Fine Mapping of a Pleiotropic Locus (BnUD1) Responsible for the Up-Curling Leaves and Downward-Pointing Siliques in Brassica napus
Abstract
1. Introduction
2. Results
2.1. Performance of the Bnud1 Mutant
2.2. Inheritance of the Up-Curling Leaf and Downward-Pointing Silique Traits
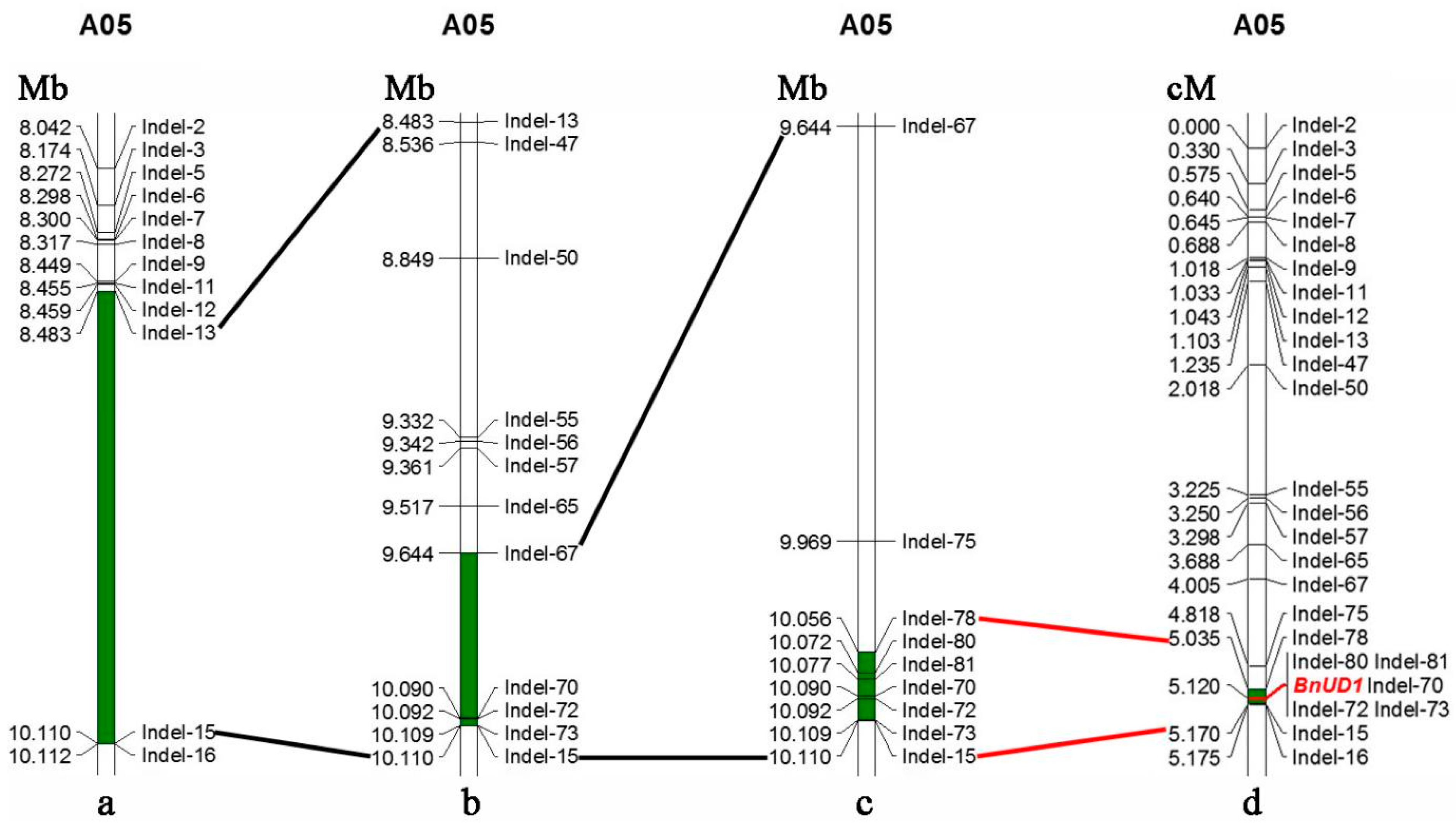
2.3. Mapping of the BnUD1 Locus
2.4. Gene Cloning
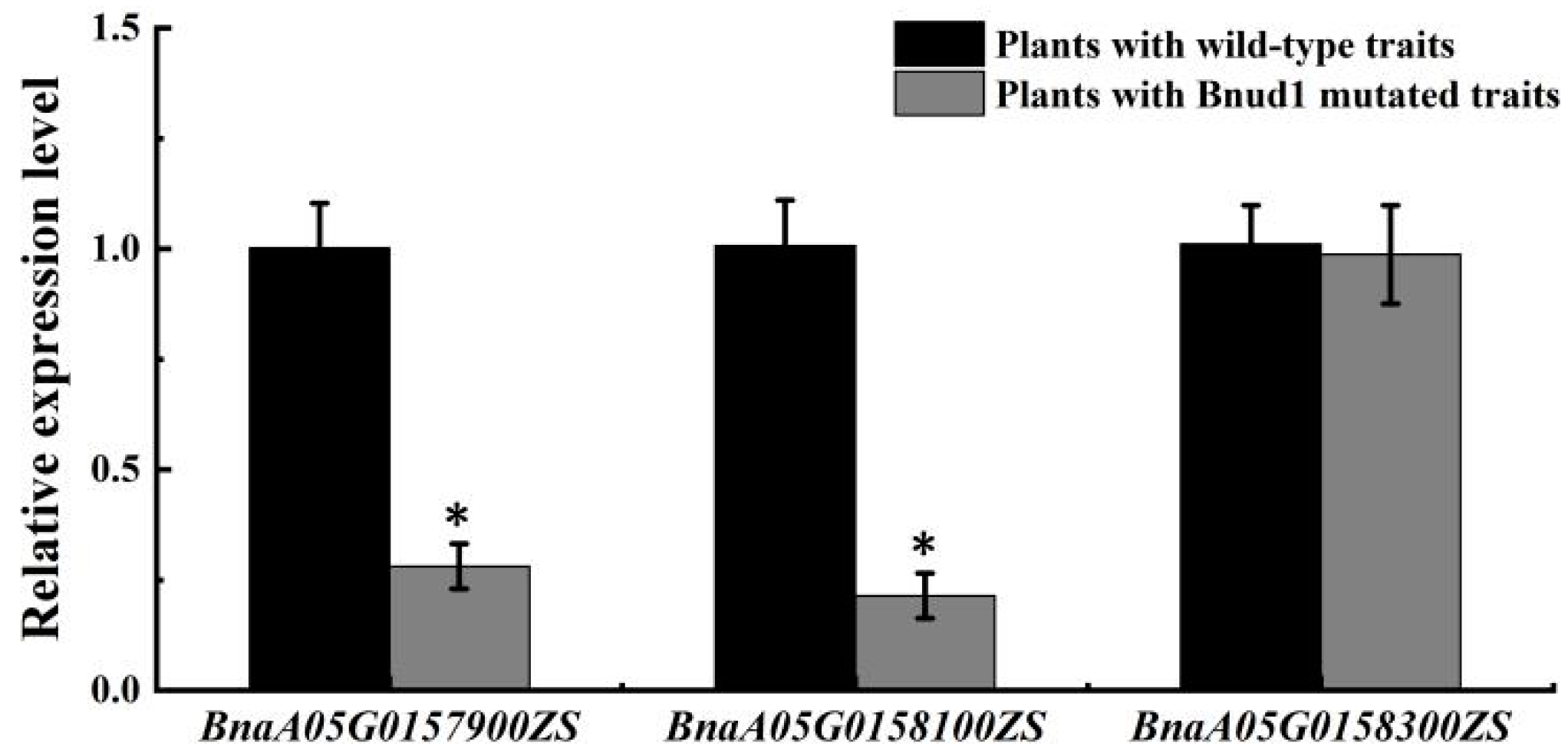
2.5. Candidate Gene Analysis
2.6. Agronomic Traits
2.7. Determination of the Chlorophyll Content and Photosynthetic Efficiency
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Genetic Analysis
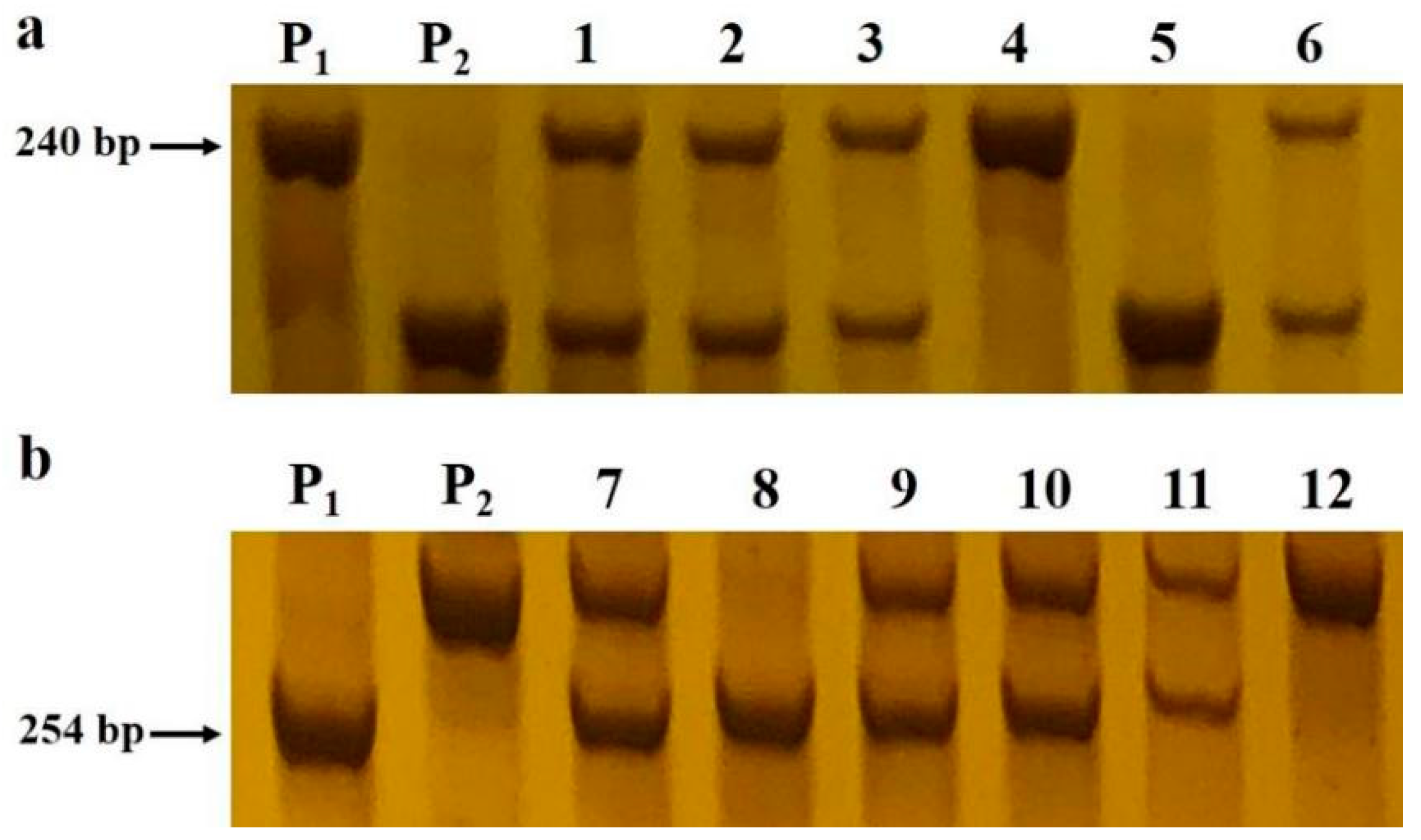
4.3. Bulked Segregant Analysis
4.4. Mapping of the BnUD1 Locus
4.5. Identification of Genes in the Mapping Interval and Comparative Sequencing
4.6. Quantitative Real-Time PCR Analysis
4.7. Agronomic Trait Analysis
4.8. Determination of the Chlorophyll Content and Photosynthetic Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fei, D.; Guan, C.; Jiao, Y. Molecular mechanisms of leaf morphogenesis. Mol. Plant 2018, 11, 1117–1134. [Google Scholar]
- Conklin, P.A.; Josh, S.; Shujie, L.; Scanlon, M.J. On the mechanisms of development in monocot and eudicot leaves. New Phytol. 2019, 221, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Shen, A.; Xiong, W.; Sun, Q.L.; Luo, Q.; Song, T.; Li, Z.L.; Luan, W.J. Overexpression of OsHox32 results in pleiotropic effects on plant type architecture and leaf development in Rice. Rice 2016, 9, 46. [Google Scholar] [CrossRef]
- Rong, F.; Chen, F.; Huang, L.; Zhang, J.; Zhang, C.; Hou, D.; Cheng, Z.; Weng, Y.; Chen, P.; Li, Y. A mutation in class III homeodomain-leucine zipper (HD-ZIP III) transcription factor results in curly leaf (cul) in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2018, 132, 113–123. [Google Scholar] [CrossRef]
- Merelo, P.; Ram, H.; Caggiano, M.P.; Ohno, C.; Heisler, M.G. Regulation of MIR165/166 by class II and class III homeodomain leucine zipper proteins establishes leaf polarity. Proc. Natl. Acad. Sci. USA 2016, 113, 11973–11978. [Google Scholar] [CrossRef]
- Zhang, G.H.; Xu, Q.; Zhu, X.D.; Qian, Q.; Xue, H.W. SHALLOT-LIKE1 is a KANADI transcription factor that modulates Rice leaf rolling by regulating leaf Abaxial cell development. Plant Cell 2009, 21, 719–735. [Google Scholar] [CrossRef]
- Huang, T.; Harrar, Y.; Lin, C.; Reinhart, B.; Newell, N.R.; Talaverarauh, F.; Hokin, S.A.; Barton, M.K.; Kerstetter, R.A. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. Plant Cell 2014, 26, 246. [Google Scholar] [CrossRef]
- Merelo, P.; Paredes, E.B.; Heisler, M.G.; Wenkel, S. The shady side of leaf development: The role of the REVOLUTA/KANADI1 module in leaf patterning and auxin-mediated growth promotion. Curr. Opin. Plant Biol. 2017, 35, 111–116. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.J.; Hwang, J.Y.; Kim, G.T.; Paek, N.C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Sato, F.; Ohme-Takagi, M. Roles of miR319 and TCP transcription factors in leaf development. Plant Physiol. 2017, 175, 874–885. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, L.; Xu, B.; Yang, L.; Ling, Q.; Wang, H.; Huang, H. Genetic interactions between leaf polarity-controlling genes and ASYMMETRIC LEAVES1 and 2 in Arabidopsis leaf patterning. Plant Cell Physiol. 2007, 48, 724–735. [Google Scholar] [CrossRef]
- Pekker, I.; Alvarez, J.P.; Eshed, Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 2005, 17, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, J.M.; Ponce, M.R.; Micol, J.L. The UCU1 Arabidopsis Gene Encodes a SHAGGY/GSK3-like Kinase Required for Cell Expansion along the Proximodistal Axis. Dev. Biol. 2002, 242, 161–173. [Google Scholar] [CrossRef]
- Prigge, M.J.; Otsuga, D.; Alonso, J.M.; Ecker, J.R.; Drews, G.N.; Clark, S.E. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 2005, 17, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Tatematsu, K.; Toyokura, K.; Miyashima, S.; Nakajima, K.; Okada, K. A molecular mechanism that confines the activity pattern of miR165 in Arabidopsis leaf primordia. Plant J. 2015, 82, 596–608. [Google Scholar] [CrossRef]
- Mallory, A.C.; Vaucheret, H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006, 38, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef]
- Shen, X.X.; He, J.Q.; Ping, Y.K.; Guo, J.X.; Hou, N.; Cao, F.G.; Li, X.W.; Geng, D.L.; Wang, S.C.; Chen, P.X.; et al. The positive feedback regulatory loop of miR160-Auxin Response Factor 17-HYPONASTIC LEAVES 1 mediates drought tolerance in apple trees. Plant Physiol. 2022, 188, 1686–1708. [Google Scholar] [CrossRef]
- Nagasaki, H.; Itoh, J.-I.; Hayashi, K.; Hibara, K.-I.; Satoh-Nagasawa, N.; Nosaka, M.; Mukouhata, M.; Ashikari, M.; Kitano, H.; Matsuoka, M.; et al. The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc. Natl. Acad. Sci. USA 2007, 104, 14867–14871. [Google Scholar] [CrossRef]
- Chen, X.J.; Qi, C.K.; Pu, H.M.; Zhang, J.F.; Gao, J.Q.; Fu, S.Z. Evaluation of lodging resistance in rapeseed (Brassica napus L.) and relationship between plant architecture and lodging resistance. Chin. J. Oil Crop Sci. 2007, 29, 54–57. [Google Scholar]
- Li, N.; Shi, J.; Wang, X.; Liu, G.; Wang, H. A combined linkage and regional association mapping validation and fine mapping of two major pleiotropic QTLs for seed weight and silique length in rapeseed (Brassica napus L.). BMC Plant Biol. 2014, 14, 114. [Google Scholar] [CrossRef]
- Khan, F.; Ali, S.; Shakeel, A.; Saeed, A.; Abbas, G. Correlation analysis of some quantitative characters in Brassica napus L. J. Agric. Res. 2006, 44, 7–14. [Google Scholar]
- Wang, X.; Chen, L.; Wang, A.; Wang, H.; Tian, J.; Zhao, X.; Chao, H.; Zhao, Y.; Zhao, W.; Xiang, J.; et al. Quantitative trait loci analysis and genome-wide comparison for silique related traits in Brassica napus. BMC Plant Biol. 2016, 16, 71. [Google Scholar] [CrossRef]
- Sadat, H.A.; Nematzadeh, G.A.; Jelodar, N.B.; Chapi, O.G. Genetic evaluation of yield and yield components at advanced generations in rapeseed (Brassica napus L.). Afr. J. Agric. Res. 2010, 5, 1958–1964. [Google Scholar]
- Samizadeh, H.; Samadi, B.Y.; Behamta, M.; Taleii, A.; Stringam, G. Study of pod length trait in doubled haploid Brassica napus population by molecular markers. J. Agric. Sci. 2007, 9, 129–136. [Google Scholar]
- Zhang, L.; Yang, G.; Liu, P.; Hong, D.; Li, S.; He, Q. Genetic and correlation analysis of silique-traits in Brassica napus L. by quantitative trait locus mapping. Theor. Appl. Genet. 2011, 122, 21–31. [Google Scholar] [CrossRef]
- Venglat, S.P.; Dumonceaux, T.; Rozwadowski, K.; Parnell, L.; Babic, V.; Keller, W.; Martienssen, R.; Selvaraj, G.; Datla, R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 4730–4735. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, C.; Long, J.; Yamaguchi, J.; Serikawa, K.; Hake, S. A knotted 1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 1994, 6, 1859–1876. [Google Scholar]
- Nari, J.; Noat, G.; Ricard, J. Pectin methylesterase, metal ions and plant cell-wall extension. Hydrolysis of pectin by plant cell-wall pectin methylesterase. Biochem. J. 1991, 279, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Röckel, N.; Wolf, S.; Kost, B.; Rausch, T.; Greiner, S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008, 53, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell Biol. 2017, 44, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, W.; Traas, J. Mechanical signaling in plant morphogenesis. Curr. Opin. Genet. Dev. 2018, 51, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef]
- Müller, K.; Levesque-Tremblay, G.; Fernandes, A.; Wormit, A.; Bartels, S.; Usadel, B.; Kermode, A. Overexpression of a pectin methylesterase inhibitor in Arabidopsis thaliana leads to altered growth morphology of the stem and defective organ separation. Plant Signal. Behav. 2013, 8, e26464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Chen, W.J.; Pu, C.; Wan, S.B.; Mao, Y.; Wang, M.; Guan, R.Z. Mapping amajor QTL responsible for dwarf architecture in Brassica napus using a single-nucleotide polymorphism marker approach. BMC Plant Biol. 2016, 16, 178. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Song, J.; Zhao, B.; Guo, C.; Wang, B.; Zhang, Q.; Wang, J.; King, G.J.; Liu, K. An auxin signaling gene BnaA3.IAA7 contributes to improved plant architecture and yield heterosis in rapeseed. New Phytol. 2019, 222, 837–851. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, B.; Li, Z.; Guo, T.; Zhao, J.; Guan, Z.; Liu, K. Identification and characterization of a new dwarf locus DS-4 encoding an aux/IAA7 protein in Brassica napus. Theor. Appl. Genet. 2019, 132, 1435–1449. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.W.; Wang, M.M.; Fan, H.; Wan, S.B.; Wang, Y.; He, J.; Guan, R.Z. Fine mapping of an up-curling leaf locus (BnUC1) in Brassica napus. BMC Plant Biol. 2019, 19, 324. [Google Scholar] [CrossRef]
- Huang, C.; Yang, M.; Shao, D.; Wang, Y.; Wan, S.; He, J.; Meng, Z.; Guan, R. Fine mapping of the BnUC2 locus related to leaf up-curling and plant semi-dwarfing in Brassica napus. BMC Genom. 2020, 21, 530. [Google Scholar] [CrossRef]
- Wan, S.; Qin, Z.; Jiang, X.; Yang, M.; Chen, W.; Wang, Y.; Ni, F.; Guan, Y.; Guan, R.Z. Identification and Fine Mapping of a Locus Related to Leaf Up-Curling Trait (Bnuc3) in Brassica napus. Int. J. Mol. Sci. 2021, 22, 11693. [Google Scholar] [CrossRef]
- Mai, Y.X.; Wang, L.; Yang, H.Q. A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 480–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Q.; Sha, L.; Li, Q.; Yang, X.; Wang, X.; Wang, S. Expression of wild-type PtrIAA14.1, a poplar aux/IAA gene causes morphological changes in Arabidopsis. Front. Plant Sci. 2015, 6, 388. [Google Scholar] [CrossRef]
- Hou, Y.; Li, H.; Zhai, L.; Xie, X.; Li, X.; Bian, S. Identification and functional characterization of the Aux/IAA gene VcIAA27 in blueberry. Plant Signal. Behav. 2020, 15, 1700327. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhu, Y.; Hawes, M.C. Effect of pectin methylesterase gene expression on pea root development. Plant Cell 1999, 11, 1129–1140. [Google Scholar] [CrossRef]
- Hasunuma, T.; Fukusaki, E.I.; Kobayashi, A. Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. J. Biotechnol. 2004, 111, 241–251. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Yang, R.; Wang, T.; Shi, W.; Li, S.; Liu, Z.; Wang, J.; Yang, Y. E3 ubiquitin ligase ATL61 acts as a positive regulator in abscisic acid mediated drought response in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 528, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.H.; Min, H.J.; Yu, S.G.; Byun, M.Y.; Oh, T.R.; Lee, A.; Yang, H.W.; Kim, W.T. OsATL38 mediates mono-ubiquitination of the 14-3-3 protein OsGF14d and negatively regulates the cold stress response in rice. J. Exp. Bot. 2022, 73, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ramonell, K. Arabidopsis Toxicos en Levadura 12 Modulates Salt Stress and ABA Responses in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 7290. [Google Scholar] [CrossRef]
- Aoyama, S.; Terada, S.; Sanagi, M.; Hasegawa, Y.; Lu, Y.; Morita, Y.; Chiba, Y.; Sato, T.; Yamaguchi, J. Membrane-localized ubiquitin ligase ATL15 functions in sugar-responsive growth regulation in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 491, 33–39. [Google Scholar] [CrossRef]
- Ramaiah, M.; Jain, A.; Yugandhar, P.; Raghothama, K.G. ATL8, a RING E3 ligase, modulates root growth and phosphate homeostasis in Arabidopsis. Plant Physiol. Biochem. 2022, 179, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lo, M.C.; Shang, C.; Kao, S.C.; Tseng, Y.Z. Role of M-line proteins in sarcomeric titin assembly during cardiac myofibrillogenesis. J. Cell. Biochem. 1998, 71, 82–95. [Google Scholar] [CrossRef]
- Qadota, H.; Matsunaga, Y.; Nguyen, K.Q.; Mattheyses, A.; Hall, D.H.; Benian, G.M. High-resolution imaging of muscle attachment structures in Caenorhabditis elegans. Cytoskeleton 2017, 74, 426–442. [Google Scholar] [CrossRef]
- Iwakawa, H.; Takahashi, H.; Machida, Y.; Machida, C. Roles of ASYMMETRIC LEAVES2 (AS2) and Nucleolar Proteins in the Adaxial–Abaxial Polarity Specification at the Perinucleolar Region in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7314. [Google Scholar] [CrossRef] [PubMed]
- Waites, R.; Hudson, A. Phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 1995, 121, 2143–2154. [Google Scholar] [CrossRef]
- Waites, R.; Selvadurai, H.R.; Oliver, I.R.; Hudson, A. The PHANTASTICA Gene Encodes a MYB Transcription Factor Involved in Growth and Dorsoventrality of Lateral Organs in Antirrhinum. Cell 1998, 93, 779–789. [Google Scholar] [CrossRef]
- McConnell, J.R.; Emery, J.; Eshed, Y.; Bao, N.; Bowman, J.; Barton, M.K. Role of PHABULOSA and PHAVOLUTA in deter-mining radial patterning in shoots. Nature 2001, 411, 709–713. [Google Scholar] [CrossRef]
- Itoh, J.I.; Hibara, K.-I.; Sato, Y.; Nagato, Y. Developmental Role and Auxin Responsiveness of Class III Homeodomain Leucine Zipper Gene Family Members in Rice. Plant Physiol. 2008, 147, 1960–1975. [Google Scholar] [CrossRef] [PubMed]
- Manuela, D.; Xu, M. Patterning a Leaf by Establishing Polarities. Front. Plant Sci. 2020, 11, 568730. [Google Scholar] [CrossRef]
- Wu, G.; Lin, W.-C.; Huang, T.; Poethig, R.S.; Springer, P.S.; Kerstetter, R.A. KANADI1 regulates adaxial-abaxial polarity in Arabidopsis by directly repressing the transcription of ASYMMETRIC LEAVES2. Proc. Natl. Acad. Sci. USA 2008, 105, 16392–16397. [Google Scholar] [CrossRef]
- Emery, J.F.; Floyd, S.K.; Alvarez, J.; Eshed, Y.; Hawker, N.P.; Izhaki, A.; Baum, S.F.; Bowman, J.L. Radial patterning of Ara-bidopsis shoots by class IIIHD-ZIP and KANADI genes. Curr. Biol. 2003, 13, 1768–1774. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950. [Google Scholar] [CrossRef]
- Kelley, D.; Arreola, A.; Gallagher, T.L.; Gasser, C.S. ETTIN (ARF3) physically interacts with KANADI proteins to form a functional complex essential for integument development and polarity determination in Arabidopsis. Development 2012, 139, 1105–1109. [Google Scholar] [CrossRef]
- Du, F.; Gong, W.; Boscá, S.; Tucker, M.; Laux, T. Dose-dependent AGO1-mediated inhibition of the miRNA165/166 pathway modulates stem cell maintenance in arabidopsis shoot apical meristem. Plant Commun. 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Kirolinko, C.; Hobecker, K.; Wen, J.; Mysore, K.S.; Niebel, A.; Blanco, F.A.; Zanetti, M.E. Auxin Response Factor 2 (ARF2), ARF3,and ARF4 Mediate Both Lateral Root and Nitrogen Fixing Nodule Development in Medicago truncatula. Front. Plant Sci. 2021, 12, 659061. [Google Scholar] [CrossRef]
- Yifhar, T.; Pekker, I.; Peled, D.; Friedlander, G.; Pistunov, A.; Sabban, M.; Wachsman, G.; Alvarez, J.P.; Amsellem, Z.; Eshed, Y. Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 un-derlies the wiry leaf syndrome. Plant Cell 2012, 24, 3575–3589. [Google Scholar] [CrossRef] [PubMed]
- Roeder, A.H.; Ferrándiz, C.; Yanofsky, M.F. The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr. Biol. 2003, 13, 1630–1635. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Roeder, A.H.; Kempin, S.A.; Gremski, K.; Østergaard, L.; Guimil, S.; Reyes, D.K.; Yanofsky, M.F. Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 2004, 116, 843–853. [Google Scholar] [CrossRef]
- Rajani, S.; Sundaresan, V. The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr. Biol. 2001, 11, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wu, B.; Feng, S.; Lü, S.; Guan, C.; Zhang, X.; Qiu, D.; Hu, Y.; Zhou, Y.; Li, C.; et al. Mechanical regulation of organ asymmetry in leaves. Nat. Plants. 2017, 3, 724–3733. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.G. Leaf Angles, Leaf Area, and Canopy Photosynthesis 1. Crop Sci. 1971, 11, 482–485. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Liu, K.; Tang, D.; Sun, M.; Li, Y.; Shen, Y.; Du, G.; Cheng, Z. Semi-Rolled Leaf2modulates rice leaf rolling by regulating abaxial side cell differentiation. J. Exp. Bot. 2016, 67, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Li, Z.; Yue, X.; Wang, B.; Chen, J.; Liu, K.D. Development of INDELs markers in oilseed rape (Brassica napus L.) using re-sequencing data. Mol. Breed. 2016, 36, 79. [Google Scholar] [CrossRef]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.K.; Li, Z.; Zhang, Q.H.; Hu, J.L.; Xiao, Y.J.; Cai, D.F.; Wu, J.S.; King, G.; Li, H.T.; et al. Dissection of the genetic architecture of three seed-quality traits and consequences for breeding in Brassica napus. Plant Biotechnol. J. 2018, 16, 1336–1348. [Google Scholar] [CrossRef]
- Ooijen, J.V. JoinMap 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma B.V.: Wageningen, The Netherlands, 2006. [Google Scholar]
- Singh, V.K.; Mangalam, A.K.; Dwivedi, S.; Naik, S. Primer premier: Program for design of degenerate primers from a protein sequence. Biotechniques 1998, 24, 318–319. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, S.; Fan, H.; Yang, M.; Li, W.; Guan, R. A sulfotransferase gene BnSOT-like1 has a minor genetic effect on seed glucosinolate content in Brassica napus. Crop J. 2020, 8, 855–865. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Frédéric, P.; Franois, J.; Higgins, D.G. The CLUSTAL_X Windows Interface. Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Huggett, J.F.; Foy, C.A.; Vladimir, B.; Kerry, E.; Garson, J.A.; Ross, H.; Jan, H.; Mikael, K.; Mueller, R.D.; Tania, N. The digital MIQE guidelines: Minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 2013, 59, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, H.H.; Zhao, H.X.; Hu, S. Comparison of two protein extraction methods for proteomic analysis of chlorophyll-deficient mutants in Brassica juncea L. Prog. Biochem. Biophys. 2010, 37, 1025–1032. [Google Scholar] [CrossRef]


| Population | UD | WT | Total | Expectation | |
|---|---|---|---|---|---|
| F1 | 30 | 0 | 30 | ||
| RF1 | 30 | 0 | 30 | ||
| F2 | 100 | 42 | 142 | 3:1 | 1.43 |
| BC1 | 58 | 70 | 128 | 1:1 | 1.13 |
| BC2 | 59 | 73 | 132 | 1:1 | 1.48 |
| BC3 | 55 | 65 | 120 | 1:1 | 0.83 |
| BC4 | 63 | 53 | 116 | 1:1 | 0.86 |
| BC5 | 74 | 60 | 134 | 1:1 | 1.46 |
| BC6 | 53 | 65 | 118 | 1:1 | 1.22 |
| BC5F3 | 527 | 207 | 734 | 3:1 | 3.56 |
| BC6F2 | 220 | 88 | 308 | 3:1 | 1.91 |
| Gene in B. napus. cv ZS11 | Gene in B. napus. cv Darmor | Homologue in A. thaliana | Gene Function |
|---|---|---|---|
| BnaA05G0157300ZS | BnaA05g14090D | AT2G20619 | Plant thionin family protein |
| BnaA05G0157400ZS | Unknown | ||
| BnaA05G0157500ZS | AT4G18570 | Tetratricopeptide repeat (TPR)-like superfamily protein | |
| BnaA05G0157600ZS | AT4G18570 | Tetratricopeptide repeat (TPR)-like superfamily protein | |
| BnaA05G0157700ZS | BnaA05g14100D | AT1G53860 | Remorin family protein |
| BnaA05G0157800ZS | BnaA05g14110D | AT1G53850 | 20S proteasome alpha subunit E1 |
| BnaA05G0157900ZS | BnaA05g14120D | AT1G53840 | Pectin methylesterase 1 |
| BnaA05G0158000ZS | BnaA05g14130D | AT1G53830 | Pectin methylesterase 2 |
| BnaA05G0158100ZS | BnaA05g14140D | AT1G53820 | RING/U-box superfamily protein |
| BnaA05G0158200ZS | BnaA05g14150D | AT5G38830 | Cysteinyl-tRNA synthetase, class Ia family protein |
| BnaA05G0158300ZS | BnaA05g14160D | AT1G53800 | Muscle M-line assembly protein |
| Trait | Plants without BnUD1 Locus | Plants with BnUD1 Locus |
|---|---|---|
| Plant height (cm) | 180.76 ± 1.95 | 144.61 ± 6.49 * |
| Branch height (cm) | 56.86 ± 5.03 | 33.70 ± 4.55 * |
| Main inflorescence length (cm) | 73.94 ± 2.44 | 66.50 ± 8.41 * |
| Stem diameter (mm) | 24.37 ± 1.85 | 23.10 ± 0.66 |
| Number of first effective branch | 8.80 ± 0.84 | 6.88 ± 0.99 * |
| Siliques of main inflorescence | 72.63 ± 6.78 | 71.60 ± 4.45 |
| Total siliques per plant | 346.20 ± 18.83 | 363.13 ± 29.86 |
| Silique length | 9.23 ± 0.63 | 9.37 ± 0.78 |
| Seeds per siliques | 26.94 ± 1.94 | 30.67 ± 2.54 * |
| 1000-seed weight (g) | 5.42 ± 0.18 | 4.13 ± 0.15 * |
| Genotype | Chl a (mg/g) | Chl b (mg/g) | Total | Chl a/b Ratio |
|---|---|---|---|---|
| Plants without BnUD1 locus | 1.59 ± 0.33 | 0.96 ± 0.32 | 2.55 ± 0.62 | 1.75 ± 0.37 |
| Plants with BnUD1 locus | 3.39 ± 0.36 * | 1.80 ± 0.23 * | 5.19 ± 0.57 * | 1.89 ± 0.27 * |
| Genotype | NPR µmol CO2 m−2 s−1 | SC mol H2O m−2 s−1 | ICC µmol CO2 mol−1 | TR mmol H2O m−2 s−1 |
|---|---|---|---|---|
| Plants without BnUD1 locus | 8.96 ± 0.48 | 0.24 ± 0.03 | 368.86 ± 4.82 | 2.46 ± 0.31 |
| Plants with BnUD1 locus | 11.89 ± 0.76 * | 0.42 ± 0.02 * | 425.50 ± 6.37 * | 2.89 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Chen, J.; Chang, Y.; Wan, S.; Zhao, Z.; Ni, F.; Guan, R. Fine Mapping of a Pleiotropic Locus (BnUD1) Responsible for the Up-Curling Leaves and Downward-Pointing Siliques in Brassica napus. Int. J. Mol. Sci. 2023, 24, 3069. https://doi.org/10.3390/ijms24043069
Yang M, Chen J, Chang Y, Wan S, Zhao Z, Ni F, Guan R. Fine Mapping of a Pleiotropic Locus (BnUD1) Responsible for the Up-Curling Leaves and Downward-Pointing Siliques in Brassica napus. International Journal of Molecular Sciences. 2023; 24(4):3069. https://doi.org/10.3390/ijms24043069
Chicago/Turabian StyleYang, Mao, Jun Chen, Yuqing Chang, Shubei Wan, Zisu Zhao, Fei Ni, and Rongzhan Guan. 2023. "Fine Mapping of a Pleiotropic Locus (BnUD1) Responsible for the Up-Curling Leaves and Downward-Pointing Siliques in Brassica napus" International Journal of Molecular Sciences 24, no. 4: 3069. https://doi.org/10.3390/ijms24043069
APA StyleYang, M., Chen, J., Chang, Y., Wan, S., Zhao, Z., Ni, F., & Guan, R. (2023). Fine Mapping of a Pleiotropic Locus (BnUD1) Responsible for the Up-Curling Leaves and Downward-Pointing Siliques in Brassica napus. International Journal of Molecular Sciences, 24(4), 3069. https://doi.org/10.3390/ijms24043069






