Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome
Abstract
1. Introduction
2. Results
2.1. Tyrosinase Inhibitory Peptide Candidates
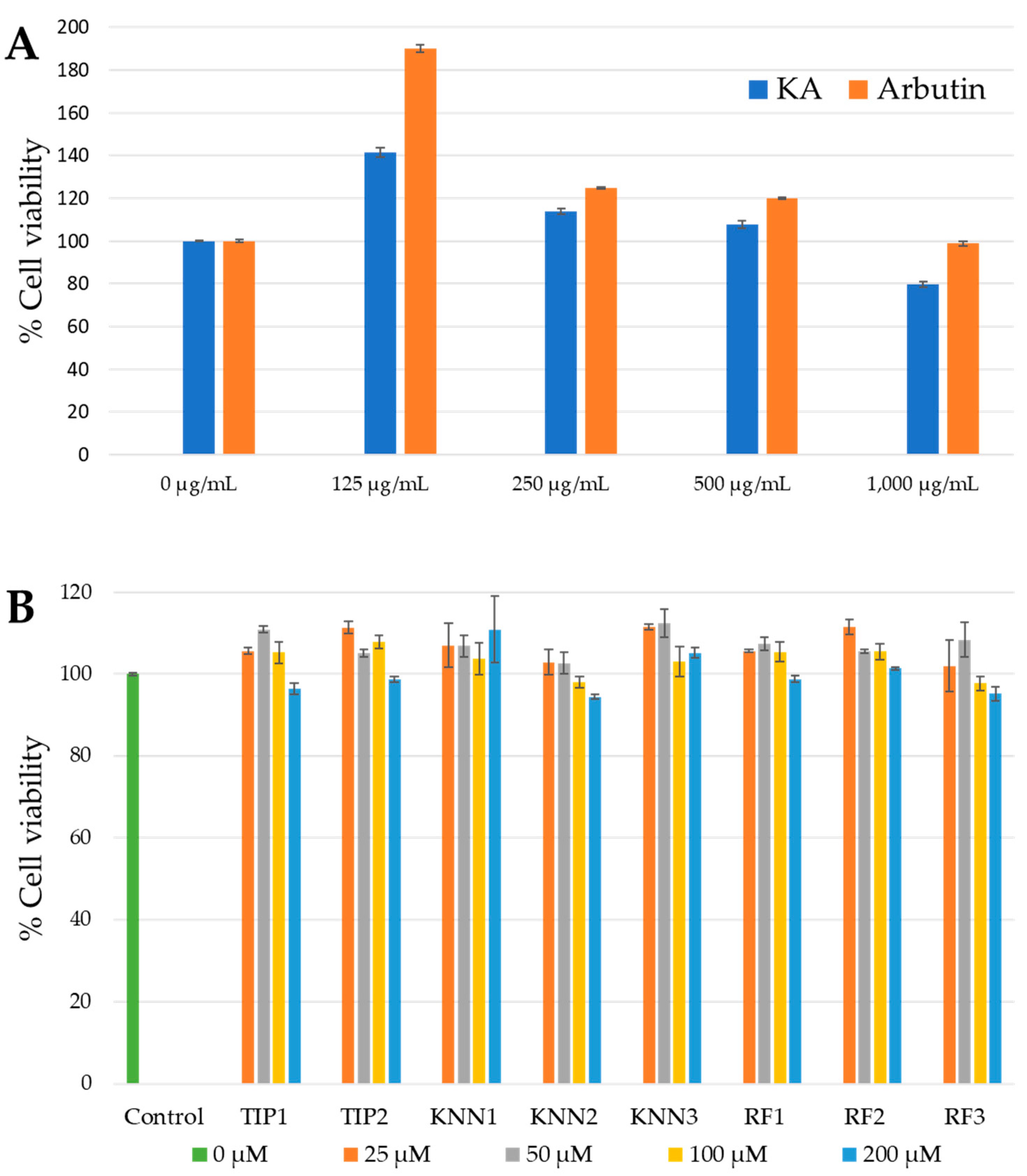
2.2. Cytotoxicity of hdTIPs on B16F10 Cell Line
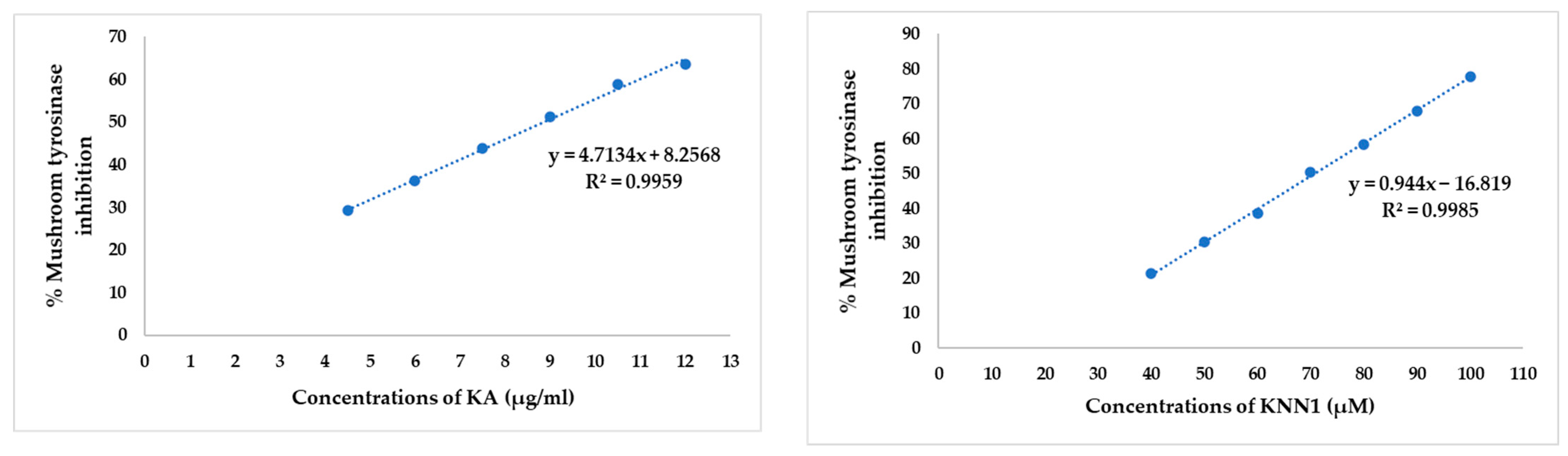
2.3. Inhibitory Effect of hdTIPs on Mushroom Tyrosinase
2.4. Inhibitory Effect of hdTIPs on Cellular Tyrosinase and Melanin Content
2.5. Effects of hdTIPs on ROS Levels and Intracellular Antioxidant Activities
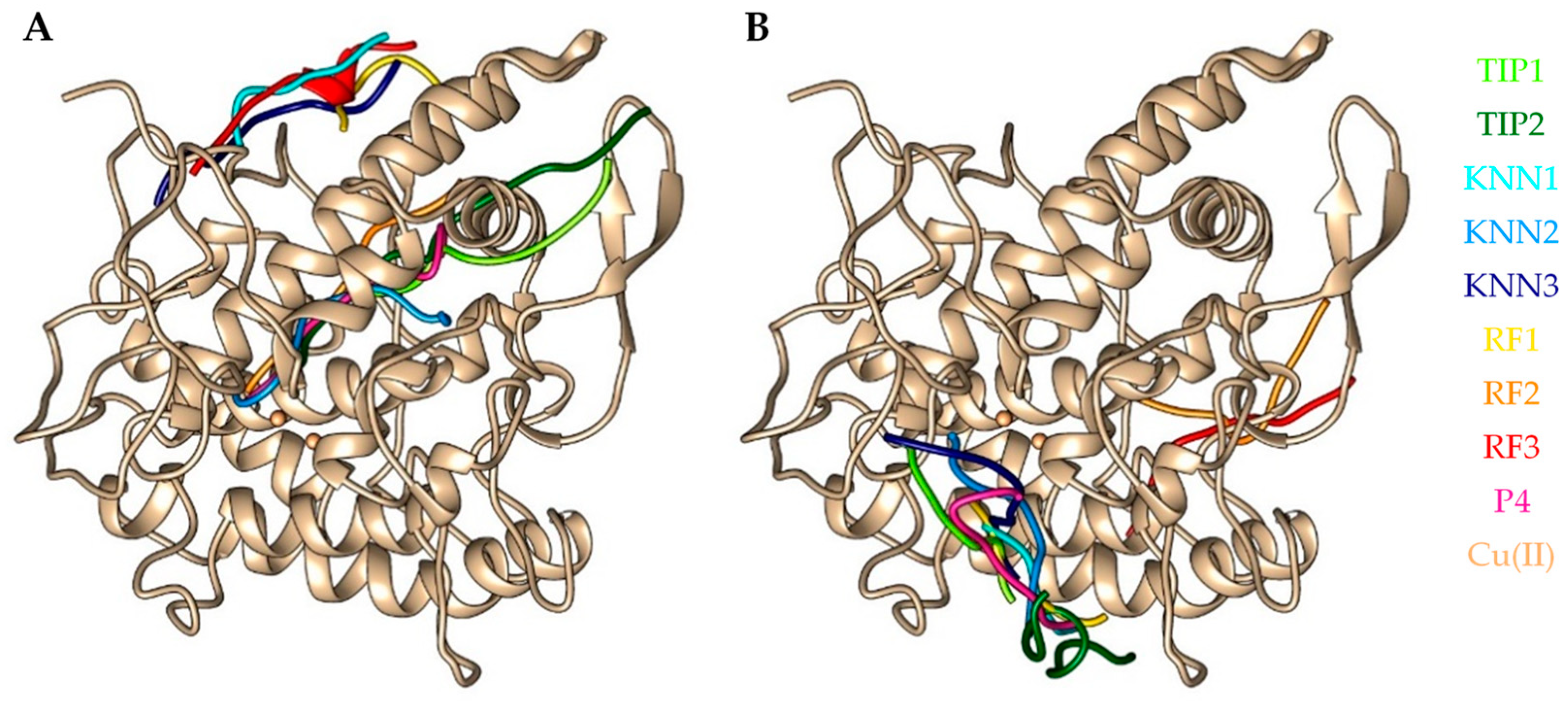
2.6. Molecular Docking Simulation of Selected hdTIPs on Tyrosinase
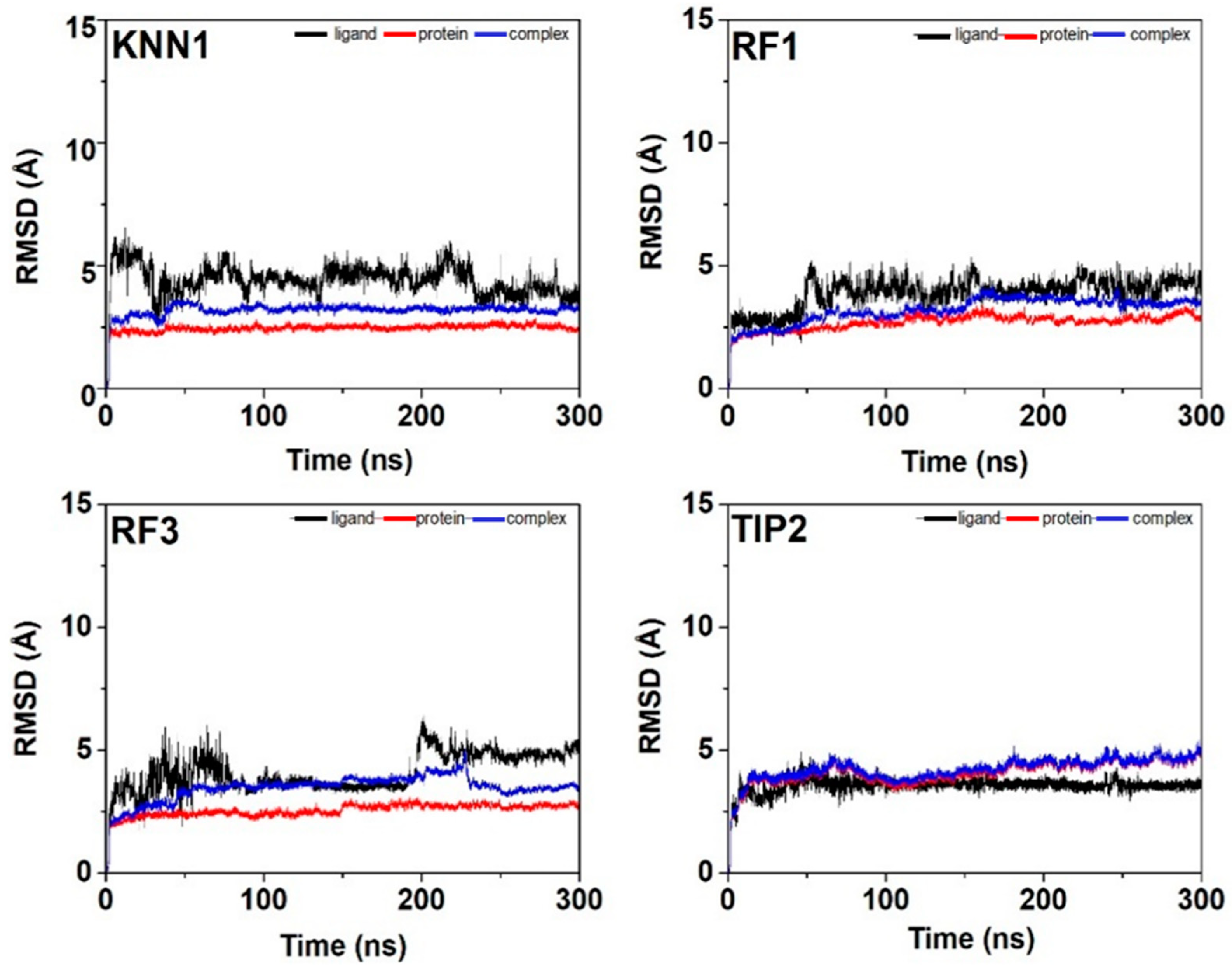
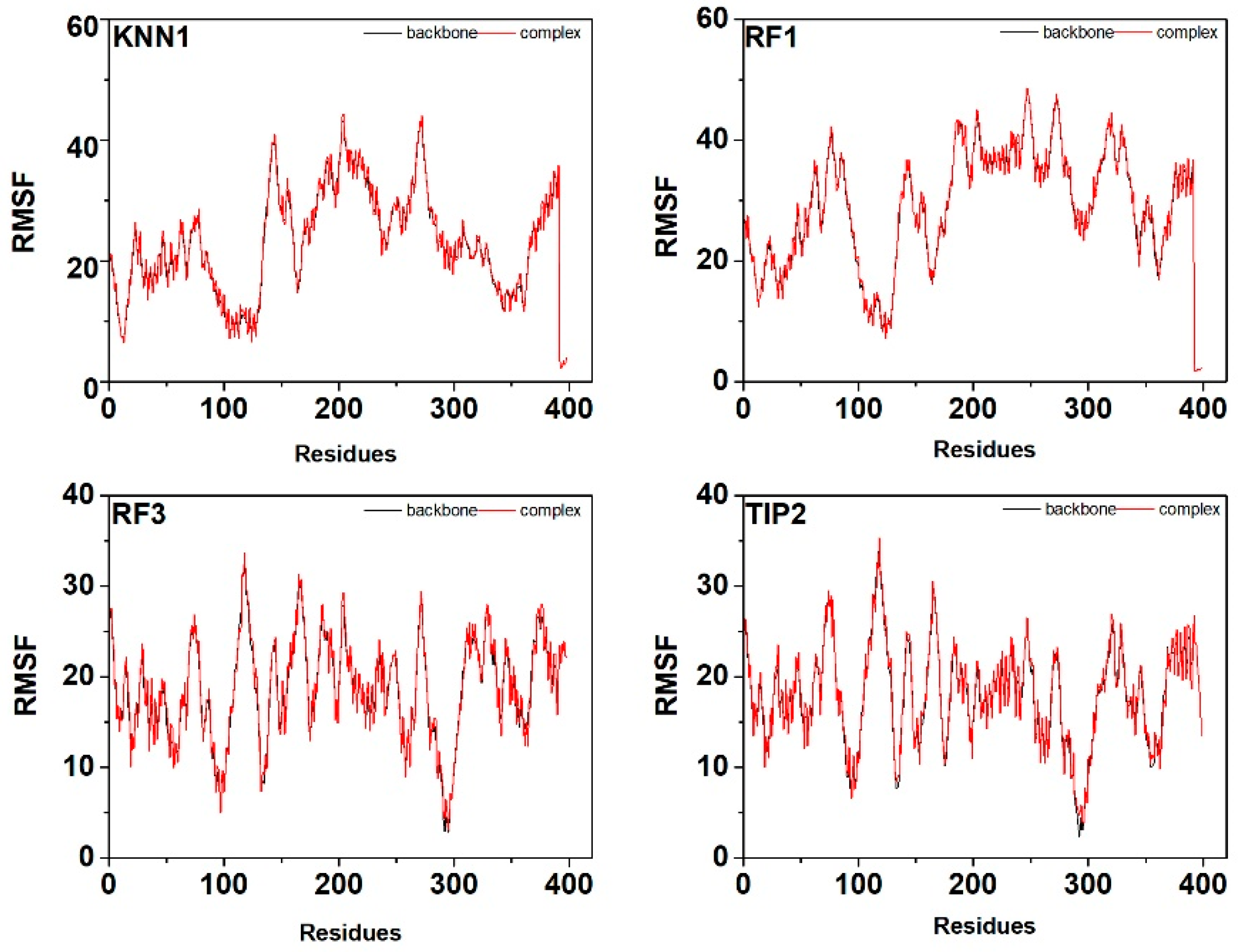
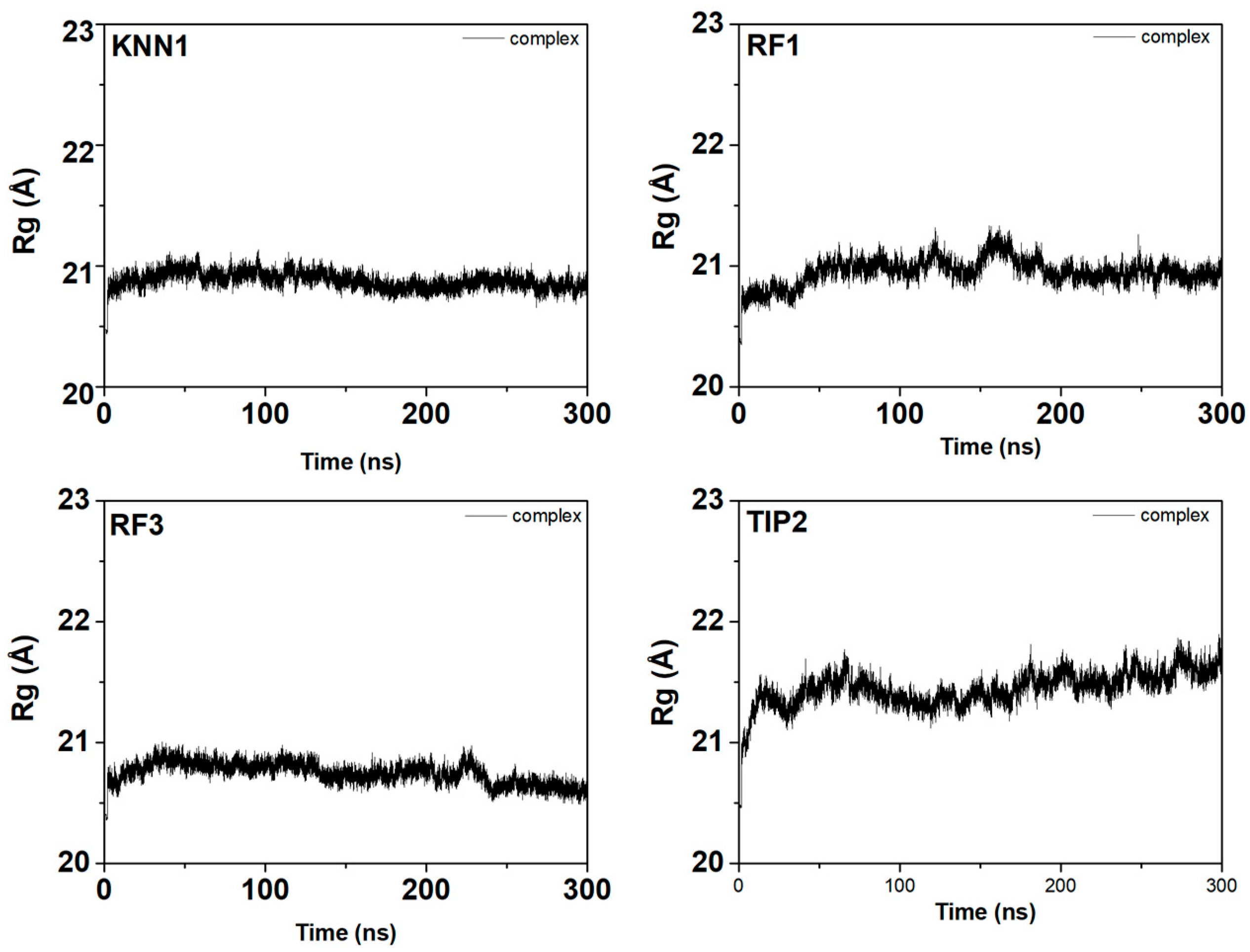
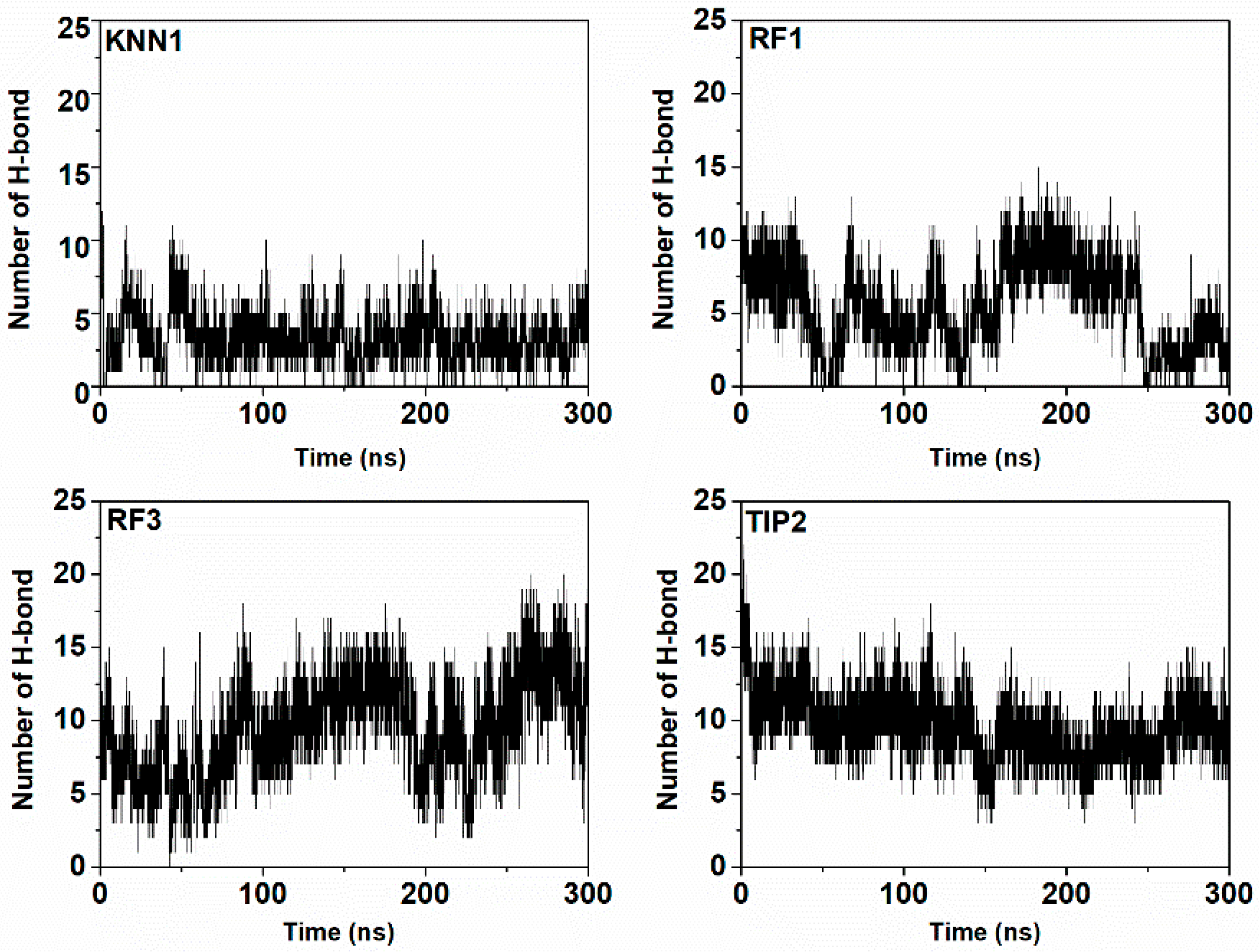
2.7. Molecular Dynamic Simulation of Tyrosinase-hdTIPs Complexes
3. Discussion
4. Materials and Methods
4.1. Biomimetic Synthetic Peptide
4.2. Melanoma Cell Culture
4.3. Cell Viability Assay (MTT Assay)
4.4. Mushroom Tyrosinase Activity Assay
4.5. Intracellular Tyrosinase Activity Assay
4.6. Melanin Content Assay
4.7. Assay of Antioxidative Enzymes Activities
4.8. Antioxidant Peptides Prediction and Molecular Docking Simulation
4.9. Molecular Dynamics Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, S. A chemist’s view of melanogenesis. Pigment. Cell Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. The chemistry of melanins and melanogenesis. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1995; pp. 93–148. [Google Scholar]
- Lynde, C.; Kraft, J.; Lynde, C. Topical treatments for melasma and postinflammatory hyperpigmentation. Ski. Ther. Lett. 2006, 11, 1–6. [Google Scholar]
- Pandya, A.G.; Guevara, I.L. Disorders of hyperpigmentation. Dermatol. Clin. 2000, 18, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Plensdorf, S.; Martinez, J. Common pigmentation disorders. Am. Fam. Physician 2009, 79, 109–116. [Google Scholar]
- Vashi, N.A.; Kundu, R. Facial hyperpigmentation: Causes and treatment. Br. J. Dermatol. 2013, 169, 41–56. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Maria-Solano, M.A.; Garcia-Molina, M.D.M.; Varon, R.; Tudela, J.; Tomas, V.; Garcia-Canovas, F. Kinetic characterization of substrate-analogous inhibitors of tyrosinase. IUBMB Life 2015, 67, 757–767. [Google Scholar] [CrossRef]
- Ochiai, A.; Tanaka, S.; Tanaka, T.; Taniguchi, M. Rice bran protein as a potent source of antimelanogenic peptides with tyrosinase inhibitory activity. J. Nat. Prod. 2016, 79, 2545–2551. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorganic Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef]
- Vanitha, M.; Soundhari, C. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti-tyrosinase activity. Int. J. Chem.Tech. Res. 2017, 10, 1156–1167. [Google Scholar]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, W.; Kooyers, T. Hydroquinone and its analogues in dermatology—A potential health risk. J. Cosmet. Dermatol. 2005, 4, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-Y.; Chen, C.-Y.; Lai, S.-T.; Chan, C.-F. Kinetics of mushroom tyrosinase and melanogenesis inhibition by N-acetyl-pentapeptides. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rendon, M.I.; Gaviria, J.I. Review of skin-lightening agents. Dermatol. Surg. 2005, 31, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Lee, E.; Hwang, I.-J.; Yim, D.; Han, J.; Lee, Y.-S.; Kim, J.-H. β-Lactoglobulin peptide fragments conjugated with caffeic acid displaying dual activities for tyrosinase inhibition and antioxidant effect. Bioconjugate Chem. 2018, 29, 1000–1005. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Yang, J.K.; Kim, J.H.; Lee, Y.S. Chemical modulation of bioactive compounds via oligopeptide or amino acid conjugation. Pept. Sci. 2013, 100, 584–591. [Google Scholar] [CrossRef]
- Castro-Jácome, T.P.; Alcántara-Quintana, L.E.; Montalvo-González, E.; Chacón-López, A.; Kalixto-Sánchez, M.A.; del Pilar Rivera, M.; López-García, U.M.; Tovar-Pérez, E.G. Skin-protective properties of peptide extracts produced from white sorghum grain kafirins. Ind. Crops Prod. 2021, 167, 113551. [Google Scholar] [CrossRef]
- Hu, Z.; Sha, X.; Zhang, L.; Huang, S.; Tu, Z. Effect of grass carp scale collagen peptide FTGML on cAMP-PI3K/Akt and MAPK signaling pathways in B16F10 melanoma cells and correlation between anti-melanin and antioxidant properties. Foods 2022, 11, 391. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S.; Li, L.; Zeng, Y.; Hu, X. The Hypopigmentation Mechanism of Tyrosinase Inhibitory Peptides Derived from Food Proteins: An Overview. Molecules 2022, 27, 2710. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- De Zoysa, M. Nutritional value, bioactive compounds, and health-promoting properties of abalone. In Marine Nutraceuticals: Prospects and Perspectives; CRC Press: Boca Raton, FL, USA, 2013; Volume 57. [Google Scholar]
- Lee, Y.-H.; Vacquier, V. Evolution and systematics in Haliotidae (Mollusca: Gastropoda): Inferences from DNA sequences of sperm lysin. Mar. Biol. 1995, 124, 267–278. [Google Scholar] [CrossRef]
- Zheng, P.; Hao, G.; Weng, W.; Ren, H. Antioxidant activities of hydrolysates from abalone viscera using subcritical water-assisted enzymatic hydrolysis. Food Bioprocess Technol. 2019, 12, 910–918. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; He, C.; Wei, H.; Ma, Y.; Xiong, H. Preparation and antioxidant activities of polysaccharides obtained from abalone viscera by combination of enzymolysis and multiple separation methods. J. Food Sci. 2020, 85, 4260–4270. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Park, S.Y.; Hwang, J.-Y.; Ahn, C.-B. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods 2015, 16, 94–103. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Zhu, B.-W.; Qiao, L.; Wu, H.-T.; Li, D.-M.; Yang, J.-F.; Murata, Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod. Process. 2012, 90, 148–154. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Tang, Y.; Zhu, B.-W.; Qin, L.; Li, D.-M.; Yang, J.-F.; Lei, K.; Murata, Y. Antioxidant activity of hydrolysates obtained from scallop (Patinopecten yessoensis) and abalone (Haliotis discus hannai Ino) muscle. Food Chem. 2012, 132, 815–822. [Google Scholar] [CrossRef]
- Suleria, H.; Masci, P.; Gobe, G.; Osborne, S. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef]
- Park, S.Y.; Je, J.-Y.; Hwang, J.-Y.; Ahn, C.-B. Abalone protein hydrolysates: Preparation, angiotensin i converting enzyme inhibition and cellular antioxidant activity. Prev. Nutr. Food Sci. 2015, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Coates, C.J.; Zhu, H.; Zhu, P.; Wu, Z.; Xie, L. Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol. 2015, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cai, Q.-F.; Tao, Z.-P.; Sun, L.-C.; Shen, J.-D.; Zhang, L.-J.; Liu, G.-M.; Cao, M.-J. Purification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from abalone (Haliotis discus hannai Ino) gonads. Eur. Food Res. Technol. 2015, 240, 137–145. [Google Scholar] [CrossRef]
- He, C.; Shao, J.; Wei, H.; Xiong, H.; Wu, G.; Ma, Y.; Wu, J. Antioxidant and immunoregulatory activity of peptides from abalone visceral protein hydrolysate. Shipin Kexue/Food Sci. 2018, 39, 206–212. [Google Scholar]
- Suleria, H.A.R.; Masci, P.P.; Addepalli, R.; Chen, W.; Gobe, G.C.; Osborne, S.A. In vitro anti-thrombotic and anti-coagulant properties of blacklip abalone (Haliotis rubra) viscera hydrolysate. Anal. Bioanal. Chem. 2017, 409, 4195–4205. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Chen, M.-F.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.-J. Boiled abalone byproduct peptide exhibits anti-tumor activity in HT1080 cells and HUVECs by suppressing the metastasis and angiogenesis in vitro. J. Agric. Food Chem. 2019, 67, 8855–8867. [Google Scholar] [CrossRef]
- De Zoysa, M.; Nikapitiya, C.; Whang, I.; Lee, J.-S.; Lee, J. Abhisin: A potential antimicrobial peptide derived from histone H2A of disk abalone (Haliotis discus discus). Fish Shellfish. Immunol. 2009, 27, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, P.; Xiao, Z.; Chen, M.-F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Jung, W.-K.; Qian, Z.-J. Antiphotoaging effect of boiled abalone residual peptide ATPGDEG on UVB-induced keratinocyte HaCaT cells. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Kuanpradit, C.; Jaisin, Y.; Jungudomjaroen, S.; Akter Mitu, S.; Puttikamonkul, S.; Sobhon, P.; Cummins, S.F. Attenuation of UV-B exposure-induced inflammation by abalone hypobranchial gland and gill extracts. Int. J. Mol. Med. 2017, 39, 1083–1090. [Google Scholar] [CrossRef]
- Kongsompong, S.; E-kobon, T.; Chumnanpuen, P. K-Nearest Neighbor and Random Forest-Based Prediction of Putative Tyrosinase Inhibitory Peptides of Abalone Haliotis diversicolor. Molecules 2021, 26, 3671. [Google Scholar] [CrossRef]
- Yamamura, T.; Onishi, J.; Nishiyama, T. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch. Dermatol. Res. 2002, 294, 349–354. [Google Scholar] [CrossRef]
- Ochiai, A.; Tanaka, S.; Imai, Y.; Yoshida, H.; Kanaoka, T.; Tanaka, T.; Taniguchi, M. New tyrosinase inhibitory decapeptide: Molecular insights into the role of tyrosine residues. J. Biosci. Bioeng. 2016, 121, 607–613. [Google Scholar] [CrossRef]
- Reddy, B.; Jow, T.; Hantash, B.M. Bioactive oligopeptides in dermatology: Part I. Exp. Dermatol. 2012, 21, 563–568. [Google Scholar] [CrossRef]
- Vishvakarma, V.K.; Singh, M.B.; Jain, P.; Kumari, K.; Singh, P. Hunting the main protease of SARS-CoV-2 by plitidepsin: Molecular docking and temperature-dependent molecular dynamics simulations. Amino Acids 2022, 54, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Kim, M.; Shin, S.; Lee, J.-A.; Park, D.; Lee, J.; Jung, E. Inhibition of melanogenesis by Gaillardia aristata flower extract. BMC Complement. Altern. Med. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef]
- Loizzo, M.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Wang, W.; Zhang, J.; Yin, J.; Le, T.; Xue, J.; Engelhardt, U.H.; Jiang, H. Kojic Acid Showed Consistent Inhibitory Activity on Tyrosinase from Mushroom and in Cultured B16F10 Cells Compared with Arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Campos, P.M.; da Silva Horinouchi, C.D.; da Silveira Prudente, A.; Cechinel-Filho, V.; de Almeida Cabrini, D.; Otuki, M.F. Effect of a Garcinia gardneriana (Planchon and Triana) Zappi hydroalcoholic extract on melanogenesis in B16F10 melanoma cells. J. Ethnopharmacol. 2013, 148, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Park, K.-T.; Lee, H.-S.; Kim, M.; Lim, Y.-H. Evaluation of the inhibition of mushroom tyrosinase and cellular tyrosinase activities of oxyresveratrol: Comparison with mulberroside A. J. Enzym. Inhib. Med. Chem. 2012, 27, 495–503. [Google Scholar] [CrossRef]
- Qiao, Z.; Koizumi, Y.; Zhang, M.; Natsui, M.; Flores, M.J.; Gao, L.; Yusa, K.; Koyota, S.; Sugiyama, T. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci. Biotechnol. Biochem. 2012, 76, 1877–1883. [Google Scholar] [CrossRef]
- Ding, C.; Hao, M.; Ma, S.; Zhang, Y.; Yang, J.; Ding, Q.; Sun, S.; Zhang, J.; Zhang, Y.; Liu, W. Identification of peptides with antioxidant, anti-lipoxygenase, anti-xanthine oxidase and anti-tyrosinase activities from velvet antler blood. LWT 2022, 168, 113889. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Mlouka, M.A.B.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Čakar, U.; Čolović, M.; Milenković, D.; Medić, B.; Krstić, D.; Petrović, A.; Đorđević, B. Protective effects of fruit wines against hydrogen peroxide—Induced oxidative stress in rat synaptosomes. Agronomy 2021, 11, 1414. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tandrasasmita, O.M.; Sundari, S.; Lai, X.; Retnoningrum, D.S.; Dijkstra, B.W.; Tjandrawinata, R.R.; Rachmawati, H. The light subunit of mushroom Agaricus bisporus tyrosinase: Its biological characteristics and implications. Int. J. Biol. Macromol. 2017, 102, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Damayanti, S.; Wijaya, C.; Tjandrawinata, R.R.; Retnoningrum, D.S.; Rachmawati, H. In silico study to develop a lectin-like protein from mushroom Agaricus bisporus for pharmaceutical application. Sci. Pharm. 2016, 84, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Chauhan, S.S.; Parthasarathi, R.; Mogili, N.S. In silico investigation and assessment of plausible novel tyrosinase inhibitory peptides from sesame seeds. LWT 2021, 147, 111619. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.-Y.; Yang, S.Y.; Choi, S.-K.; Kwon, S.J.; Cho, I.S.; Jeong, M.H.; Ho Kim, Y.; Choi, G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 78–83. [Google Scholar] [CrossRef]
- Prasertsuk, K.; Prongfa, K.; Suttiwanich, P.; Harnkit, N.; Sangkhawasi, M.; Promta, P.; Chumnanpuen, P. Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome. Molecules 2022, 28, 50. [Google Scholar] [CrossRef] [PubMed]
- Harnkit, N.; Khongsonthi, T.; Masuwan, N.; Prasartkul, P.; Noikaew, T.; Chumnanpuen, P. Virtual Screening for SARS-CoV-2 Main Protease Inhibitory Peptides from the Putative Hydrolyzed Peptidome of Rice Bran. Antibiotics 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chalongkulasak, S.; E-kobon, T.; Chumnanpuen, P. Prediction of Antibacterial Peptides against Propionibacterium acnes from the Peptidomes of Achatina fulica Mucus Fractions. Molecules 2022, 27, 2290. [Google Scholar] [CrossRef]
- Chantawannakul, J.; Chatpattanasiri, P.; Wattayagorn, V.; Kongsema, M.; Noikaew, T.; Chumnanpuen, P. Virtual Screening for Biomimetic Anti-Cancer Peptides from Cordyceps militaris Putative Pepsinized Peptidome and Validation on Colon Cancer Cell Line. Molecules 2021, 26, 5767. [Google Scholar] [CrossRef] [PubMed]
- Tachapuripunya, V.; Roytrakul, S.; Chumnanpuen, P.; E-kobon, T. Unveiling putative functions of mucus proteins and their tryptic peptides in seven gastropod species using comparative proteomics and machine learning-based bioinformatics predictions. Molecules 2021, 26, 3475. [Google Scholar] [CrossRef]
- Teerasak, E.; Thongararm, P.; Roytrakul, S.; Meesuk, L.; Chumnanpuen, P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput. Struct. Biotechnol. J. 2016, 14, 49–57. [Google Scholar]
- Weng, G.; Gao, J.; Wang, Z.; Wang, E.; Hu, X.; Yao, X.; Cao, D.; Hou, T. Comprehensive evaluation of fourteen docking programs on protein–peptide complexes. J. Chem. Theory Comput. 2020, 16, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997; Volume 12. [Google Scholar]
- McRee, D.E. Practical Protein Crystallography; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.; Dror, R.; Shaw, D. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, S.A.; Ali, A.S. Effect of purified mushroom tyrosinase on melanin content and melanogenic protein expression. Biotechnol. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jegal, J.; Chung, K.W.; Chung, H.Y.; Jeong, E.J.; Yang, M.H. The standardized extract of juniperus communis alleviates hyperpigmentation in vivo HRM-2 hairless mice and in vitro murine B16 melanoma cells. Biol. Pharm. Bull. 2017, 40, 1381–1388. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Anghel, M.; Voter, A.F. Synchronization of trajectories in canonical molecular-dynamics simulations: Observation, explanation, and exploitation. J. Chem. Phys. 2004, 120, 6363–6374. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Clementel, D.; Del Conte, A.; Monzon, A.M.; Camagni, G.F.; Minervini, G.; Piovesan, D.; Tosatto, S.C. RING 3.0: Fast generation of probabilistic residue interaction networks from structural ensembles. Nucleic Acids Res. 2022, 50, W651–W656. [Google Scholar] [CrossRef] [PubMed]


| hdTIPs | Peptide Sequences | Predicted Anti-Tyrosinase Probability | Predicted Antioxidant Scores | ||
|---|---|---|---|---|---|
| KNN Predictor | RF Predictor | Free Radical Scavenging (Rank) | Ion Chelating (Rank) | ||
| TIP1 | TASSDAWYR | 0.97 | 0.71 | 0.37 (3) | 0.21 (2) |
| TIP2 | SAPFMPDAFFRNV | 0.79 | 0.54 | 0.44 (1) | 0.21 (2) |
| KNN1 | NICECMK | 1.00 | 0.39 | 0.37 (3) | 0.25 (1) |
| KNN2 | TSQMSRSSSR | 1.00 | 0.37 | 0.27 (5) | 0.21 (2) |
| KNN3 | KKNYRVSEAYK | 1.00 | 0.32 | 0.25 (6) | 0.19 (4) |
| RF1 | SAPTFFR | 0.00 | 0.63 | 0.39 (2) | 0.21 (2) |
| RF2 | NSSLRVQSR | 0.00 | 0.60 | 0.24 (7) | 0.20 (3) |
| RF3 | SQSNSRSVSR | 0.00 | 0.52 | 0.34 (4) | 0.15 (5) |
| Protein-Peptide Complex | ∆G (kcal/mol) | Kd (M) at 25.0 °C | H-Bond Ener. (kJ/mol) | Elec. Ener. (kJ/mol) | VDW. Ener. (kJ/mol) | Molecular Docking Score (kJ/mol) |
|---|---|---|---|---|---|---|
| 2Y9X—TIP1 | −9.8 | 6.4 × 10−8 | −16.5114 | 26.7705 | −102.934 | −92.6749 |
| 2Y9X—TIP2 | −8.5 | 5.4 × 10−7 | −38.7443 | 0 | 68.545 | 29.8007 |
| 2Y9X—KNN1 | −9.8 | 6.8 × 10−8 | −11.9095 | −40.3499 | 4.3536 | −47.9058 |
| 2Y9X—KNN2 | −12.5 | 7.1 × 10−10 | −29.1007 | −32.8336 | −133.06 | −194.9942 |
| 2Y9X—KNN3 | −10.3 | 2.9 × 10−8 | −27.1574 | −17.0376 | −144.366 | −188.561 |
| 2Y9X—RF1 | −8.5 | 6.3 × 10−7 | −27.8065 | −4.7535 | −117.47 | −150.03 |
| 2Y9X—RF2 | −9.3 | 1.6 × 10−7 | −37.0432 | −35.4341 | −153.134 | −225.6113 |
| 2Y9X—RF3 | −10.6 | 1.6 × 10−8 | −31.213 | −21.6992 | −104.05 | −156.9622 |
| 2Y9X—P4 | −9.3 | 1.6 × 10−7 | −34.23 | 24.0025 | −9.2737 | −19.5013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongsompong, S.; E-kobon, T.; Taengphan, W.; Sangkhawasi, M.; Khongkow, M.; Chumnanpuen, P. Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome. Int. J. Mol. Sci. 2023, 24, 3154. https://doi.org/10.3390/ijms24043154
Kongsompong S, E-kobon T, Taengphan W, Sangkhawasi M, Khongkow M, Chumnanpuen P. Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome. International Journal of Molecular Sciences. 2023; 24(4):3154. https://doi.org/10.3390/ijms24043154
Chicago/Turabian StyleKongsompong, Sasikarn, Teerasak E-kobon, Weerasak Taengphan, Mattanun Sangkhawasi, Mattaka Khongkow, and Pramote Chumnanpuen. 2023. "Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome" International Journal of Molecular Sciences 24, no. 4: 3154. https://doi.org/10.3390/ijms24043154
APA StyleKongsompong, S., E-kobon, T., Taengphan, W., Sangkhawasi, M., Khongkow, M., & Chumnanpuen, P. (2023). Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome. International Journal of Molecular Sciences, 24(4), 3154. https://doi.org/10.3390/ijms24043154








