STS1 and STS2 Phosphatase Inhibitor Baicalein Enhances the Expansion of Hematopoietic and Progenitor Stem Cells and Alleviates 5-Fluorouracil-Induced Myelosuppression
Abstract
:1. Introduction
2. Results
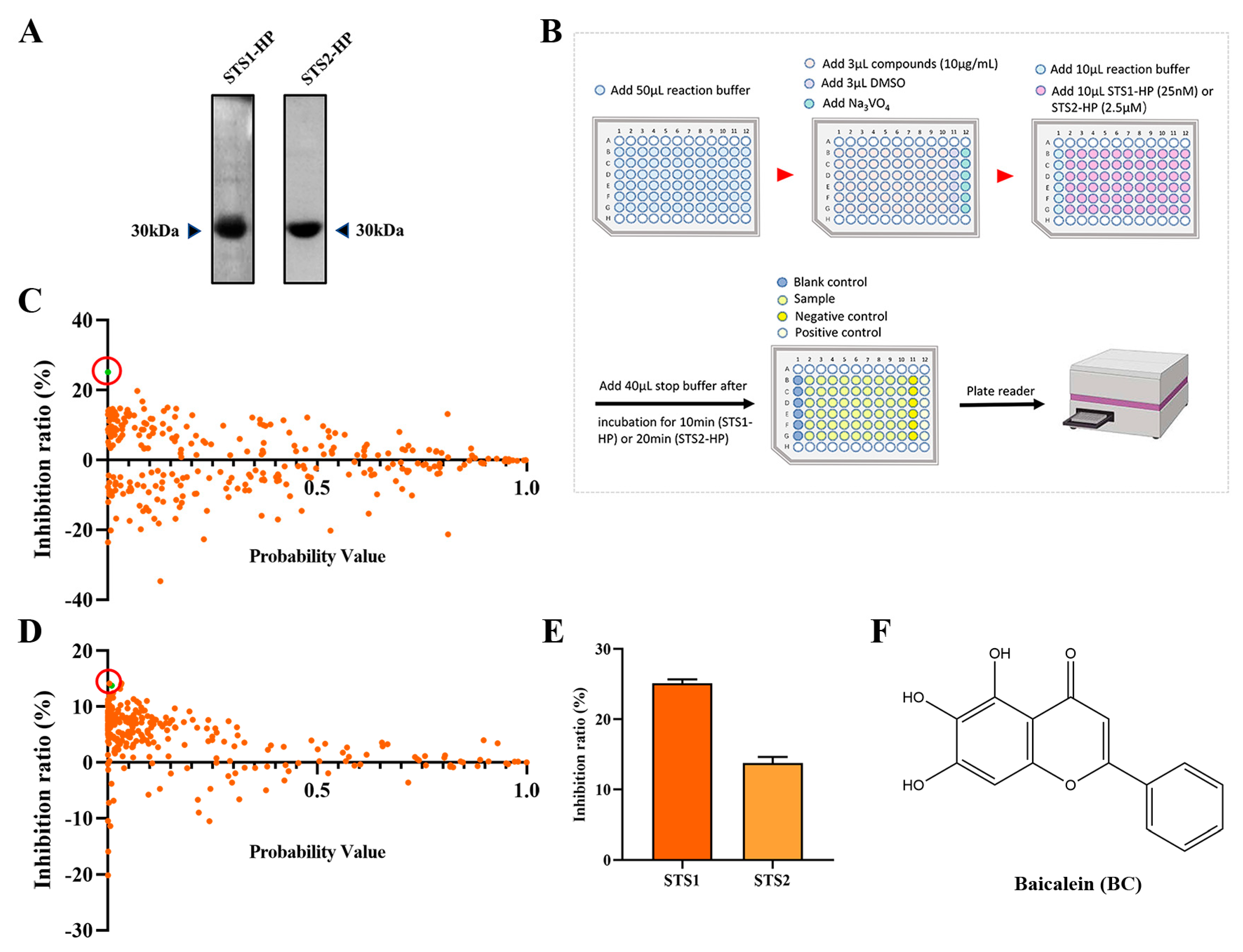
2.1. Screening STS1 and STS2 Phosphatase Inhibitors
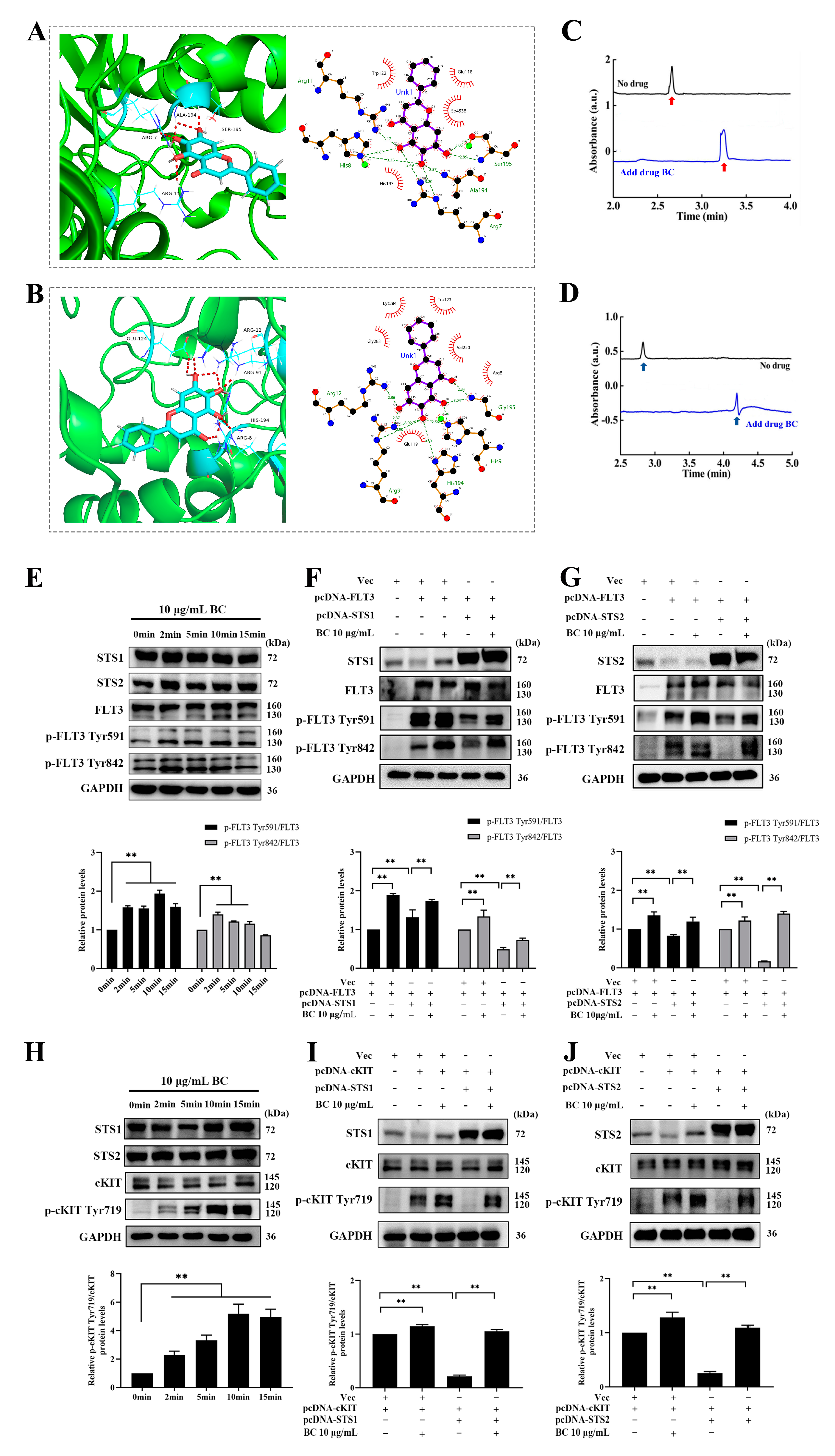
2.2. Validation of BC as a Phosphatase Inhibitor of STS1 and STS2
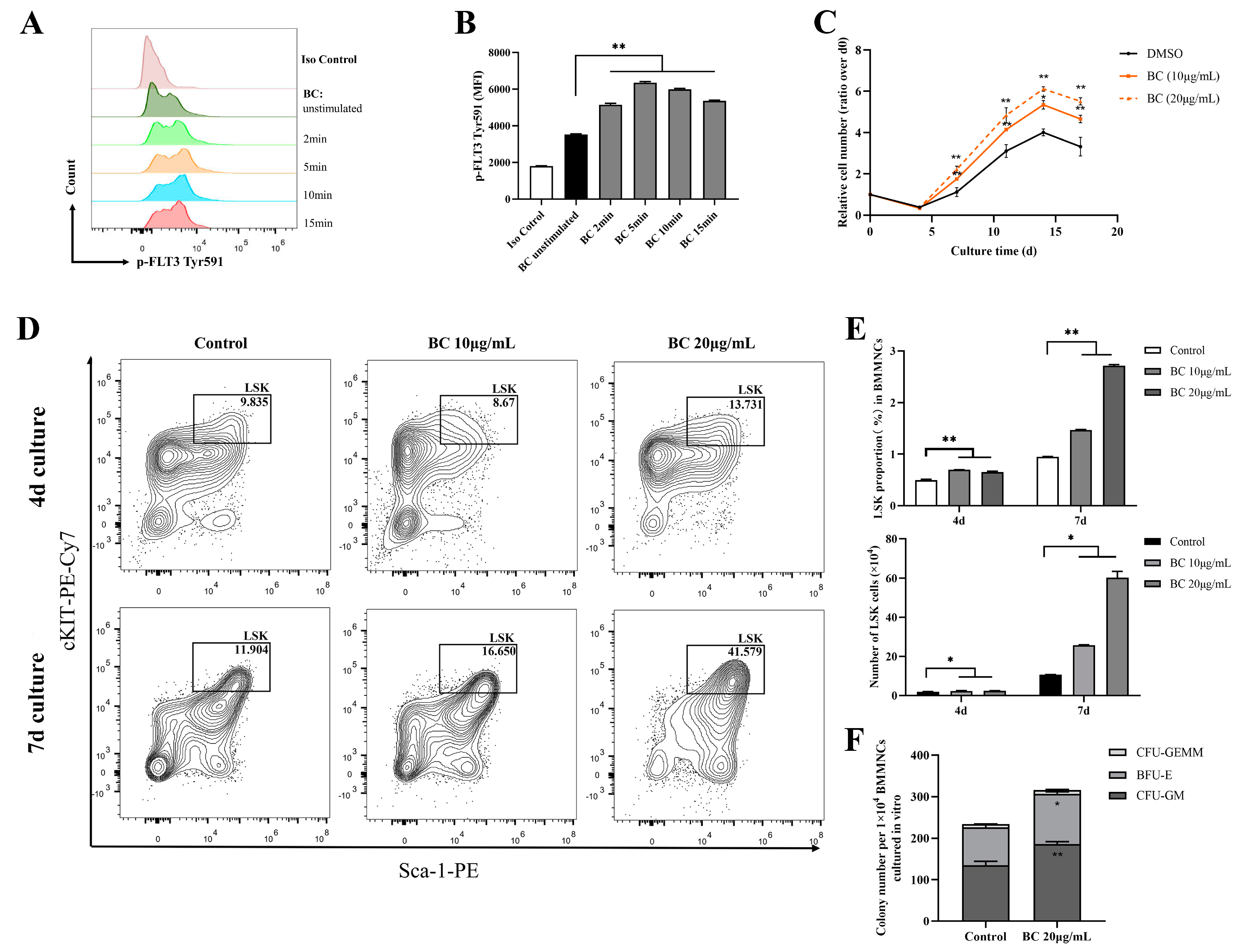
2.3. BC Treatment Promotes the Proliferation of Mouse BMMNCs and the Expansion of HPSCs In Vitro
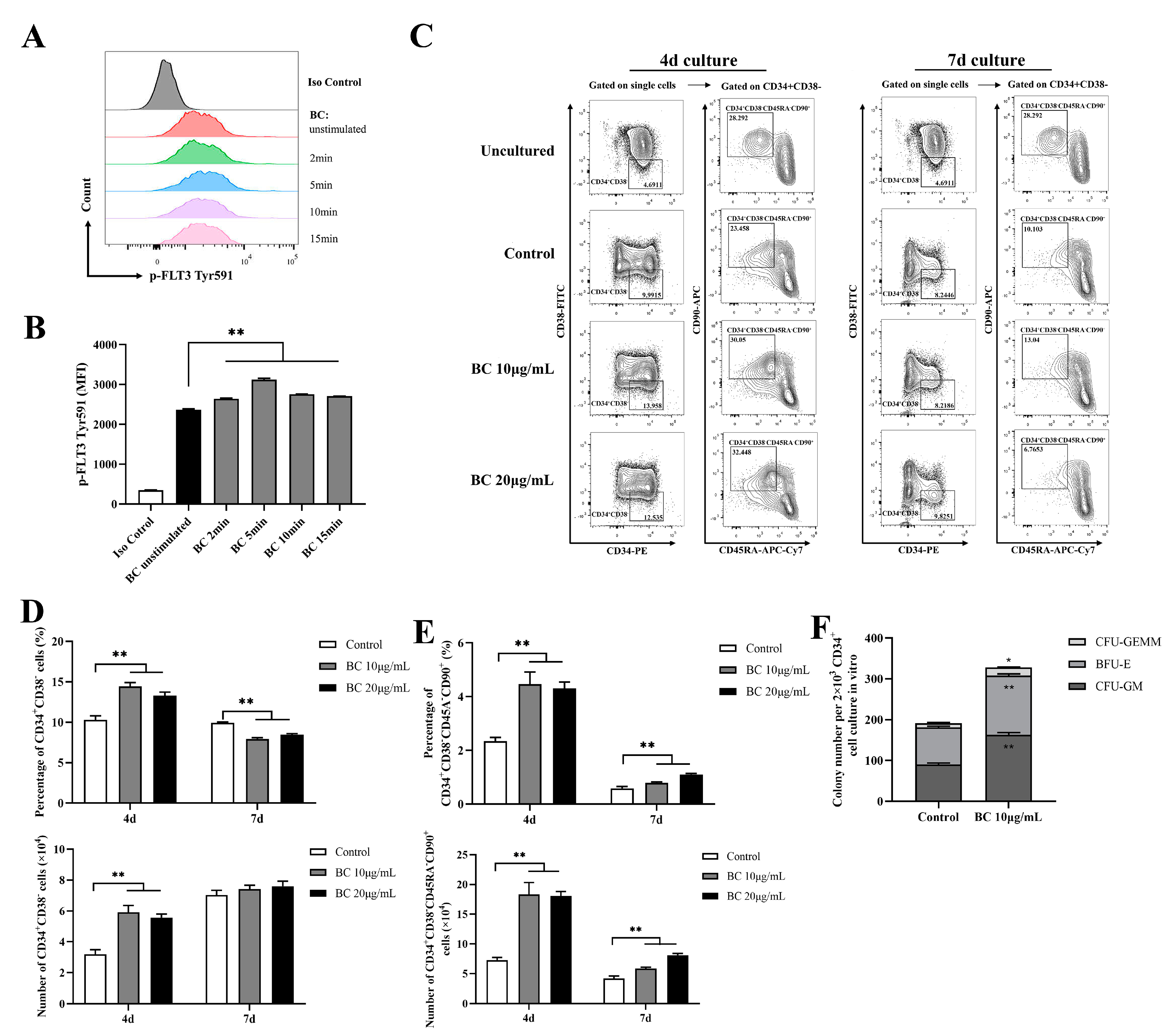
2.4. BC Treatment Enhances the In Vitro Expansion of Primitive HSCs and the Multipotency of HSPCs in Human CD34+ Umbilical Cord Blood Cells
2.5. In Vivo Administration of BC Increases the Proportion and Multilineage Differentiation Potential of Healthy Mouse Bone Marrow HSPCs
2.6. BC Pretreatment Improves the Survival and Ameliorates Myelosuppression in 5-FU-Injured Mice
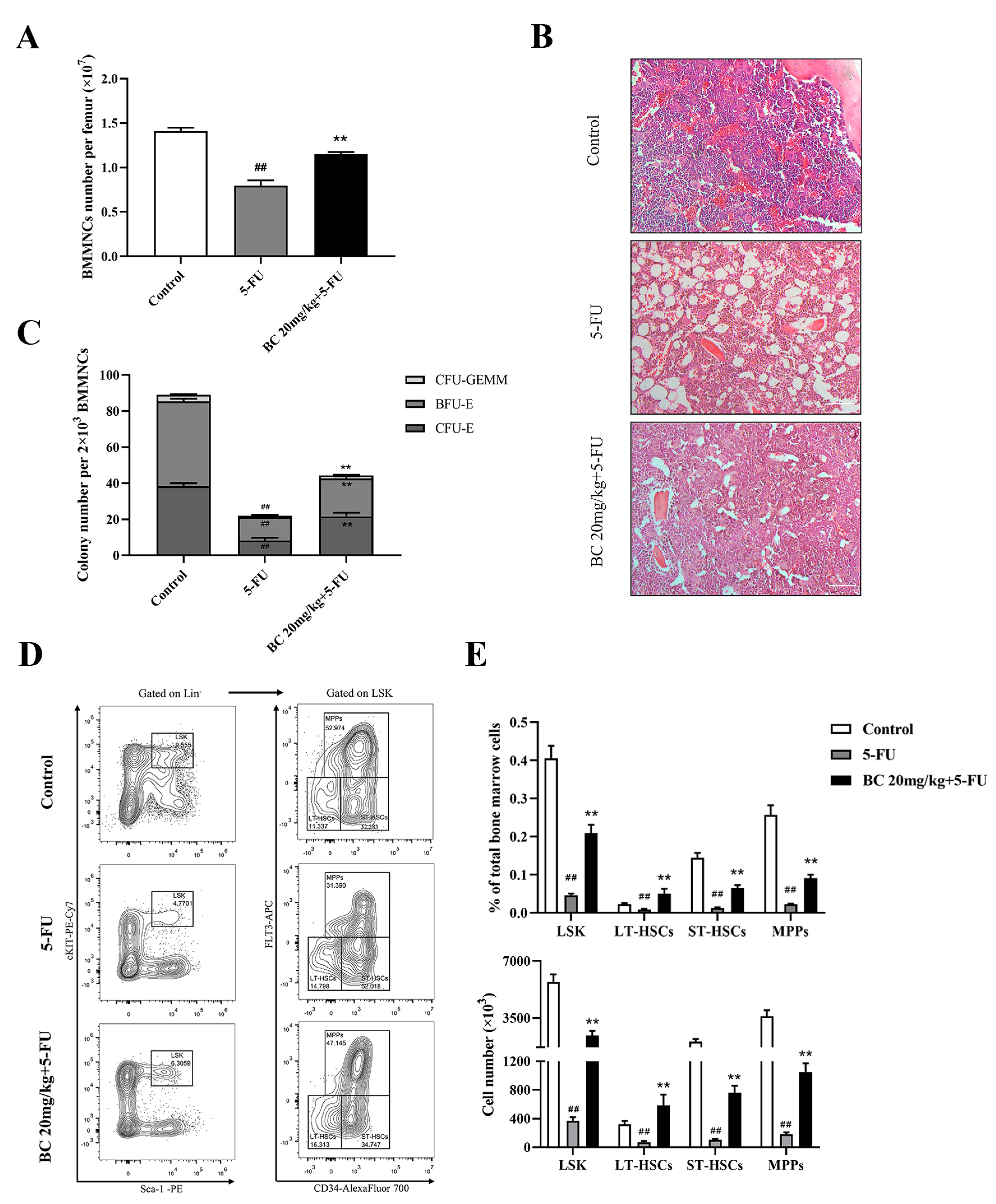
2.7. BC Ameliorates 5-FU-Induced Impairment of HSCs and Progenitor Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Plasmids and Cell Lines
4.3. Mice
4.4. Generation of STS1 and STS2 HP Domain Recombinant Proteins
4.5. Enzyme Kinetic Analysis
4.6. Screening for STS1/STS2 Phosphatase Inhibitors
4.7. Molecular Docking
4.8. Capillary Electrophoresis Assay
4.9. Plasmid Transfection and Western Blot
4.10. Mouse BMMNC Isolation and In Vitro Culture
4.11. Human CD34+ Cord Blood Cell Isolation and In Vitro Culture
4.12. Spleen Cell Isolation
4.13. Flow Cytometry Analysis
4.14. Animal Experiments
4.15. CFU Assay
4.16. Histological Analysis
4.17. Peripheral Blood Item Analysis
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobsen, S.E.W.; Nerlov, C. Haematopoiesis in the era of advanced single-cell technologies. Nat. Cell Biol. 2019, 21, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Fares, I.; Chagraoui, J.; Gareau, Y.; Gingras, S.; Ruel, R.; Mayotte, N.; Csaszar, E.; Knapp, D.J.; Miller, P.; Ngom, M.; et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 2014, 345, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: Prevention and treatment of graft-versus-host disease. Stem Cell Res. Ther. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, P.; Shen, H.; Yu, H.; Song, X.; Zhang, L.; Zhang, P.; Cheng, H.; Xie, Z.; Hao, S.; et al. Small-molecule inhibitors targeting INK4 protein p18(INK4C) enhance ex vivo expansion of haematopoietic stem cells. Nat. Commun. 2015, 6, 6328. [Google Scholar] [CrossRef] [PubMed]
- Bisi, J.E.; Sorrentino, J.A.; Roberts, P.J.; Tavares, F.X.; Strum, J.C. Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol. Cancer Ther. 2016, 15, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. BioMed Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef]
- Epstein, R.S.; Aapro, M.S.; Basu Roy, U.K.; Salimi, T.; Krenitsky, J.; Leone-Perkins, M.L.; Girman, C.; Schlusser, C.; Crawford, J. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv. Ther. 2020, 37, 3606–3618. [Google Scholar] [CrossRef]
- Mackey, M.C.; Glisovic, S.; Leclerc, J.M.; Pastore, Y.; Krajinovic, M.; Craig, M. The timing of cyclic cytotoxic chemotherapy can worsen neutropenia and neutrophilia. Br. J. Clin. Pharmacol. 2021, 87, 687–693. [Google Scholar] [CrossRef]
- Rossi, L.; Lin, K.K.; Boles, N.C.; Yang, L.; King, K.Y.; Jeong, M.; Mayle, A.; Goodell, M.A. Less is more: Unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell 2012, 11, 302–317. [Google Scholar] [CrossRef]
- Williams, K.M.; Moore, A.R.; Lucas, P.J.; Wang, J.; Bare, C.V.; Gress, R.E. FLT3 ligand regulates thymic precursor cells and hematopoietic stem cells through interactions with CXCR4 and the marrow niche. Exp. Hematol. 2017, 52, 40–49. [Google Scholar] [CrossRef]
- Young, I.C.; Wu, B.; Andricovich, J.; Chuang, S.T.; Li, R.; Tzatsos, A.; Wu, R.C.; Wu, M.Y. Differentiation of fetal hematopoietic stem cells requires ARID4B to restrict autocrine KITLG/KIT-Src signaling. Cell Rep. 2021, 37, 110036. [Google Scholar] [CrossRef]
- Li, L.; Piloto, O.; Kim, K.T.; Ye, Z.; Nguyen, H.B.; Yu, X.; Levis, M.; Cheng, L.; Small, D. FLT3/ITD expression increases expansion, survival and entry into cell cycle of human haematopoietic stem/progenitor cells. Br. J. Haematol. 2007, 137, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mackarehtschian, K.; Hardin, J.D.; Moore, K.A.; Boast, S.; Goff, S.P.; Lemischka, I.R. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity 1995, 3, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Rönnstrand, L. FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications. Physiol. Rev. 2019, 99, 1433–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Vakhrusheva, O.; Bandi, S.R.; Demirel, Ö.; Kazi, J.U.; Fernandes, R.G.; Jakobi, K.; Eichler, A.; Rönnstrand, L.; Rieger, M.A.; et al. The Phosphatases STS1 and STS2 Regulate Hematopoietic Stem and Progenitor Cell Fitness. Stem Cell Rep. 2015, 5, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Hicke, L.; Schubert, H.L.; Hill, C.P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 2005, 6, 610–621. [Google Scholar] [CrossRef]
- Musacchio, A. How SH3 domains recognize proline. Adv. Protein Chem. 2002, 61, 211–268. [Google Scholar]
- Tsygankov, A.Y. TULA-family proteins: A new class of cellular regulators. J. Cell Physiol. 2013, 228, 43–49. [Google Scholar] [CrossRef]
- Hoeller, D.; Crosetto, N.; Blagoev, B.; Raiborg, C.; Tikkanen, R.; Wagner, S.; Kowanetz, K.; Breitling, R.; Mann, M.; Stenmark, H.; et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 2006, 8, 163–169. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Y.; Weinheimer, A.S.; Kaur, N.; Carpino, N.; French, J.B. Structural and Functional Characterization of the Histidine Phosphatase Domains of Human Sts-1 and Sts-2. Biochemistry 2017, 56, 4637–4645. [Google Scholar] [CrossRef]
- Carpino, N.; Turner, S.; Mekala, D.; Takahashi, Y.; Zang, H.; Geiger, T.L.; Doherty, P.; Ihle, J.N. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity 2004, 20, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Mikhailik, A.; Ford, B.; Keller, J.; Chen, Y.; Nassar, N.; Carpino, N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol. Cell 2007, 27, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Carpino, N.; Chen, Y.; Nassar, N.; Oh, H.W. The Sts proteins target tyrosine phosphorylated, ubiquitinated proteins within TCR signaling pathways. Mol. Immunol. 2009, 46, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.W.; Schroeder, A.V.; Smith-Berdan, S.; Forsberg, E.C. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 2011, 9, 64–73. [Google Scholar] [CrossRef]
- Thoren, L.A.; Liuba, K.; Bryder, D.; Nygren, J.M.; Jensen, C.T.; Qian, H.; Antonchuk, J.; Jacobsen, S.E. Kit regulates maintenance of quiescent hematopoietic stem cells. J. Immunol. 2008, 180, 2045–2053. [Google Scholar] [CrossRef]
- Buza-Vidas, N.; Cheng, M.; Duarte, S.; Charoudeh, H.N.; Jacobsen, S.E.; Sitnicka, E. FLT3 receptor and ligand are dispensable for maintenance and posttransplantation expansion of mouse hematopoietic stem cells. Blood 2009, 113, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; Toifl, S.; Farlik, M.; Halbritter, F.; Scheicher, R.; Fischer, I.; Sexl, V.; Bock, C.; Baccarini, M. An ERK-Dependent Feedback Mechanism Prevents Hematopoietic Stem Cell Exhaustion. Cell Stem Cell 2018, 22, 879–892.e876. [Google Scholar] [CrossRef]
- Zayas, J.; George, J.; Nachtman, R.G.; Jurecic, R. Varying Levels of CD34 Expression Correlate with Maintenance and Differentiation of HSC/MPP-Like Cell Line EML. Blood 2007, 110, 4050. [Google Scholar] [CrossRef]
- Majeti, R.; Park, C.Y.; Weissman, I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 2007, 1, 635–645. [Google Scholar] [CrossRef]
- Weissman, I.L.; Shizuru, J.A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 2008, 112, 3543–3553. [Google Scholar] [CrossRef]
- Martin, S.K.; Fitter, S.; El Khawanky, N.; Grose, R.H.; Walkley, C.R.; Purton, L.E.; Ruegg, M.A.; Hall, M.N.; Gronthos, S.; Zannettino, A.C.W. mTORC1 plays an important role in osteoblastic regulation of B-lymphopoiesis. Sci. Rep. 2018, 8, 14501. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, L.; Pazhanisamy, S.K.; Li, H.; Meng, A.; Zhou, D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010, 48, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Duyvestyn, J.M.; Dagger, S.A.; Dishington, E.J.; Rinaldi, C.A.; Dovey, O.M.; Vassiliou, G.S.; Grove, C.S.; Langdon, W.Y. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci. Transl. Med. 2017, 9, eaam8060. [Google Scholar] [CrossRef] [PubMed]
- Piryani, S.O.; Kam, A.Y.F.; Kliassov, E.G.; Chen, B.J.; Spector, N.L.; Chute, J.P.; Chao, N.J.; Doan, P.L. EGF Accelerates Hematopoietic Stem Cell Regeneration Following 5-FU Chemotherapy Via G-CSF Receptor Signaling. Blood 2016, 128, 1490. [Google Scholar] [CrossRef]
- Goncalves, K.A.; Silberstein, L.; Li, S.; Severe, N.; Hu, M.G.; Yang, H.; Scadden, D.T.; Hu, G.F. Angiogenin Promotes Hematopoietic Regeneration by Dichotomously Regulating Quiescence of Stem and Progenitor Cells. Cell 2016, 166, 894–906. [Google Scholar] [CrossRef]
- Qayyum, M.I.; Ullah, S.; Rashid, U.; Sadiq, A.; Obaidullah Mahnashi, M.H.; Alshehri, O.M.; Jalal, M.M.; Alzahrani, K.J.; Halawani, I.F. Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects. Molecules 2022, 27, 6199. [Google Scholar] [CrossRef] [PubMed]
- Carpino, N.; Kobayashi, R.; Zang, H.; Takahashi, Y.; Jou, S.T.; Feng, J.; Nakajima, H.; Ihle, J.N. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 2002, 22, 7491–7500. [Google Scholar] [CrossRef]
- De Castro, R.O.; Zhang, J.; Groves, J.R.; Barbu, E.A.; Siraganian, R.P. Once phosphorylated, tyrosines in carboxyl terminus of protein-tyrosine kinase Syk interact with signaling proteins, including TULA-2, a negative regulator of mast cell degranulation. J. Biol. Chem. 2012, 287, 8194–8204. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef]
- Elmore, S.A. Enhanced histopathology of the spleen. Toxicol. Pathol. 2006, 34, 648–655. [Google Scholar] [CrossRef]
- Barreto, J.N.; McCullough, K.B.; Ice, L.L.; Smith, J.A. Antineoplastic agents and the associated myelosuppressive effects: A review. J. Pharm. Pract. 2014, 27, 440–446. [Google Scholar] [CrossRef]
- Schoedel, K.B.; Morcos, M.N.F.; Zerjatke, T.; Roeder, I.; Grinenko, T.; Voehringer, D.; Göthert, J.R.; Waskow, C.; Roers, A.; Gerbaulet, A. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 2016, 128, 2285–2296. [Google Scholar] [CrossRef]
- Wang, M.; Dong, Y.; Wu, J.; Li, H.; Zhang, Y.; Fan, S.; Li, D. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. 2020, 261, 118463. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.S.; Sharma, D.; Checker, R.; Sandur, S.K. Mitigation of radiation-induced hematopoietic injury via regulation of cellular MAPK/phosphatase levels and increasing hematopoietic stem cells. Free Radic. Biol. Med. 2014, 68, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.I.; Su, W.C.; Liu, H.F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther. 2007, 6, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Shen, S.C.; Lin, C.W.; Yang, L.Y.; Chen, Y.C. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim. Biophys. Acta 2007, 1773, 1073–1086. [Google Scholar] [CrossRef]
- Lee, S.W.; Song, G.S.; Kwon, C.H.; Kim, Y.K. Beneficial effect of flavonoid baicalein in cisplatin-induced cell death of human glioma cells. Neurosci. Lett. 2005, 382, 71–75. [Google Scholar] [CrossRef]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Lin, G.; Krajcsi, P.; Zuo, Z. Hepatic metabolism and disposition of baicalein via the coupling of conjugation enzymes and transporters-in vitro and in vivo evidences. AAPS J. 2011, 13, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; He, G.; Song, J.; Wang, S.; Xin, W.; Zhang, D.; Du, G. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia 2012, 83, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.R. Baicalin, an emerging multi-therapeutic agent: Pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica 2010, 40, 357–367. [Google Scholar] [CrossRef]
- Taiming, L.; Xuehua, J. Investigation of the absorption mechanisms of baicalin and baicalein in rats. J. Pharm. Sci. 2006, 95, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W.; et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Guo, W.; Wang, J.; Gao, J.; Peng, W.; Gu, J. Identification and high-throughput quantification of baicalein and its metabolites in plasma and urine. J. Ethnopharmacol. 2023, 301, 115853. [Google Scholar] [CrossRef]
- Pang, H.; Xue, W.; Shi, A.; Li, M.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Xiao, W.; He, G.; et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016, 36, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z.; Dong, R. Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin. Transl. Sci. 2021, 14, 2017–2024. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Gol’dberg, V.E.; Ryzhakov, V.M.; Matiash, M.G.; Stepovaia, E.A.; Boldyshev, D.A.; Litvinenko, V.I.; Dygaĭ, A.M. Dry extract of Scutellaria baicalensis as a hemostimulant in antineoplastic chemotherapy in patents with lung cancer. Eksperimental’naia I Klin. Farmakol. 1997, 60, 28–30. [Google Scholar]
- Udut, E.V.; Zhdanov, V.V.; Gur’iantseva, L.A.; Minakova, M.; Dygaĭ, A.M. Mechanisms of the erythropoiesis-stimulating effect of skullcap (Scutellaria baicalensis) extract. Eksperimental’naia I Klin. Farmakol. 2005, 68, 43–45. [Google Scholar]
- Simmons, C.W.; VanderGheynst, J.S. Transient co-expression of post-transcriptional gene silencing suppressors and beta-glucuronidase in harvested lettuce leaf tissue does not improve recombinant protein accumulation in planta. Biotechnol. Lett. 2007, 29, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Zhao, J.; Song, Q.H.; Zheng, L.H.; Fan, C.; Liu, T.T.; Bao, Y.L.; Sun, L.G.; Zhang, L.B.; Li, Y.X. Virtual screening and experimental validation of novel histone deacetylase inhibitors. BMC Pharmacol. Toxicol. 2016, 17, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, J.; Mohamed, A.C.; Han, Y.; Guo, L.; Yang, L. Efficient capillary electrophoresis separation and determination of free amino acids in beer samples. Electrophoresis 2014, 35, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xie, X.Z.; Zhou, Y.W.; Jiang, Y.Y.; Chen, Z. Effects of γ-aminobutyric acid on the thymus tissue structure, antioxidant activity, cell apoptosis, and cytokine levels in chicks under heat stress. Czech J. Anim. Sci. 2016, 61, 539–550. [Google Scholar] [CrossRef] [Green Version]


| Enzyme | Km (mM) | Kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| STS1-HP | 3.52 ± 0.44 | 67.7 ± 1.31 | (1.95 ± 0.21) × 104 |
| STS2-HP | 5.39 ± 0.52 | 0.27 ± 0.005 | 50.51 ± 4.51 |
| Goldscore | ASP | |
|---|---|---|
| BC lock with STS1-HP | 51.64 | 9.82 |
| BC lock with STS2-HP | 54.33 | 37.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, Y.; Wang, A.; Zhang, J.; Jia, C.; Yu, C.; Song, Z.; Wang, S.; Liu, L.; Yi, J.; et al. STS1 and STS2 Phosphatase Inhibitor Baicalein Enhances the Expansion of Hematopoietic and Progenitor Stem Cells and Alleviates 5-Fluorouracil-Induced Myelosuppression. Int. J. Mol. Sci. 2023, 24, 2987. https://doi.org/10.3390/ijms24032987
Li N, Wang Y, Wang A, Zhang J, Jia C, Yu C, Song Z, Wang S, Liu L, Yi J, et al. STS1 and STS2 Phosphatase Inhibitor Baicalein Enhances the Expansion of Hematopoietic and Progenitor Stem Cells and Alleviates 5-Fluorouracil-Induced Myelosuppression. International Journal of Molecular Sciences. 2023; 24(3):2987. https://doi.org/10.3390/ijms24032987
Chicago/Turabian StyleLi, Na, Yanhong Wang, Anqing Wang, Jing Zhang, Chaoran Jia, Chunlei Yu, Zhenbo Song, Shuyue Wang, Lei Liu, Jingwen Yi, and et al. 2023. "STS1 and STS2 Phosphatase Inhibitor Baicalein Enhances the Expansion of Hematopoietic and Progenitor Stem Cells and Alleviates 5-Fluorouracil-Induced Myelosuppression" International Journal of Molecular Sciences 24, no. 3: 2987. https://doi.org/10.3390/ijms24032987





