The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer
Abstract
1. Introduction
2. Results
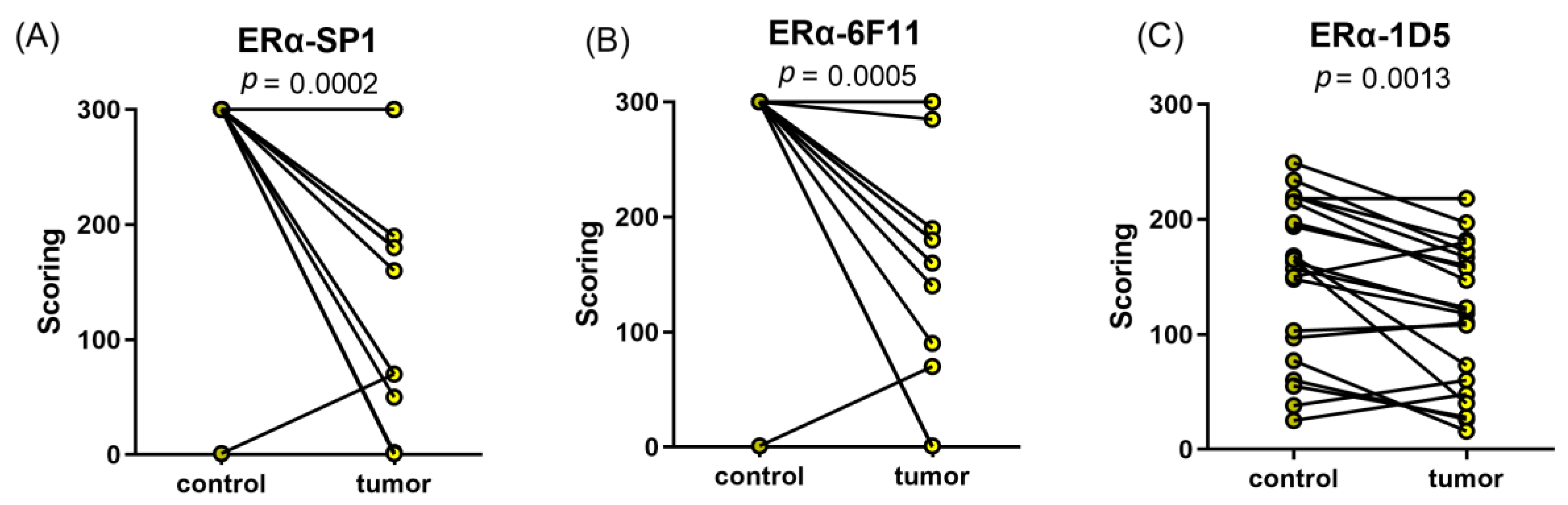
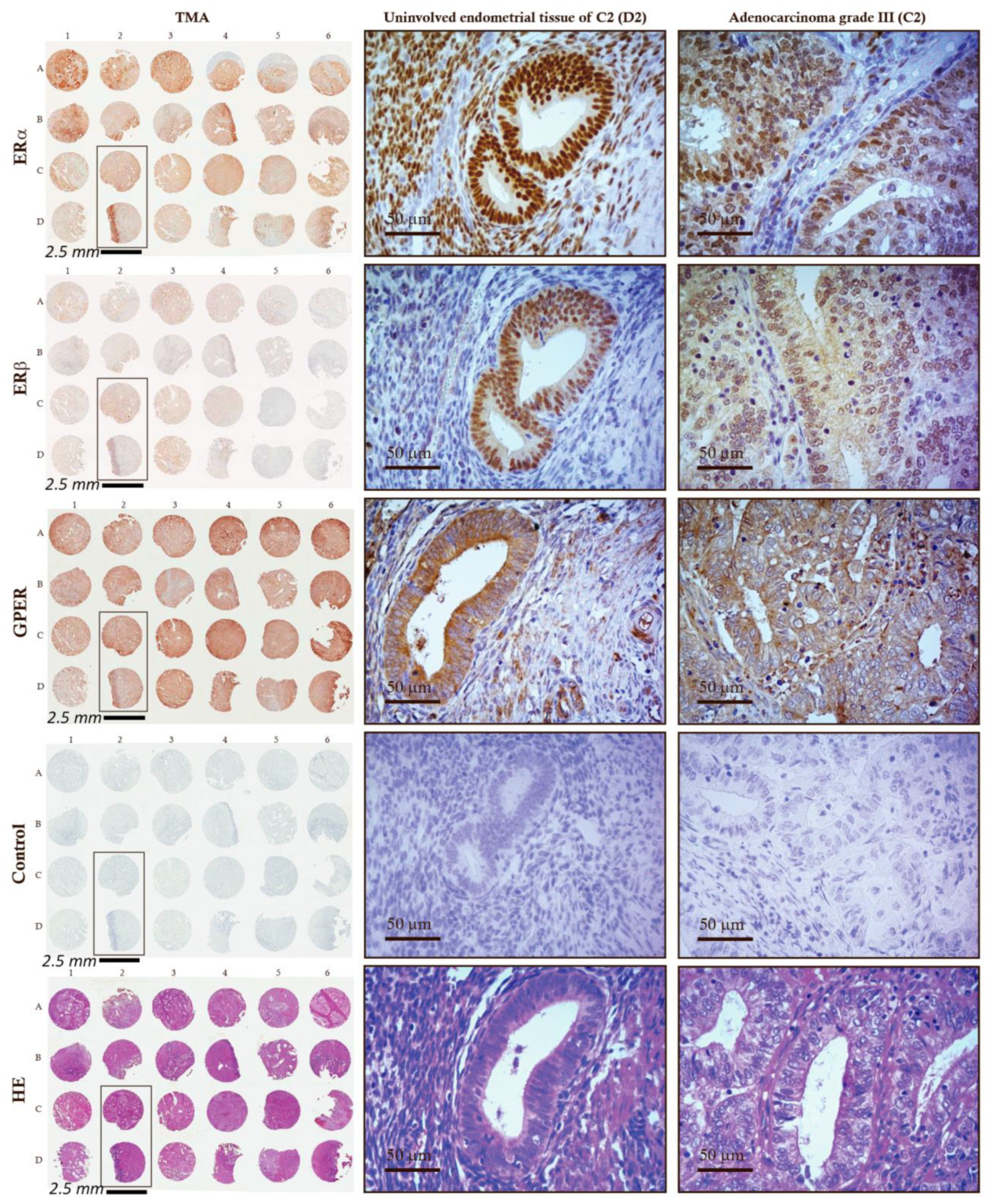
2.1. Lower mRNA and Protein Levels and IHC Scores of ERα in EC Tissues
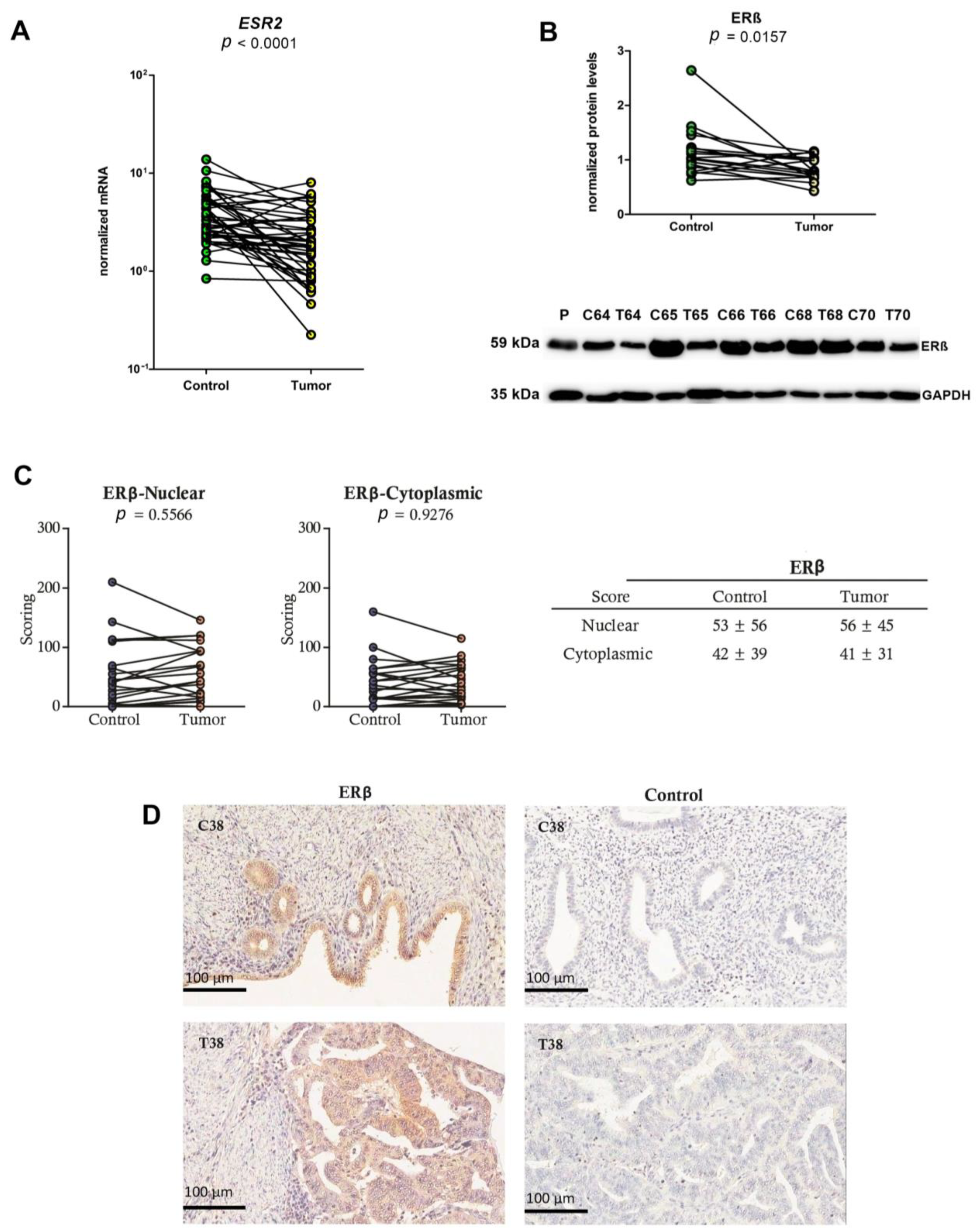
2.2. Lower mRNA and Protein Levels and Unchanged IHC Scores of ERβ in EC Tissues
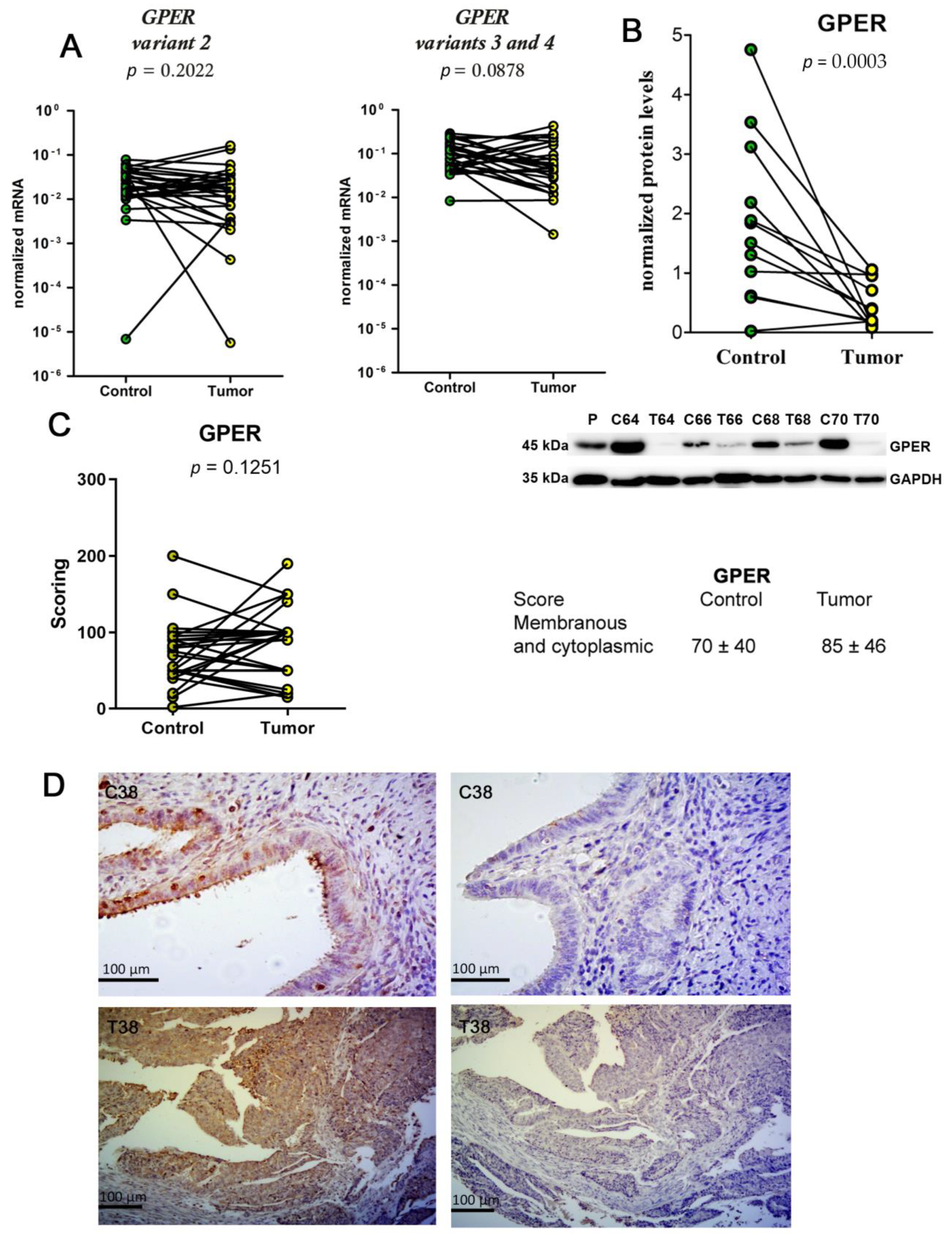
2.3. Unchanged mRNA Levels and Decreased Protein Levels of GPER in EC Tissue
2.4. ERα, ERβ, and GPER Are Co-Expressed in EC Tissue and Correlate in Their mRNA and Protein Levels
2.5. IHC Levels of ERα and GPER in Endometrioid EC Are Not Associated with Survival
3. Discussion
4. Materials and Methods
4.1. Endometrial Tissue
4.2. RNA Isolation and qPCR
4.3. Western Blotting
4.4. Immunohistochemistry (IHC)
4.5. Survival Data
4.6. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Prim. 2021, 7, 88. [Google Scholar] [CrossRef]
- Inoue, M. Current molecular aspects of the carcinogenesis of the uterine endometrium. Int. J. Gynecol. Cancer 2001, 11, 339–348. [Google Scholar] [CrossRef]
- Samarnthai, N.; Hall, K.; Yeh, I.T. Molecular profiling of endometrial malignancies. Obstet. Gynecol. Int. 2010, 2010, 162363. [Google Scholar] [CrossRef]
- Berstein, L.M.; Tchernobrovkina, A.E.; Gamajunova, V.B.; Kovalevskij, A.J.; Vasilyev, D.A.; Chepik, O.F.; Turkevitch, E.A.; Tsyrlina, E.V.; Maximov, S.J.; Ashrafian, L.A.; et al. Tumor estrogen content and clinico-morphological and endocrine features of endometrial cancer. J. Cancer Res. Clin. Oncol. 2003, 129, 245–249. [Google Scholar] [CrossRef]
- Wan, J.; Gao, Y.; Zeng, K.; Yin, Y.; Zhao, M.; Wei, J.; Chen, Q. The levels of the sex hormones are not different between type 1 and type 2 endometrial cancer. Sci. Rep. 2016, 6, 39744. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Trabert, B.; Anderson, G.L.; Falk, R.T.; Felix, A.S.; Fuhrman, B.J.; Gass, M.L.; Kuller, L.H.; Pfeiffer, R.M.; Rohan, T.E.; et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Barakat, R.R. Screening and the prevention of gynecologic cancer: Endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 363–377. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol. Cell. Endocrinol. 2013, 381, 124–139. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. Methods Mol. Biol. 2016, 1366, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Taylor, S.E.; Martin-Hirsch, P.L.; Martin, F.L. Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett. 2010, 288, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Adlanmerini, M.; Solinhac, R.; Abot, A.; Fabre, A.; Raymond-Letron, I.; Guihot, A.L.; Boudou, F.; Sautier, L.; Vessières, E.; Kim, S.H.; et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA 2014, 111, E283–E290. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.; Pesiri, V.; Leclercq, G.; Marino, M.; Acconcia, F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol. Endocrinol. 2012, 26, 762–774. [Google Scholar] [CrossRef]
- Matthews, J.; Gustafsson, J.A. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Membrane oestrogen receptor alpha signalling to cell functions. J. Physiol. 2009, 587, 5019–5023. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor. Front. Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Błachnio-Zabielska, A.; Walentowicz-Sadłecka, M.; Grabiec, M.; Knapp, P.A. Expression of estrogen receptors (α, β), cyclooxygenase-2 and aromatase in normal endometrium and endometrioid cancer of uterus. Adv. Med. Sci. 2013, 58, 96–103. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Wang, D.; Hu, L.; Zhang, G.; Zhang, L.; Chen, C. G protein-coupled receptor 30 in tumor development. Endocrine 2010, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142. [Google Scholar] [PubMed]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef]
- Hwang, N.M.; Stabile, L.P. Estrogen Receptor ß in Cancer: To ß(e) or not to ß(e)? Endocrinology 2021, 162, bqab162. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.A. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Kamal, A.M.; Bulmer, J.N. Estrogen receptor β: The guardian of the endometrium. Hum. Reprod. Update 2015, 21, 174–193. [Google Scholar] [CrossRef]
- Ranhotra, H.S. Estrogen-related receptor alpha and cancer: Axis of evil. J. Recept. Signal Transduct. Res. 2015, 35, 505–508. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Yoriki, K.; Mori, T.; Kokabu, T.; Matsushima, H.; Umemura, S.; Tarumi, Y.; Kitawaki, J. Estrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 2019, 9, 6697. [Google Scholar] [CrossRef]
- Gustafsson, J.A. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol. Sci. 2003, 24, 479–485. [Google Scholar] [CrossRef]
- Böttner, M.; Thelen, P.; Jarry, H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2014, 139, 245–251. [Google Scholar] [CrossRef]
- Chen, G.G.; Zeng, Q.; Tse, G.M. Estrogen and its receptors in cancer. Med. Res. Rev. 2008, 28, 954–974. [Google Scholar] [CrossRef]
- Wu, X.; Subramaniam, M.; Negron, V.; Cicek, M.; Reynolds, C.; Lingle, W.L.; Goetz, M.P.; Ingle, J.N.; Spelsberg, T.C.; Hawse, J.R. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J. Cell. Biochem. 2012, 113, 711–723. [Google Scholar] [CrossRef]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.M.; Zieba, A.; Ramström, M.; Söderberg, O.; et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun. 2017, 8, 15840. [Google Scholar] [CrossRef] [PubMed]
- Hawse, J.R.; Carter, J.M.; Aspros, K.G.M.; Bruinsma, E.S.; Koepplin, J.W.; Negron, V.; Subramaniam, M.; Ingle, J.N.; Rech, K.L.; Goetz, M.P. Optimized immunohistochemical detection of estrogen receptor beta using two validated monoclonal antibodies confirms its expression in normal and malignant breast tissues. Breast Cancer Res. Treat. 2020, 179, 241–249. [Google Scholar] [CrossRef]
- Saczko, J.; Michel, O.; Chwiłkowska, A.; Sawicka, E.; Mączyńska, J.; Kulbacka, J. Estrogen Receptors in Cell Membranes: Regulation and Signaling. Adv. Anat. Embryol. Cell Biol. 2017, 227, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; O’Flatharta, C.; Harvey, B.J.; Thomas, W. Membrane ERalpha-dependent activation of PKCalpha in endometrial cancer cells by estradiol. Steroids 2008, 73, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.S.; Zhang, Q.H.; Wang, Z.B.; Li, S.; Yang, C.R.; Fu, X.Q.; Hou, Y.; Wang, Z.Y.; Sheng, J.; Sun, Q.Y. ER-α36, a novel variant of ER-α, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCδ/ERK pathway. PLoS ONE 2010, 5, e15408. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhao, L.; Zhang, G.; Wang, J.; Wei, L. Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells. J. Cell. Biochem. 2009, 106, 553–562. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, J.; Wang, J. Calcium and calcium-related proteins in endometrial cancer: Opportunities for pharmacological intervention. Int. J. Biol. Sci. 2022, 18, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Okayasu, I. Changes in expression of estrogen receptors alpha and beta in relation to progesterone receptor and pS2 status in normal and malignant endometrium. Jpn. J. Cancer Res. 2000, 91, 510–518. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Fujimoto, J.; Aoki, I.; Toyoki, H.; Khatun, S.; Tamaya, T. Expression of oestrogen receptor alpha and beta in uterine endometrial and ovarian cancers. Eur. J. Cancer 2002, 38 (Suppl. 6), S74–S75. [Google Scholar] [CrossRef]
- Hu, K.; Zhong, G.; He, F. Expression of estrogen receptors ERalpha and ERbeta in endometrial hyperplasia and adenocarcinoma. Int. J. Gynecol. Cancer 2005, 15, 537–541. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Monterossi, G.; De Stefano, I.; Gargini, A.; Salerno, M.G.; Farulla, I.; Travaglia, D.; Vellone, V.G.; Scambia, G.; Gallo, D. The expression ratios of estrogen receptor α (ERα) to estrogen receptor β1 (ERβ1) and ERα to ERβ2 identify poor clinical outcome in endometrioid endometrial cancer. Hum. Pathol. 2013, 44, 1047–1054. [Google Scholar] [CrossRef]
- Mylonas, I. Prognostic significance and clinical importance of estrogen receptor alpha and beta in human endometrioid adenocarcinomas. Oncol. Rep. 2010, 24, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, J.; Zhou, X.; Liu, J.; Wang, Q.; Zhang, B. Roles of estrogen receptor α and β in the regulation of proliferation in endometrial carcinoma. Pathol. Res. Pract. 2020, 216, 153149. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.; MacPherson, S.; Brown, P.; Bombail, V.; Williams, A.R.; Anderson, R.A.; Jabbour, H.N.; Saunders, P.T. Expression of oestrogen receptors, ERalpha, ERbeta, and ERbeta variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERalpha. BMC Cancer 2009, 9, 330. [Google Scholar] [CrossRef]
- Wang, J.; Bao, W.; Qiu, M.; Liao, Y.; Che, Q.; Yang, T.; He, X.; Qiu, H.; Wan, X. Forkhead-box A1 suppresses the progression of endometrial cancer via crosstalk with estrogen receptor α. Oncol. Rep. 2014, 31, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Walker, C.J.; Goodfellow, P.J.; Hade, E.M.; Agarwal, G.; Mutch, D.; Cohn, D.E.; Suarez, A.A. Estrogen receptor-alpha as a predictive biomarker in endometrioid endometrial cancer. Gynecol. Oncol. 2016, 141, 312–317. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, W.J.; Reijnen, C.; Küsters-Vandevelde, H.V.N.; Bulten, J.; Bult, P.; Leung, S.; Visser, N.C.M.; Santacana, M.; Bronsert, P.; Hirschfeld, M.; et al. The cutoff for estrogen and progesterone receptor expression in endometrial cancer revisited: A European Network for Individualized Treatment of Endometrial Cancer collaboration study. Hum. Pathol. 2021, 109, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Trovik, J.; Wik, E.; Werner, H.M.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- van der Putten, L.J.M.; Visser, N.C.M.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Added Value of Estrogen Receptor, Progesterone Receptor, and L1 Cell Adhesion Molecule Expression to Histology-Based Endometrial Carcinoma Recurrence Prediction Models: An ENITEC Collaboration Study. Int. J. Gynecol. Cancer 2018, 28, 514–523. [Google Scholar] [CrossRef]
- Guan, J.; Xie, L.; Luo, X.; Yang, B.; Zhang, H.; Zhu, Q.; Chen, X. The prognostic significance of estrogen and progesterone receptors in grade I and II endometrioid endometrial adenocarcinoma: Hormone receptors in risk stratification. J. Gynecol. Oncol. 2019, 30, e13. [Google Scholar] [CrossRef]
- Obata, T.; Nakamura, M.; Mizumoto, Y.; Iizuka, T.; Ono, M.; Terakawa, J.; Daikoku, T.; Fujiwara, H. Dual expression of immunoreactive estrogen receptor β and p53 is a potential predictor of regional lymph node metastasis and postoperative recurrence in endometrial endometrioid carcinoma. PLoS ONE 2017, 12, e0188641. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Oprea, T.I.; Sklar, L.A.; Arterburn, J.B. The ins and outs of GPR30: A transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 2008, 109, 350–353. [Google Scholar] [CrossRef]
- Bubb, M.; Beyer, A.L.; Dasgupta, P.; Kaemmerer, D.; Sänger, J.; Evert, K.; Wirtz, R.M.; Schulz, S.; Lupp, A. Assessment of G Protein-Coupled Oestrogen Receptor Expression in Normal and Neoplastic Human Tissues Using a Novel Rabbit Monoclonal Antibody. Int. J. Mol. Sci. 2022, 23, 5191. [Google Scholar] [CrossRef]
- Vivacqua, A.; De Marco, P.; Santolla, M.F.; Cirillo, F.; Pellegrino, M.; Panno, M.L.; Abonante, S.; Maggiolini, M. Estrogenic gper signaling regulates mir144 expression in cancer cells and cancer-associated fibroblasts (cafs). Oncotarget 2015, 6, 16573–16587. [Google Scholar] [CrossRef]
- Cirillo, F.; Lappano, R.; Bruno, L.; Rizzuti, B.; Grande, F.; Guzzi, R.; Briguori, S.; Miglietta, A.M.; Nakajima, M.; Di Martino, M.T.; et al. AHR and GPER mediate the stimulatory effects induced by 3-methylcholanthrene in breast cancer cells and cancer-associated fibroblasts (CAFs). J. Exp. Clin. Cancer Res. 2019, 38, 335. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Noske, A.; Meisel, A.; Varga, Z.; Fink, D.; Imesch, P. The G protein-coupled estrogen receptor (GPER) is expressed in two different subcellular localizations reflecting distinct tumor properties in breast cancer. PLoS ONE 2014, 9, e83296. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, M.; Hartman, L.; Grabau, D.; Fornander, T.; Malmström, P.; Nordenskjöld, B.; Sgroi, D.C.; Skoog, L.; Stål, O.; Leeb-Lundberg, L.M.; et al. Lack of G protein-coupled estrogen receptor (GPER) in the plasma membrane is associated with excellent long-term prognosis in breast cancer. Breast Cancer Res. Treat. 2014, 145, 61–71. [Google Scholar] [CrossRef]
- He, Y.Y.; Cai, B.; Yang, Y.X.; Liu, X.L.; Wan, X.P. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009, 100, 1051–1061. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Schüler, S.; Lattrich, C.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor (GPER) expression in endometrial adenocarcinoma and effect of agonist G-1 on growth of endometrial adenocarcinoma cell lines. Steroids 2013, 78, 1087–1091. [Google Scholar] [CrossRef]
- Krakstad, C.; Trovik, J.; Wik, E.; Engelsen, I.B.; Werner, H.M.; Birkeland, E.; Raeder, M.B.; Øyan, A.M.; Stefansson, I.M.; Kalland, K.H.; et al. Loss of GPER identifies new targets for therapy among a subgroup of ERα-positive endometrial cancer patients with poor outcome. Br. J. Cancer 2012, 106, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.Y.; Lv, Q.Y.; Ning, C.C.; Yang, B.Y.; Shan, W.W.; Cheng, Y.L.; Gu, C.; Luo, X.Z.; Zhang, Z.B.; Chen, X.J.; et al. TET1-GPER-PI3K/AKT pathway is involved in insulin-driven endometrial cancer cell proliferation. Biochem. Biophys. Res. Commun. 2017, 482, 857–862. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Bian, Y.; Tong, H.; Qu, J.; Wang, K.; Wan, X.P. Autocrine motility factor promotes endometrial cancer progression by targeting GPER-1. Cell Commun. Signal. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Silva, C.D.; Villegas-Pineda, J.C.; Pereira-Suárez, A.L. Expression and Role of the G Protein-Coupled Estrogen Receptor (GPR30/GPER) in the Development and Immune Response in Female Reproductive Cancers. Front. Endocrinol. 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Smuc, T.; Hevir, N.; Ribic-Pucelj, M.; Husen, B.; Thole, H.; Rizner, T.L. Disturbed estrogen and progesterone action in ovarian endometriosis. Mol. Cell. Endocrinol. 2009, 301, 59–64. [Google Scholar] [CrossRef]
- Smuc, T.; Rizner, T.L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol. Cell. Endocrinol. 2009, 301, 74–82. [Google Scholar] [CrossRef] [PubMed]
- NCBI. The National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&term=ESR1 (accessed on 20 June 2022).
- NCBI. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/2099 (accessed on 25 June 2022).
- Reisenbichler, E.S.; Lester, S.C.; Richardson, A.L.; Dillon, D.A.; Ly, A.; Brock, J.E. Interobserver concordance in implementing the 2010 ASCO/CAP recommendations for reporting ER in breast carcinomas: A demonstration of the difficulties of consistently reporting low levels of ER expression by manual quantification. Am. J. Clin. Pathol. 2013, 140, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cheang, M.C.; Treaba, D.O.; Speers, C.H.; Olivotto, I.A.; Bajdik, C.D.; Chia, S.K.; Goldstein, L.C.; Gelmon, K.A.; Huntsman, D.; Gilks, C.B.; et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J. Clin. Oncol. 2006, 24, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Bevitt, D.J.; Milton, I.D.; Piggot, N.; Henry, L.; Carter, M.J.; Toms, G.L.; Lennard, T.W.; Westley, B.; Angus, B.; Horne, C.H. New monoclonal antibodies to oestrogen and progesterone receptors effective for paraffin section immunohistochemistry. J. Pathol. 1997, 183, 228–232. [Google Scholar] [CrossRef]
- Kaplan, P.A.; Frazier, S.R.; Loy, T.S.; Diaz-Arias, A.A.; Bradley, K.; Bickel, J.T. 1D5 and 6F11: An immunohistochemical comparison of two monoclonal antibodies for the evaluation of estrogen receptor status in primary breast carcinoma. Am. J. Clin. Pathol. 2005, 123, 276–280. [Google Scholar] [CrossRef]
- Paul, M.; Cholewa, K.; Mazurek, U.; Witek, A.; Wilczok, T. Estrogen receptor beta delta 6 (ER beta delta 6) isoform in human endometrial hyperplasia and adenocarcinoma. Cancer Investig. 2004, 22, 211–218. [Google Scholar] [CrossRef]
- Jarzabek, K.; Koda, M.; Walentowicz-Sadlecka, M.; Grabiec, M.; Laudanski, P.; Wolczynski, S. Altered expression of ERs, aromatase, and COX2 connected to estrogen action in type 1 endometrial cancer biology. Tumour Biol. 2013, 34, 4007–4016. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Bieche, I.; Szymczak, S.; Tozlu, S.; Lewandowski, S.; Girault, I.; Radwanska, K.; Szczylik, C.; Jakowicki, J.A.; Lidereau, R.; et al. Evaluation of mRNA expression of estrogen receptor beta and its isoforms in human normal and neoplastic endometrium. Int. J. Cancer 2004, 110, 783–787. [Google Scholar] [CrossRef]
- Kreizman-Shefer, H.; Pricop, J.; Goldman, S.; Elmalah, I.; Shalev, E. Distribution of estrogen and progesterone receptors isoforms in endometrial cancer. Diagn. Pathol. 2014, 9, 77. [Google Scholar] [CrossRef]
- Sasaki, M.; Kaneuchi, M.; Fujimoto, S.; Tanaka, Y.; Dahiya, R. Hypermethylation can selectively silence multiple promoters of steroid receptors in cancers. Mol. Cell. Endocrinol. 2003, 202, 201–207. [Google Scholar] [CrossRef]
- Felix, A.S.; Stone, R.A.; Chivukula, M.; Bowser, R.; Parwani, A.V.; Linkov, F.; Edwards, R.P.; Weissfeld, J.L. Survival outcomes in endometrial cancer patients are associated with CXCL12 and estrogen receptor expression. Int. J. Cancer 2012, 131, E114–E121. [Google Scholar] [CrossRef]
- van Weelden, W.J.; van der Putten, L.J.M.; Inda, M.A.; van Brussel, A.; Snijders, M.P.L.M.; Schriever, L.M.M.; Bulten, J.; Massuger, L.F.A.G.; van de Stolpe, A.; Pijnenborg, J.M.A. Oestrogen receptor pathway activity is associated with outcome in endometrial cancer. Br. J. Cancer 2020, 123, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, V.; Bednarikova, M.; Hausnerova, J.; Ovesna, P.; Vinklerova, P.; Minar, L.; Felsinger, M.; Jandakova, E.; Cihalova, M.; Zikan, M. A Novel Approach to Preoperative Risk Stratification in Endometrial Cancer: The Added Value of Immunohistochemical Markers. Front. Oncol. 2019, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- NCBI. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/2100 (accessed on 30 June 2022).
- Häring, J.; Skrzypczak, M.; Stegerer, A.; Lattrich, C.; Weber, F.; Görse, R.; Ortmann, O.; Treeck, O. Estrogen receptor β transcript variants associate with oncogene expression in endometrial cancer. Int. J. Mol. Med. 2012, 29, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; O’Neill, P.A.; Foster, C.S. Re: Skliris et al. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol 2002; 196: 155-162. J. Pathol. 2002, 199, 130. [Google Scholar] [CrossRef] [PubMed]
- Skliris, G.P.; Parkes, A.T.; Limer, J.L.; Burdall, S.E.; Carder, P.J.; Speirs, V. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J. Pathol. 2002, 197, 155–162. [Google Scholar] [CrossRef]
- Rizner, T.L.; Sasano, H.; Choi, M.H.; Odermatt, A.; Adamski, J. Recommendations for description and validation of antibodies for research use. J. Steroid Biochem. Mol. Biol. 2016, 156, 40–42. [Google Scholar] [CrossRef]
- Leung, Y.K.; Mak, P.; Hassan, S.; Ho, S.M. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proc Natl. Acad. Sci. USA 2006, 103, 13162–13167. [Google Scholar] [CrossRef]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, S.; Wei, M.; Vlantis, A.C.; Chan, J.Y.K.; van Hasselt, C.A.; Li, D.; Zeng, X.; Xue, L.; Tong, M.C.F.; et al. The Isoforms of Estrogen Receptor Alpha and Beta in Thyroid Cancer. Front. Oncol. 2022, 12, 916804. [Google Scholar] [CrossRef]
- Pelekanou, V.; Anastasiou, E.; Bakogeorgou, E.; Notas, G.; Kampa, M.; Garcia-Milian, R.; Lavredaki, K.; Moustou, E.; Chinari, G.; Arapantoni, P.; et al. Estrogen receptor-alpha isoforms are the main estrogen receptors expressed in non-small cell lung carcinoma. Steroids 2019, 142, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, I.; Makovitzky, J.; Friese, K.; Jeschke, U. Immunohistochemical labelling of steroid receptors in normal and malignant human endometrium. Acta Histochem. 2009, 111, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Springwald, A.; Lattrich, C.; Skrzypczak, M.; Goerse, R.; Ortmann, O.; Treeck, O. Identification of novel transcript variants of estrogen receptor α, β and progesterone receptor gene in human endometrium. Endocrine 2010, 37, 415–424. [Google Scholar] [CrossRef]
- Häring, J.; Schüler, S.; Lattrich, C.; Ortmann, O.; Treeck, O. Role of estrogen receptor β in gynecological cancer. Gynecol. Oncol. 2012, 127, 673–676. [Google Scholar] [CrossRef]
- Takama, F.; Kanuma, T.; Wang, D.; Kagami, I.; Mizunuma, H. Oestrogen receptor beta expression and depth of myometrial invasion in human endometrial cancer. Br. J. Cancer 2001, 84, 545–549. [Google Scholar] [CrossRef]
- Srijaipracharoen, S.; Tangjitgamol, S.; Tanvanich, S.; Manusirivithaya, S.; Khunnarong, J.; Thavaramara, T.; Leelahakorn, S.; Pataradool, K. Expression of ER, PR, and Her-2/neu in endometrial cancer: A clinicopathological study. Asian Pac. J. Cancer Prev. 2010, 11, 215–220. [Google Scholar]
- Jongen, V.; Briët, J.; de Jong, R.; ten Hoor, K.; Boezen, M.; van der Zee, A.; Nijman, H.; Hollema, H. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol. Oncol. 2009, 112, 537–542. [Google Scholar] [CrossRef]
- Bardin, A.; Boulle, N.; Lazennec, G.; Vignon, F.; Pujol, P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr. Relat. Cancer 2004, 11, 537–551. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- NCBI. The National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/gene/2852 (accessed on 17 June 2022).
- Du, G.Q.; Zhou, L.; Chen, X.Y.; Wan, X.P.; He, Y.Y. The G protein-coupled receptor GPR30 mediates the proliferative and invasive effects induced by hydroxytamoxifen in endometrial cancer cells. Biochem. Biophys. Res. Commun. 2012, 420, 343–349. [Google Scholar] [CrossRef]
- Vivacqua, A.; Bonofiglio, D.; Recchia, A.G.; Musti, A.M.; Picard, D.; Andò, S.; Maggiolini, M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol. Endocrinol. 2006, 20, 631–646. [Google Scholar] [CrossRef]
- van Weelden, W.J.; Reijnen, C.; Pijnenborg, J.M. Predictive value of estrogen and progesterone receptors in endometrial hyperplasia and cancer. Acta Obstet. Gynecol. Scand. 2020, 99, 139. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M.M.; Lydon, J.P.; O’Malley, B.W. Steroid receptor coactivators as therapeutic targets in the female reproductive system. J. Steroid Biochem. Mol. Biol. 2015, 154, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hevir, N.; Sinkovec, J.; Rižner, T.L. Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in endometrial cancer: Lower levels of CYP1B1 and increased expression of S-COMT. Mol. Cell. Endocrinol. 2011, 331, 158–167. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Sannino, P.; Shousha, S. Demonstration of oestrogen receptors in paraffin wax sections of breast carcinoma using the monoclonal antibody 1D5 and microwave oven processing. J. Clin. Pathol. 1994, 47, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Lausen, B. Maxstat: Maximally Selected Rank Statistics; R Package Version 07-25; R News; 2002; Volume 2, pp. 3–5. Available online: https://cran.r-project.org/web/packages/maxstat/maxstat.pdf (accessed on 10 September 2022).
- Welsh, A.W.; Lannin, D.R.; Young, G.S.; Sherman, M.E.; Figueroa, J.D.; Henry, N.L.; Ryden, L.; Kim, C.; Love, R.R.; Schiff, R.; et al. Cytoplasmic estrogen receptor in breast cancer. Clin. Cancer Res. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Bogina, G.; Zamboni, G.; Sapino, A.; Bortesi, L.; Marconi, M.; Lunardi, G.; Coati, F.; Massocco, A.; Molinaro, L.; Pegoraro, C.; et al. Comparison of anti-estrogen receptor antibodies SP1, 6F11, and 1D5 in breast cancer: Lower 1D5 sensitivity but questionable clinical implications. Am. J. Clin. Pathol. 2012, 138, 697–702. [Google Scholar] [CrossRef]
- Hevir, N.; Trošt, N.; Debeljak, N.; Rižner, T.L. Expression of estrogen and progesterone receptors and estrogen metabolizing enzymes in different breast cancer cell lines. Chem. Biol. Interact. 2011, 191, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Trošt, N.; Hevir, N.; Rižner, T.L.; Debeljak, N. Correlation between erythropoietin receptor(s) and estrogen and progesterone receptor expression in different breast cancer cell lines. Int. J. Mol. Med. 2013, 31, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Flouriot, G.; Brand, H.; Denger, S.; Metivier, R.; Kos, M.; Reid, G.; Sonntag-Buck, V.; Gannon, F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000, 19, 4688–4700. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.W.; Collins, J.M.; Ling, D.; Wang, D. Highly variable expression of ESR1 splice variants in human liver: Implication in the liver gene expression regulation and inter-person variability in drug metabolism and liver related diseases. J. Mol. Genet. Med. 2019, 13, 434. [Google Scholar] [PubMed]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HPRT1 | 5′ CCTGGCGTCGTGATTAGTG3′ | 5′TGAGGAATAAACACCCTTTCCA3′ |
| POLR2A | 5′CAAGTTCAACCAAGCCATTG3′ | 5′GTGGCAGGTTCTCCAAGG3′ |
| ESR2 isoforms a, g | 5′GGCATGGAACATCTGCTCAAC3′ | 5′CACACTGGAGTTCACGCTTC3′ |
| ESR2 isoform f | 5′TCCTGGTATCCAGTGCATCG3′ | 5′TTTCATTGCCCACATGCAAGG3′ |
| ESR2 isoform b, d, h, k, l | 5′GGACTGGGATTGTGTGGTC3′ | 5′TAGGCATCGGCATTTCCCCT3′ |
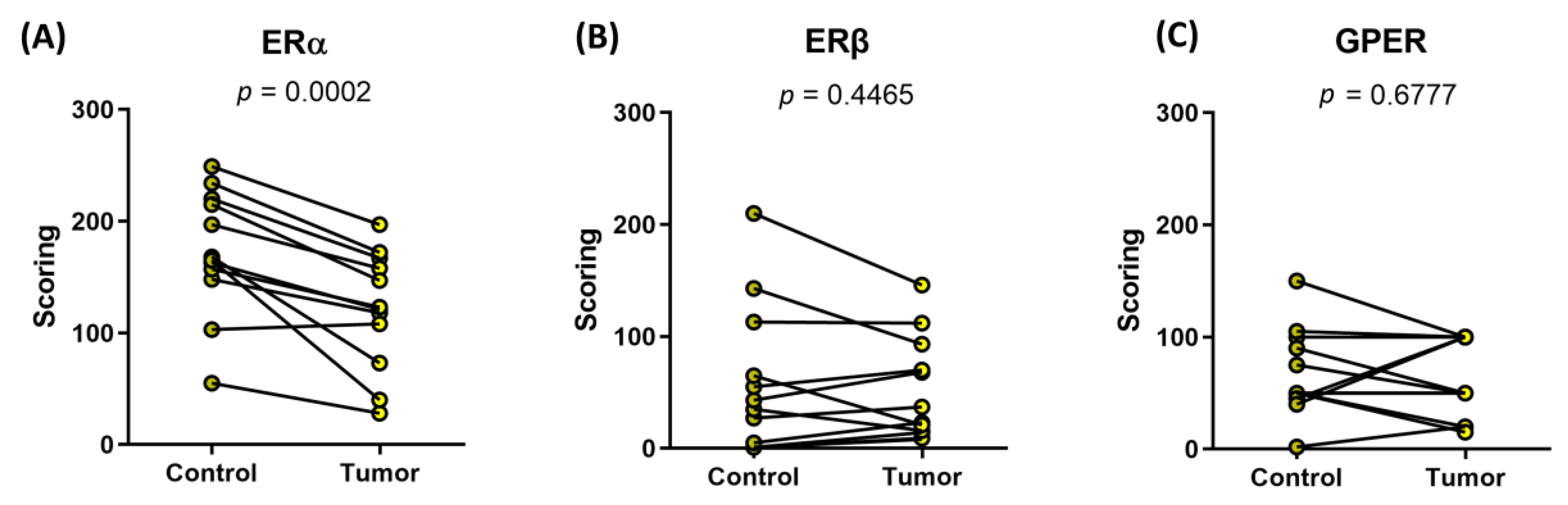
| (A) Our Cohort | ERα | ERβ | GPER |
| Control | 173 ± 56 | 58 ± 66 | 67 ± 39 |
| Tumor | 121 ± 52 | 51 ± 46 | 63 ± 35 |
| p = 0.0002 | p = 0.4465 | p = 0.6777 | |
| (B) Commercial tissue microarrays | ERα | ERβ | GPER |
| Control | 234 ± 96 | 39 ± 69 | 151 ± 54 |
| Tumor | 73 ± 82 | 52 ± 70 | 132 ± 51 |
| p = 0.0078 | p = 0.8438 | p = 0.2930 |
| mRNA (qPCR) | Proteins (Western Blotting) | IHC (Our Cohort) | IHC (Commercial TMAs) | |
|---|---|---|---|---|
| ERα/ERβ | rs = 0.5124, p < 0.0001 | rs = 0.2782, p = 0.1003 | c: rs = 0.4293, p = 0.0046 | rs = 0.1670, p = 0.5079 |
| n: rs = 0.1059, p = 0.5043 | ||||
| ERα/GPER | GPER 2, rs = 0.2374, p = 0.0781 | rs = 0.6777, p < 0.0001 | rs = 0.1333, p = 0.6860 | rs = 0.4563, p = 0.0570 |
| GPER 3,4, rs = 0.4688, p = 0.0003 | ||||
| ERβ/GPER | GPER 2, rs = 0.1297, p = 0.3406 | rs = 0.5598, p = 0.0004 | rs = 0.1666, p = 0.6101 | rs = −0.04187, p = 0.8690 |
| GPER 3,4, rs = 0.3375, p = 0.0110 | ||||
| GPER 2/3,4 | rs = 0.7193, p < 0.0001 | n/a | n/a | n/a |
| Assay | Isoforms | Antibodies | Isoforms | ||
|---|---|---|---|---|---|
| ERα/ESR1 | Hs00174860_m1 | 1, 2 and 4 | WB, IHC | SP1 | 1, 2, 3 |
| IHC | 1D5 | 1, 2, 3 and 5 | |||
| IHC | 6F11 | n/a | |||
| ERβ/ESR2 | Hs01100353_m1 | 1, 2, 3, 5, 6 | WB | ab3576 | 1, 5, 6 |
| IHC | 14C8 | 1, 2, 3, 5, 6 | |||
| IHC (neg.) | PPG5/10 | 1 | |||
| GPER/GPER | Hs00173506_m1 | 1 | HPA027052 | 1 | |
| Hs01116133_m1 | 1 | WB, IHC |
| Gene | Assay ID | Gene Name |
|---|---|---|
| ESR1 | Hs00174860_m1 | Estrogen receptor 1 |
| ESR2 | Hs01100353_m1 | Estrogen receptor 2 (ER beta) |
| GPER | Hs00173506_m1 | G-protein–coupled estrogen receptor 1 (GPER) (gene variant 2) |
| GPER | Hs01116133_m1 | G-protein–coupled estrogen receptor 1 (GPER) (gene variants 3 and 4) |
| HPRT1 | Hs99999909_m1 | Hypoxanthine phosphoribosyltransferase 1 (Lesch-Nyhan syndrome) |
| POLR2A | Hs00172187_m1 | Polymerase (RNA) II (DNA directed) polypeptide A, 220kDa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojnik, M.; Sinreih, M.; Anko, M.; Hevir-Kene, N.; Knific, T.; Pirš, B.; Grazio, S.F.; Rižner, T.L. The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 3009. https://doi.org/10.3390/ijms24033009
Hojnik M, Sinreih M, Anko M, Hevir-Kene N, Knific T, Pirš B, Grazio SF, Rižner TL. The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(3):3009. https://doi.org/10.3390/ijms24033009
Chicago/Turabian StyleHojnik, Marko, Maša Sinreih, Maja Anko, Neli Hevir-Kene, Tamara Knific, Boštjan Pirš, Snježana Frković Grazio, and Tea Lanišnik Rižner. 2023. "The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer" International Journal of Molecular Sciences 24, no. 3: 3009. https://doi.org/10.3390/ijms24033009
APA StyleHojnik, M., Sinreih, M., Anko, M., Hevir-Kene, N., Knific, T., Pirš, B., Grazio, S. F., & Rižner, T. L. (2023). The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer. International Journal of Molecular Sciences, 24(3), 3009. https://doi.org/10.3390/ijms24033009







