Multiple-P450 Gene Co-Up-Regulation in the Development of Permethrin Resistance in the House Fly, Musca domestica
Abstract
:1. Introduction
2. Results
2.1. Cytochrome P450 Genes and Their Transcriptome Expression Profiles in M. domestica
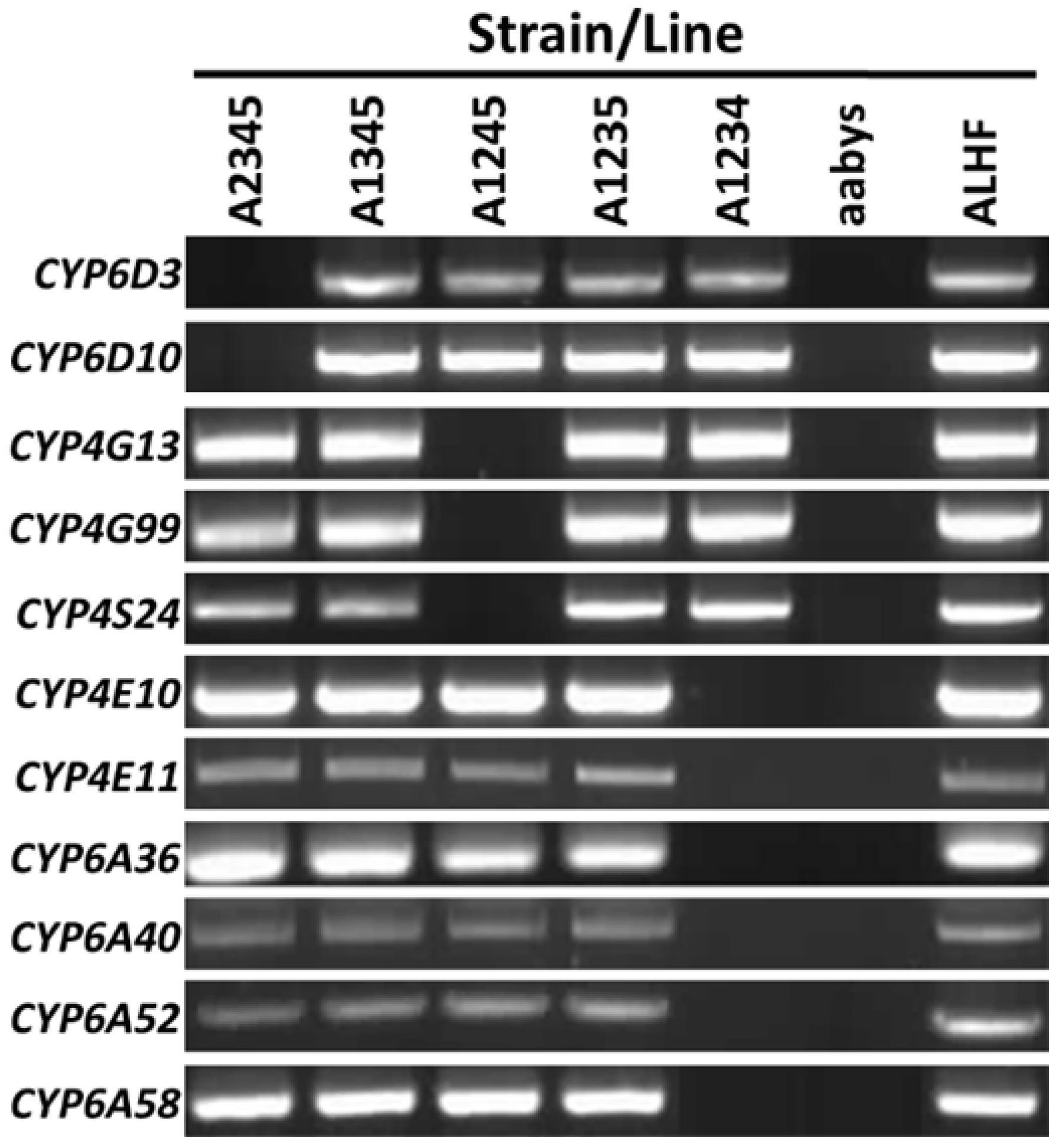
2.2. Autosome Location and Co-Up-Regulation of P450 Gene Expression in Insecticide-Resistant House Flies
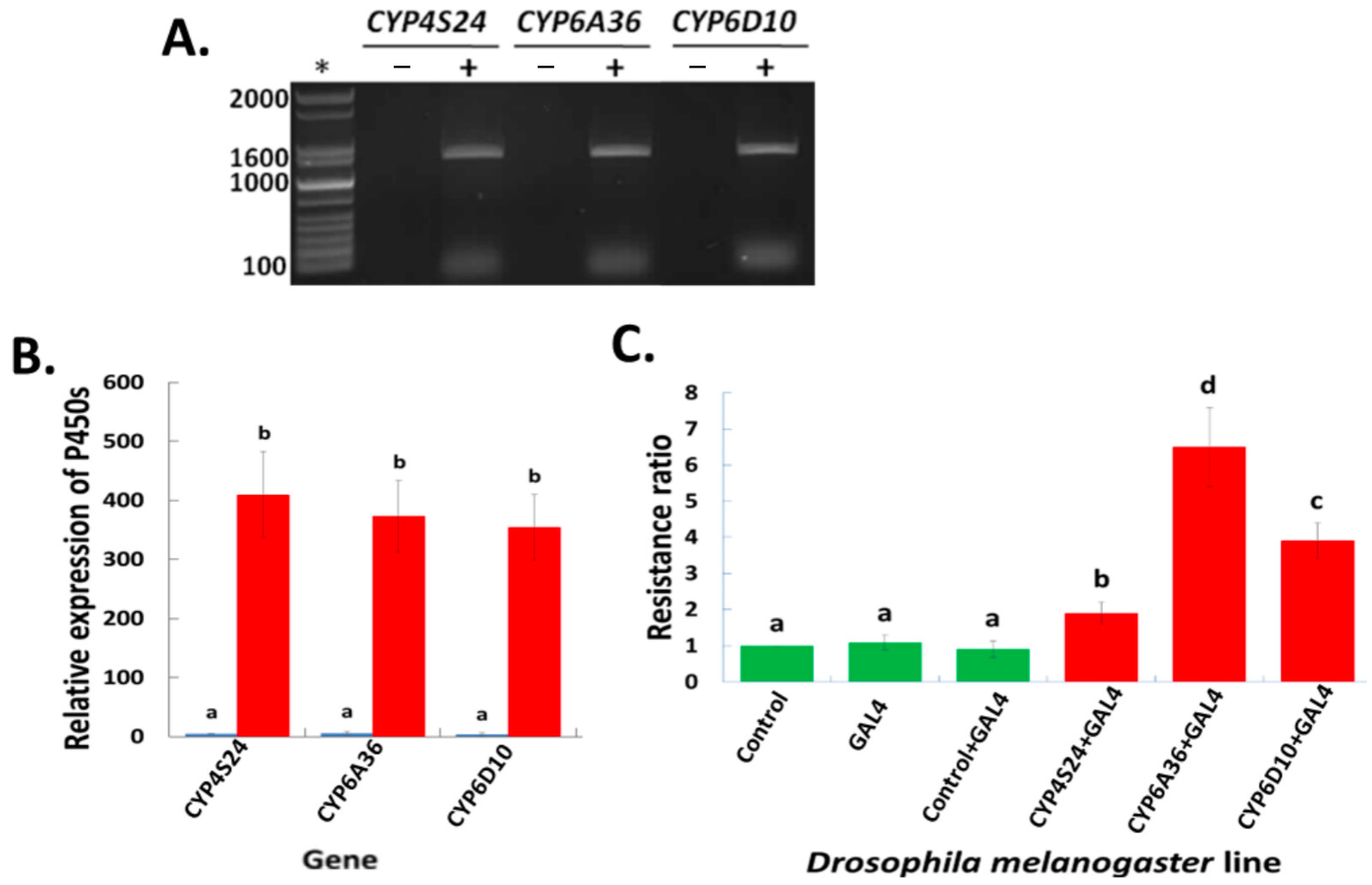
2.3. Up-Regulated P450 Genes in Transgenic D. melanogaster—An Alternative/Independent Approach to Defining P450 Gene Functions
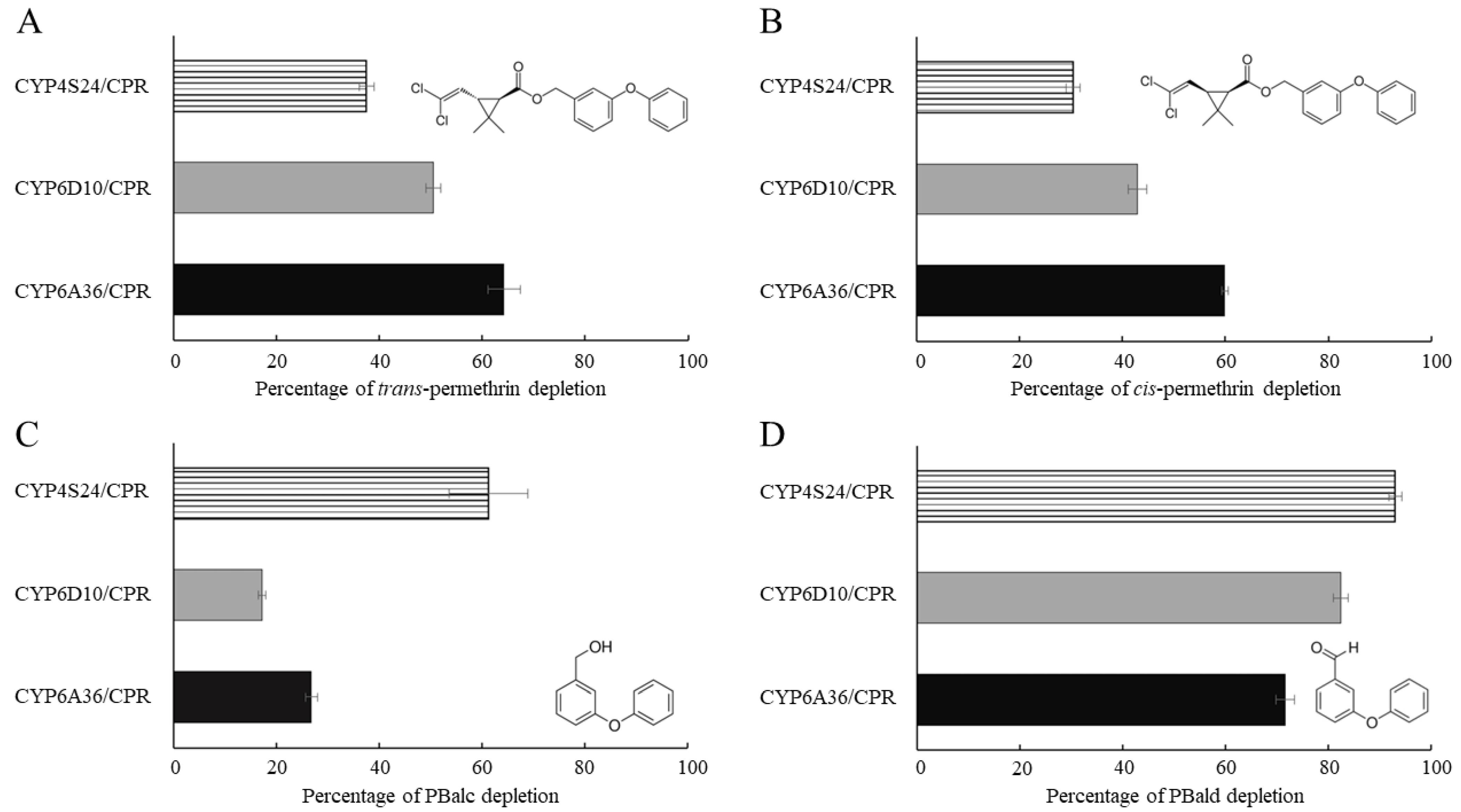
2.4. Functional Expression of P450s and In Vitro Metabolism Studies
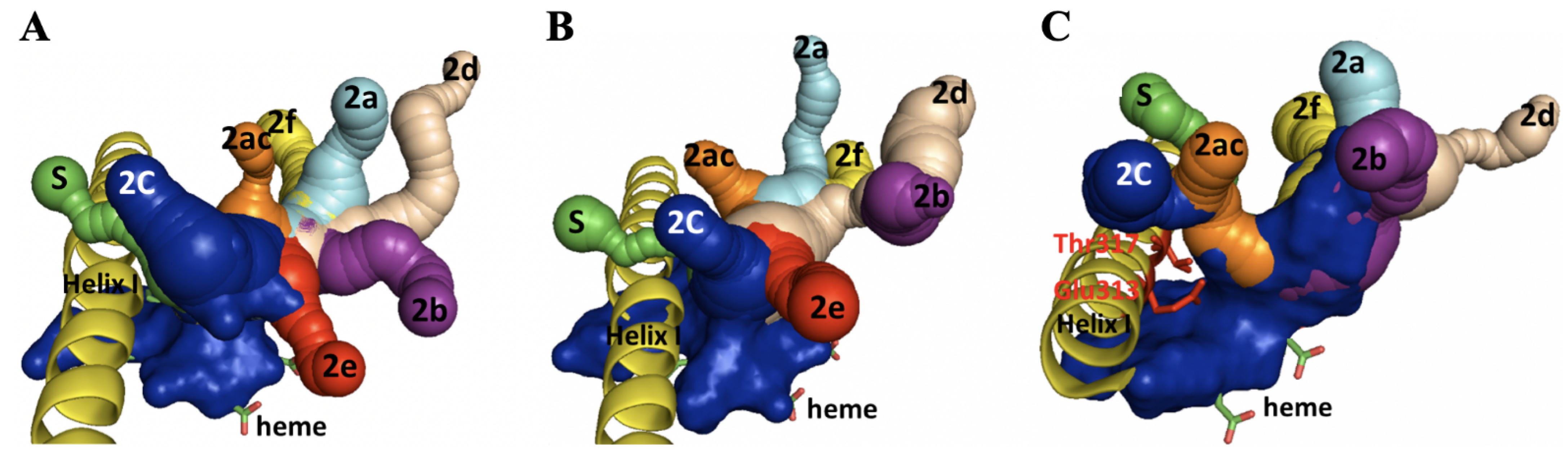
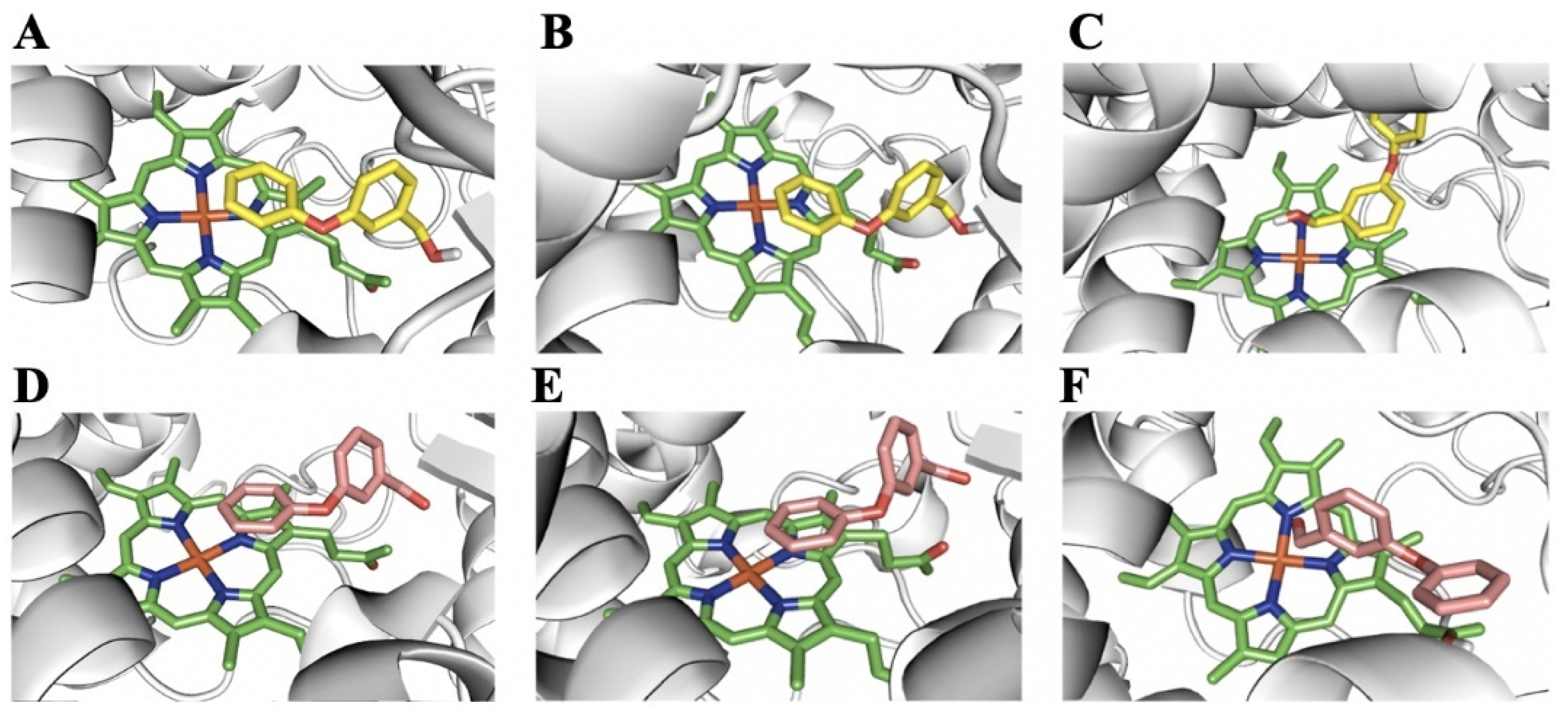
2.5. Homology Modeling and Substrate Docking
3. Discussion
4. Materials and Methods
4.1. House Fly Strains and Lines
4.2. Quantitative Real-Time PCR (qRT-PCR)
4.3. Autosomal Linkage Analysis of the P450 Genes in M. domestica
4.4. Construction of Transgenic D. melanogaster and Toxicity of Permethrin for the Transgenic Lines
4.5. Recombinant Protein Production
4.6. P450/CPR Activity Determination
4.7. P450/CPR-Mediated In Vitro Metabolism of Permethrin, 3-Phenoxybenzyl Alcohol (PBalc) and 3-Phenoxybenzaldehyde (PBald)
4.8. In Silico Modeling and Substrate Docking Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s—Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Phys. 2015, 120, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of g-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culex quinquefasciatus. Sci. Rep. 2014, 4, 6474. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Brun-Barale, A.; Héma, O.; Martin, T.; Suraporn, S.; Audant, P.; Sezutsu, H.; Feyereisen, R. Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera. Pest Manag. Sci. 2010, 66, 900–909. [Google Scholar] [CrossRef]
- Yang, T.; Liu, N. Genome analysis of cytochrome p450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e29418. [Google Scholar] [CrossRef]
- Gong, Y.; Li, T.; Zhang, L.; Gao, X.; Liu, N. Permethrin induction of multiple cytochrome p450 genes in insecticide resistant mosquitoes, Culex quinquefasciatus. Int. J. Biol. Sci. 2013, 9, 863–871. [Google Scholar] [CrossRef]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.I.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Phys. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Yang, T.; Li, T.; Feng, X.; Li, M.; Liu, S.; Liu, N. Multiple cytochrome P450 genes: Conferring high levels of permethrin resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2021, 11, 9041. [Google Scholar] [CrossRef]
- Li, M.; Reid, W.R.; Zhang, L.; Scott, J.G.; Gao, X.; Kristensen, M.; Liu, N. A whole transcriptomal linkage analysis of gene co-regulation in insecticide resistant house flies, Musca domestica. BMC Genom. 2013, 14, 803. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Warren, W.C.; Beukeboom, L.W.; Bopp, D.; Clark, A.G.; Giers, S.D.; Hediger, M.; Jones, A.K.; Kasai, S.; Leichter, C.A.; et al. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. 2014, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Vontas, J.; Van Leeuwen, T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Curr. Opin. Insect Sci. 2018, 27, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Lebedev, G.; Kontsedalov, S.; Ben-Yakir, D.; Ghanim, M. A de novo transcriptomics approach reveals genes involved in thrips tabaci resistance to spinosad. Insects 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Everitt, A.; Cornel, A.J.; Hormozdiari, F.; Lanzaro, G.C. Genetic variation associated with increased insecticide resistance in the malaria mosquito, Anopheles coluzzii. Parasites Vectors 2018, 11, 225. [Google Scholar] [CrossRef]
- Simma, E.A.; Dermauw, W.; Balabanidou, V.; Snoeck, S.; Bryon, A.; Clark, R.M.; Yewhalaw, D.; Vontas, J.; Duchateau, L.; Van Leeuwen, T. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag. Sci. 2019, 75, 1808–1818. [Google Scholar] [CrossRef]
- Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Wondji, M.J.; Irving, H.; Tchoupo, M.; Hearn, J.; Weedall, G.D.; Riveron, J.M.; Wondji, C.S. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 2019, 10, 4652. [Google Scholar] [CrossRef]
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House fly–microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef]
- Freeman, J.C.; Ross, D.H.; Scott, J.G. Insecticide resistance monitoring of house fly populations from the United States. Pestic. Biochem. Phys. 2019, 158, 61–68. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect cytochrome P450. In Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; Volume 4, pp. 1–77. [Google Scholar]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Pavlidi, N.; Monastirioti, M.; Daborn, P.; Livadaras, I.; Van Leeuwen, T.; Vontas, J. Transgenic expression of the Aedes aegypti CYP9J28 confers pyrethroid resistance in Drosophila melanogaster. Pestic. Biochem. Phys. 2012, 104, 132–135. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, L.; Chen, S.; Wu, Y. Functional expression of Helicoverpa armigera CYP9A12 and CYP9A14 in Saccharomyces cerevisiae. Pestic. Biochem. Phys. 2008, 92, 101–105. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, N. Differential expression of CYP6A5 and CYP6A5v2 in pyrethroid-resistant house flies, Musca domestica. Arch. Insect Biochem. Physiol. 2008, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, C.; He, L.; Li, M.; Zhang, L.; Zhang, L.; Liu, H.; Liu, N. Autosomal interactions and mechanisms of pyrethroid resistance in house flies, Musca domestica. Int. J. Biol. Sci. 2011, 7, 902–911. [Google Scholar] [CrossRef]
- Liu, N.; Scott, J.G. Genetics of resistance to pyrethroid insecticides in the house fly, Musca domestica. Pestic. Biochem. Phys. 1995, 52, 116–124. [Google Scholar] [CrossRef]
- Liu, N.; Yue, X. Genetics of pyrethroid resistance in a strain (ALHF) of house flies (Diptera: Muscidae). Pestic. Biochem. Phys. 2001, 70, 151–158. [Google Scholar] [CrossRef]
- Gong, Y.; Li, T.; Feng, Y.; Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci. Rep. 2017, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Chandor-Proust, A.; Bibby, J.; Régent-Kloeckner, M.; Roux, J.; Guittard-Crilat, E.; Poupardin, R.; Riaz, M.A.; Paine, M.; Dauphin-Villemant, C.; Reynaud, S.; et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem. J. 2013, 455, 75–85. [Google Scholar] [CrossRef]
- Godin, S.J.; Scollon, E.J.; Hughes, M.F.; Potter, P.M.; DeVito, M.J.; Ross, M.K. Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: Differential oxidative and hydrolytic metabolism by humans and rats. Drug Metab. Dispos. 2006, 34, 1764–1771. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sugihara, K.; Sone, T.; Isobe, M.; Ohta, S.; Kitamura, S. The in vitro metabolism of a pyrethroid insecticide, permethrin, and its hydrolysis products in rats. Toxicology 2007, 235, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.K.; Borazjani, A.; Edwards, C.C.; Potter, P.M. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 2006, 71, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Somwang, P.; Yanola, J.; Suwan, W.; Walton, C.; Lumjuan, N.; Prapanthadara, L.; Somboon, P. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol. Res. 2011, 109, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Takaku, T.; Mikata, K.; Matsui, M.; Nishioka, K.; Isobe, N.; Kaneko, H. In vitro metabolism of trans-permethrin and its major metabolites, PBalc and PBacid, in humans. J. Agric. Food Chem. 2011, 59, 5001–5005. [Google Scholar] [CrossRef] [PubMed]
- Tange, S.; Fujimoto, N.; Uramaru, N.; Sugihara, K.; Ohta, S.; Kitamura, S. In vitro metabolism of cis- and trans-permethrin by rat liver microsomes, and its effect on estrogenic and anti-androgenic activities. Environ. Toxicol. Pharmacol. 2014, 37, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Winn, P.J.; Wade, R.C. The ins and outs of cytochrome P450s. Biochim. Biophys. Acta 2007, 1770, 390–401. [Google Scholar] [CrossRef]
- Otyepka, M.; Berka, K.; Anzenbacher, P. I Is there a relationship between the substrate preferences and structural flexibility of cytochromes P450? Curr. Drug Metab. 2012, 13, 130–142. [Google Scholar] [CrossRef]
- Stevenson, B.J.; Bibby, J.; Pignatelli, P.; Muangnoicharoen, S.; O’Neill, P.M.; Lian, L.-Y.; Müller, P.; Nikou, D.; Steven, A.; Hemingway, J.; et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011, 41, 492–502. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genom 2013, 14, 174. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Bass, C.; Williamson, M.S.; Kaussmann, M.; Wölfel, K.; Gutbrod, O.; Nauen, R. Molecular and functional characterization of CYP6BQ23, a cytochrome P450 conferring resistance to pyrethroids in European populations of pollen beetle, Meligethes aeneus. Insect Biochem. Mol. Biol. 2014, 45, 18–29. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Riveron, J.M.; Stott, R.; Irving, H.; Wondji, C.S. The cytochrome P450 CYP6P4 is responsible for the high pyrethroid resistance in knockdown resistance-free Anopheles arabiensis. Insect Biochem. Mol. Biol. 2016, 68, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Karunker, I.; Morou, E.; Nikou, D.; Nauen, R.; Sertchook, R.; Stevenson, B.J.; Paine, M.J.; Morin, S.; Vontas, J. Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem. Mol. Biol. 2009, 39, 697–706. [Google Scholar] [CrossRef]
- Liu, N.; Scott, J.G. Phenobarbital induction of CYP6D1 is due to a trans acting factor on autosome 2 in house flies, Musca domestica. Insect Mol. Biol. 1997, 6, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Carino, F.A.; Koener, J.F.; Plapp Jr, F.W.; Feyereisen, R. Constitutive overexpression of the cytochrome P450 gene CYP6A1 in a house fly strain with metabolic resistance to insecticides. Insect Biochem. Mol. Biol. 1994, 24, 411–418. [Google Scholar] [CrossRef]
- Pavek, P.; Dvorak, Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 2008, 9, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Wang, L.; Liu, S.; Wu, S.; Yang, Y.; Feyereisen, R.; Wu, Y. CYP6AE gene cluster knockout in Helicoverpa armigera reveals role in detoxification of phytochemicals and insecticides. Nat. Commun. 2018, 9, 4820. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Scott, J.G. Genetic analysis of factors controlling high-level expression of cytochrome P450, CYP6D1, cytochrome b5, P450 reductase, and monooxygenase activities in LPR house flies, Musca domestica. Biochem. Genet. 1996, 34, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Dombrowski, S.M.; Waters, L.C.; Ganguly, R. Three Second Chromosome-Linked Clustered Cyp6 Genes Show Differential Constitutive and Barbital-Induced Expression in DDT-Resistant and Susceptible Strains of Drosophila Melanogaster. Gene 1996, 180, 165–171. [Google Scholar] [CrossRef]
- Maitra, S.; Dombrowski, S.M.; Basu, M.; Raustol, O.; Waters, L.C.; Ganguly, R. Factors on the third chromosome affect the level of Cyp6a2 and Cyp6a8 expression in Drosophila melanogaster. Gene 2000, 248, 147–156. [Google Scholar] [CrossRef]
- Fotoukkiaii, S.M.; Wybouw, N.; Kurlovs, A.H.; Tsakireli, D.; Pergantis, S.A.; Clark, R.M.; Vontas, J.; Leeuwen, T.V. High-resolution genetic mapping reveals cis-regulatory and copy number variation in loci associated with cytochrome P450-mediated detoxification in a generalist arthropod pest. PLoS Genet. 2021, 17, e1009422. [Google Scholar] [CrossRef]
- Papapostolou, K.M.; Riga, M.; Samantsidis, G.-R.; Skoufa, E.; Balabanidou, V.; Van Leeuwen, T.; Vontas, J. Over-expression in cis of the midgut P450 CYP392A16 contributes to abamectin resistance in Tetranychus urticae. Insect Biochem. Mol. Biol. 2022, 142, 103709. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Boey, A.; Lumb, C.; Willoughby, L.; Batterham, P.; Daborn, P.J. Induction of a detoxification gene in Drosophila melanogaster requires an interaction between tissue specific enhancers and a novel cis-regulatory element. Insect Biochem. Mol. Biol. 2011, 41, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, C.; Yang, T.; Zhang, L.; He, L.; Xi, Z.; Bian, G.; Liu, N. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2016, 5, 17772. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, S.; Zhang, Y.; He, Y.; Du, M.; O’Reilly, A.O.; Wu, S.; Yang, Y.; Wu, Y. Single amino acid variations drive functional divergence of cytochrome P450s in Helicoverpa species. Insect Biochem. Mol. Biol. 2022, 146, 103796. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Brown, R.P.; Berenbaum, M.R.; Schuler, M.A. Conserved regulatory elements in the promoters of two allelochemical-inducible cytochrome P450 genes differentially regulate transcription. Insect Biochem. Mol. Biol. 2004, 34, 1129–1139. [Google Scholar] [CrossRef]
- Mohammed, B.R.; Kawe, M.S.; Nwachukwu, O.M.; Jegede, O.C.; Agbede, R.I.; Finn, R.D. Understanding the mechanisms involved in the regulation of cytochrome P450 gene expression in Drosophila melanogaster (Diptera: Drosophilidae). Entomol. Ornithol. Herpetol. 2017, 6, 1. [Google Scholar] [CrossRef]
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19. [Google Scholar] [CrossRef]
- Li, T.; Liu, N. The function of g-protein-coupled receptor-regulatory cascade in southern house mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 862–870. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription Factors, CncC and Maf, Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 47–56. [Google Scholar] [CrossRef]
- Pu, J.; Sun, H.; Wang, J.; Wu, M.; Wang, K.; Denholm, I.; Han, Z. Multiple cis-acting elements involved in up-regulation of a cytochrome P450 gene conferring resistance to deltamethrin in smal brown planthopper, Laodelphax striatellus (Fallén). Insect Biochem. Mol. Biol. 2016, 78, 20–28. [Google Scholar] [CrossRef]
- Palli, S.R. CncC/Maf-mediated xenobiotic response pathway in insects. Arch. Insect Biochem. Physiol. 2020, 104, e21674. [Google Scholar] [CrossRef]
- Smith, L.B.; Tyagi, R.; Kasai, S.; Scott, J.G. CYP-mediated permethrin resistance in Aedes aegypti and evidence for trans-regulation. PLoS Negl. Trop. Dis. 2018, 12, e0006933. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, H.; Hu, S.; Ren, M.; Wei, Q.; Tian, X.; Elzaki, M.E.A.; Bass, C.; Su, J.; Palli, S.R. Changes in both trans- and cis-regulatory elements mediate insecticide resistance in a lepidopteron pest, Spodoptera exigua. PLoS Genet. 2021, 17, e1009403. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Sun, L.; Du, H.; Moural, T.W.; Bai, H.; Liu, P.; Zhu, F. Physiological functions of a methuselah-like G protein coupled receptor in Lymantria dispar Linnaeus. Pestic. Biochem. Phys. 2019, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, P.; Yu, X.-Y.; Chen, C.; Yu, J.; Shi, L.-N.; Hong, S.-C.; Zhou, D.; Chang, X.-L.; Wang, W.-J.; et al. Functional characterization of an arrestin gene on insecticide resistance of Culex pipiens pallens. Parasites Vectors 2012, 5, 134. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; You, C.; Zeng, X.; Gao, X. The role of G protein-coupled receptor-related genes in cytochrome P450-mediated resistance of the house fly, Musca domestica (Diptera: Muscidae), to imidacloprid. Insect Mol. Biol. 2020, 29, 92–103. [Google Scholar] [CrossRef]
- Sundaram, V.; Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Phil. Trans. R. Soc. 2020, 375, 20190347. [Google Scholar] [CrossRef]
- Moschetti, R.; Palazzo, A.; Lorusso, P.; Viggiano, L.; Marsano, R.M. “What you need, baby, I got it”: Transposable elements as suppliers of cis-operating sequences in Drosophila. Biology 2020, 9, 25. [Google Scholar] [CrossRef]
- Chen, S.; Li, X. Transposable elements are enriched within or in close proximity to xenobiotic-metabolizing cytochrome P450 genes. BMC Evol. Biol. 2007, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Bogwitz, M.R.; McCart, C.; Andrianopoulos, A.; Ffrench-Constant, R.H.; Batterham, P.; Daborn, P.J. Cis-regulatory elements in the Accord retrotransposon result in tissue-specific expression of the Drosophila melanogaster insecticide resistance gene Cyp6g1. Genetics 2007, 175, 1071–1977. [Google Scholar] [CrossRef] [PubMed]
- Schlenke, T.A.; Begun, D.J. Strong selective sweep associated with a transposon insertion in Drosophila simulans. Proc. Natl. Acad. Sci. USA 2004, 101, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Wilding, C.S.; Smith, I.; Lynd, A.; Yawson, A.E.; Weetman, D.; Paine, M.J.; Donnelly, M.J. A cis-regulatory sequence driving metabolic insecticide resistance in mosquitoes: Functional characterization and signatures of selection. Insect Biochem. Mol. Biol. 2012, 42, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Marsano, R.M.; Caizzi, R.; Moschetti, R.; Junakovic, N. Evidence for a functional interaction between the Bari1 transposable element and the cytochrome P450 cyp12a4 gene in Drosophila melanogaster. Gene 2005, 357, 122–128. [Google Scholar] [CrossRef]
- Schuler, M.A.; Berenbaum, M.R. Structure and function of cytochrome P450s in insect adaptation to natural and synthetic toxins: Insights gained from molecular modeling. J. Chem. Ecol. 2013, 39, 1232–1245. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Jenwitheesuk, E.; Rongnoparut, P. Homology modeling of mosquito cytochrome P450 enzymes involved in pyrethroid metabolism: Insights into differences in substrate selectivity. BMC Res. Notes 2011, 4, 321. [Google Scholar] [CrossRef]
- Liu, N.; Yue, X. Insecticide resistance and cross-resistance in the house fly (Diptera: Muscidae). J. Econ. Entomol. 2000, 93, 1269–1275. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Herrmann, M.G.; Moss, A.A.; Rasmussen, R.P. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 1997, 22, 130–138. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. A Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhu, F.; Feng, J.-N.; Zhang, L.; Liu, N. Characterization of two novel cytochrome P450 genes in insecticide-resistant house-flies. Insect Mol. Biol. 2008, 17, 27–37. [Google Scholar] [CrossRef]
- Gong, Y.; Li, T.; Li, Q.; Liu, S.; Liu, N. The central role of multiple P450 genes and their co-factor CPR in the development of permethrin resistance in the mosquito Culex quinquefasciatus. Front. Physiol. 2022, 12, 802584. [Google Scholar] [CrossRef]
- Feng, X.; Liu, N. Functional analyses of house fly carboxylesterases involved in insecticide resistance. Front. Physiol. 2020, 11, 595009. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Zhang, Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure 2011, 19, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Sippl, M.J. Recognition of errors in three-dimensional structures of proteins. Proteins 1993, 17, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Chovancova, E.; Pavelka, A.; Benes, P.; Strnad, O.; Brezovsky, J.; Kozlikova, B.; Gora, A.; Sustr, V.; Klvana, M.; Medek, P. CAVER 3.0: A tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 2012, 8, e1002708. [Google Scholar] [CrossRef]
- Medek, P.; Beneš, P.; Sochor, J. Computation of tunnels in protein molecules using Delaunay triangulation. J. WSCG 2007, 15, 107–114. [Google Scholar]
- Kleywegt, G.J.; Zou, J.Y.; Kjeldgaard, M.; Jones, T.A.; Around, O. International Tables for Crystallography; Kluwer: Dordrecht, The Netherlands, 2001; Volume F, Chapter 17.1; pp. 353–356, 366–367. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. ZINC: A Free Tool to Discover Chemistry for Biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, O. S Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 1992, 267, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. The PyMOL Molecular Graphics System. 2002. Available online: https://www.pymol.org (accessed on 15 May 2022).


| CYP Clan | CYP Family * | Number of CYP Members |
|---|---|---|
| Clan 2 | CYP18 | 1 |
| CYP304 | 1 | |
| CYP305 | 1 | |
| CYP306 | 1 | |
| Clan 3 | CYP6 | 27 |
| CYP9 | 6 | |
| CYP28 | 3 | |
| CYP310 | 1 | |
| CYP317 | 1 | |
| CYP438A4 | 1 | |
| Clan 4 | CYP4 | 23 |
| CYP311 | 1 | |
| CYP3073 | 3 | |
| CYP313 | 2 | |
| CYP318 | 1 | |
| Mitochondrial CYP | CYP12 | 9 |
| CYP301 | 1 | |
| CYP302 | 1 | |
| CYP314 | 1 | |
| CYP315 | 1 |
| Relative Gene Expression in Strains/Lines § | ||||||
|---|---|---|---|---|---|---|
| Gene | ALHF | A2345 | A1345 | A1245 | A1235 | A1234 |
| CYP4E10 | 274.42 ± 22.40 | 125.67 ± 10.39 * | 144.62 ± 12.62 * | 257.81 ± 23.61 | 268.61 ± 25.8 | 66.6 ± 6.74 * |
| CYP4E11 | 5.86 ± 0.55 | 6.31 ± 0.48 | 2.61 ± 0.17 * | 5.99 ± 0.71 | 5.80 ± 0.36 | 2.71 ± 0.26 * |
| CYP4G13 | 2.13 ± 0.13 | 1.04 ± 0.10 * | 1.22 ± 0.09 * | 1.04 ± 0.04 * | 2.01 ± 0.12 | 1.91 ± 0.08 |
| CYP4G99 | 4.52 ± 0.31 | 2.12 ± 0.22 * | 4.28 ± 0.41 | 1.38 ± 0.13 * | 4.43 ± 0.45 | 4.43 ± 0.23 |
| CYP4S24 | 2.94 ± 0.08 | 1.03 ± 0.05 * | 1.14 ± 0.06 * | 0.83 ± 0.03 * | 2.83 ± 0.49 | 3.04 ± 0.53 |
| CYP6A36 | 7.03 ± 0.64 | 2.60 ± 0.21 * | 2.80 ± 0.41 * | 6.63 ± 0.62 | 7.01 ± 1.04 | 1.72 ± 0.19 * |
| CYP6A40 | 2.95 ± 0.22 | 2.90 ± 0.32 | 1.16 ± 0.10 * | 2.99 ± 0.41 | 2.68 ± 0.36 | 1.17 ± 0.05 * |
| CYP6A52 | 2.74 ± 0.15 | 2.50 ± 0.22 | 2.70 ± 0.17 | 2.54 ± 0.08 | 3.01 ± 0.24 | 0.81 ± 0.03 * |
| CYP6A58 | 4.32 ± 1.02 | 1.32 ± 0.23 * | 1.86 ± 0.36 * | 5.63 ± 2.17 | 4.19 ± 0.22 | 0.64 ± 0.15 * |
| CYP6D3 | 2.40 ± 0.13 | 1.38 ± 0.07 * | 1.34 ± 0.06 * | 2.25 ± 0.13 | 2.40 ± 0.37 | 2.34 ± 0.18 |
| CYP6D10 | 6.24 ± 0.63 | 2.55 ± 0.37 * | 3.06 ± 0.55 * | 5.99 ± 0.25 | 6.19 ± 0.51 | 2.62 ± 0.32 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Feng, X.; Reid, W.R.; Tang, F.; Liu, N. Multiple-P450 Gene Co-Up-Regulation in the Development of Permethrin Resistance in the House Fly, Musca domestica. Int. J. Mol. Sci. 2023, 24, 3170. https://doi.org/10.3390/ijms24043170
Li M, Feng X, Reid WR, Tang F, Liu N. Multiple-P450 Gene Co-Up-Regulation in the Development of Permethrin Resistance in the House Fly, Musca domestica. International Journal of Molecular Sciences. 2023; 24(4):3170. https://doi.org/10.3390/ijms24043170
Chicago/Turabian StyleLi, Ming, Xuechun Feng, William R. Reid, Fang Tang, and Nannan Liu. 2023. "Multiple-P450 Gene Co-Up-Regulation in the Development of Permethrin Resistance in the House Fly, Musca domestica" International Journal of Molecular Sciences 24, no. 4: 3170. https://doi.org/10.3390/ijms24043170
APA StyleLi, M., Feng, X., Reid, W. R., Tang, F., & Liu, N. (2023). Multiple-P450 Gene Co-Up-Regulation in the Development of Permethrin Resistance in the House Fly, Musca domestica. International Journal of Molecular Sciences, 24(4), 3170. https://doi.org/10.3390/ijms24043170







