Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites
Abstract
1. Introduction
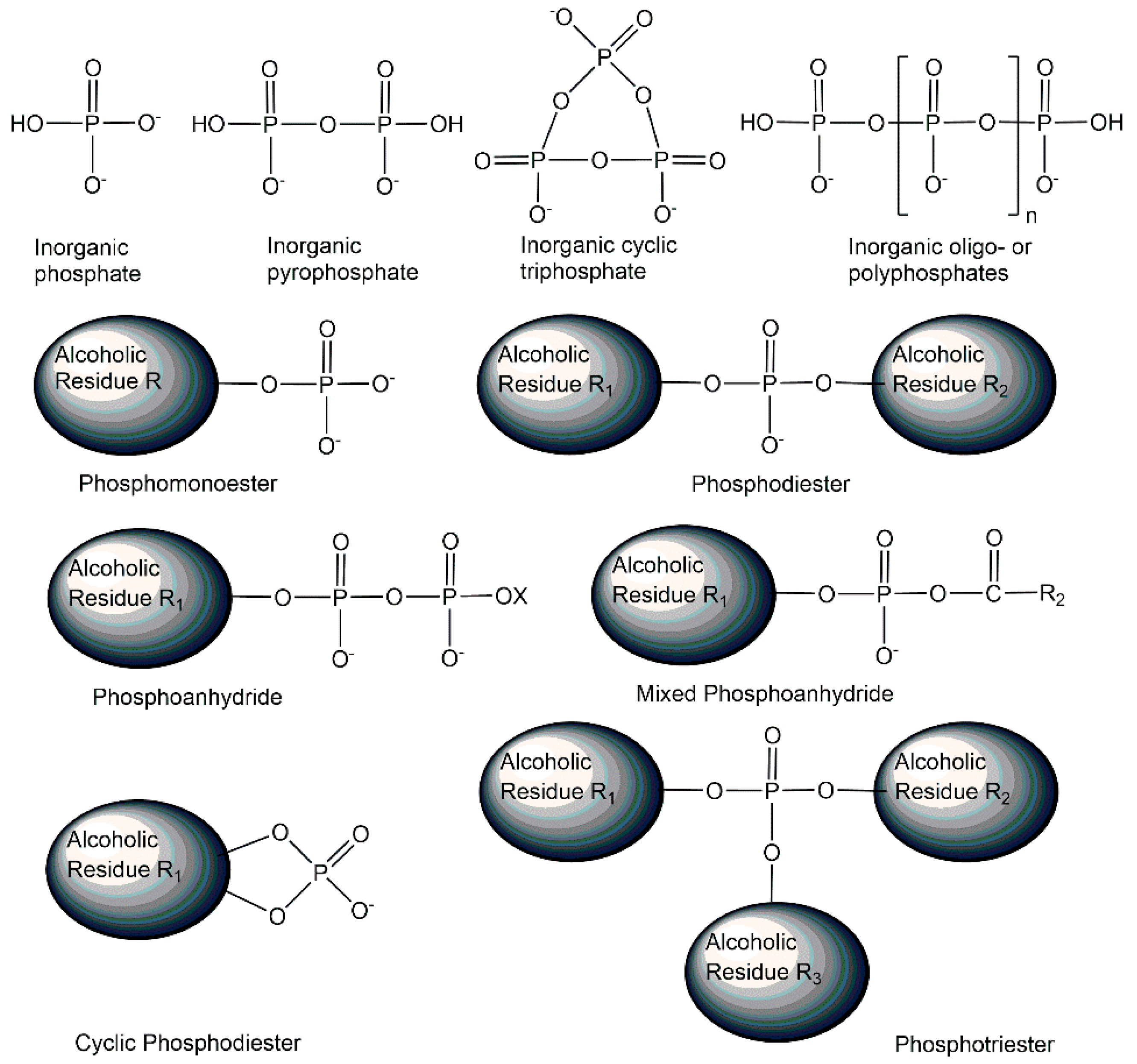
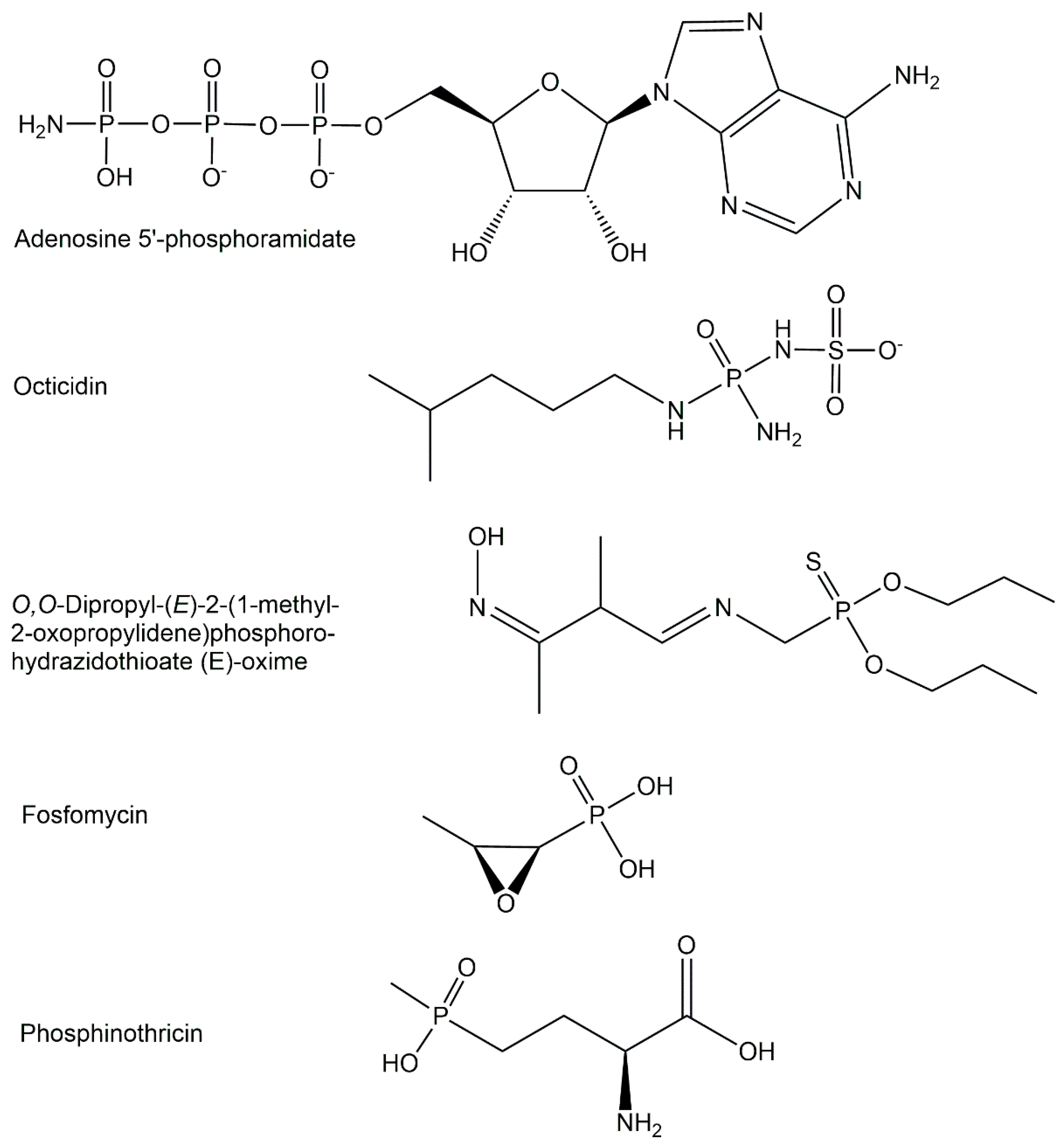
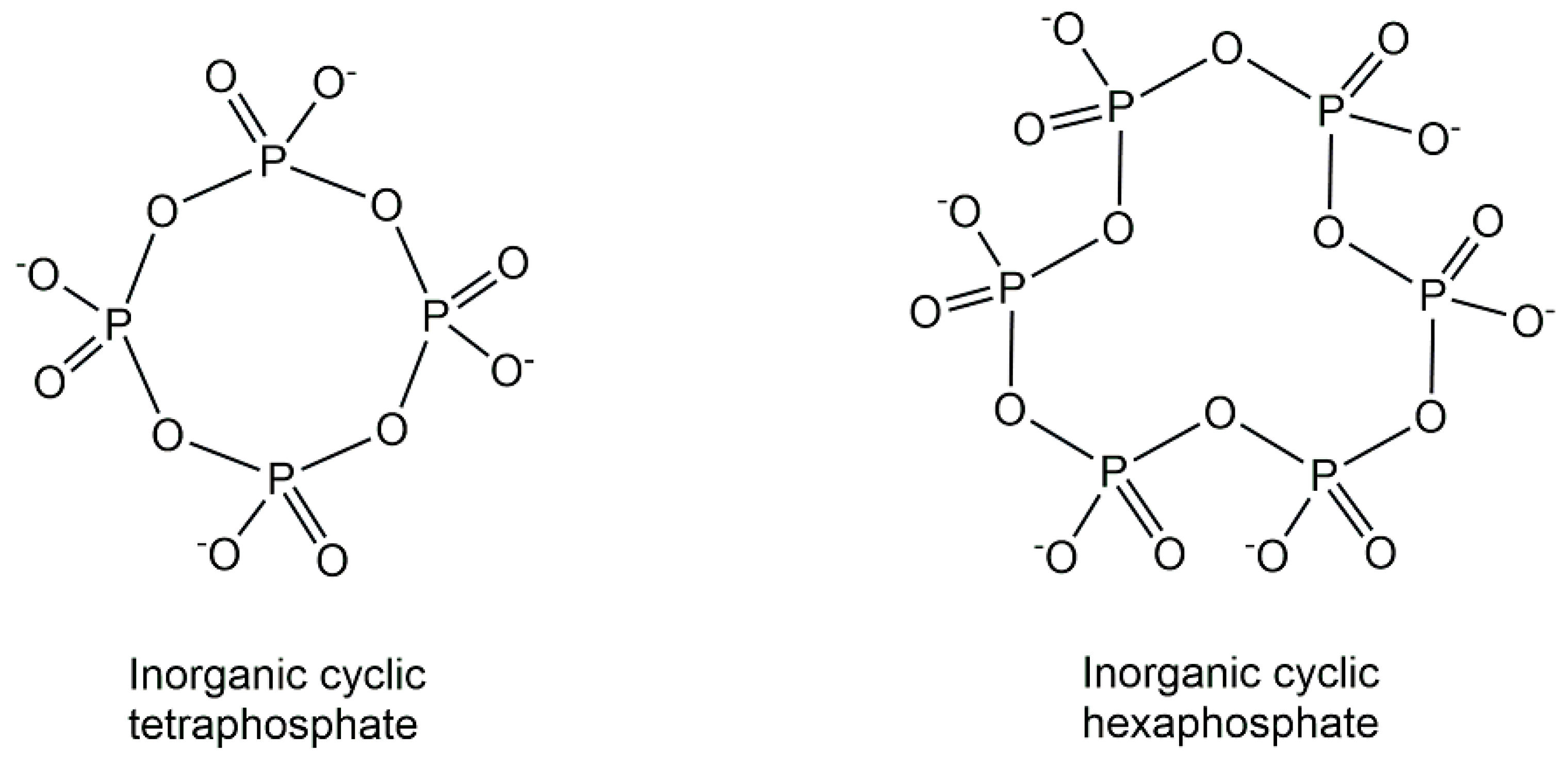
2. Structures of Biologically Active Phosphometabolites
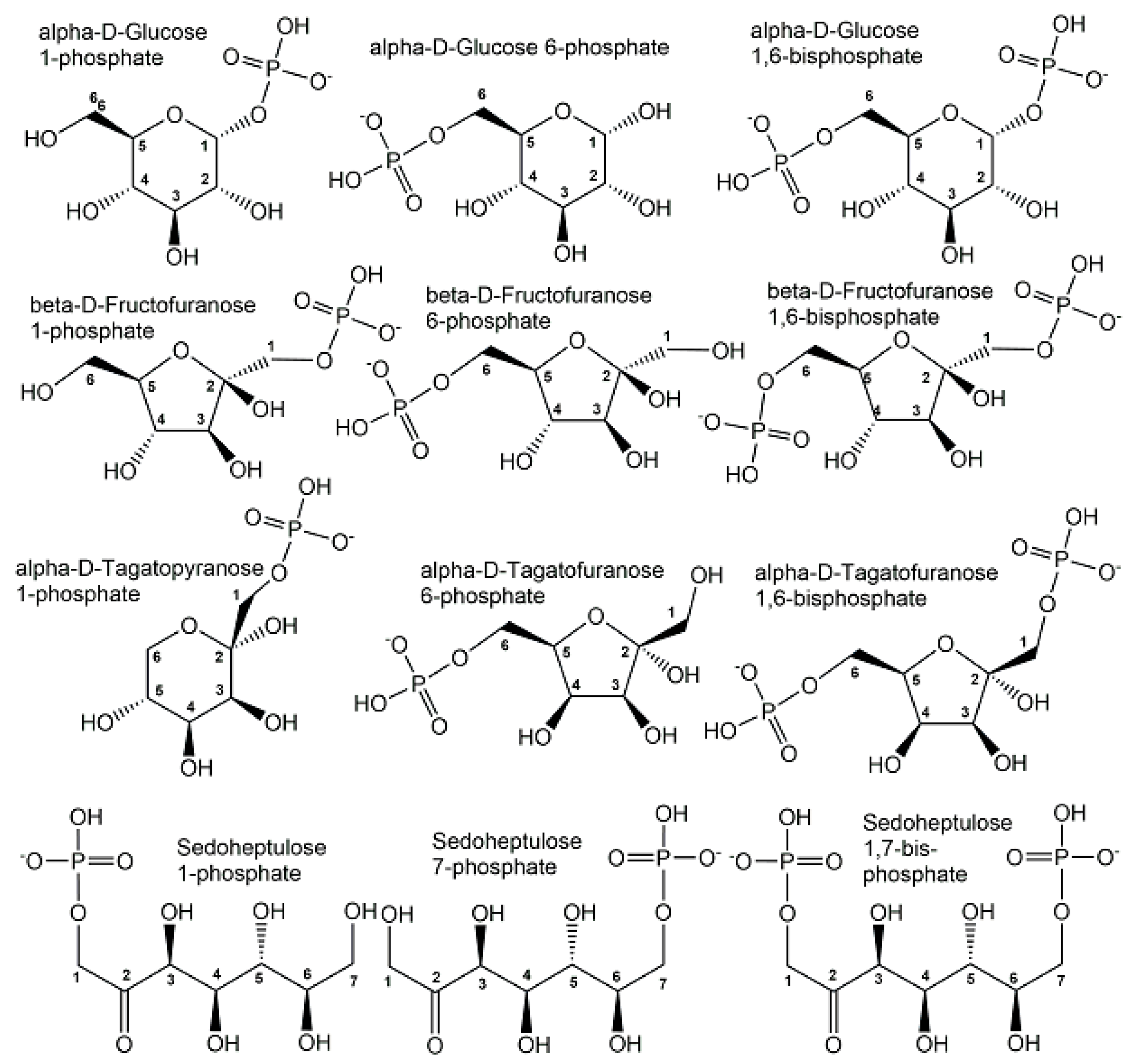
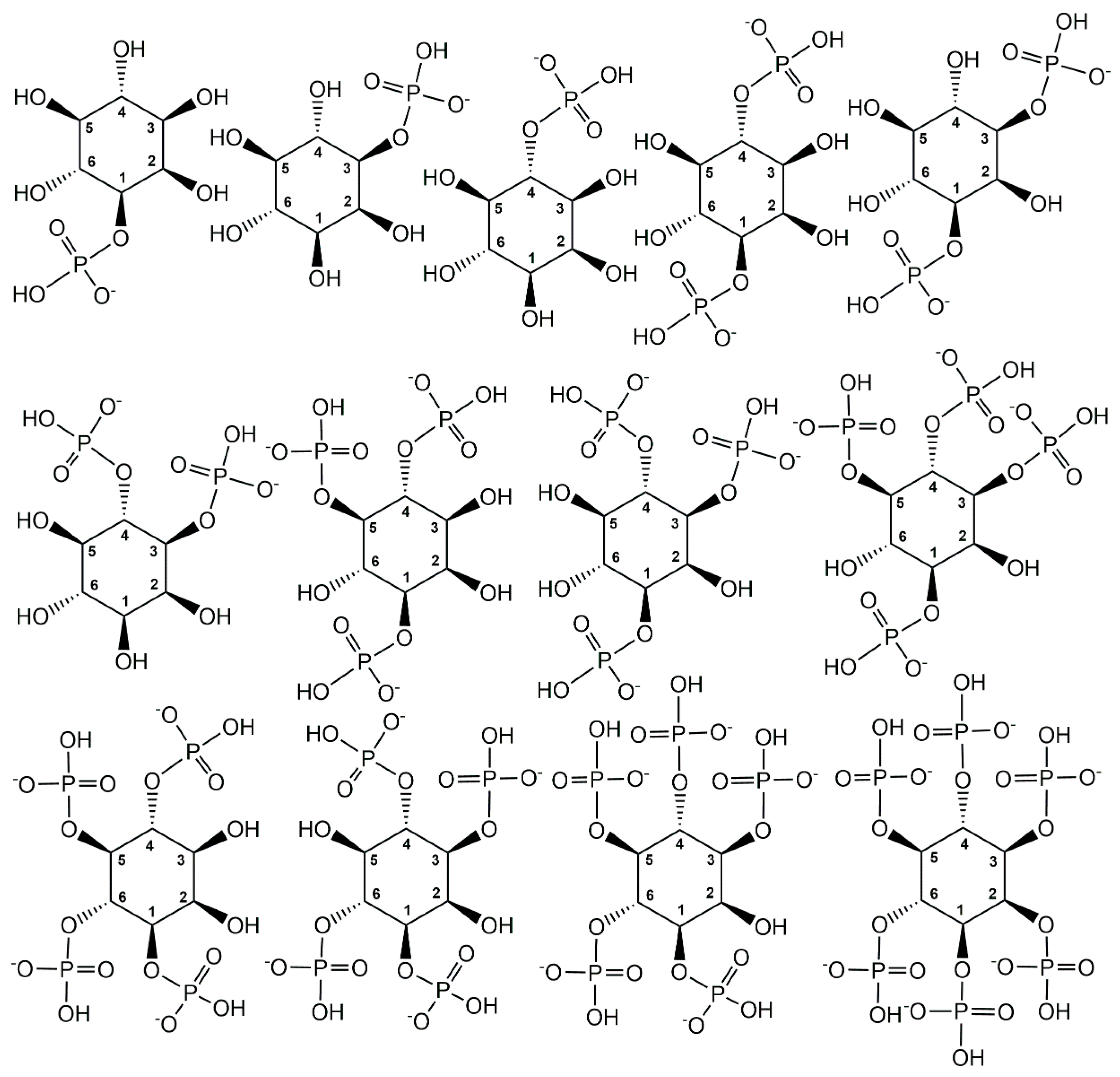
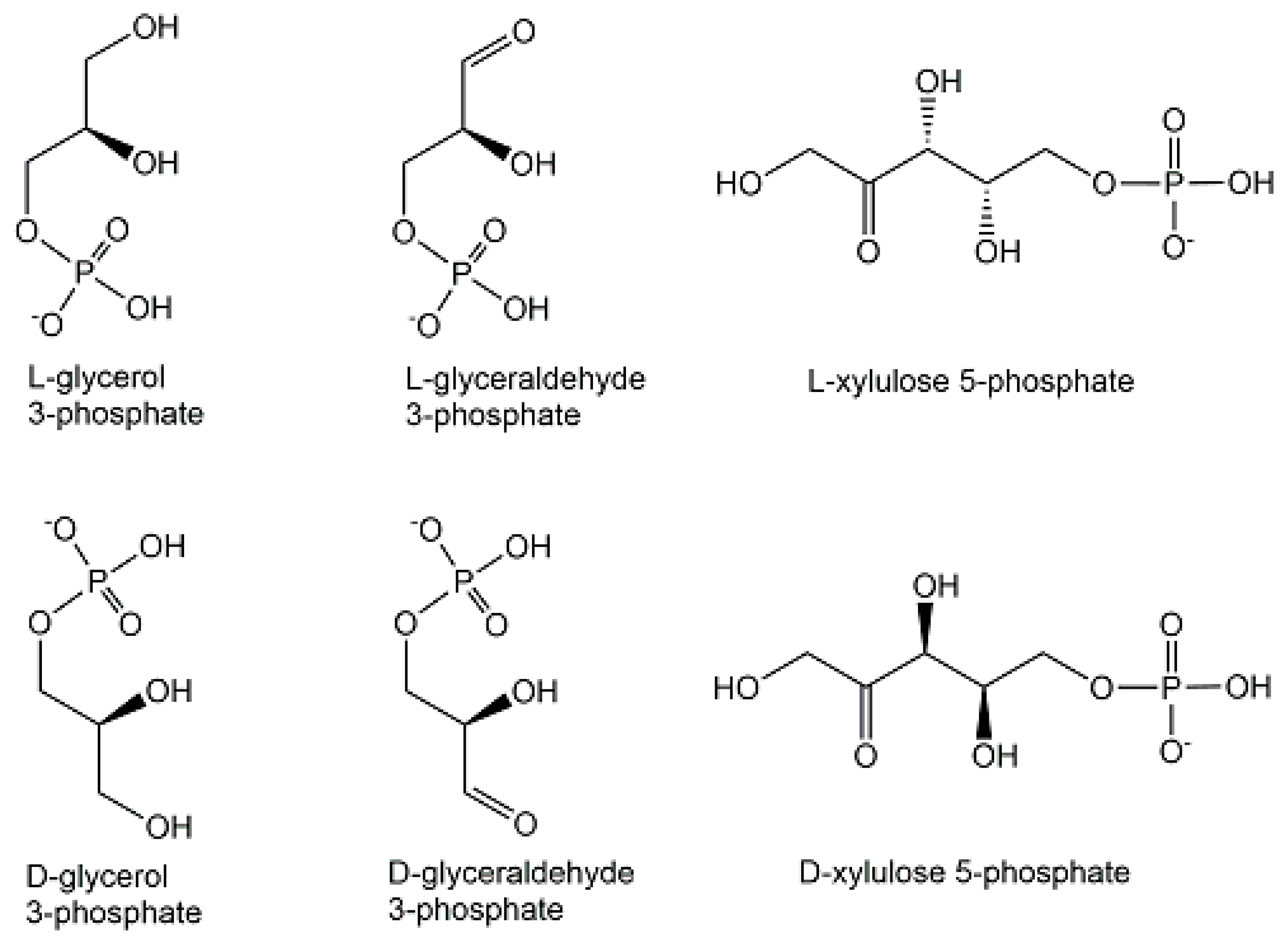
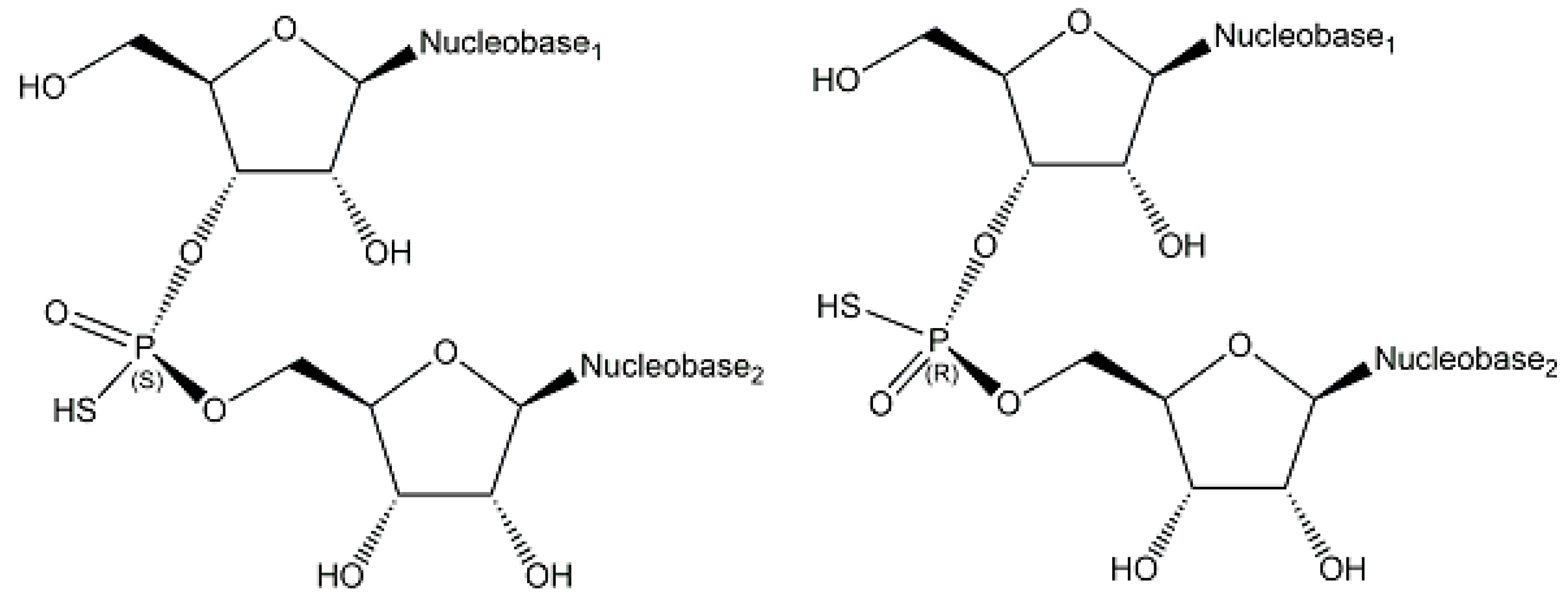
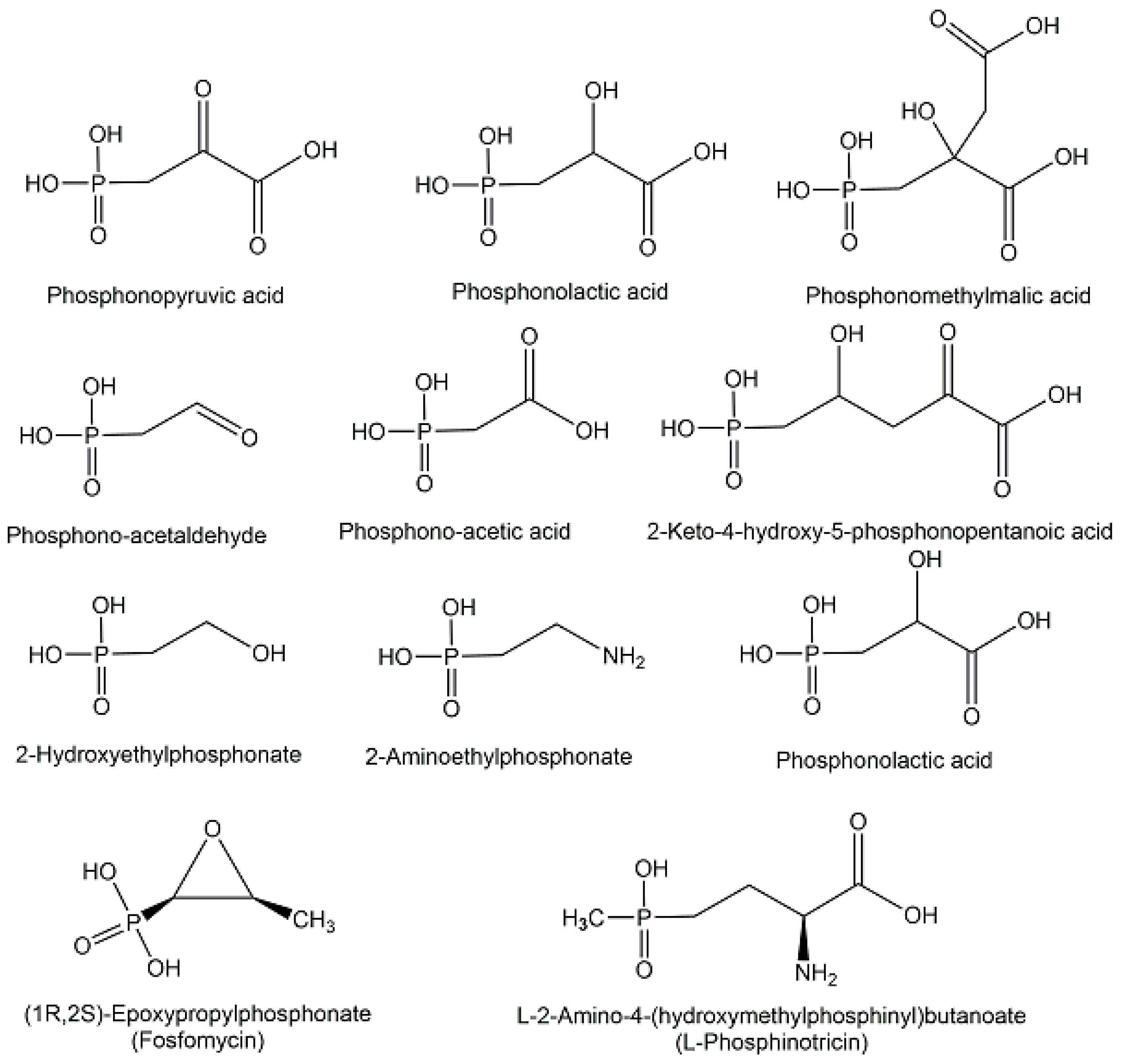
2.1. Endogenous Phosphometabolites
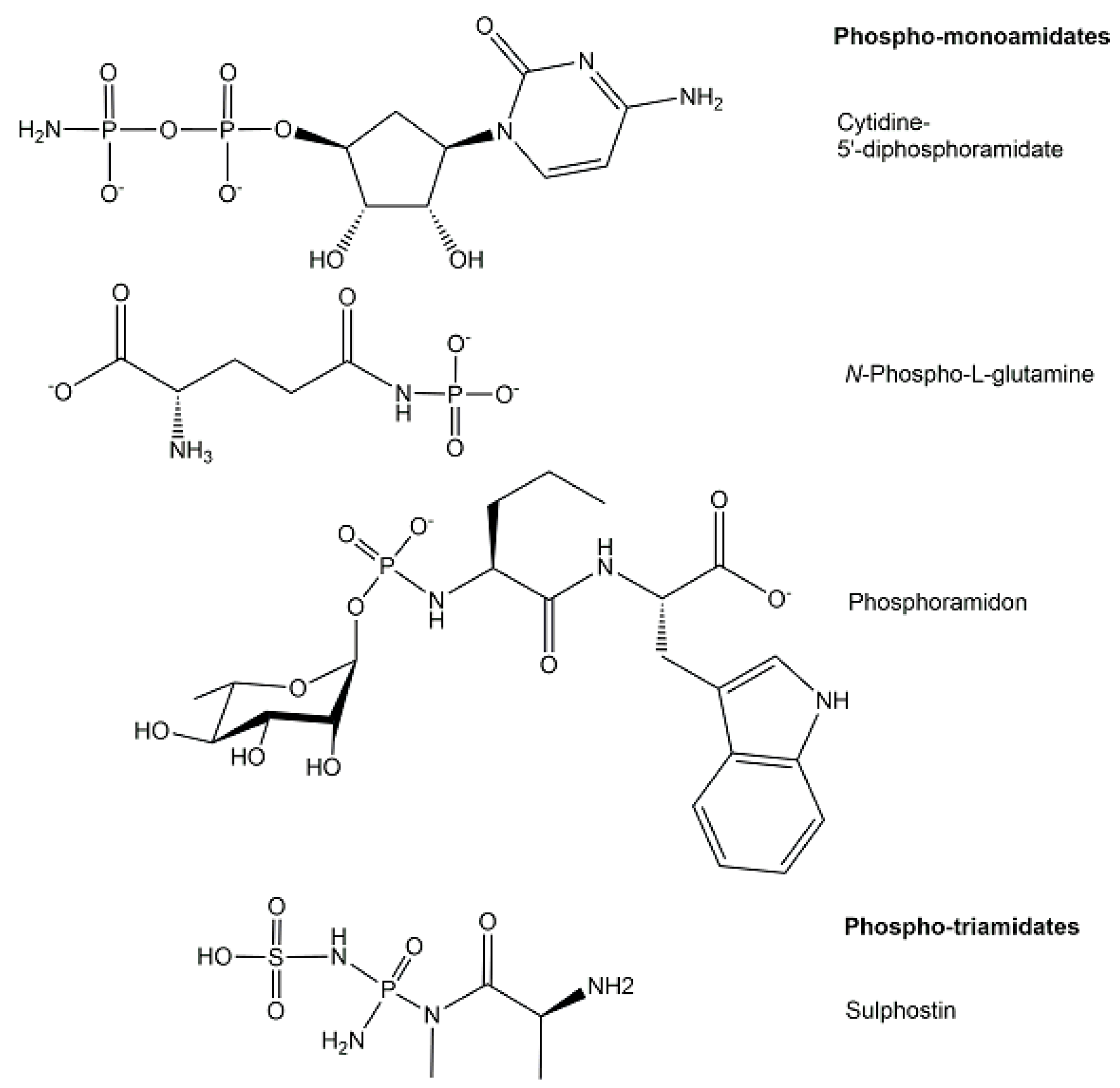
2.2. Exogenous Phosphometabolites
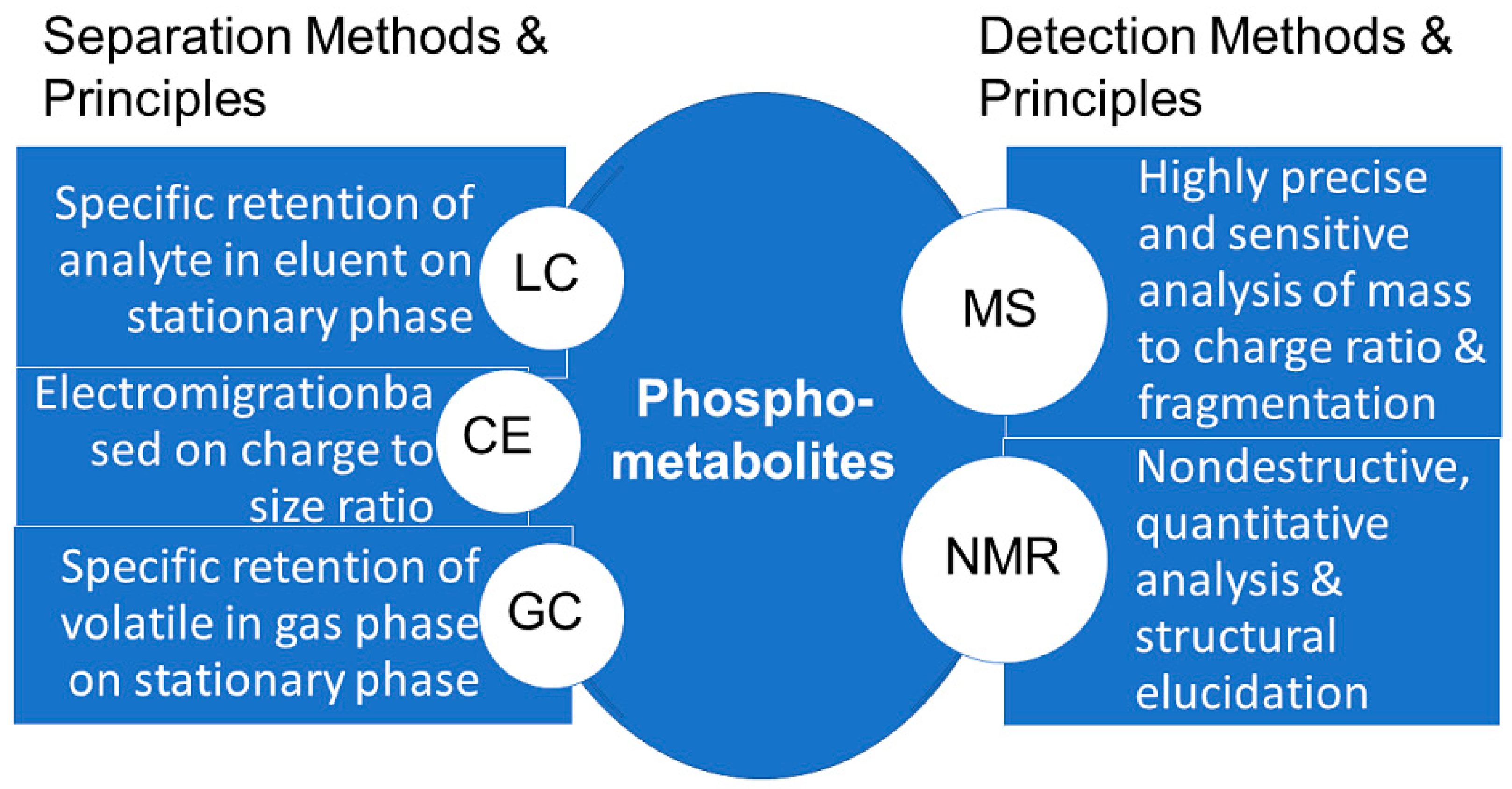
3. Analysis of Biologically Active Phosphometabolites
3.1. NMR Methodologies for the Analysis of Phosphometabolites
3.2. MS Methodologies for the Analysis of Phosphometabolites
3.3. Discovery and Structural Identification of Novel Phosphometabolites
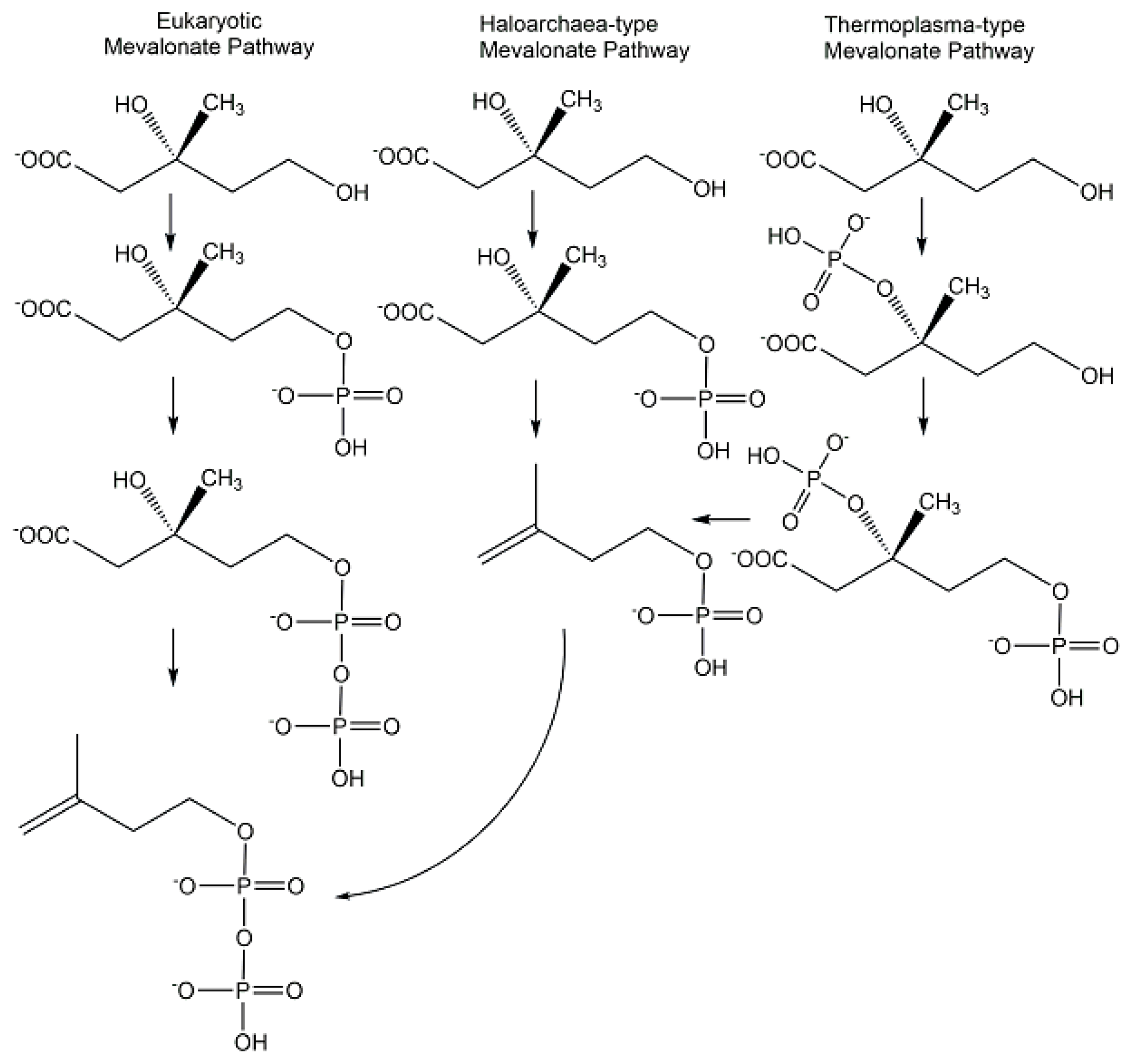
4. Synthesis of Biologically Active Phosphometabolites
4.1. Chemical Methods of Synthesis
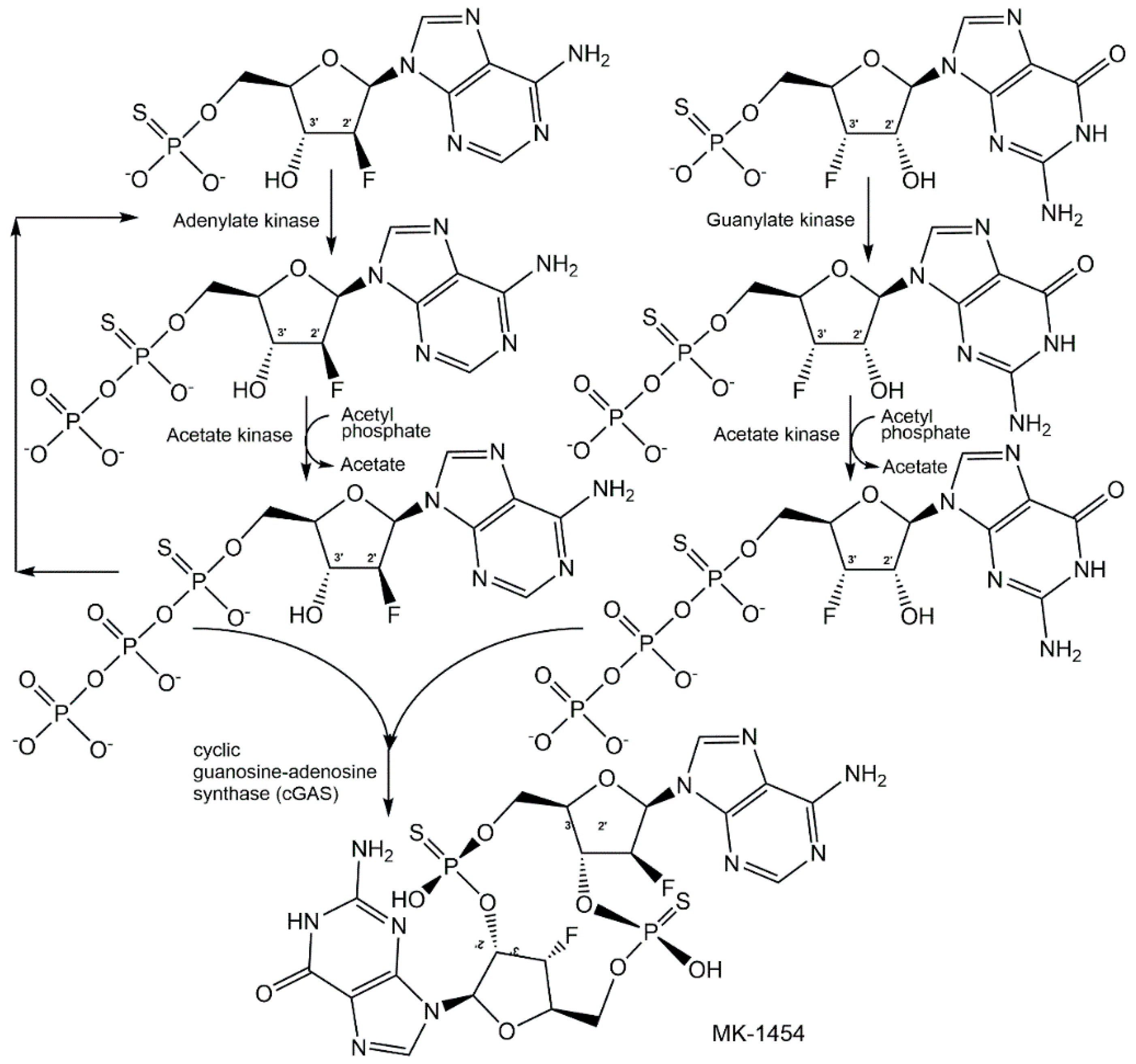
4.2. Biological Methods of Synthesis
5. Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, C.T. The Chemical Biology of Phosphorus; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Ashley, K.; Cordell, D.; Mavinic, D. A brief history of phosphorus: From the philosopher’s stone to nutrient recovery and reuse. Chemosphere 2011, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.R. Where There’s Life, There’s Phosphorus; Kageyama, M., Nakamura, K., Oshima, T., Eds.; Japan Science Society Press: Tokyo, Japan, 1981; pp. 275–279. [Google Scholar]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segre, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134. [Google Scholar] [CrossRef]
- Westheimer, F.H. Why nature chose phosphates. Science 1987, 235, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Bowler, M.W.; Cliff, M.J.; Waltho, J.P.; Blackburn, G.M. Why did Nature select phosphate for its dominant roles in biology? New J. Chem. 2010, 34, 784–794. [Google Scholar] [CrossRef]
- Kamerlin, S.C.L.; Sharma, P.K.; Prasad, R.B.; Warshel, A. Why nature really chose phosphate. Quart. Rev. Biophys. 2013, 46, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Knouse, K.W.; Flood, D.T.; Vantourout, J.C.; Schmidt, M.A.; Mcdonald, I.M.; Eastgate, M.D.; Baran, P.S. Nature chose phosphates and chemists should too: How emerging P (V) methods can augment existing strategies. ACS Cent. Sci. 2021, 7, 1473–1485. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Geeson, M.B.; Cummins, C.C. Let’s Make White Phosphorus Obsolete. ACS Cent. Sci. 2020, 6, 848–860. [Google Scholar] [CrossRef]
- Walsh, C.T.; Tu, B.P.; Tang, Y. Eight Kinetically Stable but Thermodynamically Activated Molecules that Power Cell Metabolism. Chem Rev. 2018, 118, 1460–1494. [Google Scholar] [CrossRef]
- Mironov, N.; Atfi, A.; Razzaque, M.S. Phosphate Burden and Organ Dysfunction. Front. Aging 2022, 3, 890985. [Google Scholar] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Sampson, J.M.; Atlas, Z. Redox chemistry in the phosphorus biogeochemical cycle. Proc. Natl. Acad. Sci. USA 2014, 111, 15468–15473. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth-Science Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Fernández-García, C.; Coggins, A.J.; Powner, M.W. A Chemist’s Perspective on the Role of Phosphorus at the Origins of Life. Life 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Bains, W.; Petkowski, J.J.; Sousa-Silva, C.; Seager, S. Trivalent Phosphorus and Phosphines as Components of Biochemistry in Anoxic Environments. Astrobiology 2019, 19, 885–902. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of Prebiotic Phosphorylation. Chem. Rev. 2020, 120, 4690–4706. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Bains, W.; Seager, S. Natural Products Containing ‘Rare’ Organophosphorus Functional Groups. Molecules 2019, 24, 866. [Google Scholar] [CrossRef]
- Jessen, H.J.; Dürr-Mayer, T.; Haas, T.M.; Ripp, A.; Cummins, C.C. Lost in Condensation: Poly-, Cyclo-, and Ultraphosphates. Acc. Chem. Res. 2021, 54, 4036–4050. [Google Scholar] [CrossRef]
- Mandala, A.V.S.; Loh, D.M.; Shepard, S.M.; Geeson, M.B.; Sergeyev, I.V.; Nocera, D.G.; Cummins, C.C.; Hong, M. Bacterial Phosphate Granules Contain Cyclic Polyphosphates: Evidence from 31P Solid-State NMR. J. Am. Chem. Soc. 2020, 142, 18407–18421. [Google Scholar] [CrossRef] [PubMed]
- Dürr-Mayer, T.; Qiu, D.; Eisenbeis, V.B.; Steck, N.; Häner, M.; Hofer, A.; Mayer, A.; Siegel, J.S.; Baldridge, K.K.; Jessen, H.J. The chemistry of branched condensed phosphates. Nat. Commun. 2021, 12, 5368. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ropek, N.; Melillo, B.; Schreiber, S.L.; Cravatt, B.F.; Vinogradova, E.V. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Key advances in biocatalytic phosphorylations in the last two decades: Biocatalytic syntheses in vitro andbiotransformations in vivo (in humans). Biotechnol. J. 2021, 16, 2000090. [Google Scholar] [CrossRef]
- Shimada, H.; Yamagishi, A. Stdability of Heterochiral Hybrid Membrane Made of Bacterial sn-G3P Lipids and Archaeal sn-G1P Lipids. Biochemistry 2011, 50, 4114–4120. [Google Scholar] [CrossRef]
- Erb, T.J.; Zarzycki, J. A short history of RubisCO: The rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Curr. Opin. Biotechnol. 2018, 49, 100–107. [Google Scholar] [CrossRef]
- Luo, S.; Lin, P.B.; Nieh, L.-Y.; Liao, G.-B.; Tang, P.-W.; Chen, C.; Liao, J.C. A cell-free self-replenishing CO2-fixing system. Nat. Catal. 2022, 5, 154–162. [Google Scholar] [CrossRef]
- Schwander, T.; von Borzyskowski, L.S.; Burgener, S.; Socorro Cortina, N.; Erb, T.J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 2016, 354, 900–904. [Google Scholar] [CrossRef]
- Gomelsky, M. cAMP, c-di-GMP, c-di-AMP and now cGMP: Bacteria use them all! Mol. Microbiol. 2011, 79, 562–565. [Google Scholar] [CrossRef]
- Tal, N.; Morehouse, B.R.; Millman, A.; Stokar-Avihail, A.; Avraham, C.; Fedorenko, T.; Yirmiya, E.; Herbst, E.; Brand, A.; Mehlman, T.; et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 2021, 184, 5728–5739. [Google Scholar] [CrossRef]
- Yu, D.; Song, W.; Tan, E.Y.J.; Liu, L.; Cao, Y.; Jirschitzka, J.; Li, E.; Logemann, E.; Xu, C.; Huang, S.; et al. TIR domains of plant immune receptors are 2′, 3′-cAMP/cGMP synthetases mediating cell death. Cell 2022, 185, 2370–2386. [Google Scholar] [CrossRef] [PubMed]
- Essuman, K.; Milbrandt, J.; Dangl, J.L.; Nishimura, M.T. Shared TIR enzymatic functions regulate cell death and immunity across the tree of life. Science 2022, 377, eabo0001. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Melamed, S.; Millman, A.; Shulman, G.; Oppenheimer-Shaanan, Y.; Kacen, A.; Doron, S.; Amitai, G.; Sorek, R. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 2019, 574, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Wang, X. The phosphoanhydrides bond: One cornerstone of life. Biochemist 2019, 41, 22–27. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Phosphorus Compounds of Natural Origin: Prebiotic, Stereochemistry, Application. Symmetry 2021, 13, 889. [Google Scholar] [CrossRef]
- Pietrowska-Borek, M.; Dobrogojski, J.; Wojdyła-Mamoń, A.M.; Romanowska, J.; Gołębiewska, J.; Borek, S.; Murata, K.; Ishihara, A.; Pedreño, M.A.; Guranowski, A. Nucleoside 5’-Phosphoramidates Control the Phenylpropanoid Pathway in Vitis vinifera Suspension-Cultured Cells. Int. J. Mol. Sci. 2021, 22, 13567. [Google Scholar] [CrossRef]
- Taylor, Z.W.; Raushel, F.M. Cytidine diphosphoramidate kinase: An enzyme required for the biosynthesis of the O-methyl phosphoramidate modification in the capsular polysaccharides of Campylobacter jejuni. Biochemistry 2018, 57, 2238–2244. [Google Scholar] [CrossRef]
- Umezawa, S.; Tatsuta, K.; Izawa, O.; Tsuchiya, T.; Umezawa, H. A new microbial metabolite phosphoramidon (isolation and structure). Tetrahedron Lett. 1972, 1, 97–100. [Google Scholar] [CrossRef]
- Baulig, A.; Helmle, I.; Bader, M.; Wolf, F.; Kulik, A.; Al-Dilaimi, A.; Wibberg, D.; Kalinowski, J.; Gross, H.; Kaysser, L. Biosynthetic reconstitution of deoxysugar phosphoramidate metalloprotease inhibitors using an N–P-bond-forming kinase. Chem. Sci. 2019, 10, 4486–4490. [Google Scholar] [CrossRef] [PubMed]
- Roush, R.F.; Nolan, E.M.; Löhr, F.; Walsh, C.T. Maturation of an Escherichia coli Ribosomal Peptide Antibiotic by ATP-Consuming N-P Bond Formation in Microcin C7. J. Am. Chem. Soc. 2008, 130, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Bertran-Vicente, J.; Penkert, M.; Nieto-Garcia, O.; Jeckelmann, J.M.; Schmieder, P.; Krause, E.; Hackenberger, C.P.R. Chemoselective synthesis and analysis of naturally occurring phosphorylated cysteine peptides. Nat. Commun. 2016, 7, 12703. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Vergin, K.L.; Giovannoni, S.J.; Chan, S.W.; DeMott, M.S.; Taghizadeh, K.; Cordero, O.X.; Cutler, M.; Timberlake, S.; et al. DNA phosphorothioation is widespread and quantized in bacterial genomes. Proc. Natl. Acad. Sci. USA 2011, 108, 2963–2968. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, S.; Deng, Z.; Dedon, P.C.; Chen, S. DNA phosphorothioate modification—A new multi-functional epigenetic system in bacteria. FEMS Microbiol. Rev. 2019, 43, 109–122. [Google Scholar] [CrossRef]
- Li, S.; Horsman, G.P. An inventory of early branch points in microbial phosphonate biosynthesis. Microbial. Genom. 2022, 8, 000781. [Google Scholar] [CrossRef]
- Falagas, M.A.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use Beyond Urinary Tract and Gastrointestinal Infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, Q.; Li, S.; Deng, Z.; Gao, J. The intriguing biology and chemistry of fosfomycin: The only marketed phosphonate antibiotic. RSC Adv. 2019, 9, 42204–42218. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and clinical application of phosphorus-containing drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The role of the phosphorus atom in drug design. ChemMedChem 2019, 14, 190–216. [Google Scholar] [CrossRef] [PubMed]
- Fulmali, A.; Bharate, S.S. Phosphate moiety in FDA-approved pharmaceutical salts and prodrugs. Drug Dev. Res. 2022, 83, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Mehellou, Y.; Rattan, H.S.; Balzarini, J. The ProTide Prodrug Technology: From the Concept to the Clinic. J. Med. Chem. 2018, 61, 2211–2226. [Google Scholar] [CrossRef] [PubMed]
- Slusarczyk, M.; Serpi, M.; Ghazaly, E.; Kariuki, B.M.; McGuigan, C.; Pepper, C. Single Diastereomers of the Clinical Anticancer ProTide Agents NUC-1031 and NUC-3373 Preferentially Target Cancer Stem Cells In Vitro. J. Med. Chem. 2021, 64, 8179–8193. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Malde, R.; Mykura, R.C.; Baker, A.T.; Taher, T.E.; Le Duff, C.S.; Willcox, B.E.; Mehellou, Y. Synthesis and biological evaluation of (E)-4-hydroxy-3-methylbut-2-enyl phosphate (HMBP) aryloxy triester phosphoramidate prodrugs as activators of Vγ9/Vδ2 T-cell immune responses. J. Med. Chem. 2018, 61, 2111–2117. [Google Scholar] [CrossRef]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nature Rev. Drug Discovery 2010, 9, 883–897. [Google Scholar] [CrossRef]
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457. [Google Scholar] [CrossRef]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.-J.; Card, D.; Keohane, C.A.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Albert, R.; Hinterding, K.; Brinkmann, V.; Guerini, D.; Müller-Hartwieg, C.; Knecht, H.; Simeon, C.; Streiff, M.; Wagner, T.; Welzenbach, K.; et al. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J. Med. Chem. 2005, 48, 5373–5377. [Google Scholar] [CrossRef]
- US Food & Drug Administration. GILENYA (Fingolimod) Capsules. Available online: www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022527Orig1s000TOC.cfm (accessed on 9 January 2023).
- US Food & Drug Administration. FDA Expands Approval of Gilenya to Treat Multiple Sclerosis in Pediatric Patients. 2018. Available online: www.fda.gov/news-events/press-announcements/fda-expands-approval-gilenya-treat-multiple-sclerosis-pediatric-patients (accessed on 9 January 2023).
- Benson, A.A.; Bassham, J.A.; Calvin, M.; Goodale, T.C.; Haas, V.A.; Stepka, W. The Path of Carbon in Photosynthesis. V. Paper Chromatography and Radioautography of the Products. J. Am. Chem. Soc. 1950, 72, 1710–1718. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Su, X.; Klein, M.S.; Lewis, I.A.; Fiehn, O.; Rabinowitz, J.D. Metabolite Measurement: Pitfalls to Avoid and Practices to Follow. Annu. Rev. Biochem. 2017, 86, 277–304. [Google Scholar] [CrossRef]
- Qiu, D.; Wilson, M.S.; Eisenbeis, V.B.; Harmel, R.K.; Riemer, E.; Haas, T.M.; Wittwer, C.; Jork, N.; Gu, C.; Shears, S.B.; et al. Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Commun. 2020, 11, 6035. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Cheng, L.L.; Copié, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B.; et al. NMR and Metabolomics—A Roadmap for the Future. Metabolites 2022, 12, 678. [Google Scholar] [CrossRef]
- Bhinderwala, F.; Roth, H.E.; Noel, H.; Feng, D.; Powers, R. Chemical shift variations in common metabolites. J. Magn. Res. 2022, 345, 107335. [Google Scholar] [CrossRef]
- Hoult, D.; Busby, S.; Gadian, D.; Radda, G.K.; Richards, R.E.; Seeley, P.J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 1974, 252, 285–287. [Google Scholar] [CrossRef]
- Bhinderwala, F.; Evans, P.; Jones, K.; Laws, B.R.; Smith, T.G.; Morton, M.; Powers, R. Phosphorus NMR and Its Application to Metabolomics. Anal. Chem. 2020, 92, 9536–9545. [Google Scholar] [CrossRef]
- Cox, N.; Kuemmerle, R.; Millard, P.; Cahoreau, E.; Francois, J.-M.; Parrou, J.-L.; Lippens, G. Integrated pH Measurement during Reaction Monitoring with Dual Reception 1H−31P NMR Spectroscopy. Anal. Chem. 2019, 91, 3959–3963. [Google Scholar] [CrossRef]
- Cox, N.; Millard, P.; Charlier, C.; Lippens, G. Improved NMR Detection of Phospho-Metabolites in a Complex Mixture. Anal. Chem. 2021, 93, 4818–4824. [Google Scholar] [CrossRef]
- Bernardo-Seisdedos, G.; Bilbao, J.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; Gutierrez de Juan, V.; Bizkarguenaga, M.; Mateos, B.; Fondevila, M.F.; Abril-Fornaguera, J.; Diercks, T.; et al. Metabolic landscape of the mouse liver by quantitative 31P nuclear magnetic resonance analysis of the phosphorome. Hepatology 2021, 74, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Groenke, K.; Takors, R.; Wandrey, C.; Oldiges, M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography–mass spectrometry. J. Chrom. A 2007, 1147, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Stafsnes, M.H.; Røst, L.M.; Bruheim, P. Improved phosphometabolome profiling applying isotope dilution strategy and capillary ion chromatography-tandem mass spectrometry. J. Chrom. B 2018, 1083, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, P.; Delmotte, N.; Vorholt, J.A. Nanoscale Ion-Pair Reversed-Phase HPLC-MS for Sensitive Metabolome Analysis. Anal. Chem. 2011, 83, 850–855. [Google Scholar] [CrossRef]
- Buescher, J.M.; Moco, S.; Sauer, U.; Zamboni, N. Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Fast and Robust Quantification of Anionic and Aromatic Metabolites. Anal. Chem. 2010, 82, 4403–4412. [Google Scholar] [CrossRef]
- Li, P.; Su, M.; Chatterjee, M.; Lämmerhofer, M. Targeted analysis of sugar phosphates from glycolysis pathway by phosphate methylation with liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2022, 1221, 340099. [Google Scholar] [CrossRef]
- Liu, F.-L.; Ye, T.-T.; Ding, J.-J.; Yin, X.-M.; Yang, X.-K.; Huang, W.-H.; Yuan, B.-F.; Feng, Y.-Q. Chemical Tagging Assisted Mass Spectrometry Analysis Enables Sensitive Determination of Phosphorylated Compounds in a Single Cell. Anal. Chem. 2021, 93, 6848–6856. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Liu, F.-L.; Yuan, B.-F.; Liu, T.-G.; Feng, Y.-Q. Comprehensive Profiling of Phosphomonoester Metabolites in Saccharomyces cerevisiae by the Chemical Isotope Labeling−LC−MS Method. J. Proteome Res. 2022, 22, 114–122. [Google Scholar] [CrossRef]
- Sakamaki, H.; Uchida, T.; Lima, L.W.; Takeuchi, T. Evaluation of column hardware on liquid chromatography–mass spectrometry of phosphorylated compounds. J. Chrom. A 2015, 1381, 125–131. [Google Scholar] [CrossRef]
- DeLano, M.; Walter, T.H.; Lauber, M.A.; Gilar, M.; Jung, M.C.; Nguyen, J.M.; Boissel, C.; Patel, A.V.; Bates-Harrison, A.; Wyndham, K.D. Using hybrid organic–inorganic surface technology to mitigate analyte interactions with metal surfaces in UHPLC. Anal. Chem. 2021, 93, 5773–5781. [Google Scholar] [CrossRef]
- Iwata, T.A.; Ishino, Y.; Aoki, J.; Kono, N. Advances in phosphoinositide profiling by mass spectrometry. Med. Mass Spectrom. 2022, 6, 93–100. [Google Scholar]
- Palyzová, A.; Guschina, I.A.; Rezanka, T. Chiral analysis of glycerol phosphates—Can bacteria biosynthesize heterochiral phospholipid membranes. J. Chrom. A 2022, 1676, 463267. [Google Scholar] [CrossRef]
- Arrivault, S.; Guenther, M.; Fry, S.C.; Fuenfgeld, M.M.F.F.; Veyel, D.; Mettler-Altmann, T.; Stitt, M.; Lunn, J.E. Synthesis and Use of Stable-Isotope-Labeled Internal Standards for Quantification of Phosphorylated Metabolites by LC−MS/MS. Anal. Chem. 2015, 87, 6896–6904. [Google Scholar] [CrossRef]
- Patacq, C.; Chaudet, N.; Letisse, F. Absolute Quantification of ppGpp and pppGpp by Double-Spike Isotope Dilution Ion Chromatography−High-Resolution Mass Spectrometry. Anal. Chem. 2018, 90, 10715–10723. [Google Scholar] [CrossRef]
- Soga, T. Advances in capillary electrophoresis mass spectrometry for metabolomics. Trends Anal. Chem. 2023, 158, 116883. [Google Scholar] [CrossRef]
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a metabolic alternative to the classical mevalonate pathway. Elife 2013, 2, e00672. [Google Scholar] [CrossRef]
- VanNice, J.C.; Skaff, D.A.; Keightley, A.; Addo, J.K.; Wyckoff, G.J.; Miziorko, H.M. Identification in Haloferax volcanii of Phosphomevalonate Decarboxylase and Isopentenyl Phosphate Kinase as Catalysts of the Terminal Enzyme Reactions in an Archaeal Alternate Mevalonate Pathway. J. Bact. 2014, 196, 1055–1063. [Google Scholar] [CrossRef]
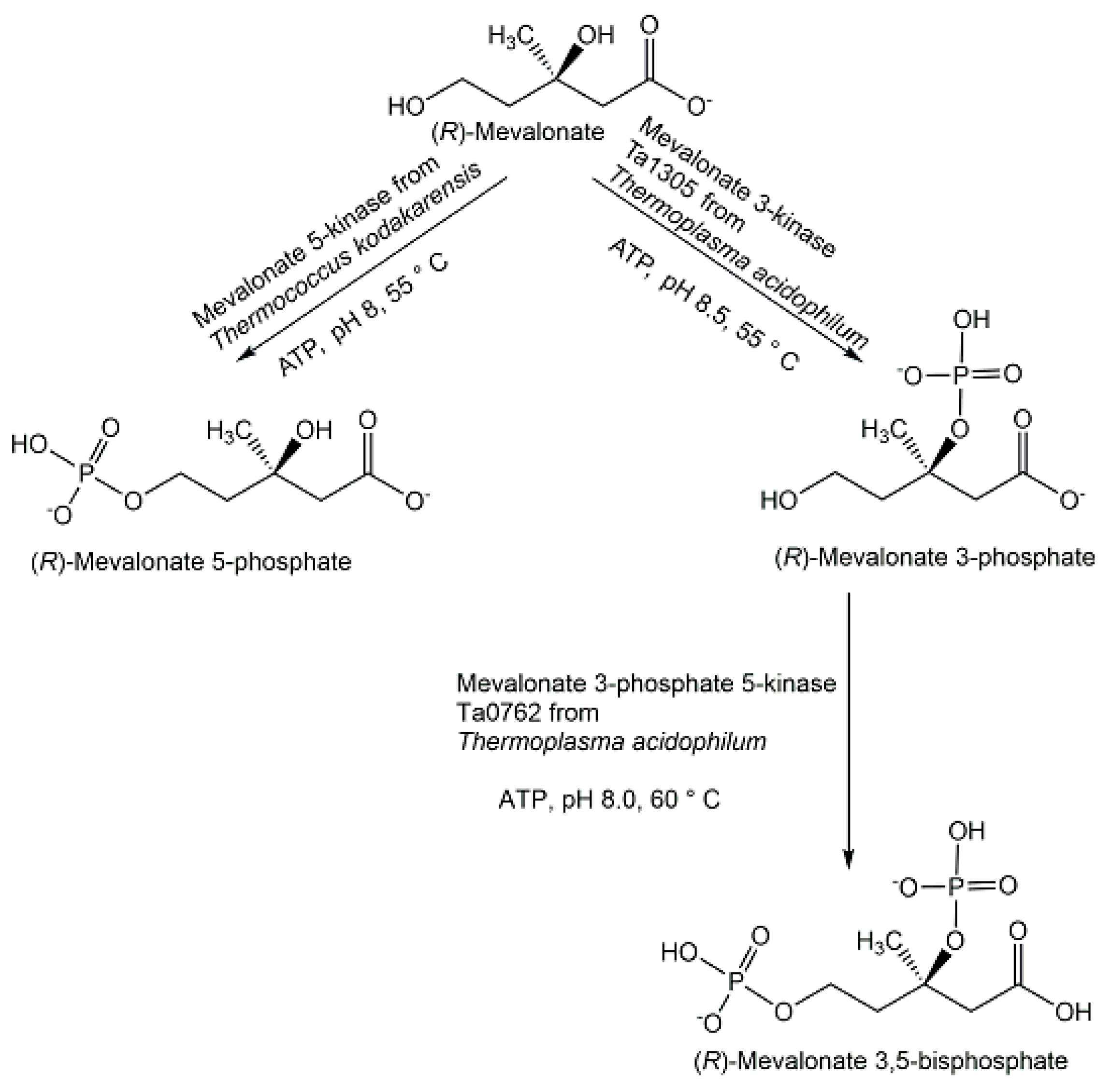
- Azami, Y.; Hattori, A.; Nishimura, H.; Kawaide, H.; Yoshimura, T.; Hemmi, H. (R)-Mevalonate 3-Phosphate Is an Intermediate of the Mevalonate Pathway in Thermoplasma acidophilum. J. Biol. Chem. 2014, 289, 15957–15967. [Google Scholar] [CrossRef]
- Hayakawa, H.A.; Motoyama, K.; Sobue, F.; Ito, T.; Kawaide, H.; Yoshimura, T.; Hemmi, H. Modified mevalonate pathway of the archaeon Aeropyrum pernix proceeds via trans-anhydromevalonate 5-phosphate. Proc. Natl. Acad. Sci. USA 2018, 115, 10034–10039. [Google Scholar] [CrossRef]
- Yoshida, R.; Yoshimura, T.; Hemmi, H. Reconstruction of the “Archaeal” Mevalonate Pathway from the Methanogenic Archae-on Methanosarcina mazei in Escherichia coli Cells. Appl. Environ. Microbiol. 2020, 86, e02889-19. [Google Scholar] [CrossRef]
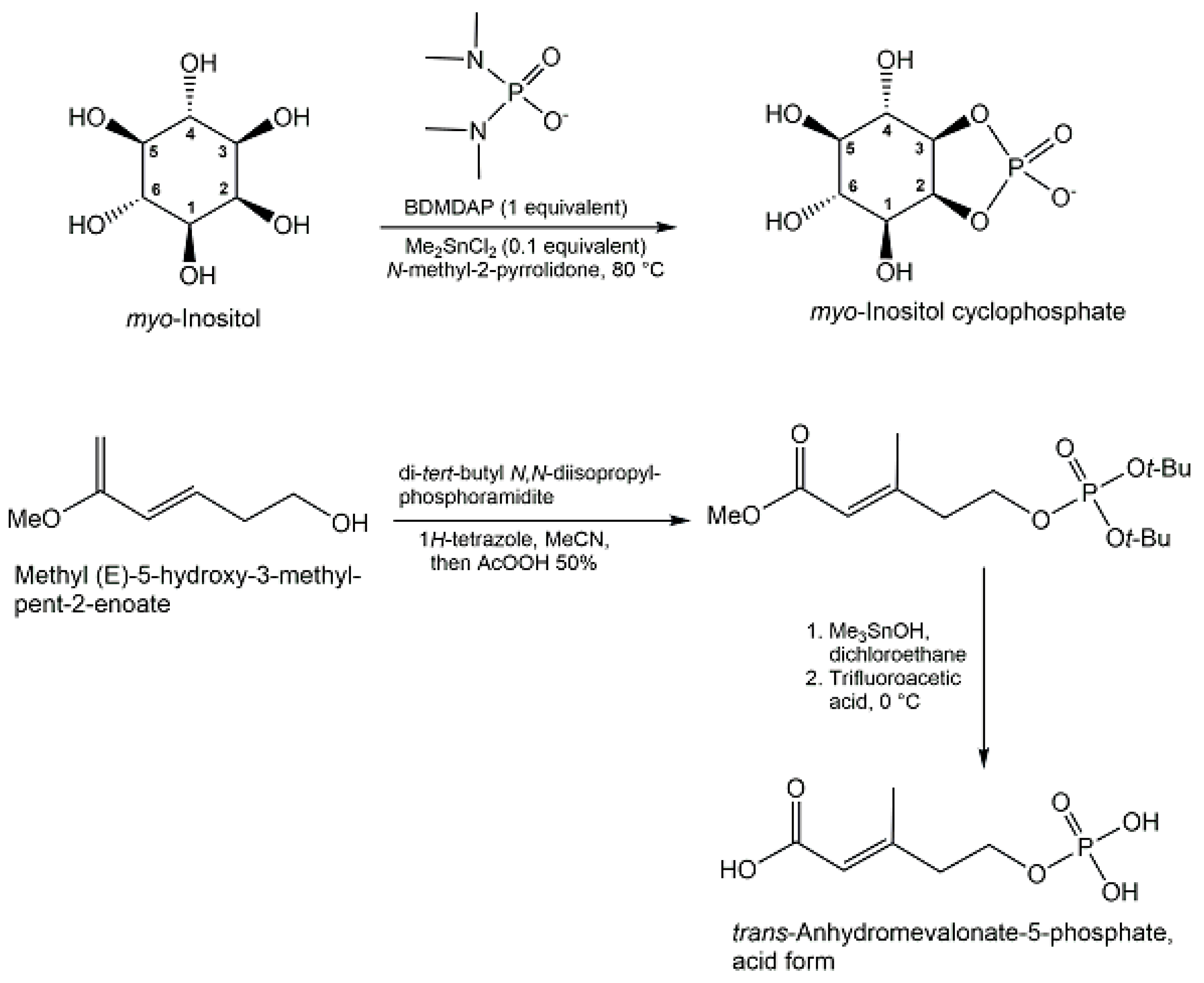
- Yasuno, Y.; Nakayama, A.; Saito, K.; Kitsuwa, K.; Okamura, H.; Komeyama, M.; Hemmi, H.; Shinada, T. Total Synthesis and Structure Confirmation of trans-Anhydromevalonate-5-phosphate, a Key Biosynthetic Intermediate of the Archaeal Mevalonate Pathway. J. Nat. Prod. 2021, 84, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K. The Discovery of Adenosine Triphosphate and the Establishment of Its Structure. J. Hist. Biol. 1991, 24, 145–154. [Google Scholar]
- Baddiley, J.; Michelson, A.M.; Todd, A.R. Synthesis of adenosine triphosphate. Nature 1948, 161, 761–762. [Google Scholar] [CrossRef]
- Lipmann, F. Enzymatic Synthesis of Acetyl Phosphate. J. Biol. Chem. 1944, 155, 55–70. [Google Scholar] [CrossRef]
- Jones, M.E.; Lipmann, F. Chemical and enzymatic synthesis of carbamyl phosphate. Proc. Natl. Acad. Sci. USA 1960, 46, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Gauss, D.; Schönenberger, B.; Molla, G.S.; Kinfu, B.M.; Chow, J.; Liese, A.; Streit, W.R.; Wohlgemuth, R. Biocatalytic phosphorylation of metabolites. In Applied Biocatalysis–From Fundamental Science to Industrial Applications; Hilterhaus, L., Liese, A., Kettling, U., Antranikian, G., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 147–177. [Google Scholar]
- Wohlgemuth, R.; Liese, A.; Streit, W. Biocatalytic phosphorylations of metabolites: Past, present, and future. Trends Biotechnol. 2017, 35, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Guntha, S.; Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 2000, 39, 2281–2285. [Google Scholar] [CrossRef]
- Yadav, M.; Krishnamurthy, R. Bis(dimethylamino)phosphorodiamidate: A Reagent for the Regioselective Cyclophosphorylation of cis-Diols Enabling One-Step Access to High-Value Target Cyclophosphates. Org. Lett. 2019, 21, 7400–7404. [Google Scholar] [CrossRef]
- Smith, J.P.; Obligacion, J.V.; Dance, Z.E.; Lomont, J.P.; Ralbovsky, N.M.; Bu, X.; Mann, B.F. Investigation of Lithium Acetyl Phosphate Synthesis Using Process Analytical Technology. Org. Proc. Res. Dev. 2021, 25, 1402–1413. [Google Scholar] [CrossRef]
- Depken, C.; Krätzschmar, F.; Rieger, R.; Rode, K.; Breder, A. Photocatalytic Aerobic Phosphatation of Alkenes. Angew. Chem. Int. Ed. 2018, 57, 2459–2463. [Google Scholar] [CrossRef]
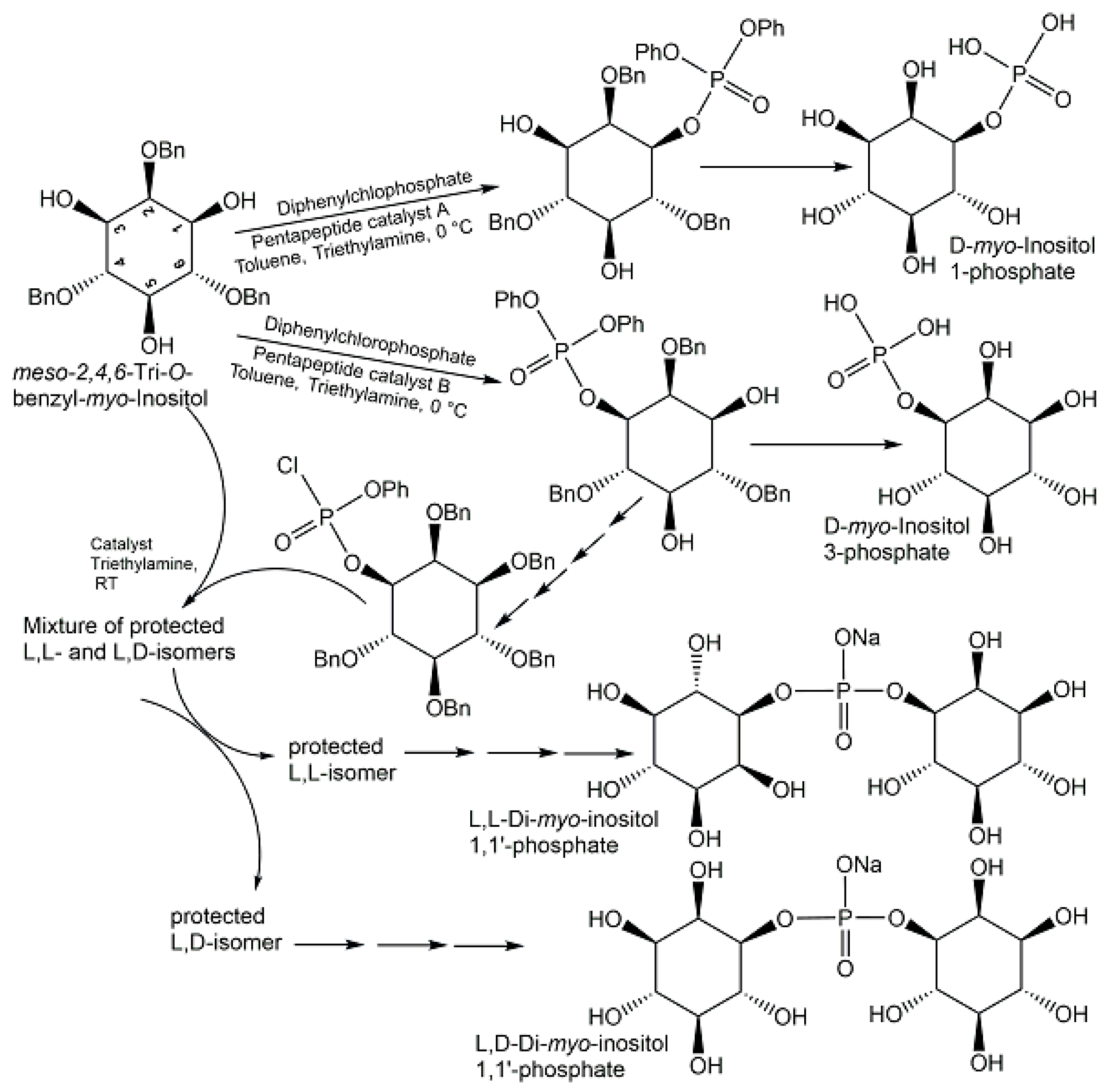
- Sculimbrene, B.R.; Morgan, A.J.; Miller, S.J. Nonenzymatic peptide-based catalytic asymmetric phosphorylation of inositol derivatives. Chem. Comm. 2003, 15, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.M.; Wei, Y.; Roberts, M.F.; Miller, S.J. Asymmetric Syntheses of L,L-and L,D-Di-myo-inositol-1, 1′-phosphate and their Behavior as Stabilizers of Enzyme Activity at Extreme Temperatures. Angew. Chem. Int. Ed. 2009, 48, 4158–4161. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Miller, S.J. Asymmetric catalysis at a distance: Catalytic, site-selective phosphorylation of teicoplanin. J. Am. Chem. Soc. 2013, 135, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Featherston, A.L.; Kwon, Y.; Pompeo, M.M.; Engl, O.D.; Leahy, D.K.; Miller, S.J. Catalytic asymmetric and stereodivergent oligonucleotide synthesis. Science 2021, 371, 702–707. [Google Scholar] [CrossRef]
- Boyer, P.D. Energy, life, and ATP (Nobel lecture). Angew. Chem. Int. Ed. 1998, 37, 2296–2307. [Google Scholar] [CrossRef]
- Walker, J.E. ATP synthesis by rotary catalysis (Nobel Lecture). Angew. Chem. Int. Ed. 1998, 37, 2308–2319. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Reconstitution of F0F1-ATPase-based biomimetic systems. Nat. Rev. Chem. 2019, 3, 361–374. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Yu, F.; Fei, J.; Li, Q.; Dong, M.; Li, J. Oriented Nanoarchitectonics of Bacteriorhodopsin for Enhancing ATP Generation in a FoF1-ATPase-Based Assembly System. Angew. Chem. Int. Ed. 2022, 61, e202116220. [Google Scholar]
- Maruyama, A.; Fujio, T. ATP Production from Adenine by a Self-coupling Enzymatic Process: High-level Accumulation under Ammonium-limited Conditions. Biosci. Biotechnol. Biochem. 2001, 65, 644–650. [Google Scholar] [CrossRef]
- Mordhorst, S.; Andexer, J.N. Round, round we go–strategies for enzymatic cofactor regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef]
- Gauss, D.; Schoenenberger, B.; Wohlgemuth, R. Chemical and enzymatic methodologies for the synthesis of enantiomerically pure glyceraldehyde 3-phosphates. Carbohydr. Res. 2014, 389, 18–24. [Google Scholar] [CrossRef]
- Molla, G.S.; Kinfu, B.M.; Chow, J.; Streit, W.; Wohlgemuth, R.; Liese, A. Bioreaction engineering leading to efficient synthesis of L-glyceraldehyd-3-phosphate. Biotechnol. J. 2017, 12, 1600625. [Google Scholar] [CrossRef] [PubMed]
- Gauss, D.; Sánchez-Moreno, I.; Oroz-Guinea, I.; García-Junceda, E.; Wohlgemuth, R. Phosphorylation catalyzed by dihydroxyacetone kinase. Eur. J. Org. Chem. 2018, 23, 2892–2895. [Google Scholar] [CrossRef]
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259. [Google Scholar] [CrossRef]
- Hardt, N.; Kinfu, B.M.; Chow, J.; Schoenenberger, B.; Streit, W.R.; Obkircher, M.; Wohlgemuth, R. Biocatalytic Asymmetric Phosphorylation Catalyzed by Recombinant Glycerate-2-Kinase. ChemBioChem 2017, 18, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Matsumi, R.; Hellriegel, C.; Schoenenberger, B.; Milesi, T.; Van Der Oost, J.; Wohlgemuth, R. Biocatalytic asymmetric phosphorylation of mevalonate. RSC Adv. 2014, 4, 12989–12994. [Google Scholar] [CrossRef]
- Vinokur, J.M.; Korman, T.P.; Cao, Z.; Bowie, J.U. Evidence of a Novel Mevalonate Pathway in Archaea. Biochemistry 2014, 53, 25–4161. [Google Scholar] [CrossRef]
- Aoki, M.; Vinokur, J.; Motoyama, K.; Ishikawa, R.; Collazo, M.; Cascio, D.; Sawaya, M.R.; Ito, T.; James, U.; Bowie, J.U.; et al. Crystal structure of mevalonate 3,5-bisphosphate decarboxylase reveals insight into the evolution of decarboxylases in the mevalonate metabolic pathways. J. Biol. Chem. 2022, 298, 102111. [Google Scholar] [CrossRef]
- Motoyama, K.; Sobue, F.; Kawaide, H.; Yoshimura, T.; Hemmi, H. Conversion of mevalonate 3-kinase into 5-phosphomevalonate 3-kinase by single amino acid mutations. Appl. Env. Microbiol. 2019, 85, e00256-19. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Recombinant AroL-Catalyzed Phosphorylation for the Efficient Synthesis of Shikimic Acid 3-Phosphate. Biotechnol. J. 2018, 13, 1700529. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Shikimate Kinase-Catalysed Phosphorylations and Synthesis of Shikimic Acid 3-Phosphate by AroL-Catalysed Phosphorylation of Shikimic Acid. In Applied Biocatalysis: The Chemist’s Enzyme Toolbox; Whittall, J., Sutton, P.W., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2021; pp. 386–393. [Google Scholar]
- Knappmann, B.R.; El-Nawawy, M.A.; Schlegel, H.G.; Kula, M.-R. Microbial synthesis of 3-deoxy-d-erythro-hex-2-ulosonic acid 6-phosphate. Carbohydrate Res. 1993, 242, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lamble, H.J.; Theodossis, A.; Milburn, C.C.; Taylor, G.L.; Bull, S.D.; Hough, D.W.; Danson, M.J. Promiscuity in the part-phosphorylative Entner–Doudoroff pathway of the archaeon Sulfolobus solfataricus. FEBS Lett. 2005, 579, 6865–6869. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, I.; Trachtmann, N.; Ilhan, S.; Hélaine, V.; Lemaire, M.; Guérard-Hélaine, C.; Sprenger, G.A. 2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity. Molecules 2019, 24, 2393. [Google Scholar] [CrossRef] [PubMed]
- Krevet, S.; Shen, L.; Bohnen, T.; Schoenenberger, B.; Meier, R.; Obkircher, M.; Bangert, K.; Koehling, R.; Allenspach, E.; Wohlgemuth, R.; et al. Enzymatic Synthesis of 2-Keto-3-Deoxy-6-Phosphogluconate by the 6-Phosphogluconate-Dehydratase From Caulobacter crescentus. Front. Bioeng. Biotechnol. 2020, 8, 185. [Google Scholar] [CrossRef]
- Hardt, N.; Kind, S.; Schoenenberger, B.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Facile synthesis of D-xylulose-5-phosphate and L-xylulose-5-phosphate by xylulokinase-catalyzed phosphorylation. Biocat. Biotrans. 2020, 38, 35–45. [Google Scholar] [CrossRef]
- Hardt, N.; Kind, S.; Schoenenberger, B.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Kinase-Catalysed Phosphorylations of Xylulose Substrates and Synthesis of Xylulose-5-Phosphate Enantiomers. In Applied Biocatalysis: The Chemist’s Enzyme Toolbox; Whittall, J., Sutton, P.W., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2021; pp. 397–401. [Google Scholar]
- Wen, L.; Huang, K.; Liu, Y.; Wang, P.G. Facile enzymatic synthesis of phosphorylated ketopentoses. ACS Catal. 2016, 6, 1649–1654. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Kind, S.; Meier, R.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Efficient biocatalytic synthesis of D-tagatose 1, 6-diphosphate by LacC-catalysed phosphorylation of D-tagatose 6-phosphate. Biocat. Biotrans. 2020, 38, 53–63. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Kind, S.; Meier, R.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Kinase-Catalysed Phosphorylations of Ketohexose Phosphates and LacC-Catalysed Synthesis of D-Tagatose-1,6-Diphosphate Lithium Salt. In Applied Biocatalysis: The Chemist’s Enzyme Toolbox; Whittall, J., Sutton, P.W., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2021; pp. 393–397. [Google Scholar]
- Sánchez-Moreno, I.; Hélaine, V.; Poupard, N.; Charmantray, F.; Légeret, B.; Hecquet, L.; García-Junceda, E.; Wohlgemuth, R.; Guérard-Hélaine, C.; Lemaire, M. One-pot cascade reactions using fructose-6-phosphate aldolase: Efficient synthesis of d-arabinose 5-phosphate, d-fructose 6-phosphate and analogues. Adv. Synth. Catal. 2012, 354, 1725–1730. [Google Scholar] [CrossRef]
- Hélaine, V.; Mahdi, R.; Sudhir Babu, G.V.; de Berardinis, V.; Wohlgemuth, R.; Lemaire, M.; Guérard-Hélaine, C. Straightforward Synthesis of Terminally Phosphorylated L-Sugars via Multienzymatic Cascade Reactions. Adv. Synth. Catal. 2015, 357, 1703–1708. [Google Scholar] [CrossRef]
- Schell, U.; Wohlgemuth, R.; Ward, J.M. Synthesis of pyridoxamine 5′-phosphate using an MBA: Pyruvate transaminase as biocatalyst. J. Mol. Catal. B Enzym. 2009, 59, 279–285. [Google Scholar] [CrossRef]
- Qian, X.-L.; Dai, Y.-S.; Li, C.X.; Pan, J.; Xu, J.-H.; Mu, B. Enzymatic synthesis of high-titer nicotinamide mononucleotide with a new nicotinamide riboside kinase and an efficient ATP regeneration system. Bioresour. Bioprocess. 2022, 9, 26. [Google Scholar] [CrossRef]
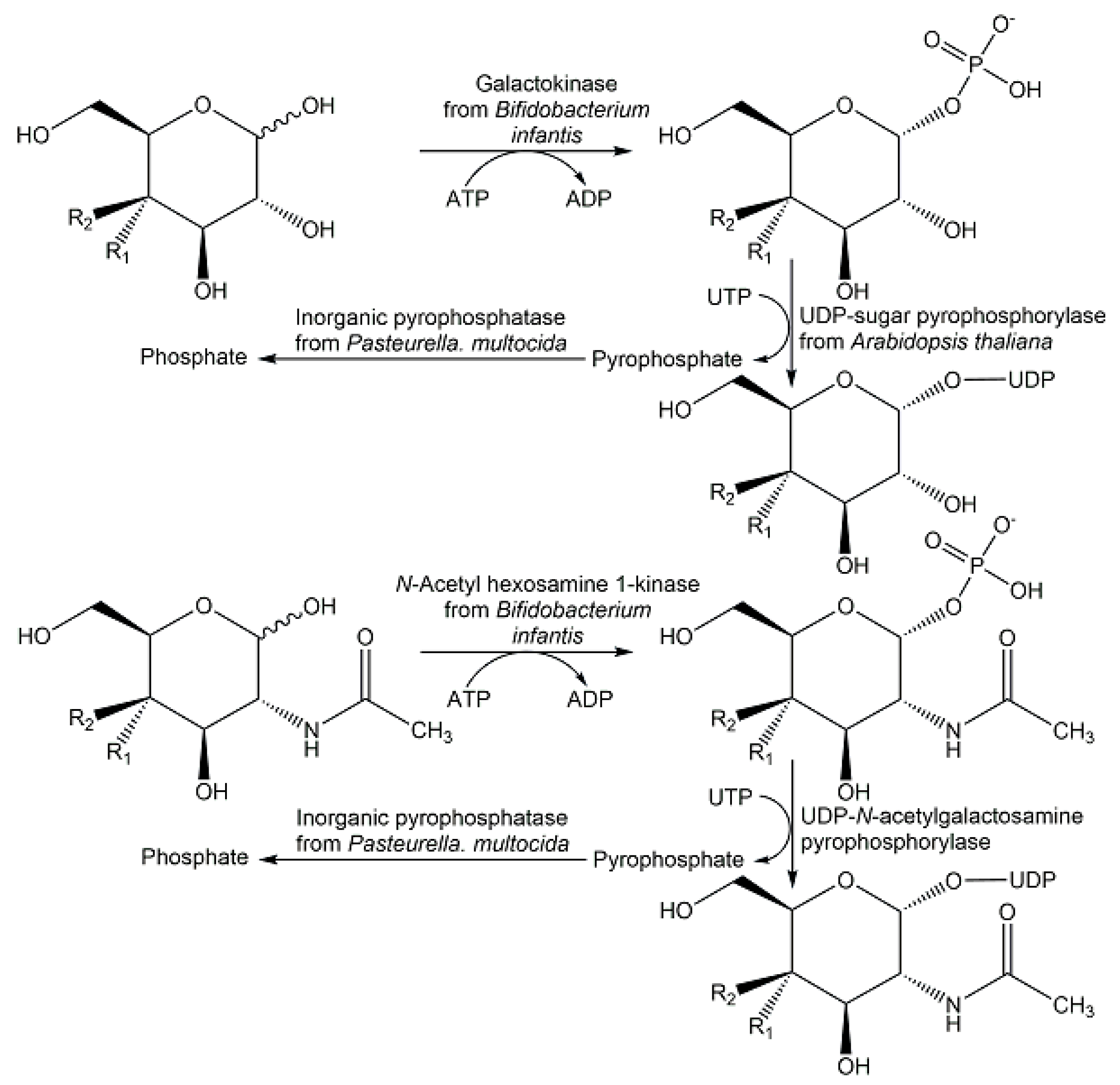
- Frohnmeyer, H.; Elling, L. Enzyme cascades for the synthesis of nucleotide sugars: Updates to recent production strategies. Carbohydrate Res. 2023, 523, 108727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Wang, Y.; Qu, J.; Liu, X.W.; Wang, P.G.; Fang, J. Gram-scale production of sugar nucleotides and their derivatives. Green Chem. 2021, 23, 2628–2633. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Milesi, T.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Synthesis of Nω-Phospho-L-arginine by Biocatalytic Phosphorylation of L-Arginine. ChemCatChem 2017, 9, 121–126. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Milesi, T.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Phosphoramidates by Kinase-Catalysed Phosphorylation and Arginine Kinase-Catalysed Synthesis of Nω-Phospho-L-Arginine. In Applied Biocatalysis: The Chemist’s Enzyme Toolbox; Whittall, J., Sutton, P.W., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2021; pp. 401–407. [Google Scholar]
- Bartsch, T.; Becker, M.; Rolf, J.; Rosenthal, K.; Lütz, S. Biotechnological production of cyclic dinucleotides—Challenges and opportunities. Biotechnol. Bioeng. 2022, 119, 677–684. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, Q.; Wang, X.; Lu, Y.; Li, Y.; Yuan, H.; Zhu, J.; Zhu, D. Highly Efficient Preparation of Cyclic Dinucleotides via Engineering of Dinucleotide Cyclases in Escherichia coli. Front. Microbiol. 2019, 10, 2111. [Google Scholar] [CrossRef]
- Benkovics, T.; Peng, F.; Phillips, E.M.; An, C.; Bade, R.S.; Chung, C.K.; Dance, Z.E.; Fier, P.S.; Forstater, J.H.; Liu, Z.; et al. Diverse Catalytic Reactions for the Stereoselective Synthesis of Cyclic Dinucleotide MK-1454. J. Am. Chem. Soc. 2022, 144, 5855–5863. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Liu, Z.; Andresen, B.M.; Marzijarani, N.S.; Moore, J.C.; Marshall, N.C.; Borra-Garske, M.; Obligacion, J.V.; Fier, P.S.; Peng, F.; et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature 2022, 603, 439–444. [Google Scholar] [CrossRef]
- Winston, M.S.; Poirier, M.; Liu, Z.; Peng, F.; Humphrey, G.R.; McIntosh, J.A.; Reibarkh, M.; Wang, F.; Guetschow, E.D.; Castro, S.; et al. pH-Switchable Phase-Transfer Agents for Host Cell Protein Rejection in the Cascaded Biocatalytic Synthesis of an Active Pharmaceutical Ingredient. Org. Proc. Res. Dev, 2022; in press. [Google Scholar]
- Wohlgemuth, R.; Littlechild, J. Complexity reduction and opportunities in the design, integration and intensification of biocatalytic processes for metabolite synthesis. Front. Bioeng. Biotechnol. 2022, 10, 958606. [Google Scholar] [CrossRef]
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as Key to Sustainable Industrial Chemistry. ChemSusChem 2022, 15, e202102709. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlgemuth, R. Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites. Int. J. Mol. Sci. 2023, 24, 3150. https://doi.org/10.3390/ijms24043150
Wohlgemuth R. Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites. International Journal of Molecular Sciences. 2023; 24(4):3150. https://doi.org/10.3390/ijms24043150
Chicago/Turabian StyleWohlgemuth, Roland. 2023. "Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites" International Journal of Molecular Sciences 24, no. 4: 3150. https://doi.org/10.3390/ijms24043150
APA StyleWohlgemuth, R. (2023). Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites. International Journal of Molecular Sciences, 24(4), 3150. https://doi.org/10.3390/ijms24043150