Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association
Abstract
1. Introduction
2. Immunological Characteristics
2.1. Immunological Mechanism of Periodontitis
2.2. Immunological Mechanism of COVID-19
2.3. Similar Pathways of Immune Response in Periodontitis and COVID-19
2.3.1. NF-κB Pathway
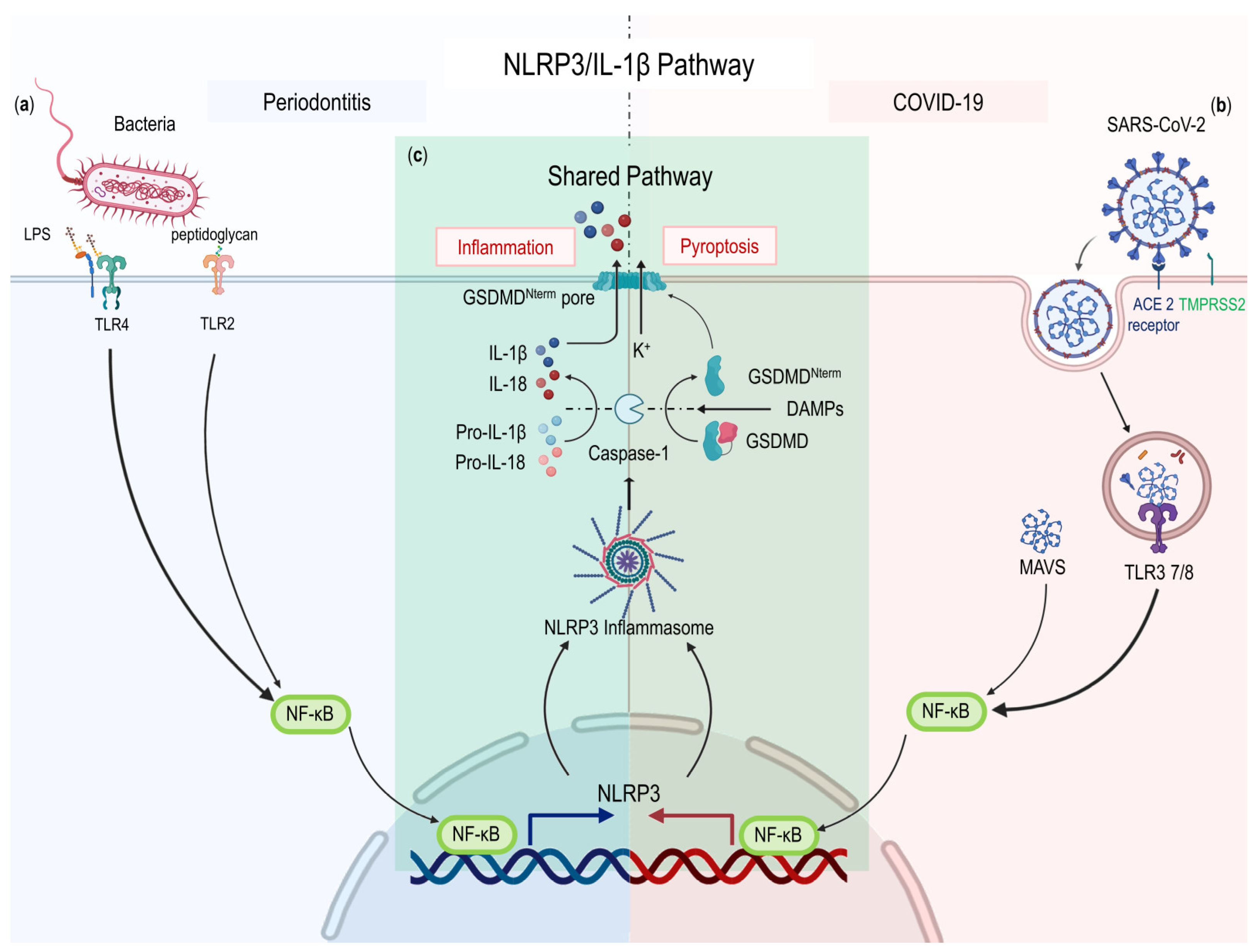
2.3.2. NLRP3/IL-1β Pathway
2.3.3. IL-6 Signaling Pathway
3. Association between Periodontitis and COVID-19
3.1. Common Risk Factors
3.1.1. Gender
3.1.2. Lifestyle
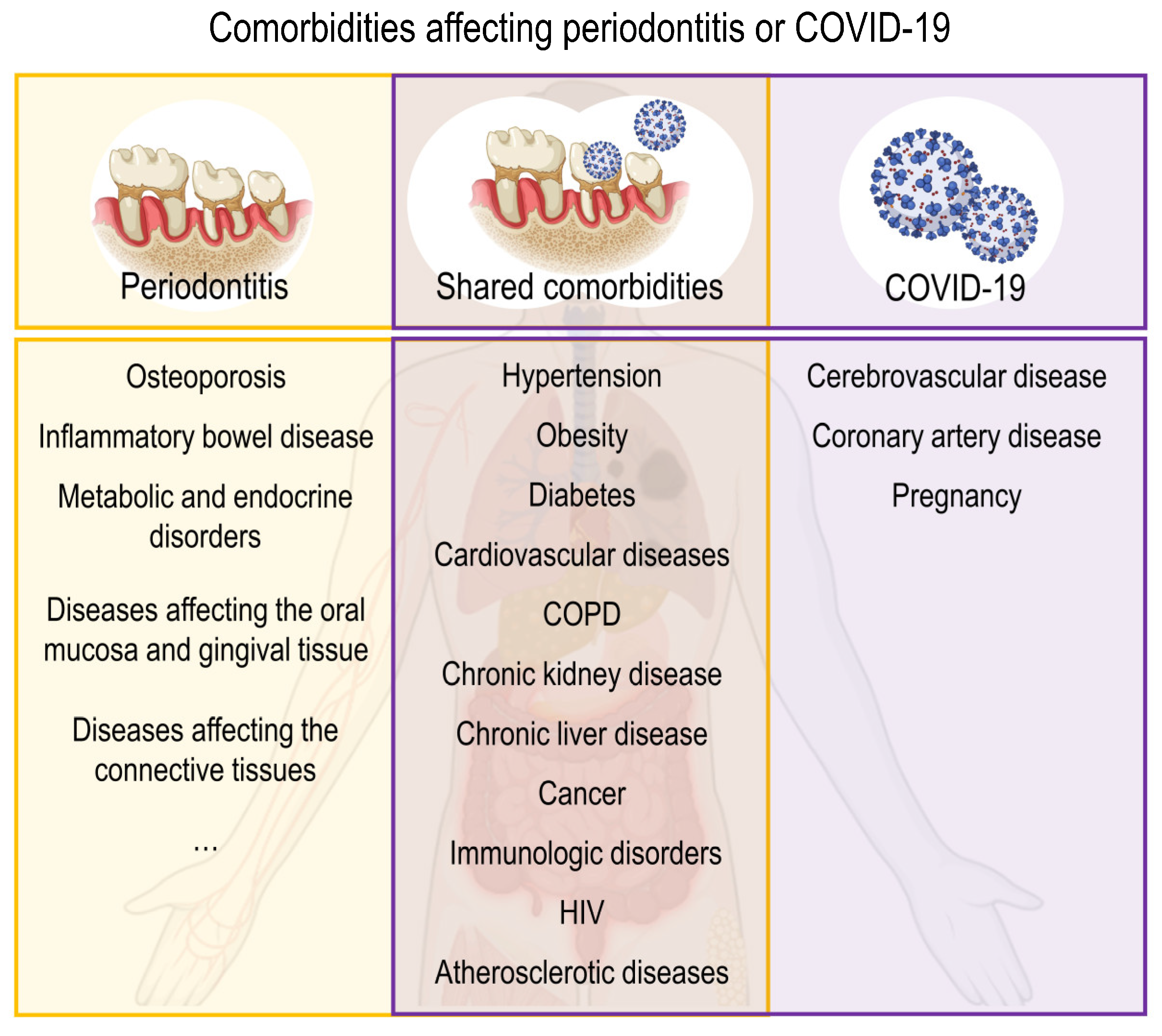
3.1.3. Comorbidities
3.2. Impact of Periodontitis on COVID-19
3.2.1. The Oral Cavity Is a Reservoir for SARS-CoV-2
3.2.2. Oral Microorganisms Are Significant Contributors to COVID-19
3.2.3. Association between Periodontitis and COVID-19 in Clinical Studies
3.3. Impact of COVID-19 on Periodontitis
3.3.1. Effects of Virus
3.3.2. Stress during the COVID-19 Outbreak and Lockdown
4. Possible Interventions and Potential Targets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2020, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Lane, H.C.; Redfield, R.R. Covid-19—Navigating the Uncharted. N. Engl. J. Med. 2020, 382, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly epidemiological update on COVID-19—4 January 2023 Edition 124. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---covid-19 (accessed on 4 January 2023).
- Wang, Y.; Perlman, S. COVID-19: Inflammatory Profile. Annu. Rev. Med. 2022, 73, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A case-control study on the association between periodontitis and coronavirus disease (COVID-19). J. Periodontol. 2021, 93, 584–590. [Google Scholar] [CrossRef]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef]
- Gupta, S.; Mohindra, R.; Singla, M.; Khera, S.; Sahni, V.; Kanta, P.; Soni, R.K.; Kumar, A.; Gauba, K.; Goyal, K.; et al. The clinical association between Periodontitis and COVID-19. Clin. Oral Investig. 2021, 26, 1361–1374. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Tervahartiala, T.; Sorsa, T.; Sakellari, D. Is There a Link between COVID-19 and Periodontal Disease? A Narrative Review. Eur. J. Dent. 2022, 16, 514–520. [Google Scholar] [CrossRef]
- Tamimi, F.; Altigani, S.; Sanz, M. Periodontitis and coronavirus disease 2019. Periodontol. 2000 2022, 89, 207–214. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Brenchley, L.; Moutsopoulos, N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol. Rev. 2018, 287, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontol. 2000 2019, 82, 78–92. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2019, 20, 95–112. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernández, M.; Tervahartiala, T.; Leppilahti, J.; Gürsoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- De Morais, E.F.; Pinheiro, J.; Leite, R.B.; Santos, P.P.D.A.; Barboza, C.A.G.; Freitas, R.A. Matrix metalloproteinase-8 levels in periodontal disease patients: A systematic review. J. Periodontal Res. 2017, 53, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Shin, M.-S.; Kim, M.-S.; Ahn, Y.-B. Incipient periodontitis and salivary molecules among Korean adults: Association and screening ability. J. Clin. Periodontol. 2016, 43, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Miranda, T.S.; de Freitas Figueiredo, N.; Figueiredo, L.C.; da Silva, H.D.P.; Rocha, F.R.G.; Duarte, P.M. Cytokine profiles of healthy and diseased sites in individuals with periodontitis. Arch. Oral Biol. 2020, 120, 104957. [Google Scholar] [CrossRef]
- El-Sayed, K.M.F.; Elahmady, M.; Adawi, Z.; Aboushadi, N.; Elnaggar, A.; Eid, M.; Hamdy, N.; Sanaa, D.; Dörfer, C.E. The periodontal stem/progenitor cell inflammatory-regenerative cross talk: A new perspective. J. Periodontal Res. 2018, 54, 81–94. [Google Scholar] [CrossRef]
- Sahingur, S.E.; Yeudall, W.A. Chemokine Function in Periodontal Disease and Oral Cavity Cancer. Front. Immunol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E.; et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Karbuz, A.; Gervais, A.; Tayoun, A.A.; Aiuti, A.; Belot, A.; Bolze, A.; Gaudet, A.; Bondarenko, A.; et al. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- LaForge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Hoiland, R.L.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Assessing the importance of interleukin-6 in COVID-19. Lancet Respir. Med. 2021, 9, e13. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sahu, S.K.; Cano, M.; Kuppuswamy, V.; Bajwa, J.; McPhatter, J.N.; Pine, A.; Meizlish, M.L.; Goshua, G.; Chang, C.H. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabh2259. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Zingariello, M.; Cavalcanti, M.; Merciaro, I.; Pizzicannella, J.; De Isla, N.; Caputi, S.; Ballerini, P.; Trubiani, O. MyD88/ERK/NFkB pathways and pro-inflammatory cytokines release in periodontal ligament stem cells stimulated by Porphyromonas gingivalis. Eur. J. Histochem. 2017, 61, 2791. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Sommer, M.E.L.; Dalia, R.A.; Nogueira, A.V.B.; Cirelli, J.A.; Vinolo, M.A.R.; Fachi, J.L.; Oliveira, C.A.; Andrade, T.A.M.; Mendonca, F.A.S.; Santamaria, M., Jr.; et al. Immune response mediated by Th1/IL-17/caspase-9 promotes evolution of periodontal disease. Arch. Oral. Biol. 2019, 97, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Moutsopoulos, N.M. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci. Immunol. 2020, 5, eaau4594. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kappaB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-kappaB hyper-activation and inflammation. Signal. Transduct. Target Ther. 2021, 6, 167. [Google Scholar] [CrossRef]
- E Ingraham, N.; Lotfi-Emran, S.; Thielen, B.; Techar, K.; Morris, R.S.; Holtan, S.G.; Dudley, R.A.; Tignanelli, C. Immunomodulation in COVID-19. Lancet Respir. Med. 2020, 8, 544–546. [Google Scholar] [CrossRef]
- Kircheis, R.; Haasbach, E.; Lueftenegger, D.; Heyken, W.T.; Ocker, M.; Planz, O. NF-kappaB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020, 11, 598444. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Lv, C.; Chen, Y.; Zhao, W.; Li, W.; Chen, H.; Wang, H.; Sun, W.; Yuan, H. NLRP3 regulates alveolar bone loss in ligature-induced periodontitis by promoting osteoclastic differentiation. Cell Prolif. 2020, 54, e12973. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Williams, R.C. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J. Periodontol. 2021, 93, 135–145. [Google Scholar] [CrossRef]
- Isaza-Guzmán, D.M.; Medina-Piedrahíta, V.M.; Gutiérrez-Henao, C.; Tobón-Arroyave, S.I. Salivary Levels of NLRP3 Inflammasome-Related Proteins as Potential Biomarkers of Periodontal Clinical Status. J. Periodontol. 2017, 88, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Song, J.; Oh, S.; Kim, J.; Lee, M.; Piao, X.; Yang, J.; Kim, O.; Kim, T.; Kim, S.; et al. Targeting NLRP3 Inflammasome Reduces Age-Related Experimental Alveolar Bone Loss. J. Dent. Res. 2020, 99, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Kapila, Y.; Berdeli, A.; Cooper, P.R. Inflammasomes and their regulation in periodontal disease: A review. J. Periodontal Res. 2020, 55, 473–487. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1beta is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391, Erratum in Nat. Rev. Immunol. 2020, 20, 448. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457, Correction in Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Möller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 2016, 18, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J. Old dog, new tricks: IL-6 cluster signaling promotes pathogenic TH17 cell differentiation. Nat. Immunol. 2016, 18, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch. Oral Biol. 2020, 122, 104997. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Botelho, J.; Lopes, J.; Patrão, M.; Alves, R.; Chambrone, L.; Alcoforado, G.; Mendes, J.J. Periodontitis Impact in Interleukin-6 Serum Levels in Solid Organ Transplanted Patients: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, W.X.; Meng, H.X.; Zhan, Y.L.; Hou, J.X. Pro-inflammatory cytokine interleukin-6-induced hepcidin, a key mediator of periodontitis-related anemia of inflammation. J. Periodontal Res. 2021, 56, 690–701. [Google Scholar] [CrossRef]
- Cruz, A.S.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Gil Castro, A.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, P.; Zhao, Y.; Zhuang, Z.; Wang, Z.; Song, R.; Zhang, J.; Liu, C.; Gao, Q.; Xu, Q.; et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity 2020, 53, 685–696.e3. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Lee, T.C. IL-6 blockade for COVID-19: A global scientific call to arms. Lancet Respir. Med. 2021, 9, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Harbour, S.N.; DiToro, D.F.; Witte, S.J.; Zindl, C.L.; Gao, M.; Schoeb, T.R.; Jones, G.W.; Jones, S.A.; Hatton, R.D.; Weaver, C.T. TH17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020, 5, eaaw2262. [Google Scholar] [CrossRef]
- Dutzan, N.; Kajikawa, T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, eaat0797. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.H.A.; Rivas, A.C.A.; Costa, K.F.; Oliveira, L.F.F.; Suzuki, K.T.; Messora, M.R.; Ricoldi, M.S.; de Almeida, A.L.G.; Taba, M. Specific inhibition of IL-6 receptor attenuates inflammatory bone loss in experimental periodontitis. J. Periodontol. 2021, 92, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kilian, C.; Turner, J.E.; Bosurgi, L.; Roedl, K.; Bartsch, P.; Gnirck, A.C.; Cortesi, F.; Schultheiss, C.; Hellmig, M.; et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021, 6, eabf6692. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-D.; Ding, M.; Dong, X.; Zhang, J.-J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Danielsen, A.C.; Lee, K.M.; Boulicault, M.; Rushovich, T.; Gompers, A.; Tarrant, A.; Reiches, M.; Shattuck-Heidorn, H.; Miratrix, L.W.; Richardson, S.S. Sex disparities in COVID-19 outcomes in the United States: Quantifying and contextualizing variation. Soc. Sci. Med. 2022, 294, 114716. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual dimorphism in COVID-19: Potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Costa, F.O.; Cota, L.O.M. Cumulative smoking exposure and cessation associated with the recurrence of periodontitis in periodontal maintenance therapy: A 6-year follow-up. J. Periodontol. 2019, 90, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Patanavanich, R.; Glantz, S.A. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2020, 93, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; von Ende, A.; Tan, P.S.; Sallis, H.M.; Lindson, N.; Coupland, C.A.C.; Munafò, M.R.; Aveyard, P.; Hippisley-Cox, J.; Hopewell, J.C. Smoking and COVID-19 outcomes: An observational and Mendelian randomisation study using the UK Biobank cohort. Thorax 2021, 77, 65–73. [Google Scholar] [CrossRef]
- Chick, J. Alcohol and COVID-19. Alcohol Alcohol. 2020, 55, 341–342. [Google Scholar] [CrossRef]
- Gay, I.C.; Tran, D.T.; Paquette, D.W. Alcohol intake and periodontitis in adults aged >/=30 years: NHANES 2009-2012. J. Periodontol. 2018, 89, 625–634. [Google Scholar] [CrossRef]
- De Souza, D.-M.; Rodrigues, V.-A.; Silva, A.-D.A.; Gonsalves, V.-S.; Pereira, K.A.; Nishioka, R.-S.; De Carvalho, C. Influence of different alcohol intake frequencies on alveolar bone loss in adult rats: A sem study. J. Clin. Exp. Dent. 2018, 10, e852–e857. [Google Scholar] [CrossRef]
- Dubey, S.; Biswas, P.; Ghosh, R.; Chatterjee, S.; Dubey, M.J.; Chatterjee, S.; Lahiri, D.; Lavie, C.J. Psychosocial impact of COVID-19. Diabetes Metab. Syndr. 2020, 14, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Calina, D.; Hartung, T.; Mardare, I.; Mitroi, M.; Poulas, K.; Tsatsakis, A.; Rogoveanu, I.; Docea, A.O. COVID-19 pandemic and alcohol consumption: Impacts and interconnections. Toxicol. Rep. 2021, 8, 529–535. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S219–S229. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal disease–related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Sahingur, S.E. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontol. 2000 2022, 89, 125–141. [Google Scholar] [CrossRef]
- Parsegian, K.; Randall, D.; Curtis, M.; Ioannidou, E. Association between periodontitis and chronic kidney disease. Periodontol. 2000 2022, 89, 114–124. [Google Scholar] [CrossRef]
- Ryder, M.I.; Shiboski, C.; Yao, T.-J.; Moscicki, A.-B. Current trends and new developments in HIV research and periodontal diseases. Periodontol. 2000 2019, 82, 65–77. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Alam, M.R.; Kabir, M.R.; Reza, S. Comorbidities might be a risk factor for the incidence of COVID-19: Evidence from a web-based survey. Prev. Med. Rep. 2021, 21, 101319. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Ardizzone, S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid-19 Infection? J. Crohn’s Colitis 2020, 14, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Perez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Nakamura, T.; Shima, K.; Noguchi, K.; Chiba, N.; Matsuguchi, T. Periodontitis promotes the expression of gingival transmembrane serine protease 2 (TMPRSS2), a priming protease for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J. Oral Biosci. 2022, 64, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sena, K.; Furue, K.; Setoguchi, F.; Noguchi, K. Altered expression of SARS-CoV-2 entry and processing genes by Porphyromonas gingivalis-derived lipopolysaccharide, inflammatory cytokines and prostaglandin E2 in human gingival fibroblasts. Arch. Oral Biol. 2021, 129, 105201. [Google Scholar] [CrossRef]
- Campisi, G.; Bizzoca, M.E.; Muzio, L.L. COVID-19 and periodontitis: Reflecting on a possible association. Head Face Med. 2021, 17, 16. [Google Scholar] [CrossRef]
- Berton, F.; Rupel, K.; Florian, F.; Biasotto, M.; Pallavicini, A.; Di Lenarda, R. Dental calculus-a reservoir for detection of past SARS-CoV-2 infection. Clin. Oral Investig. 2021, 25, 5113–5114. [Google Scholar] [CrossRef]
- Gomes, S.C.; da Fonseca, J.G.; Miller, L.M.; Manenti, L.; Angst, P.D.M.; Lamers, M.L.; Brum, I.S.; Nunes, L.N. SARS-CoV-2 RNA in dental biofilms: Supragingival and subgingival findings from inpatients in a COVID-19 intensive care unit. J. Periodontol. 2022, 93, 1476–1485. [Google Scholar] [CrossRef]
- Gupta, S.; Mohindra, R.; Chauhan, P.K.; Singla, V.; Goyal, K.; Sahni, V.; Gaur, R.; Verma, D.K.; Ghosh, A.; Soni, R.K.; et al. SARS-CoV-2 Detection in Gingival Crevicular Fluid. J. Dent. Res. 2021, 100, 187–193. [Google Scholar] [CrossRef]
- Matuck, B.F.; Dolhnikoff, M.; Maia, G.V.A.; Sendyk, D.I.; Zarpellon, A.; Gomes, S.C.; Duarte-Neto, A.N.; Pinho, J.R.R.; Gomes-Gouvêa, M.S.; Sousa, S.C.M.; et al. Periodontal tissues are targets for Sars-Cov-2: A post-mortem study. J. Oral Microbiol. 2020, 13, 1848135. [Google Scholar] [CrossRef]
- Gomes, S.C.; Fachin, S.; da Fonseca, J.G.; Angst, P.D.M.; Lamers, M.L.; da Silva, I.S.B.; Nunes, L.N. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J. Clin. Periodontol. 2021, 48, 880–885. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J. Oral Sci. 2021, 63, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Scannapieco, F.A.; Sethi, S. Oral-lung microbiome interactions in lung diseases. Periodontol. 2000 2020, 83, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xiao, Y.; Kang, L.; Ma, W.; Shi, L.; Zhang, L.; Zhou, Z.; Yang, J.; Zhong, J.; Yang, D.; et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Sampson, V. The role of oral bacteria in COVID-19. Lancet Microbe 2020, 1, e105. [Google Scholar] [CrossRef]
- Lee, W.; Fu, E.; Li, C.; Huang, R.; Chiu, H.; Cheng, W.; Chen, W. Association between periodontitis and pulmonary function based on the Third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Periodontol. 2020, 47, 788–795. [Google Scholar] [CrossRef]
- Costa, C.A.; Vilela, A.C.S.; Oliveira, S.A.; Gomes, T.D.; Andrade, A.A.C.; Leles, C.R.; Costa, N.L. Poor oral health status and adverse COVID-19 outcomes: A preliminary study in hospitalized patients. J. Periodontol. 2022, 93, 1889–1901. [Google Scholar] [CrossRef]
- Gupta, S.; Saarikko, M.; Pfützner, A.; Räisänen, I.T.; Sorsa, T. Compromised periodontal status could increase mortality for patients with COVID-19. Lancet Infect. Dis. 2022, 22, 314. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Aggarwal, T.; Lamba, A.K.; Faraz, F.; Tandon, S. Viruses: Bystanders of periodontal disease. Microb. Pathog. 2017, 102, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; Collman, R.G.; Mominkhan, D.; Wang, Y. Viruses, periodontitis, and comorbidities. Periodontol. 2000 2022, 89, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, P.; Slots, J. Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol. 2000 2019, 82, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Woolley, J. Necrotizing periodontal disease: Oral manifestation of COVID-19. Oral Dis. 2020, 27, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Turna, J.; Zhang, J.; Lamberti, N.; Patterson, B.; Simpson, W.; Francisco, A.P.; Bergmann, C.G.; Van Ameringen, M. Anxiety, depression and stress during the COVID-19 pandemic: Results from a cross-sectional survey. J. Psychiatr. Res. 2021, 137, 96–103. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Z.; Wu, L.; Zhu, Z.; Zhang, F.; Shang, Z.; Jia, Y.; Gu, J.; Zhou, Y.; Wang, Y.; et al. Prevalence and risk factors for acute posttraumatic stress disorder during the COVID-19 outbreak. J. Affect. Disord. 2021, 283, 123–129. [Google Scholar] [CrossRef]
- Rossi, R.; Socci, V.; Talevi, D.; Mensi, S.; Niolu, C.; Pacitti, F.; Di Marco, A.; Rossi, A.; Siracusano, A.; Di Lorenzo, G. COVID-19 Pandemic and Lockdown Measures Impact on Mental Health Among the General Population in Italy. Front. Psychiatry 2020, 11, 790. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Decker, A.M.; Kapila, Y.L.; Wang, H.L. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol. 2000 2021, 87, 94–106. [Google Scholar] [CrossRef]
- Sabbah, W.; Gomaa, N.; Gireesh, A. Stress, allostatic load, and periodontal diseases. Periodontol. 2000 2018, 78, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; de Wit, E. Antiviral agents for the treatment of COVID-19: Progress and challenges. Cell Rep. Med. 2022, 3, 100549. [Google Scholar] [CrossRef] [PubMed]
- Cattoni, F.; Tete, G.; D’Orto, B.; Bergamaschi, A.; Polizzi, E.; Gastaldi, G. Comparison of hygiene levels in metal-ceramic and stratified zirconia in prosthetic rehabilitation on teeth and implants: A retrospective clinical study of a three-year follow-up. J. Biol. Regul. Homeost. Agents 2021, 35, 41–49. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Sun, W.; Wang, K.; Li, W.; Lin, J.; Gong, J.; Wang, L. Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association. Int. J. Mol. Sci. 2023, 24, 3012. https://doi.org/10.3390/ijms24033012
Qi M, Sun W, Wang K, Li W, Lin J, Gong J, Wang L. Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association. International Journal of Molecular Sciences. 2023; 24(3):3012. https://doi.org/10.3390/ijms24033012
Chicago/Turabian StyleQi, Manlin, Wenyue Sun, Kun Wang, Wen Li, Jinying Lin, Jing Gong, and Lin Wang. 2023. "Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association" International Journal of Molecular Sciences 24, no. 3: 3012. https://doi.org/10.3390/ijms24033012
APA StyleQi, M., Sun, W., Wang, K., Li, W., Lin, J., Gong, J., & Wang, L. (2023). Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association. International Journal of Molecular Sciences, 24(3), 3012. https://doi.org/10.3390/ijms24033012





