Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects
Abstract
1. Introduction
2. Results
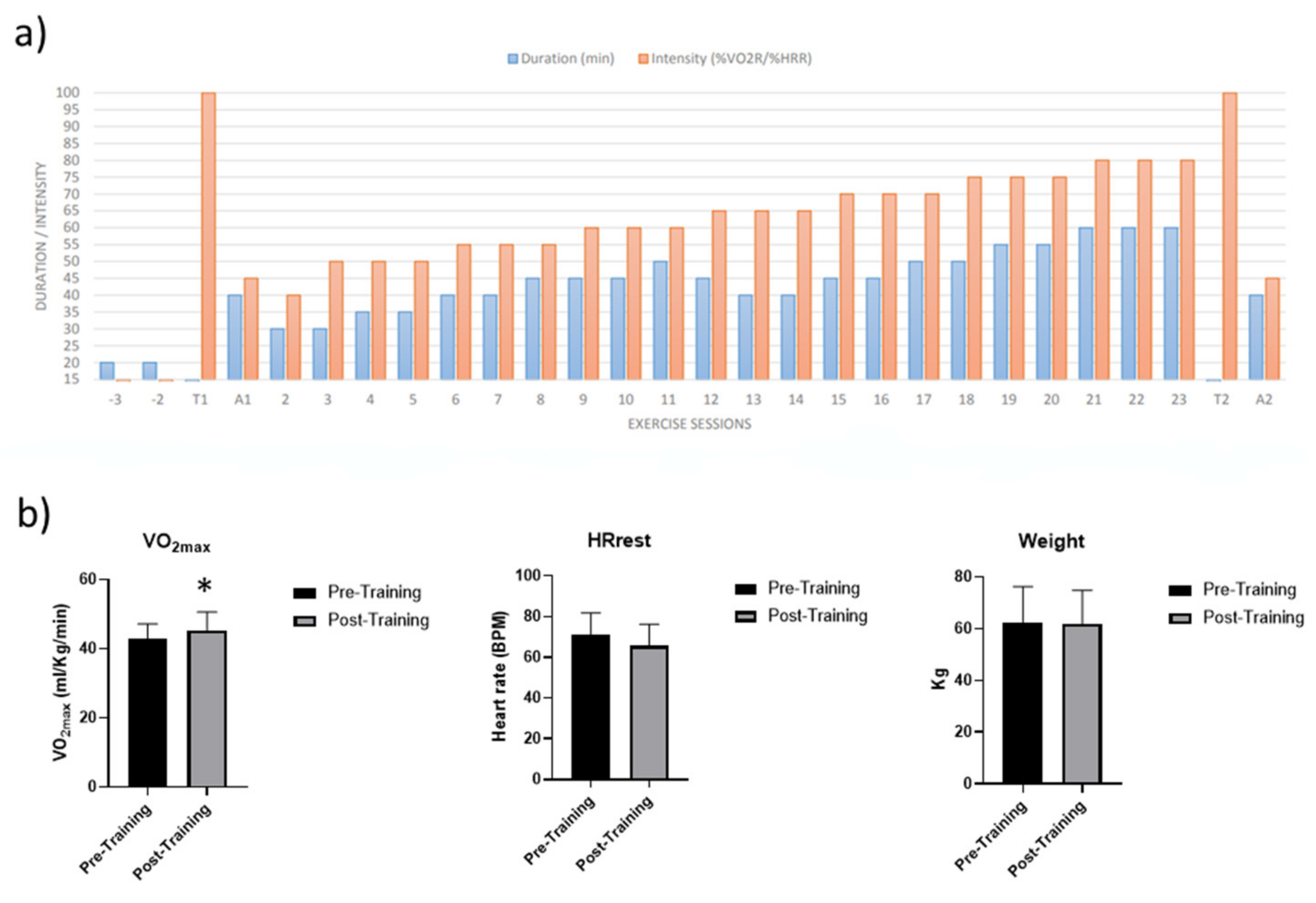
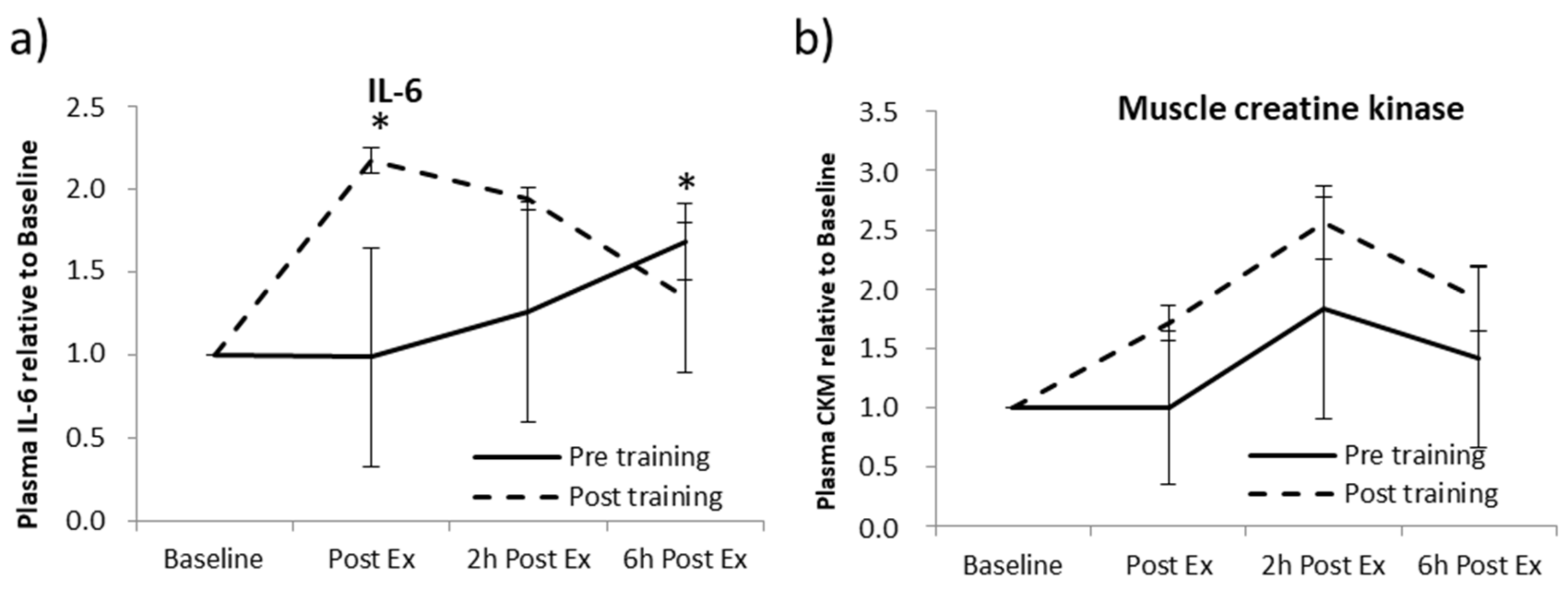
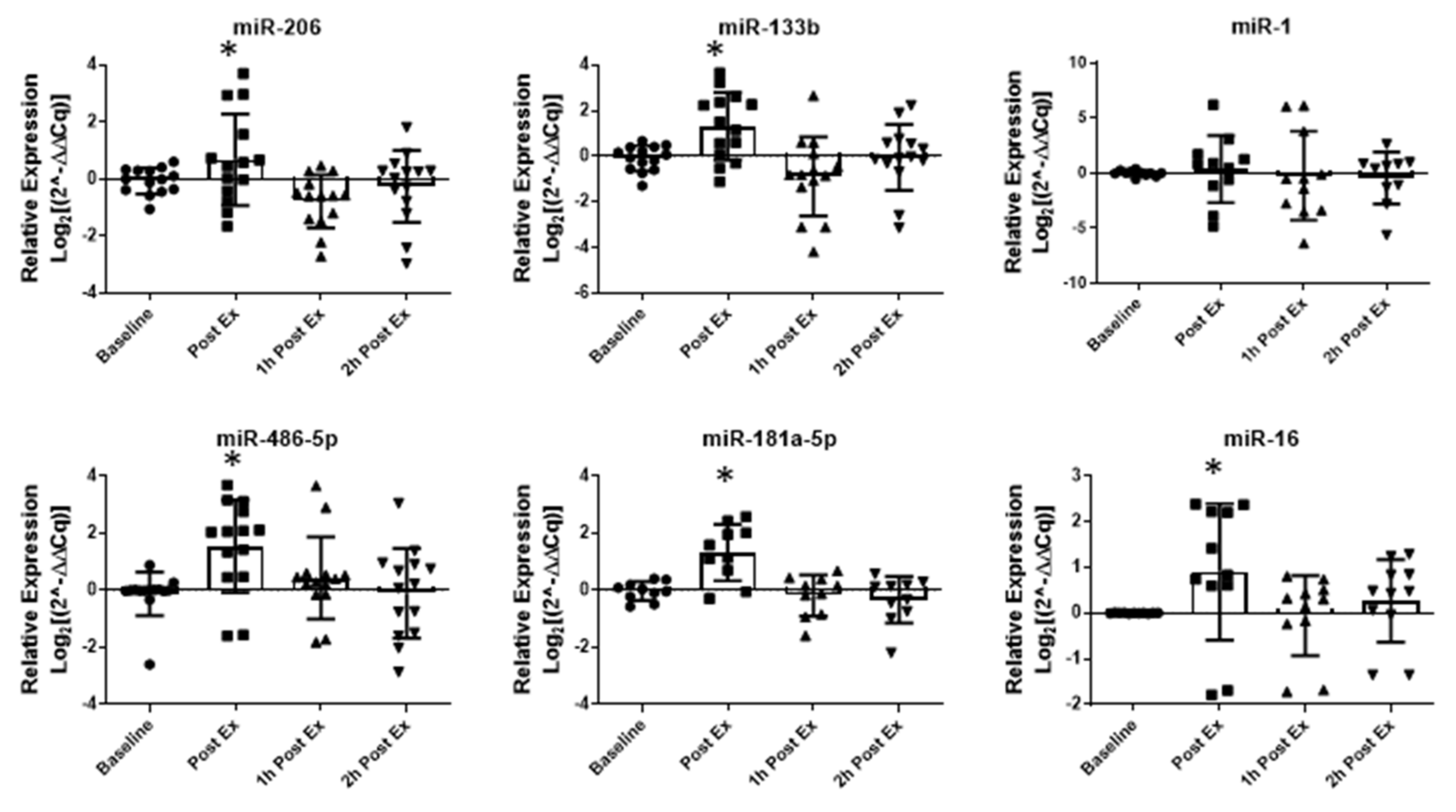
2.1. Acute Aerobic Exercise (AAE) and Aerobic Training (AT)
2.2. Acute Maximal Aerobic Exercise (AMAE)
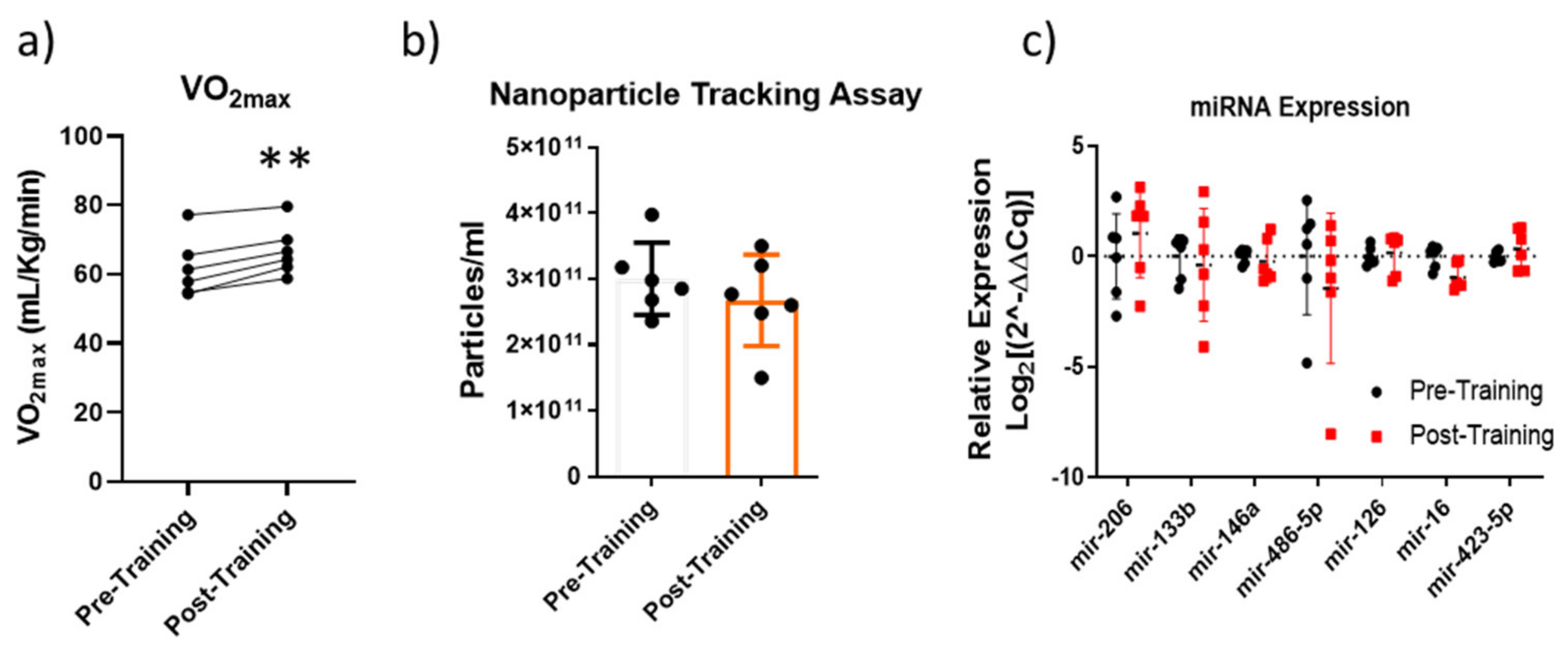
2.3. Altitude Aerobic Training (AAT)
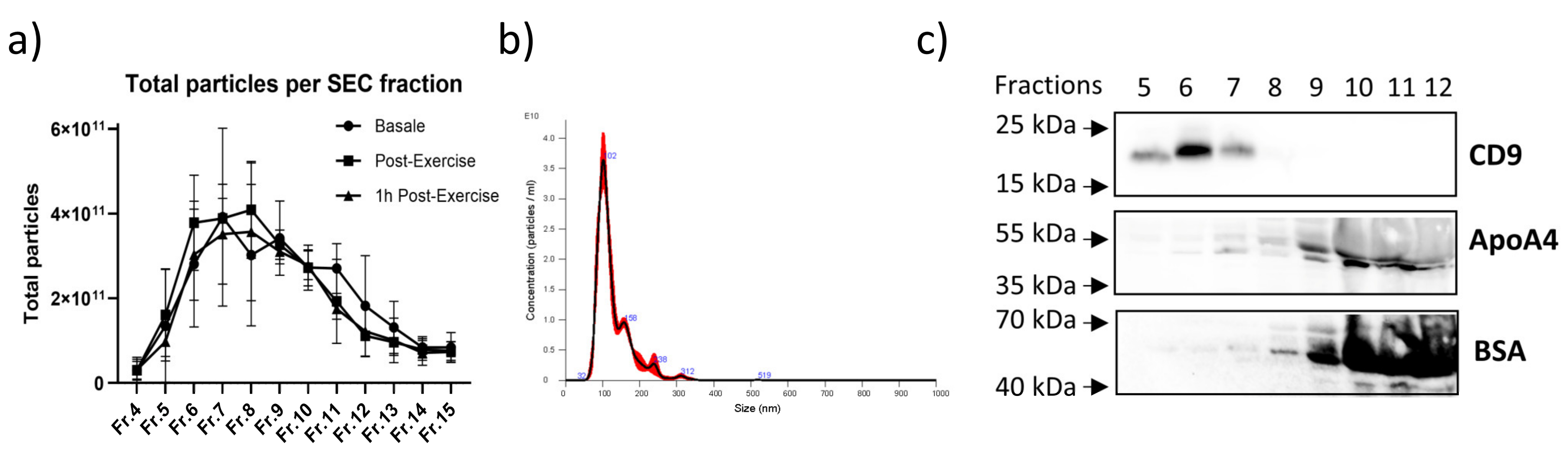
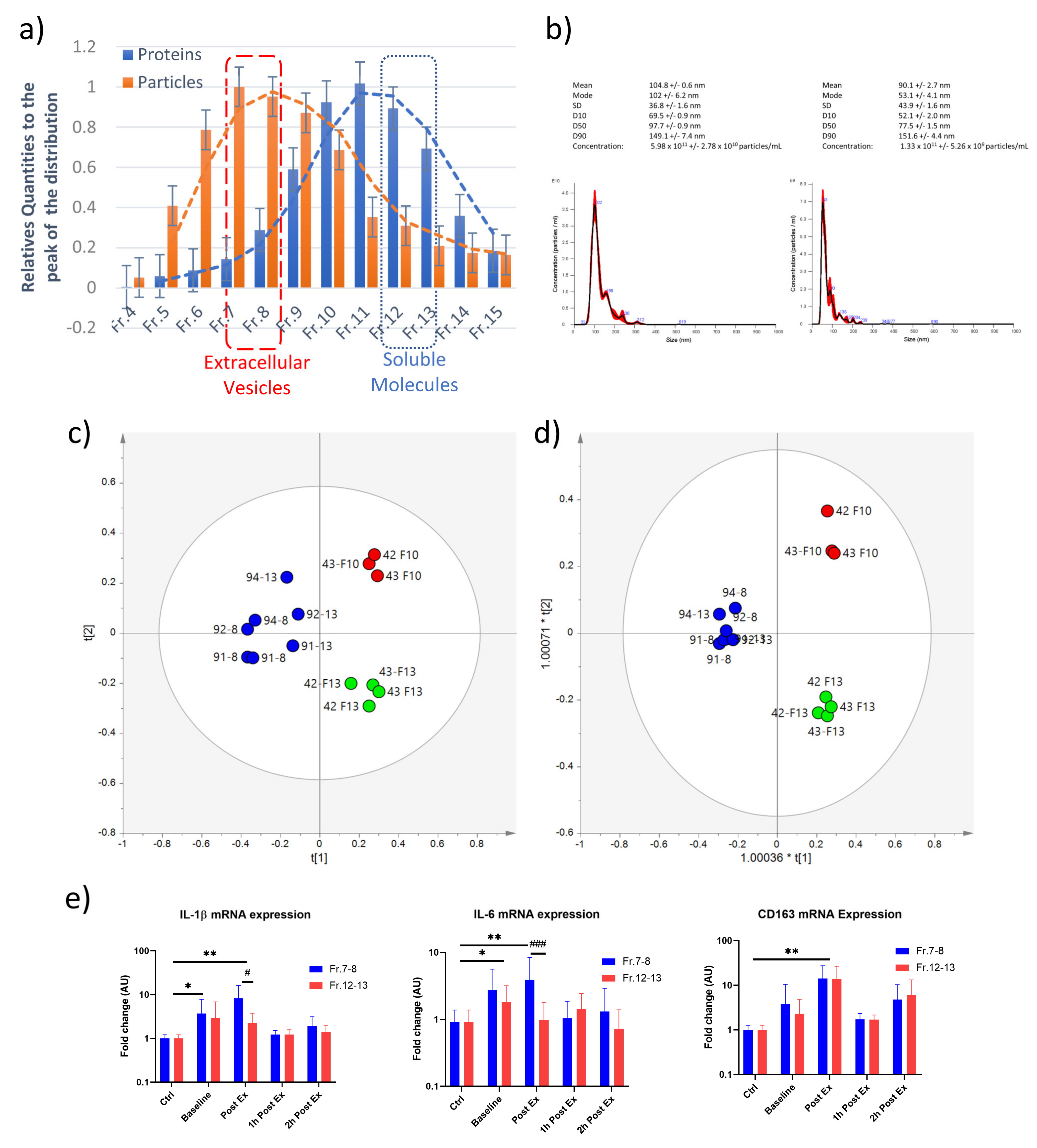
2.4. Characterization of the Circulating EVs Released in Response to an Aerobic Acute Exercise
2.5. Inflammatory Activity of Exercise-Derived EVs on THP-1 Cell Line
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Study Population
4.3. Acute Aerobic Exercise (AAE) and Aerobic Training (AT)
4.4. Acute Maximal Aerobic Exercise (AMAE)
4.5. Altitude Aerobic Training (AAT)
4.6. Blood Sampling
4.7. IL-6 and CMK Activity ELISA Assays
4.8. Extracellular Vesicle Isolation
4.9. Nanoparticle Tracking Assay (NTA)
4.10. Cytofluorimetric Analysis
4.11. miRNA and DNA Quantifications
4.12. MACSPlex Analysis
4.13. NMR Data Processing
4.14. THP-1 Treatment and Gene Expression Analysis
4.15. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise Benefits in Cardiovascular Disease: Beyond Attenuation of Traditional Risk Factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Frazzitta, G.; Balbi, P.; Maestri, R.; Bertotti, G.; Boveri, N.; Pezzoli, G. The Beneficial Role of Intensive Exercise on Parkinson Disease Progression. Am. J. Phys. Med. Rehabil. 2013, 92, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Ladiges, W.C. Exercise Enhances Wound Healing and Prevents Cancer Progression during Aging by Targeting Macrophage Polarity. Mech. Ageing Dev. 2014, 139, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the Management of Obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Xie, W.-Q.; Men, C.; He, M.; Li, Y.; Lv, S. The Effect of MicroRNA-Mediated Exercise on Delaying Sarcopenia in Elderly Individuals. Dose-Response 2020, 18, 155932582097454. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential Effects of Aerobic Exercise, Resistance Training and Combined Exercise Modalities on Cholesterol and the Lipid Profile: Review, Synthesis and Recommendations. Sport. Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Antunes, J.M.M.; Ferreira, R.M.P.; Moreira-Gonçalves, D. Exercise Training as Therapy for Cancer-Induced Cardiac Cachexia. Trends Mol. Med. 2018, 24, 709–727. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Bortoluzzi, S.; Scannapieco, P.; Cestaro, A.; Danieli, G.A.; Schiaffino, S. Computational Reconstruction of the Human Skeletal Muscle Secretome. Proteins Struct. Funct. Bioinform. 2005, 62, 776–792. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef]
- Trovato, E.; di Felice, V.; Barone, R. Extracellular Vesicles: Delivery Vehicles of Myokines. Front. Physiol. 2019, 10, 522. [Google Scholar] [CrossRef]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E.-M. Physical Exercise Induces Rapid Release of Small Extracellular Vesicles into the Circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying MiRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 Myoblasts Release Micro-Vesicles Containing MtDNA and Proteins Involved in Signal Transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Chikh, K.; Pesenti, S.; Euthine, V.; Granjon, A.; Errazuriz, E.; Lefai, E.; Vidal, H.; Rome, S. Myotube-Derived Exosomal MiRNAs Downregulate Sirtuin1 in Myoblasts during Muscle Cell Differentiation. Cell Cycle 2014, 13, 78–89. [Google Scholar] [CrossRef]
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and Systemic Molecular Responses to a Single Flywheel Based Iso-Inertial Training Session in Resistance-Trained Men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Lovett, J.A.C.; Durcan, P.J.; Myburgh, K.H. Investigation of Circulating Extracellular Vesicle MicroRNA Following Two Consecutive Bouts of Muscle-Damaging Exercise. Front. Physiol. 2018, 9, 1149. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; Visavadiya, N.P.; de Paz, J.A.; Whitehurst, M.; Cuevas, M.J.; González-Gallego, J.; Huang, C.-J. Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma MiR-146a-5p and CfDNA in the Elderly. Nutrients 2021, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma Exosomal MiRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, Mechanism, Function, and Recent Clinical Trials. Biochim. Et Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A Family of MicroRNAs Encoded by Myosin Genes Governs Myosin Expression and Muscle Performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef]
- Mccarthy, J. MicroRNA-206: The Skeletal Muscle-Specific MyomiR. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2008, 1779, 682–691. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-Specific MicroRNA MiR-206 Promotes Muscle Differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef]
- Singh, G.B.; Cowan, D.B.; Wang, D.-Z. Tiny Regulators of Massive Tissue: MicroRNAs in Skeletal Muscle Development, Myopathies, and Cancer Cachexia. Front. Oncol. 2020, 10, 598964. [Google Scholar] [CrossRef]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-β Regulates MiR-206 and MiR-29 to Control Myogenic Differentiation through Regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-Kinase/Akt Signaling by Muscle-Enriched MicroRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- Liu, H.-C.; Han, D.-S.; Hsu, C.-C.; Wang, J.-S. Circulating MicroRNA-486 and MicroRNA-146a Serve as Potential Biomarkers of Sarcopenia in the Older Adults. BMC Geriatr. 2021, 21, 86. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.T.; Zeng, N.; Blenkiron, C.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Circulatory Exosomal MiRNA Following Intense Exercise Is Unrelated to Muscle and Plasma MiRNA Abundances. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E723–E733. [Google Scholar] [CrossRef]
- Silver, J.L.; Alexander, S.E.; Dillon, H.T.; Lamon, S.; Wadley, G.D. Extracellular Vesicular MiRNA Expression Is Not a Proxy for Skeletal Muscle MiRNA Expression in Males and Females Following Acute, Moderate Intensity Exercise. Physiol. Rep. 2020, 8, e14520. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Arcangeletti, M.; Guescini, M.; Cesarini, E.; Stocchi, V.; Degli Esposti, M.; Papa, S. Fas Signalling Promotes Intercellular Communication in T Cells. PLoS ONE 2012, 7, e35766. [Google Scholar] [CrossRef]
- Asakura, A.; Seale, P.; Girgis-Gabardo, A.; Rudnicki, M.A. Myogenic Specification of Side Population Cells in Skeletal Muscle. J. Cell Biol. 2002, 159, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, E.W.I.; Hillen, B.; Mayr, K.; Simon, P.; Krämer-Albers, E.-M.; Brahmer, A. Kinetics and Topology of DNA Associated with Circulating Extracellular Vesicles Released during Exercise. Genes 2021, 12, 522. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The MiRNA Plasma Signature in Response to Acute Aerobic Exercise and Endurance Training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, Á.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.A.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating Inflammatory MiRNA Signature in Response to Different Doses of Aerobic Exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.F.; Wang, C.; Yin, X.; Tian, D.; Lu, Q.J.; Zhang, C.Y.; Chen, X.; Ma, J.Z. Similar Responses of Circulating MicroRNAs to Acute High-Intensity Interval Exercise and Vigorous-Intensity Continuous Exercise. Front. Physiol. 2016, 7, 102. [Google Scholar] [CrossRef]
- Gomes, C.P.C.; Oliveira, G.P., Jr.; Madrid, B.; Almeida, J.A.; Franco, O.L.; Pereira, R.W. Circulating MiR-1, MiR-133a, and MiR-206 Levels Are Increased after a Half-Marathon Run. Biomarkers 2014, 19, 585–589. [Google Scholar] [CrossRef]
- Mooren, F.C.; Viereck, J.; Krüger, K.; Thum, T. Circulating Micrornas as Potential Biomarkers of Aerobic Exercise Capacity. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef]
- Aoi, W.; Ichikawa, H.; Mune, K.; Tanimura, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Muscle-Enriched MicroRNA MiR-486 Decreases in Circulation in Response to Exercise in Young Men. Front. Physiol. 2013, 4, 80. [Google Scholar] [CrossRef]
- Rome, S.; Forterre, A.; Mizgier, M.L.; Bouzakri, K. Skeletal Muscle-Released Extracellular Vesicles: State of the Art. Front. Physiol. 2019, 10, 929. [Google Scholar] [CrossRef]
- Kirby, T.J.; McCarthy, J.J. MicroRNAs in Skeletal Muscle Biology and Exercise Adaptation. Free Radic. Biol. Med. 2013, 64, 95–105. [Google Scholar] [CrossRef]
- Miura, P.; Amirouche, A.; Clow, C.; Bélanger, G.; Jasmin, B.J. Brain-Derived Neurotrophic Factor Expression Is Repressed during Myogenic Differentiation by MiR-206. J. Neurochem. 2012, 120, 230–238. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef]
- Das, U.N. Circulating Microparticles in Septic Shock and Sepsis-Related Complications. Minerva Anestesiol. 2019, 85, 571–576. [Google Scholar] [CrossRef]
- Reich, N.; Beyer, C.; Gelse, K.; Akhmetshina, A.; Dees, C.; Zwerina, J.; Schett, G.; Distler, O.; Distler, J.H.W. Microparticles Stimulate Angiogenesis by Inducing ELR+ CXC-Chemokines in Synovial Fibroblasts. J. Cell Mol. Med. 2011, 15, 756–762. [Google Scholar] [CrossRef]
- Guescini, M.; Maggio, S.; Ceccaroli, P.; Battistelli, M.; Annibalini, G.; Piccoli, G.; Sestili, P.; Stocchi, V. Extracellular Vesicles Released by Oxidatively Injured or Intact C2C12 Myotubes Promote Distinct Responses Converging toward Myogenesis. Int. J. Mol. Sci. 2017, 18, 2488. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.; Zhou, Q.; Cheng, Y.; Che, L.; Xu, J.; Xiao, J.; Shen, Z.; Bei, Y. Dynamic Regulation of Circulating MicroRNAs During Acute Exercise and Long-Term Exercise Training in Basketball Athletes. Front. Physiol. 2018, 9, 282. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. MiR-21 and MiR-146a: The MicroRNAs of Inflammaging and Age-Related Diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
- Seiler, K.S.; Kjerland, G.O. Quantifying Training Intensity Distribution in Elite Endurance Athletes: Is There Evidence for an “Optimal” Distribution? Scand. J. Med. Sci. Sport. 2006, 16, 49–56. [Google Scholar] [CrossRef]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L.R. Evaluation of Three Portable Blood Lactate Analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef]
- Bentley, D.J.; Newell, J.; Bishop, D. Incremental Exercise Test Design and Analysis. Sport. Med. 2007, 37, 575–586. [Google Scholar] [CrossRef]
- Newell, J.; Higgins, D.; Madden, N.; Cruickshank, J.; Einbeck, J.; McMillan, K.; McDonald, R. Software for Calculating Blood Lactate Endurance Markers. J. Sport. Sci. 2007, 25, 1403–1409. [Google Scholar] [CrossRef]
- Kenneally, M.; Casado, A.; Santos-Concejero, J. The Effect of Periodization and Training Intensity Distribution on Middle- and Long-Distance Running Performance: A Systematic Review. Int. J. Sport. Physiol. Perform. 2018, 13, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, Scaling, and Transformations: Improving the Biological Information Content of Metabolomics Data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Prusis, P.; Lundstedt, T.; Wikberg, J.E.S. Proteo-Chemometrics Analysis of MSH Peptide Binding to Melanocortin Receptors. Protein Eng. Des. Sel. 2002, 15, 305–311. [Google Scholar] [CrossRef][Green Version]
- Eastment, H.; Krzanowski, W. Cross-validatory choice of the number of components from a principal component analysis. Technometrics 1982, 24, 73–77. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Adosraku, R.K.; Choi, G.T.; Constantinou-Kokotos, V.; Anderson, M.M.; Gibbons, W.A. NMR lipid profiles of cells, tissues, and body fluids: Proton NMR analysis of human erythrocyte lipids. J. Lipid Res. 1994, 35, 1925–1931. [Google Scholar] [CrossRef]
- Bonzom, P.M.; Nicolaou, A.; Zloh, M.; Baldeo, W.; Gibbons, W.A. NMR lipid profile of Agaricus bisporus. Phytochemistry 1999, 50, 1311–1321. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, S.; Canonico, B.; Ceccaroli, P.; Polidori, E.; Cioccoloni, A.; Giacomelli, L.; Ferri Marini, C.; Annibalini, G.; Gervasi, M.; Benelli, P.; et al. Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects. Int. J. Mol. Sci. 2023, 24, 3039. https://doi.org/10.3390/ijms24033039
Maggio S, Canonico B, Ceccaroli P, Polidori E, Cioccoloni A, Giacomelli L, Ferri Marini C, Annibalini G, Gervasi M, Benelli P, et al. Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects. International Journal of Molecular Sciences. 2023; 24(3):3039. https://doi.org/10.3390/ijms24033039
Chicago/Turabian StyleMaggio, Serena, Barbara Canonico, Paola Ceccaroli, Emanuela Polidori, Andrea Cioccoloni, Luca Giacomelli, Carlo Ferri Marini, Giosuè Annibalini, Marco Gervasi, Piero Benelli, and et al. 2023. "Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects" International Journal of Molecular Sciences 24, no. 3: 3039. https://doi.org/10.3390/ijms24033039
APA StyleMaggio, S., Canonico, B., Ceccaroli, P., Polidori, E., Cioccoloni, A., Giacomelli, L., Ferri Marini, C., Annibalini, G., Gervasi, M., Benelli, P., Fabbri, F., Del Coco, L., Fanizzi, F. P., Giudetti, A. M., Lucertini, F., & Guescini, M. (2023). Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects. International Journal of Molecular Sciences, 24(3), 3039. https://doi.org/10.3390/ijms24033039











