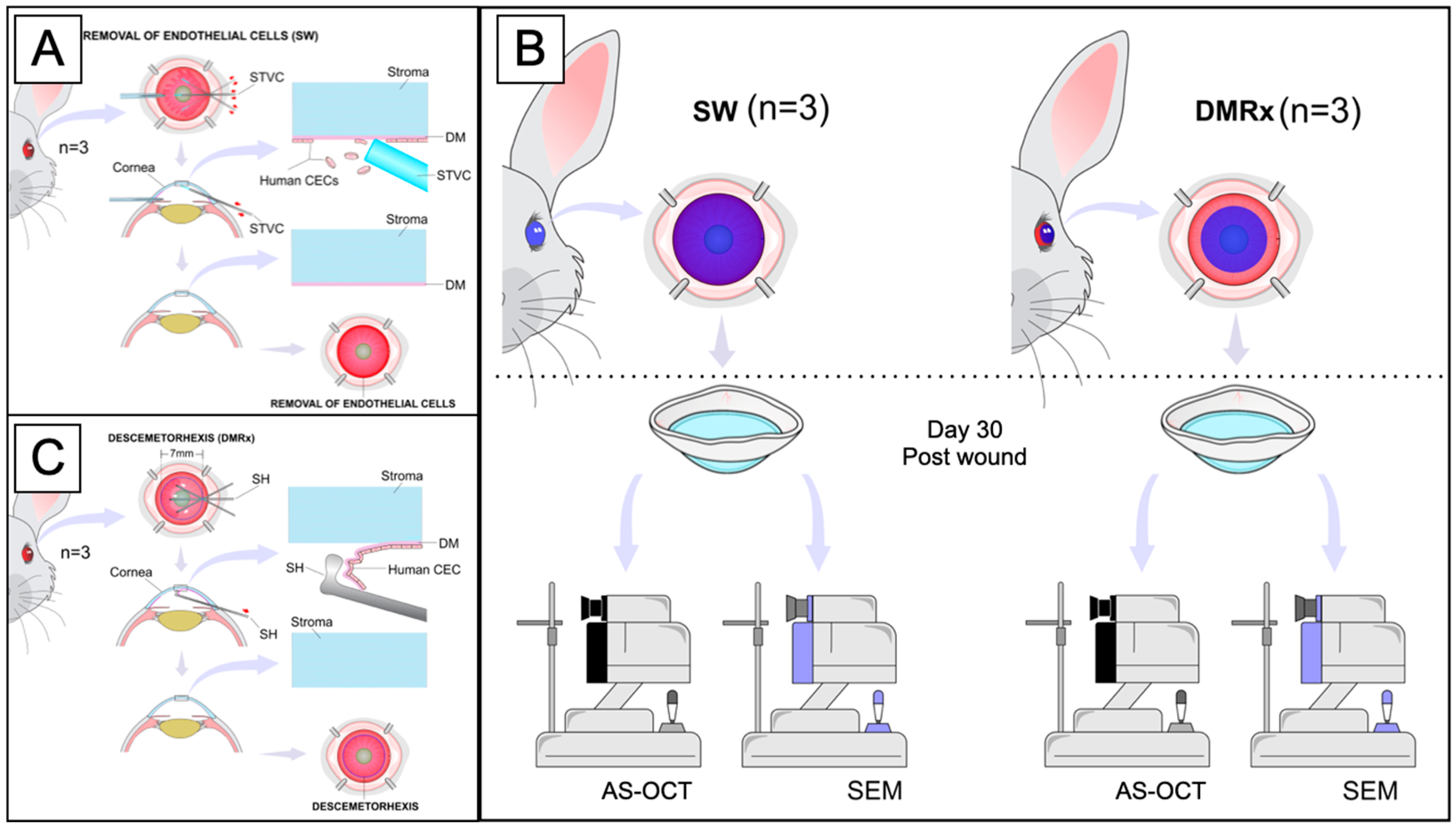
2.4. In Vivo Experimental Wound Models
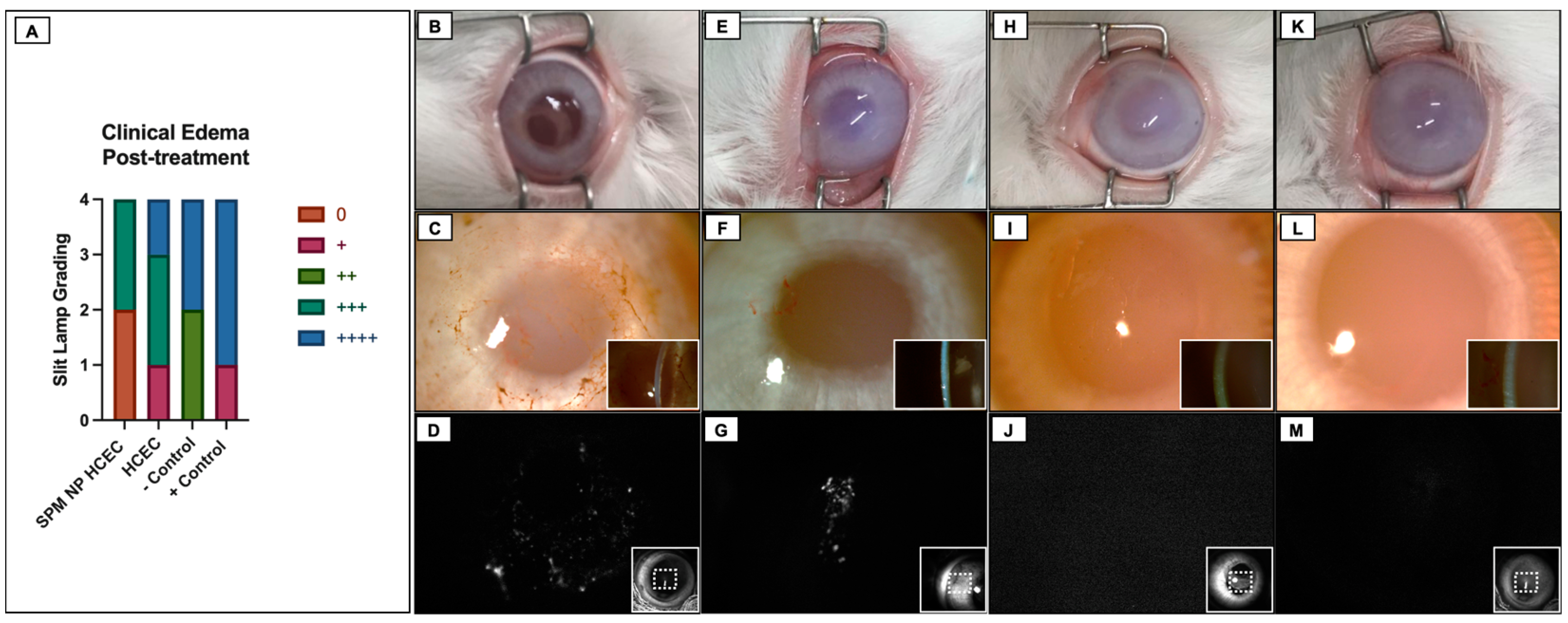
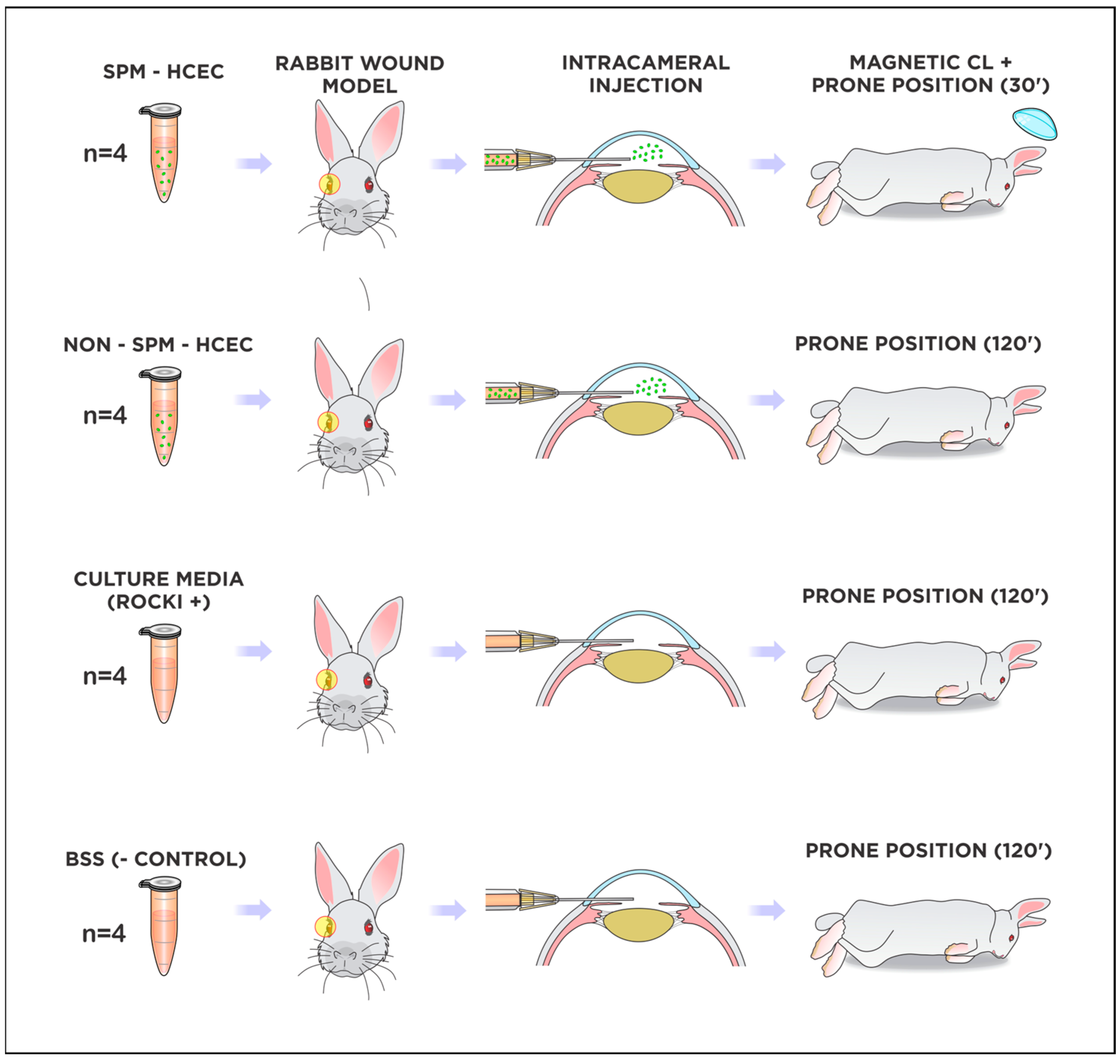
2.5. Cultured HCEC Injections in Rabbits
2.6. Fluorescence Tracking of Adherent Cultured HCEC after Injections
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Corneal Endothelial Cells
4.2. ROCK Inhibitor Treatment
4.3. HCEC Superparamagnetic Embedding
4.4. Fluorescence Confirmation of Cultured HCEC Attachment to Recipient Corneas: Ex Vivo Wound Model
4.5. In Vivo Endothelial Regeneration with Injected HCEC in White New Zealand Rabbits
4.5.1. Endothelial Wound Model
4.5.2. Experimental Treatment with Cultured Cell Injections and Fluorescence Confirmation of HCEC Adhesion
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Klyce, S.D. Endothelial pump and barrier function. Exp. Eye Res. 2020, 198, 108068. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Tassignon, M.J.; Zakaria, N. A review of the evidence for in vivo corneal endothelial regeneration. Surv. Ophthalmol. 2018, 63, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Tello, A.; Gutierrez, Á.J. Human corneal endothelium regeneration: Effect of ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4971–4973. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, Cd012097. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W., Jr. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2020, 82, 100904. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Sun, D.; Xie, L. Histological evaluation of corneal scar formation in pseudophakic bullous keratopathy. PLoS ONE 2012, 7, e39201. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Laiton, A.N.; Salcedo, S.L.L. Indications and techniques of corneal transplantation in a referral center in Colombia, South America (2012–2016). Int. Ophthalmol. 2019, 39, 1723–1733. [Google Scholar] [CrossRef]
- Moriyama, A.S.; Dos Santos Forseto, A.; Pereira, N.C.; Ribeiro, A.C.; de Almeida, M.C.; Figueras-Roca, M.; Casaroli-Marano, R.P.; Mehta, J.S.; Hofling-Lima, A.L. Trends in Corneal Transplantation in a Tertiary Hospital in Brazil. Cornea 2021, 41, 857–866. [Google Scholar] [CrossRef]
- Matthaei, M.; Sandhaeger, H.; Hermel, M.; Adler, W.; Jun, A.S.; Cursiefen, C.; Heindl, L.M. Changing Indications in Penetrating Keratoplasty: A Systematic Review of 34 Years of Global Reporting. Transplantation 2017, 101, 1387–1399. [Google Scholar] [CrossRef]
- Ang, M.J.; Chamberlain, W.; Lin, C.C.; Pickel, J.; Austin, A.; Rose-Nussbaumer, J. Effect of Unilateral Endothelial Keratoplasty on Vision-Related Quality-of-Life Outcomes in the Descemet Endothelial Thickness Comparison Trial (DETECT): A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 747–754. [Google Scholar] [CrossRef]
- Grottone, G.T.; Pereira, N.C.; Gomes, J.A. Endothelial keratoplasty: Evolution and horizons. Arq. Bras. Oftalmol. 2012, 75, 439–446. [Google Scholar] [CrossRef]
- Zafar, S.; Parker, J.S.; de Kort, C.; Melles, G.; Sikder, S. Perceived difficulties and barriers to uptake of Descemet’s membrane endothelial keratoplasty among surgeons. Clin. Ophthalmol. 2019, 13, 1055–1061. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Ang, M.; Moriyama, A.; Colby, K.; Sutton, G.; Liang, L.; Sharma, N.; Hjortdal, J.; Lam, D.S.C.; Williams, G.P.; Armitage, J.; et al. Corneal transplantation in the aftermath of the COVID-19 pandemic: An international perspective. Br. J. Ophthalmol. 2020, 104, 1477–1481. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mudgil, N.; Wadgave, Y.; Mishra, S.S. Corneal transplantation during COVID-19 pandemic: Need for special considerations-A live review. AIMS Public Health 2021, 8, 186–195. [Google Scholar] [CrossRef]
- Baum, J.L.; Niedra, R.; Davis, C.; Yue, B.Y. Mass culture of human corneal endothelial cells. Arch. Ophthalmol. 1979, 97, 1136–1140. [Google Scholar] [CrossRef]
- Smeringaiova, I.; Utheim, T.P.; Jirsova, K. Ex vivo expansion and characterization of human corneal endothelium for transplantation: A review. Stem Cell Res. Ther. 2021, 12, 554. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef]
- Peh, G.S.; Toh, K.P.; Wu, F.Y.; Tan, D.T.; Mehta, J.S. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS ONE 2011, 6, e28310. [Google Scholar] [CrossRef]
- Redbrake, C.; Becker, J.; Salla, S.; Stollenwerk, R.; Reim, M. The influence of the cause of death and age on human corneal metabolism. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3553–3556. [Google Scholar]
- Ho, W.T.; Chang, J.S.; Su, C.C.; Chang, S.W.; Hu, F.R.; Jou, T.S.; Wang, I.J. Inhibition of matrix metalloproteinase activity reverses corneal endothelial-mesenchymal transition. Am. J. Pathol. 2015, 185, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.T.; Su, C.C.; Chang, J.S.; Chang, S.W.; Hu, F.R.; Jou, T.S.; Wang, I.J. In Vitro and In Vivo Models to Study Corneal Endothelial-mesenchymal Transition. J. Vis. Exp. 2016, 114, e54329. [Google Scholar] [CrossRef]
- Lee, J.G.; Ko, M.K.; Kay, E.P. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp. Eye Res. 2012, 95, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Peh, G.S.L.; Mehta, J.S. Translational issues for human corneal endothelial tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2425–2442. [Google Scholar] [CrossRef]
- Macsai, M.S.; Shiloach, M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea 2019, 38, 529–534. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Fuquen, J.P.; Rodriguez-Barrientos, C.A.; Grice, J.M. ROCK Inhibitor (Ripasudil) as Coadjuvant After Descemetorhexis Without an Endothelial Graft. Cornea 2017, 36, e38–e40. [Google Scholar] [CrossRef]
- Franceschino, A.; Dutheil, F.; Pereira, B.; Watson, S.L.; Chiambaretta, F.; Navel, V. Descemetorhexis Without Endothelial Keratoplasty in Fuchs Endothelial Corneal Dystrophy: A Systematic Review and Meta-Analysis. Cornea 2021, 41, 815–825. [Google Scholar] [CrossRef]
- Okumura, N.; Kakutani, K.; Inoue, R.; Matsumoto, D.; Shimada, T.; Nakahara, M.; Kiyanagi, Y.; Itoh, T.; Koizumi, N. Generation and Feasibility Assessment of a New Vehicle for Cell-Based Therapy for Treating Corneal Endothelial Dysfunction. PLoS ONE 2016, 11, e0158427. [Google Scholar] [CrossRef]
- Okumura, N.; Kinoshita, S.; Koizumi, N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea 2014, 33 (Suppl. 11), S37–S41. [Google Scholar] [CrossRef]
- Okumura, N.; Matsumoto, D.; Fukui, Y.; Teramoto, M.; Imai, H.; Kurosawa, T.; Shimada, T.; Kruse, F.; Schlotzer-Schrehardt, U.; Kinoshita, S.; et al. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS ONE 2018, 13, e0191306. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ang, H.P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.Y.; Lin, S.J.; Bhogal, M.; Liu, Y.C.; Tan, D.T.; et al. Regulatory Compliant Tissue-Engineered Human Corneal Endothelial Grafts Restore Corneal Function of Rabbits with Bullous Keratopathy. Sci. Rep. 2017, 7, 14149. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Bostan, C.; Theriault, M.; Forget, K.J.; Doyon, C.; Cameron, J.D.; Proulx, S.; Brunette, I. In Vivo Functionality of a Corneal Endothelium Transplanted by Cell-Injection Therapy in a Feline Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1620–1634. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef]
- Moysidis, S.N.; Alvarez-Delfin, K.; Peschansky, V.J.; Salero, E.; Weisman, A.D.; Bartakova, A.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kador, K.E.; Kunzevitzky, N.J.; et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomedicine 2015, 11, 499–509. [Google Scholar] [CrossRef]
- Cornell, L.E.; Wehmeyer, J.L.; Johnson, A.J.; Desilva, M.N.; Zamora, D.O. Magnetic Nanoparticles as a Potential Vehicle for Corneal Endothelium Repair. Mil. Med. 2016, 181, 232–239. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Yanagi, Y.; Usui, T.; Ono, K.; Araie, M.; Amano, S. Sphere therapy for corneal endothelium deficiency in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3128–3135. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First Eleven Cases Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2020, 128, 504–514. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Peh, G.S.L.; Adnan, K.; Mehta, J.S. Translational and Regulatory Challenges of Corneal Endothelial Cell Therapy: A Global Perspective. Tissue Eng. Part B Rev. 2022, 28, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.; Ruggeri, A.; Thieme, H.; Meltendorf, C. Impact of temporary hyperthermia on corneal endothelial cell survival during organ culture preservation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Gorovoy, I.R.; Cui, Q.N.; Gorovoy, M.S. Donor tissue characteristics in preparation of DMEK grafts. Cornea 2014, 33, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Ruzza, A.; Romano, V.; Favaro, E.; Baruzzo, M.; Salvalaio, G.; Grassetto, A.; Ferrari, S.; Ponzin, D. Descemet Membrane Endothelial Keratoplasty Learning Curve for Graft Preparation in an Eye Bank Using 645 Donor Corneas. Cornea 2018, 37, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Schlotzer-Schrehardt, U.; Bachmann, B.O.; Tourtas, T.; Cursiefen, C.; Zenkel, M.; Rossler, K.; Kruse, F.E. Reproducibility of graft preparations in Descemet’s membrane endothelial keratoplasty. Ophthalmology 2013, 120, 1769–1777. [Google Scholar] [CrossRef]
- Heinzelmann, S.; Huther, S.; Bohringer, D.; Eberwein, P.; Reinhard, T.; Maier, P. Influence of donor characteristics on descemet membrane endothelial keratoplasty. Cornea 2014, 33, 644–648. [Google Scholar] [CrossRef]
- Peh, G.S.; Beuerman, R.W.; Colman, A.; Tan, D.T.; Mehta, J.S. Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 2011, 91, 811–819. [Google Scholar] [CrossRef]
- Vianna, L.M.; Stoeger, C.G.; Galloway, J.D.; Terry, M.; Cope, L.; Belfort, R., Jr.; Jun, A.S. Risk factors for eye bank preparation failure of Descemet membrane endothelial keratoplasty tissue. Am. J. Ophthalmol. 2015, 159, 829–834.e822. [Google Scholar] [CrossRef]
- Pereira, N.C.; Forseto, A.D.S.; Santos, M.S.D.; Grottone, G.; Santos, A.; Gomes, J.A.P. Descemet’s membrane endothelial keratoplasty with a simplified technique and low complication rate: The samba technique. Arq. Bras. De Oftalmol. 2018, 81, 130–136. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Migeon, B.R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell 1975, 5, 11–17. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Amano, S. Selective isolation of young cells from human corneal endothelium by the sphere-forming assay. Tissue Eng. Part C Methods 2010, 16, 803–812. [Google Scholar] [CrossRef]
- Walshe, J.; Harkin, D.G. Serial explant culture provides novel insights into the potential location and phenotype of corneal endothelial progenitor cells. Exp. Eye Res. 2014, 127, 9–13. [Google Scholar] [CrossRef]
- Sobottka Ventura, A.C.; Engelmann, K.; Bohnke, M. Fetal calf serum protects cultured porcine corneal endothelial cells from endotoxin-mediated cell damage. Ophthalmic. Res. 1999, 31, 416–425. [Google Scholar] [CrossRef]
- Engelmann, K.; Bohnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Li, W.; Sabater, A.L.; Chen, Y.T.; Hayashida, Y.; Chen, S.Y.; He, H.; Tseng, S.C. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 614–620. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Q.; Sun, H.; Zhang, Y.; Tighe, S.; Xu, L.; Zhu, Y. Advances in culture, expansion and mechanistic studies of corneal endothelial cells: A systematic review. J. Biomed. Sci. 2019, 26, 2. [Google Scholar] [CrossRef]
- Bartakova, A.; Alvarez-Delfin, K.; Weisman, A.D.; Salero, E.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kunzevitzky, N.J.; Goldberg, J.L. Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2749–2762. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Tighe, S.; Chen, S.L.; John, T.; Kao, W.Y.; Tseng, S.C. Engineering of Human Corneal Endothelial Grafts. Curr. Ophthalmol. Rep. 2015, 3, 207–217. [Google Scholar] [CrossRef]
- Okumura, N.; Nakano, S.; Kay, E.P.; Numata, R.; Ota, A.; Sowa, Y.; Sakai, T.; Ueno, M.; Kinoshita, S.; Koizumi, N. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Investig. Ophthalmol. Vis. Sci. 2014, 55, 318–329. [Google Scholar] [CrossRef]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Patel, S.V.; Bachman, L.A.; Hann, C.R.; Bahler, C.K.; Fautsch, M.P. Human corneal endothelial cell transplantation in a human ex vivo model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Rovati, L.; Docchio, F. Autofluorescence methods in ophthalmology. J. Biomed. Opt. 2004, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Atkins, M.; Dalal, R.; Kuzmenko, O.; Chang, K.C.; Sun, C.B.; Benatti, C.A.; Rak, D.J.; Nahmou, M.; Kunzevitzky, N.J.; et al. Magnetic Human Corneal Endothelial Cell Transplant: Delivery, Retention, and Short-Term Efficacy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Molina, E.; Chocarro-Wrona, C.; Martinez-Moreno, D.; Marchal, J.A.; Boulaiz, H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics 2020, 12, 1051. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef]
- Grottone, G.T.; Loureiro, R.R.; Covre, J.; Rodrigues, E.B.; Gomes, J.A. ARPE-19 cell uptake of small and ultrasmall superparamagnetic iron oxide. Curr. Eye Res 2014, 39, 403–410. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Peh, G.; George, B.L.; Seah, X.Y.; Primalani, N.K.; Adnan, K.; Mehta, J.S. Predicative Factors for Corneal Endothelial Cell Migration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 338–348. [Google Scholar] [CrossRef]
- Melles, G.R.; Wijdh, R.H.; Nieuwendaal, C.P. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis). Cornea 2004, 23, 286–288. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandeira, F.; Grottone, G.T.; Covre, J.L.; Cristovam, P.C.; Loureiro, R.R.; Pinheiro, F.I.; Casaroli-Marano, R.P.; Donato, W.; Gomes, J.Á.P. A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach. Int. J. Mol. Sci. 2023, 24, 2982. https://doi.org/10.3390/ijms24032982
Bandeira F, Grottone GT, Covre JL, Cristovam PC, Loureiro RR, Pinheiro FI, Casaroli-Marano RP, Donato W, Gomes JÁP. A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach. International Journal of Molecular Sciences. 2023; 24(3):2982. https://doi.org/10.3390/ijms24032982
Chicago/Turabian StyleBandeira, Francisco, Gustavo Teixeira Grottone, Joyce Luciana Covre, Priscila Cardoso Cristovam, Renata Ruoco Loureiro, Francisco Irochima Pinheiro, Ricardo Pedro Casaroli-Marano, Waleska Donato, and José Álvaro Pereira Gomes. 2023. "A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach" International Journal of Molecular Sciences 24, no. 3: 2982. https://doi.org/10.3390/ijms24032982
APA StyleBandeira, F., Grottone, G. T., Covre, J. L., Cristovam, P. C., Loureiro, R. R., Pinheiro, F. I., Casaroli-Marano, R. P., Donato, W., & Gomes, J. Á. P. (2023). A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach. International Journal of Molecular Sciences, 24(3), 2982. https://doi.org/10.3390/ijms24032982