Endometrial Microbiota and Immune Tolerance in Pregnancy
Abstract
:1. Introduction
- Provide protection from tissue infection by competing with pathogenic bacteria;
2. Endometrial Microbiota and Its Variation during Lifespan
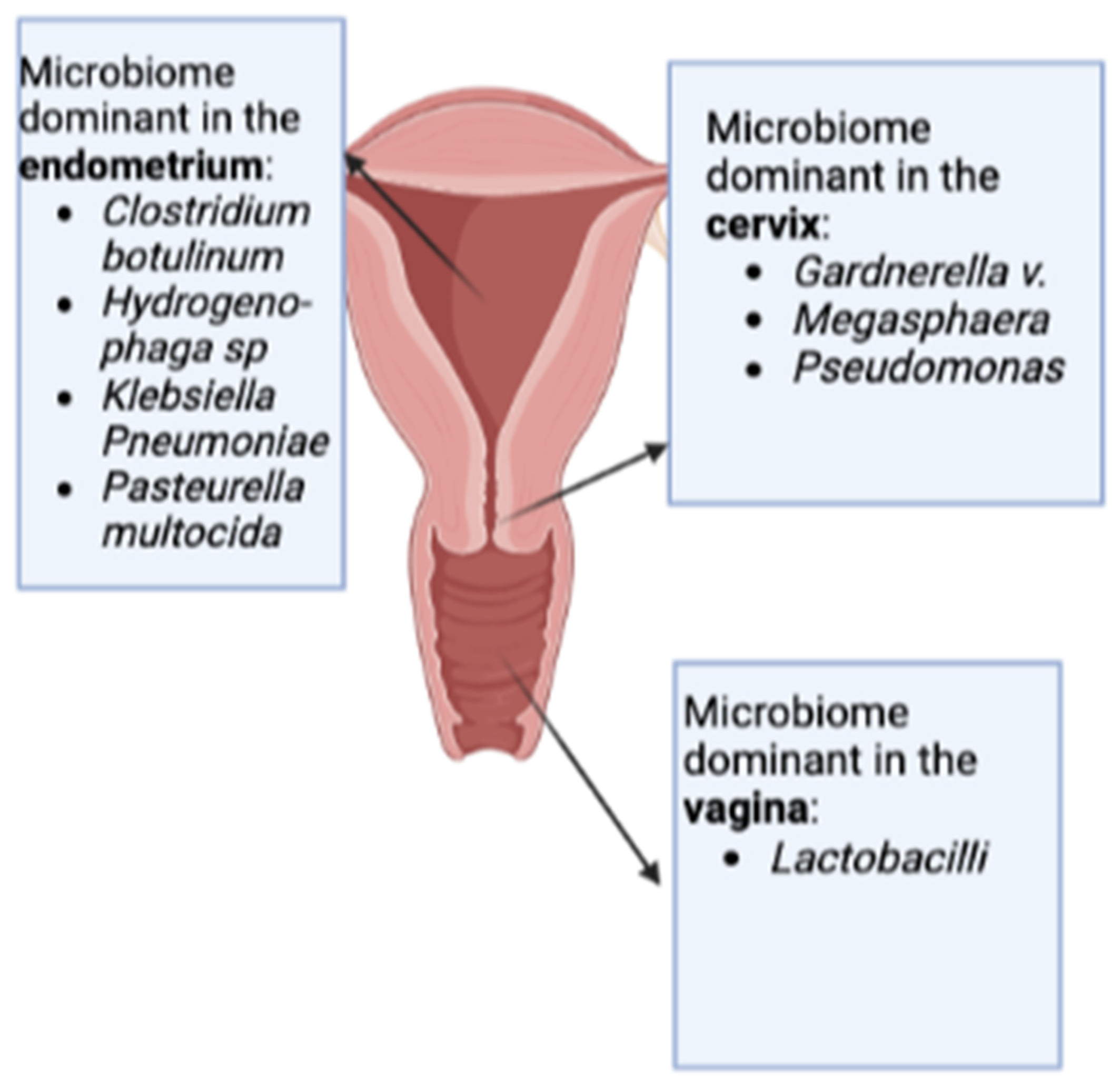
2.1. The Beginning—Initial Studies on the Topic: Emerging Differences Comparing Vaginal and Endometrial Microbiomes
2.2. Results from an Infertile Population and Women Undergoing ART Therapy
| Author, Year | Country | Number and Type of Patients | Sample (Method) | Findings |
|---|---|---|---|---|
| Moore, 2000 [32] | USA | 91 women undergoing IVF | ET (Embryo transfer catheter tips) | Live birth rate (LBR):
|
| Moreno, 2016 [24] | Spain | 35 infertile women undergoing IVF | EF (Aspiration with catheter) | 3 poor DNA quality 17 LDM 15 NLDM associated with: significantly lower implantation, pregnancy, ongoing pregnancy and LBR |
| Tao, 2017 [25] | USA | 70 women undergoing IVF | ET (Embryo transfer catheter tips) | 90% Lactobacillus abundance in 33 patients (47.1%) |
| Kyono, 2018 [26] | Japan | 102 infertile women:
| EF (IUI catheter) | 90% Lactobacillus abundance in:
|
| Wee, 2018 [27] | Australia | 16 infertile women; 15 controls | ET (Biopsy during hysteroscopy) | Lactobacillus more abundant in controls |
| Kyono, 2019 [30] | Japan | 92 women undergoing IVF | EF (IUI catheter) | LDM in 47 cases (51.1%); NLDM in 45 cases (48.9%) |
| Kitaya, 2019 [33] | Japan | 28 women with history of RIF (RIF group) vs. 18 infertile women undergoing IVF (non-RIF group) | EF (Curette) | Higher α-diversity in endometrium compared to vagina in both groups LDM:
|
| Carosso, 2020 [28] | Italy | 15 infertile women undergoing IVF pre- and post-COS | ET (Embryo transfer catheter tips) | Lactobacillus reduced post-COS Prevotella significantly increased post-COS Atopobium significantly increased post-COS |
| Kadogami 2020 [34] | Japan | 392 women with history of RIF | EF (Pipette) | LD:
|
| Riganelli, 2020 [35] | Italy | 34 infertile women undergoing ART | ET (Pipelle catheter covered by the IUI catheter) | Non-pregnant women (30/34) with increased levels of Lactobacillus species and significantly increased relative abundances of Kocuria dechangensis |
| Diaz-Martinez, 2021 [36] | Spain | 48 infertile women undergoing IVF | ET (Brush) | Delftia spp., Anaerobacillus spp., and Ralstonia spp. more abundant in endometrium compared to vagina Lactobacillus spp., Gardnerella spp., Burkholderia spp., and Anaerobacillus spp. more abundant in pregnant women Streptococcus spp., Ralstonia spp., Prevotella spp., and Delftia spp. more abundant in non-pregnant women In RIF patients:
|
| Ichiyama, 2021 [37] | Japan | 145 women with history of RIF 21 controls | ET (Pipette) | 14 genera, including Atopobium, Burkholderia, Delftia, Gardnerella, and Prevotella, in RIF group vs. Lactobacillus abundances similar in RIF and control groups |
| Chen 2021, [38] | China | 94 infertile women undergoing IVF:
| EF (IUI catheter) | Women affected by chronic endometritis (CE):
|
| Moreno, 2022 [29] | Spain, USA, Turkey, Canada, Japan, Mexico, Malaysia, Argentina | 342 infertile women undergoing IVF | ET and EF (Cannula of Cornier and catheter, respectively) | Lactobacillus strongly correlated with LBR Gardnerella, Klebsiella, and Streptococcus significantly more abundant in non-pregnant patients Klebsiella and Staphylococcus correlated with clinical miscarriage |
| Reschini, 2022 [31] | Italy | 53 women undergoing IVF | EF (Double-lumen embryo transfer catheter) | Lactobacillus most represented in 16 patients (30%) 90% Lactobacillus abundance in 4 patients (8%) Higher endometrial biodiversity among pregnant women |
| Bednarska-Czerwińska, 2022 [39] | Poland | 142 infertile women undergoing IVF | EF (Endometrial swab) | 22 bacterial strains identified:
|
| Sezer, 2022 [40] | Turkey | 26 women with unexplained infertility vs. 26 controls (age-matched) | EF (Endometrial swabs) | QLac/TBM (cutoff of 70.5%) had sensitivity of 84.6% in the infertility diagnosis |
| Kitaya, 2022 [41] | Japan | 117 women with history of RIF vs. 55 infertile women (non-RIF group) | EF (Endometrial curette biopsy) | NLDM:
|
| Keburiya, 2022 [42] | Russia | 130 infertile women undergoing IVF:
| ET (Embryo transfer catheter tips) | Group I:
|
2.3. Microbiome Sampling Direct from the Uterine Cavity during Surgery
2.4. First Meta-Transcriptomic Analysis
2.5. Age and Hormonal Influence on Endometrial Microbiome Composition
3. Endometrial Receptivity and Immune Tolerance in Pregnancy
3.1. Introduction
3.2. Immune Cells and Cytokine Involvement According to Reproductive Phase and Gestational Age
3.3. Uterine NK Cells
3.4. Fetal Escape from Alloantigen Response
4. How Could Microbiota Modulate Immune Tolerance during Pregnancy?
4.1. Endometrial Microbiome during Embryo Implantation
4.2. Role of Bacteroides Fragilis
4.3. Role of Lactobacilli
5. Endometrial Microbiota Dysbiosis and Adverse Reproductive Outcomes
5.1. Recurrent Implantation Failure (RIF)
5.2. Recurrent Pregnancy Loss (RPL)
5.3. Preterm Birth (PTB)
6. Methodological and Sampling Considerations
6.1. Introduction
- -
- A double-sheathed embryo transfer catheter;
- -
- An intrauterine insemination catheter;
- -
- A transcervical sheathed brush device.
- (1)
- Sampling blank controls, to allow the detection of contaminant DNA introduced during the sampling procedure (e.g., swabs and plastic bags) and any tools used to store or to transport the samples from the sampling site to the laboratory;
- (2)
- DNA-extraction blank controls, to monitor contaminant DNA contents in the process from extraction to sequencing;
- (3)
- No-template amplification controls, to monitor contaminant DNA in the process of library preparation and sequencing.
- (1)
- DNA-extraction positive controls, to monitor DNA-extraction efficiency: the advice is to use a positive control of known concentration that is relevant to your study and experimental questions;
- (2)
- Positive amplification controls, using a titration of DNA from a known organism type to be processed during the library preparation step.
6.2. Fluid or Tissue?
6.3. Storing Methods
6.4. Study Planning
- To take samples at identical time points;
- To take samples longitudinally from the same subject (especially if in pregnancy);
- To use PERMANOVA for the statistical analysis;
- To make publicly available the following details: the characteristics of the study population, the sample type, the collection method, and the data processing and analysis workflow.
6.5. Ethical Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIH HMP Working Group; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.; Bonazzi, V.; McEwen, J.; Wetterstrand, K.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, P.K.; Teisala, K.; Punnonen, R.; Miettinen, A.; Lehtinen, M.; Paavonen, J. Anatomic sites of upper genital tract infection. Obstet. Gynecol. 1985, 66, 384–390. Available online: https://pubmed.ncbi.nlm.nih.gov/3160987/ (accessed on 24 November 2022). [PubMed]
- Sirota, I.; Zarek, S.M.; Segars, J.H. Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology. Semin. Reprod. Med. 2014, 32, 35. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Hooper, L.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.-Y.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Ilicic, M.; Zakar, T.; Paul, J.W. The Regulation of Uterine Function during Parturition: An Update and Recent Advances. Reprod. Sci. 2020, 27, 3–28. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [Green Version]
- Idelevich, A.; Vilella, F. Mother and Embryo Cross-Communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; di Simone, N.; Campagnolo, L.; Fazleabas, A. Clinical consequences of defective decidualization. Tissue Cell 2021, 72, 101586. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Rashid, N.A.; Lalitkumar, S.; Lalitkumar, P.G.; Gemzell-Danielsson, K. Endometrial receptivity and human embryo implantation. Am. J. Reprod. Immunol. 2011, 66 (Suppl. 1), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kyono, K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J. Assist. Reprod. Genet. 2019, 36, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Laniewski, P.; Gomez, A.; Hire, G.; So, M.; Herbst-Kralovetz, M.M. Human Three-Dimensional Endometrial Epithelial Cell Model to Study Host Interactions with Vaginal Bacteria and Neisseria gonorrhoeae. Infect. Immun. 2017, 85, e01049-16. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Eschenbach, D.A.; Rosene, K.; Tompkins, L.S.; Watkins, H.; Gravett, M.G. Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. J. Infect. Dis. 1986, 153, 1038–1045. [Google Scholar] [CrossRef]
- Hemsell, D.L.; Obregon, V.L.; Heard, M.C. Endometrial bacteria in asymptomatic, nonpregnant women. J. Reprod. Med. 1989, 34, 872–874. Available online: https://europepmc.org/article/med/2585386 (accessed on 30 November 2022).
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, M.F.; Werne, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T., III; Rajchel, J.; Bedard, J.; Newby, R., Jr.; Scott, R.T.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microb. J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.; et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Reschini, M.; Benaglia, L.; Ceriotti, F.; Borroni, R.; Ferrari, S.; Castiglioni, M.; Guarneri, D.; Porcaro, L.; Vigano’, P.; Somigliana, E.; et al. Endometrial microbiome: Sampling, assessment, and possible impact on embryo implantation. Sci. Rep. 2022, 12, 8467. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef] [PubMed]
- Kadogami, D.; Nakaoka, Y.; Morimoto, Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod. Biol. 2020, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Diaz-martínez, M.D.C.; Bernabeu, A.; Lledó, B.; Carratalá-Munuera, C.; Quesada, J.; Lozano, F.; Ruiz, V.; Morales, R.; Llácer, J.; Ten, J.; et al. Impact of the Vaginal and Endometrial Microbiome Pattern on Assisted Reproduction Outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef]
- Chen, W.; Wei, K.; He, X.; Wei, J.; Yang, L.; Li, L.; Chen, T.; Tan, B. Identification of Uterine Microbiota in Infertile Women Receiving in vitro Fertilization with and without Chronic Endometritis. Front. Cell. Dev. Biol. 2021, 9, 693267. [Google Scholar] [CrossRef]
- ABednarska-Czerwińska; Czerwiński, M.; Morawiec, E.; Łach, A.; Ziaja, A.; Kusaj, A.; Strączyńska, P.; Sagan, D.; Boroń, D.; Grabarek, B.O. Marking the Profile of the Microflora of the Endometrium and Uterine Cervix in Women as a Potential Factor Determining the Effectiveness of In Vitro Fertilization. J. Clin. Med. 2022, 11, 3348. [Google Scholar] [CrossRef]
- Sezer, O.; Çalışkan, C.S.; Celik, S.; Kilic, S.S.; Kuruoglu, T.; Ustun, G.U.; Yurtcu, N. Assessment of vaginal and endometrial microbiota by real-time PCR in women with unexplained infertility. J. Obstet. Gynaecol. Res. 2022, 48, 129–139. [Google Scholar] [CrossRef]
- Kitaya, K.; Ishikawa, T. Genital tract dysbiosis in infertile women with a history of repeated implantation failure and pilot study for reproductive outcomes following oral enteric coating lactoferrin supplementation. Arch. Gynecol. Obstet. 2022, 306, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Keburiya, L.K.; Smolnikova, V.Y.; Priputnevich, T.V.; Muravieva, V.V.; Gordeev, A.B.; Trofimov, D.Y.; Shubina, E.S.; Kochetkova, T.O.; Rogacheva, M.S.; Kalinina, E.A.; et al. Does the uterine microbiota affect the reproductive outcomes in women with recurrent implantation failures? BMC Womens Health 2022, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, C.; Wei, W.; Wang, Z.; Dai, J.; Hao, L.; Song, L.; Zhang, X.; Zeng, L.; Du, H.; et al. The metagenome of the female upper reproductive tract. Gigascience 2018, 7, giy107. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Ceci, O.; Manzari, C.; Fosso, B.; Volpicella, M.; Ferrari, A.; Fiorella, P.; Pesole, G.; Cicinell, E.; Ceci, L.R. Human Endometrial Microbiota at Term of Normal Pregnancies. Genes 2019, 10, 971. [Google Scholar] [CrossRef]
- Younge, N.; McCann, J.R.; Ballard, J.; Plunkett, C.; Akhtar, S.; Araújo-Pérez, F.; Murtha, A.; Brandon, D.; Seed, P.C. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 2019, 4, e127806. [Google Scholar] [CrossRef]
- Sola-Leyva, A.; Andrés-León, E.; Molina, N.; Terron-Camero, L.C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gonzalvo, M.C.; Sánchez, R.; Ruíz, S.; Martínez, L.; et al. Mapping the entire functionally active endometrial microbiota. Hum. Reprod. 2021, 36, 1021–1031. [Google Scholar] [CrossRef]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Ma, X.; Du, L.; Jia, Z.; Cui, X.; Yu, L.; Yang, J.; Xiao, L.; Zhang, B.; et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021, 12, 4191. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Brooks, J.P.; Edwards, D.J.; Blithe, D.L.; Fettweis, J.M.; Serrano, M.G.; Sheth, N.U.; Strauss, J.F.; Buck, G.A.; Jefferson, K.K. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 2017, 95, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achilles, S.L.; Austin, M.N.; Meyn, L.A.; Mhlanga, F.; Chirenje, Z.M.; Hillier, S.L. Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet. Gynecol. 2018, 218, 622.e1–622.e10. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Luongo, F.P.; Haxhiu, A.; Piomboni, P.; Luddi, A. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell 2021, 73, 101656. [Google Scholar] [CrossRef] [PubMed]
- Gnainsky, Y.; Granot, I.; Aldo, P.; Barash, A.; Or, Y.; Mor, G.; Dekel, N. Biopsy-induced inflammatory conditions improve endometrial receptivity: The mechanism of action. Reproduction 2015, 149, 75–85. [Google Scholar] [CrossRef]
- Soares, M.J.; Chakraborty, D.; Kubota, K.; Renaud, S.J.; Rumi, M.A.K. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int. J. Dev. Biol. 2014, 58, 247–259. [Google Scholar] [CrossRef]
- Tilburgs, T.; Roelen, D.L.; van der Mast, B.J.; de Groot-Swings, G.M.; Kleijburg, C.; Scherjon, S.A.; Claas, F. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 2008, 180, 5737–5745. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Ernerudh, J. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am. J. Reprod. Immunol. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Gori, S.; Soczewski, E.; Fernández, L.; Grasso, E.; Gallino, L.; Merech, F.; Colado, A.; Borge, M.; Leirós, C.P.; Salamone, G.; et al. Decidualization Process Induces Maternal Monocytes to Tolerogenic IL-10-Producing Dendritic Cells (DC-10). Front. Immunol. 2020, 11, 1571. [Google Scholar] [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M. Immunological determinants of implantation success. Int. J. Dev. Biol. 2014, 58, 205–217. [Google Scholar] [CrossRef]
- van der Zwan, A.; Bi, K.; Norwitz, E.R.; Crespo, Â.C.; Claas, F.H.J.; Strominger, J.L.; Tilburgs, T. Mixed signature of activation and dysfunction allows human decidual CD8+ T cells to provide both tolerance and immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntire, R.H.; Hunt, J.S. Antigen presenting cells and HLA-G—A review. Placenta 2005, 26 (Suppl. A), S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Scaife, P.J.; Bulmer, J.N.; Robson, S.C.; Innes, B.A.; Searle, R.F. Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol. Reprod. 2006, 75, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, L.; Ritschel, S.; Bertoja, A.Z.; Fest, S.; Hontsu, S.; Ueha, S.; Matsushima, K.; Leber, J.; et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur. J. Immunol. 2006, 36, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Taams, L.S.; van Amelsfort, J.M.R.; Tiemessen, M.M.; Jacobs, K.M.G.; Jong, E.C.; Akbar, A.N.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum. Immunol. 2005, 66, 222–230. [Google Scholar] [CrossRef]
- Lim, H.W.; Hillsamer, P.; Banham, A.H.; Kim, C.H. Cutting edge: Direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 2005, 175, 4180–4183. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, M.Q.; Duan, J.; Fan, D.X.; Jin, L.P. IL-25 promotes Th2 bias by upregulating IL-4 and IL-10 expression of decidual γδT cells in early pregnancy. Exp. Ther. Med. 2018, 15, 1855–1862. [Google Scholar] [CrossRef]
- RSearle, F.; Jones, R.K.; Bulmer, J.N. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol. Reprod. 1999, 60, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Moretta, L.; Moretta, A.; Mingari, M.C. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011, 32, 517–523. [Google Scholar] [CrossRef]
- Benichou, G.; Yamada, Y.; Aoyama, A.; Madsen, J.C. Natural killer cells in rejection and tolerance of solid organ allografts. Curr. Opin. Organ. Transplant. 2011, 16, 47–53. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Ishitani, A.; Sageshima, N.; Lee, N.; Dorofeeva, N.; Hatake, K.; Marquardt, H.; Geraghty, D.E. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J. Immunol. 2003, 171, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Bianchi, R.; Pallotta, M.T.; Orabona, C.; Albini, E.; Iacono, A.; Belladonna, M.L.; Vacca, C.; Fallarino, F.; Macchiarulo, A.; et al. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity 2017, 46, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Guleria, I.; Sayegh, M.H. Maternal acceptance of the fetus: True human tolerance. J. Immunol. 2007, 178, 3345–3351. [Google Scholar] [CrossRef]
- Rogers, A.M.; Boime, I.; Connolly, J.; Cook, J.; Russell, J. Maternal—Fetal tolerance is maintained despite transgene—Driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur. J. Immunol. 1998, 28, 3479–3487. [Google Scholar] [CrossRef]
- Erlebacher, A.; Vencato, D.; Price, K.A.; Zhang, D.; Glimcher, L.H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Investig. 2007, 117, 1399–1411. [Google Scholar] [CrossRef]
- Erlebacher, A. Immune surveillance of the maternal/fetal interface: Controversies and implications. Trends Endocrinol. Metab. 2010, 21, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000, 101, 455–458. [Google Scholar] [CrossRef]
- Collins, M.K.; Tay, C.S.; Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Investig. 2009, 119, 2062–2073. [Google Scholar] [CrossRef]
- Barnea, E.R.; Hayrabedyan, S.; Todorova, K.; Almogi-Hazan, O.; Or, R.; Guingab, J.; McElhinney, J.; Fernandez, N.; Barder, T. PreImplantation factor (PIF*) regulates systemic immunity and targets protective regulatory and cytoskeleton proteins. Immunobiology 2016, 221, 778–793. [Google Scholar] [CrossRef]
- Di Simone, N.; Di Nicuolo, F.; Marana, R.; Castellani, R.; Ria, F.; Veglia, M.; Scambia, G.; Surbek, D.; Barnea, E.; Mueller, M. Synthetic PreImplantation Factor (PIF) prevents fetal loss by modulating LPS induced inflammatory response. PLoS ONE 2017, 12, e0180642. [Google Scholar] [CrossRef]
- Mueller, M.; Zhou, J.; Yang, L.; Gao, Y.; Wu, F.; Schoeberlein, A.; Surbek, D.; Barnea, E.R.; Paidas, M.; Huang, Y. PreImplantation factor promotes neuroprotection by targeting microRNA let-7. Proc. Natl. Acad. Sci. USA 2014, 111, 13882–13887. [Google Scholar] [CrossRef]
- Barnea, E.R.; Kirk, D.; Todorova, K.; McElhinney, J.; Hayrabedyan, S.; Fernández, N. PIF direct immune regulation: Blocks mitogen-activated PBMCs proliferation, promotes TH2/TH1 bias, independent of Ca(2+). Immunobiology 2015, 220, 865–875. [Google Scholar] [CrossRef]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; García-Gómez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef]
- Frew, L.; Stock, S.J. Antimicrobial peptides and pregnancy. Reproduction 2011, 141, 725–735. [Google Scholar] [CrossRef]
- Radtke, A.L.; Quayle, A.J.; Herbst-Kralovetz, M.M. Microbial products alter the expression of membrane-associated mucin and antimicrobial peptides in a three-dimensional human endocervical epithelial cell model. Biol. Reprod. 2012, 87, 1–10. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazmanian, S.K.; Cui, H.L.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, M.S.; Noronha, F.M.F.; Arruda, M.O.; Costa, P.D.S.; Bomfim, M.R.Q.; Monteiro, A.S.; Ferro, T.A.F.; Fernandes, E.S.; Girón, J.A.; Monteiro-Neto, V. Lactobacillus fermentum ATCC 23271 Displays In vitro Inhibitory Activities against Candida spp. Front. Microbiol. 2016, 7, 1722. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Esmaeili, S.A.; Mahmoudi, M.; Rezaieyazdi, Z.; Sahebari, M.; Tabasi, N.; Sahebkar, A.; Rastin, M. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J. Cell Biochem. 2018, 119, 7865–7872. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Li, B.; Sun, Y.; Jin, T.; Feng, M.; Ni, Y.; Liu, M. Lactobacillus rhamnosus GR-1 Alleviates Escherichia coli-Induced Inflammation via NF-κB and MAPKs Signaling in Bovine Endometrial Epithelial Cells. Front. Cell Infect. Microbiol. 2022, 12, 809674. [Google Scholar] [CrossRef]
- Li, H.; Zang, Y.; Wang, C.; Li, H.; Fan, A.; Han, C.; Xue, F. The Interaction Between Microorganisms, Metabolites, and Immune System in the Female Genital Tract Microenvironment. Front. Cell Infect. Microbiol. 2020, 10, 609488. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Challis, J.R.G.; Reid, G.; Kim, S.O.; Bocking, A.D. Probiotic Lactobacillus rhamnosus GR-1 supernatant prevents lipopolysaccharide-induced preterm birth and reduces inflammation in pregnant CD-1 mice. Am. J. Obstet. Gynecol. 2014, 211, 44.e1–44.e12. [Google Scholar] [CrossRef]
- Kim, B.; Shynlova, O.; Lye, S. Probiotic Lactobacillus rhamnosus GR-1 is a unique prophylactic agent that suppresses infection-induced myometrial cell responses. Sci. Rep. 2019, 9, 4698. [Google Scholar] [CrossRef]
- Ventimiglia, M.S.; Valeff, N.J.; Albán, M.P.; Paturlanne, J.M.; Juriol, L.; Quadrana, F.; Cecotti, M.; Malamud, M.; Dibo, M.J.; Serradell, M.D.L.; et al. Probiotic Lactobacillus kefiri prevents endotoxin-induced preterm birth and stillbirth in mice. Reproduction 2021, 161, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Escobes, I.; Iloro, I.; Osinalde, N.; Corral, B.; Ibañez-Perez, J.; Exposito, A.; Prieto, B.; Elortza, F.; Matorras, R. Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum. Reprod. 2018, 33, 1898–1906. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am. J. Reprod. Immunol. 2014, 72, 386–391. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open. 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Van Dijk, M.M.; Kolte, A.M.; Limpens, J.; Kirk, E.; Quenby, S.; van Wely, M.; Goddijn, M. Recurrent pregnancy loss: Diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum. Reprod. Update 2020, 26, 356–367. [Google Scholar] [CrossRef]
- Carp, H.J. Recurrent Pregnancy Loss Causes, Controversies, and Treatment, 3rd ed.; CRC Press: London, UK, 2020; Volume 29, Available online: https://www.routledge.com/Recurrent-Pregnancy-Loss-Causes-Controversies-and-Treatment/Carp/p/book/9781138325654 (accessed on 19 January 2023).
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: A nested case-control study. Reprod. Biomed. Online 2022, 45, 1021–1031. [Google Scholar] [CrossRef]
- Liu, F.-T.; Yang, S.; Yang, Z.; Zhou, P.; Peng, T.; Yin, J.; Ye, Z.; Shan, H.; Yu, Y.; Li, R. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol. Spectr. 2022, 10, e0046222. [Google Scholar] [CrossRef] [PubMed]
- Masucci, L.; D’Ippolito, S.; De Maio, F.; Quaranta, G.; Mazzarella, R.; Bianco, D.M.; Castellani, R.; Inversetti, A.; Sanguinetti, M.; Gasbarrini, A.; et al. Celiac Disease Predisposition and Genital Tract Microbiota in Women Affected by Recurrent Pregnancy Loss. Nutrients 2023, 15, 221. [Google Scholar] [CrossRef]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jørgensen, J.S.; Menon, R. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef]
- Molina, N.M.; Sola-Leyva, A.; Haahr, T.; Aghajanova, L.; Laudanski, P.; Castilla, J.A.; Altmäe, S. Analysing endometrial microbiome: Methodological considerations and recommendations for good practice. Hum. Reprod. 2021, 36, 859–879. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, K.W.K.; Ko, E.Y.-L.; Chen, X.; Huang, J.; Tsui, S.K.-W.; Li, T.C.; Chim, S.S.-C. Systematic Comparison of Bacterial Colonization of Endometrial Tissue and Fluid Samples in Recurrent Miscarriage Patients: Implications for Future Endometrial Microbiome Studies. Clin. Chem. 2018, 64, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Timing | Immune Cell Assets | Cytokines |
|---|---|---|
| Mid-ovulatory | Increasing levels of:
| |
| Implantation | Increasing numbers of NK cells [55] (progesterone-mediated). Increasing numbers of Treg cells [56,57]. Shift to M2 macrophages [58], induced by growth factors released by the trophoblast uDCs expressing fewer CD83 and CD86 costimulatory molecules [59] | Thanks to progesterone, decreasing levels of GM-CSF and IL-1 [60] and increasing levels of IL-8 [60]. Thanks to estrogen, increasing levels of:
|
| First and second trimester | Treg effects [64,65,66,67,68,69] (peak during second trimester):
| Treg effects [64,65,66,67,68,69] Increasing levels of:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inversetti, A.; Zambella, E.; Guarano, A.; Dell’Avanzo, M.; Di Simone, N. Endometrial Microbiota and Immune Tolerance in Pregnancy. Int. J. Mol. Sci. 2023, 24, 2995. https://doi.org/10.3390/ijms24032995
Inversetti A, Zambella E, Guarano A, Dell’Avanzo M, Di Simone N. Endometrial Microbiota and Immune Tolerance in Pregnancy. International Journal of Molecular Sciences. 2023; 24(3):2995. https://doi.org/10.3390/ijms24032995
Chicago/Turabian StyleInversetti, Annalisa, Enrica Zambella, Alice Guarano, Marinella Dell’Avanzo, and Nicoletta Di Simone. 2023. "Endometrial Microbiota and Immune Tolerance in Pregnancy" International Journal of Molecular Sciences 24, no. 3: 2995. https://doi.org/10.3390/ijms24032995





