Acquisition of Immune Privilege in GBM Tumors: Role of Prostaglandins and Bile Salts
Abstract
:1. Introduction
2. Results
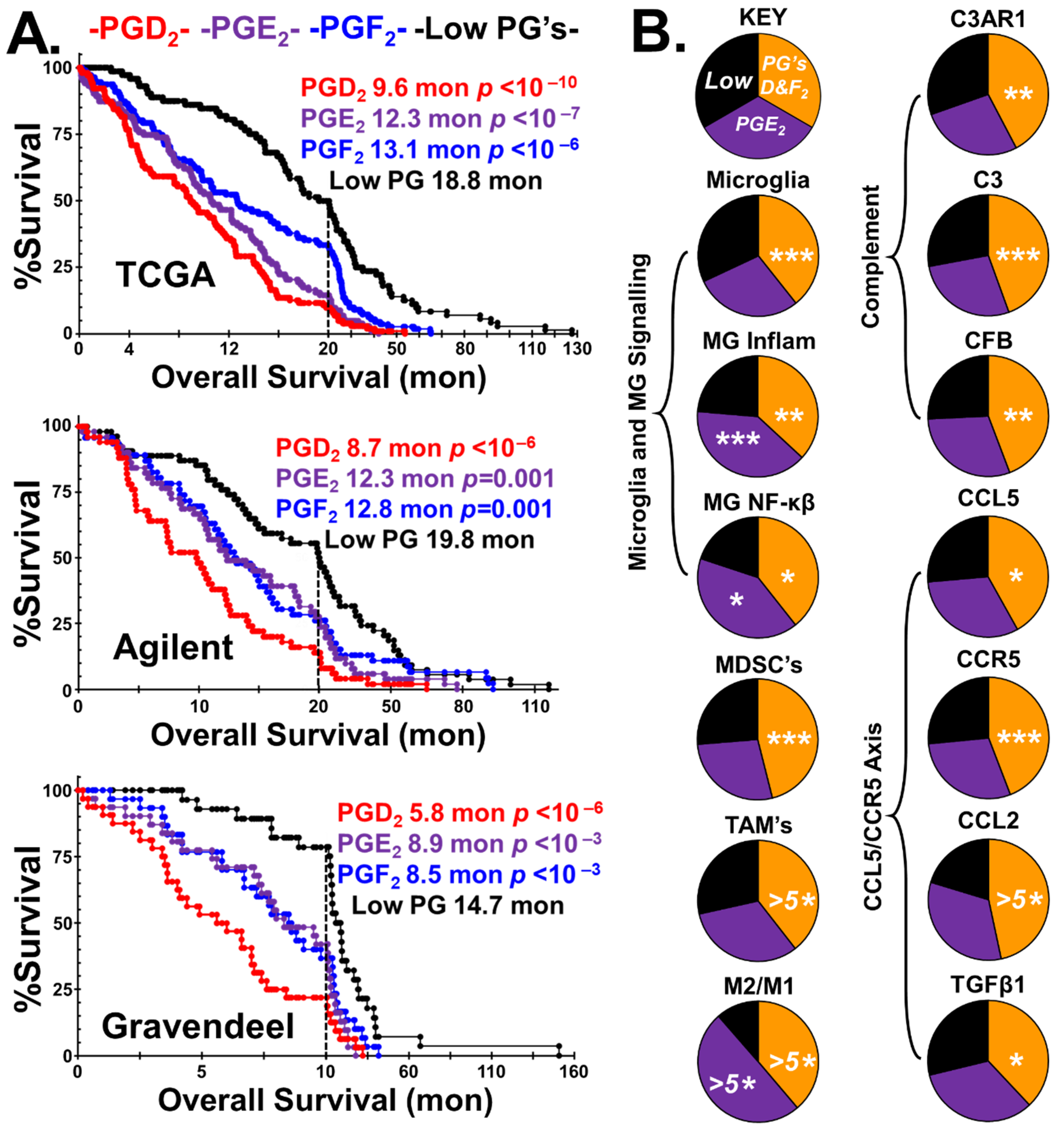
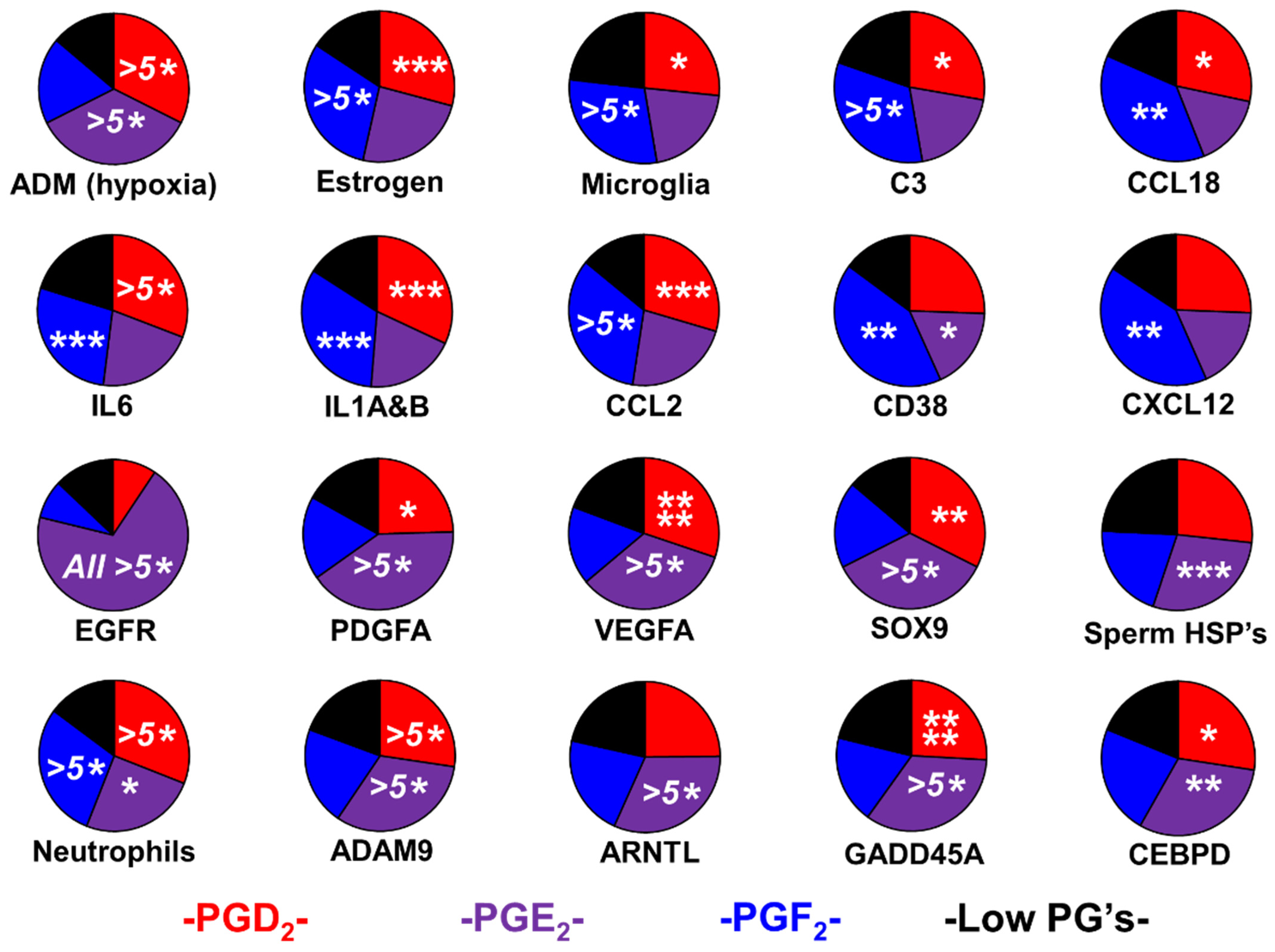
2.1. Prostaglandin Sculpting of the Tumor Immunological Phenotypes
2.2. Evidence for Sexual Hijacking in the PGE2 Phenotype
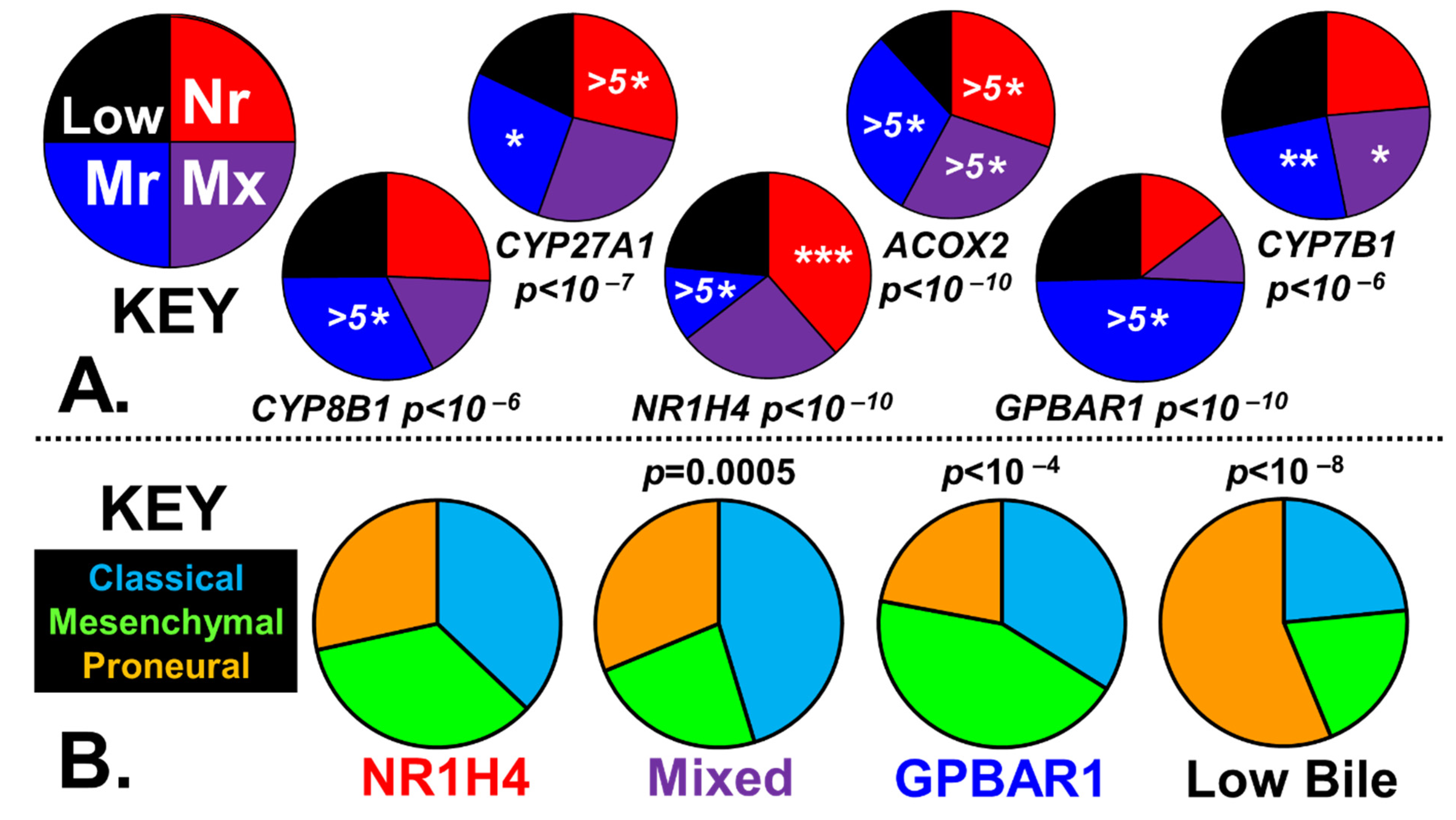
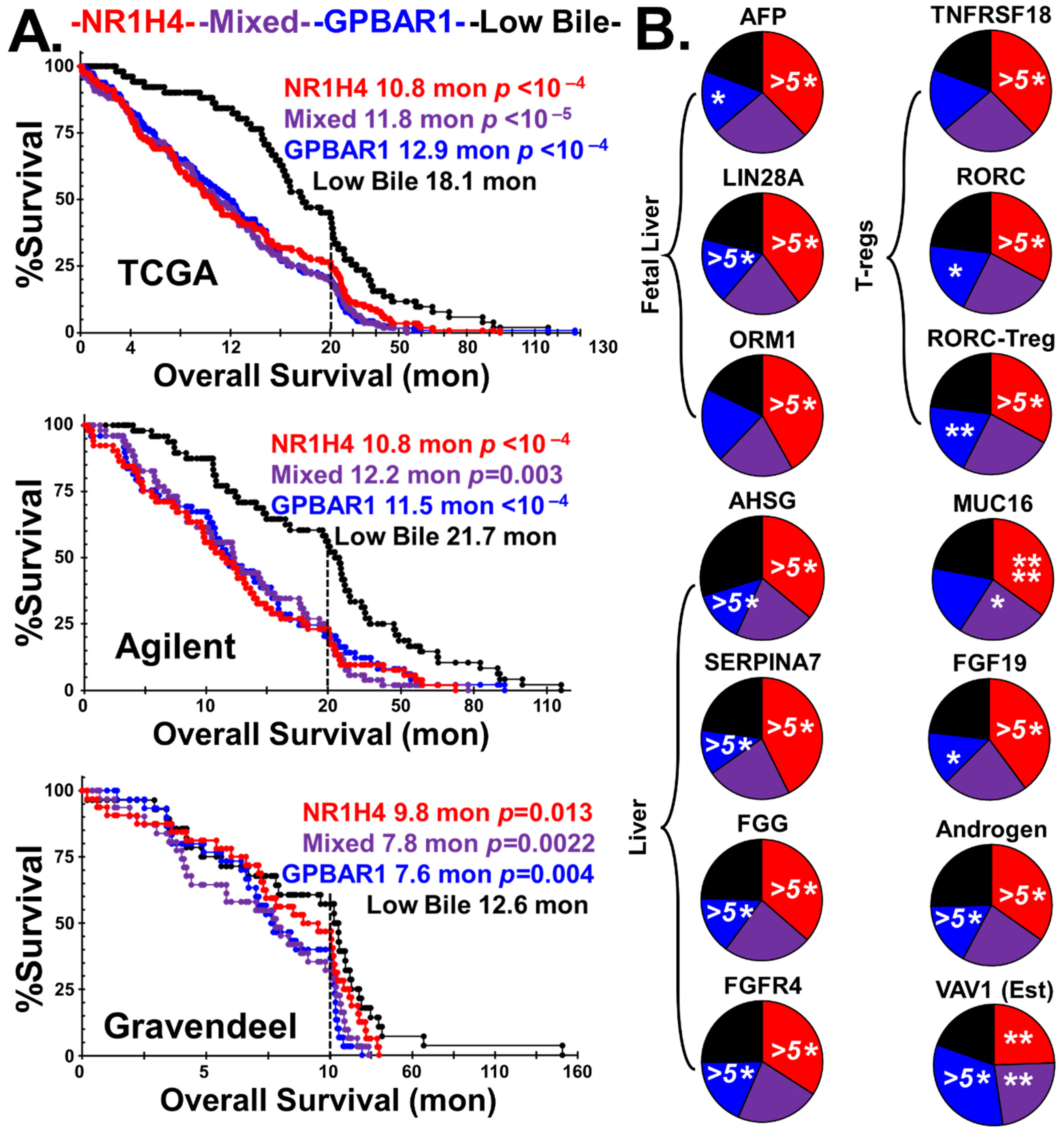
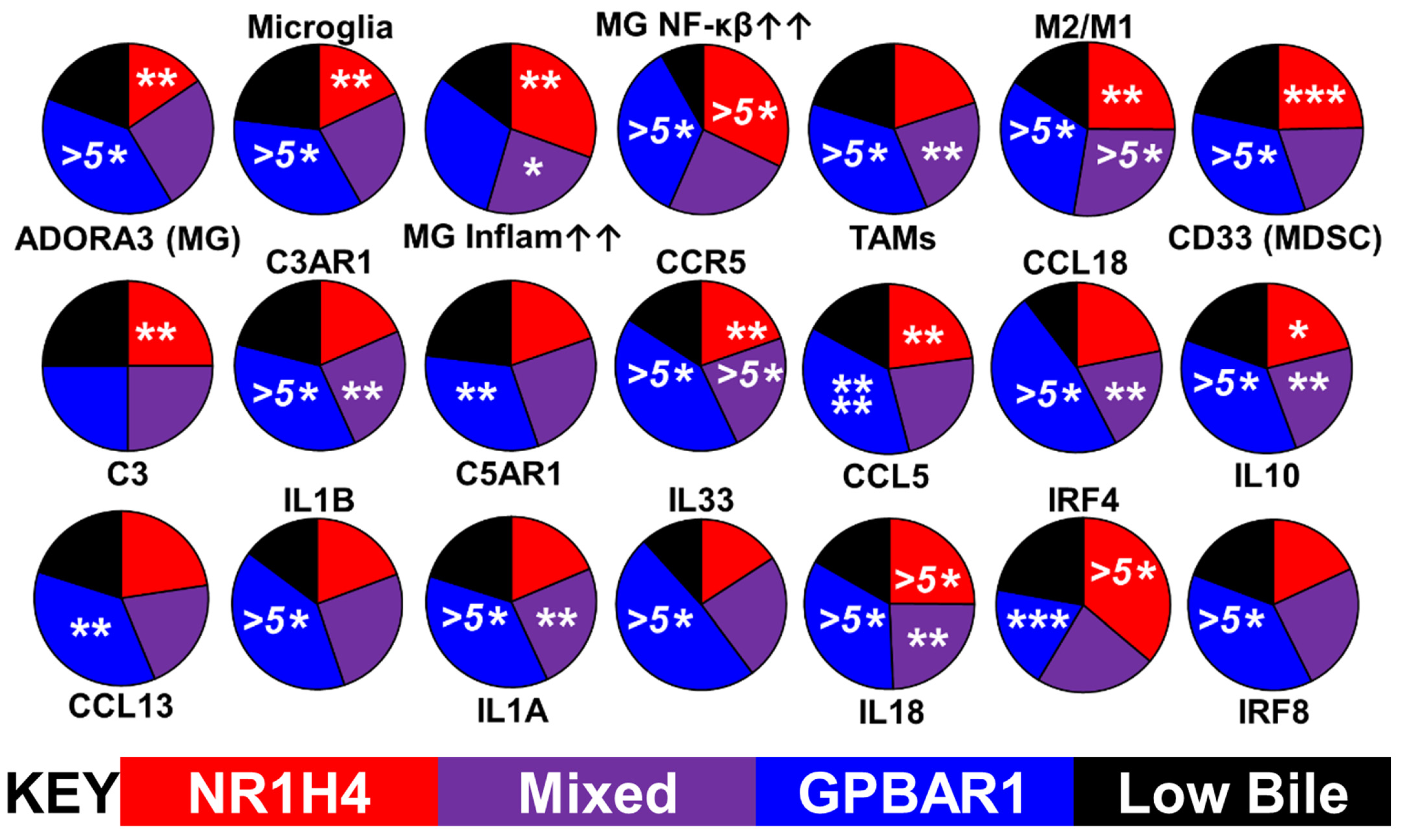
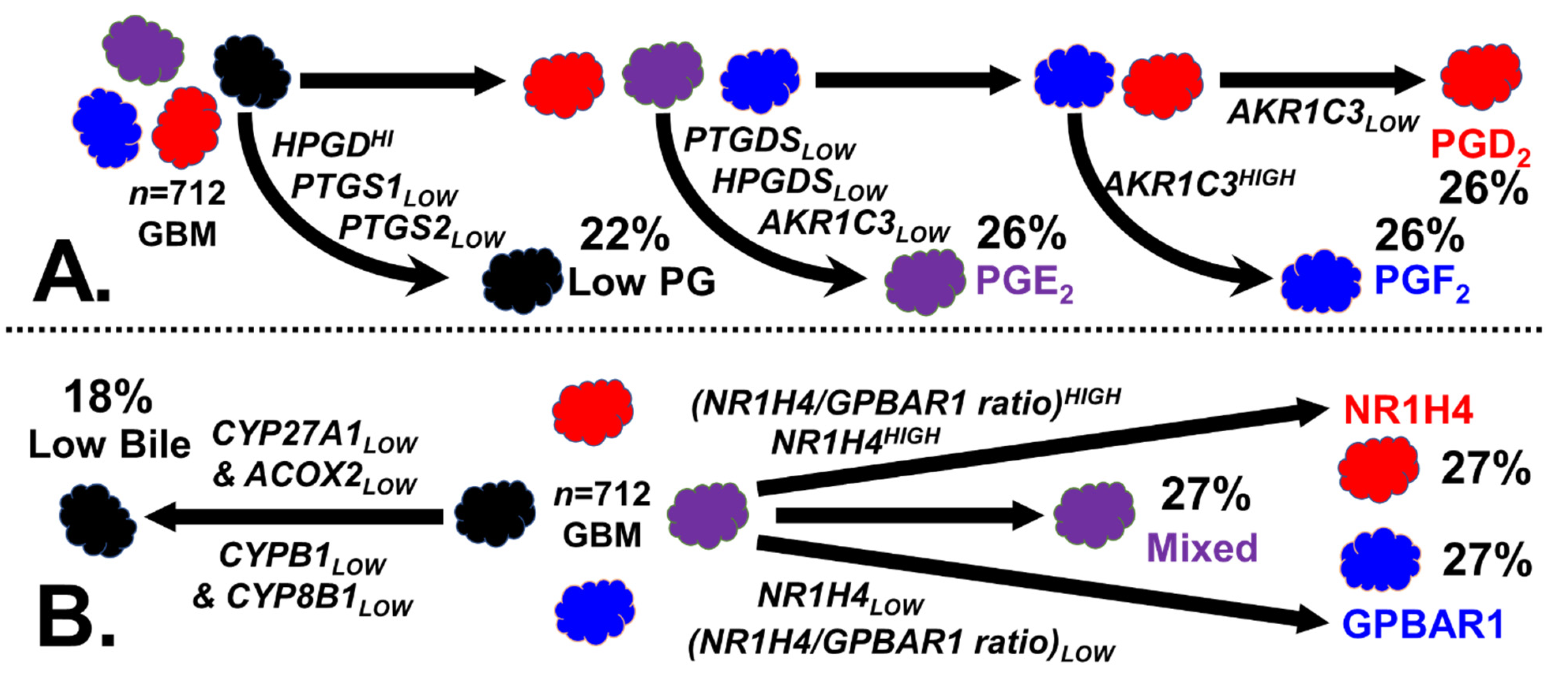
2.3. Bile Salt Sculpting of the Tumor Immunological Phenotypes
3. Discussion
3.1. Androgens, Estrogens, Prostaglandins, and Bile
3.2. Adult vs. Fetal Bile Salt Synthesis
3.3. Diagnosis, Tracking, and Treatment of GBM with Vaccines
4. Materials and Methods
4.1. GBM Transcriptomes
4.2. Stratification of GBM Phenotypes
4.2.1. Normalization of Transcript Levels
4.2.2. Stratification of GBM Tumors by Prostaglandins
4.2.3. Stratification of GBM Tumors by Bile Synthesis and Receptors
4.3. Estimation of Infiltration by Immune Cells, Polarization, and Reproduction-Related Markers
4.3.1. Microglia Levels and Activation Status
4.3.2. Infiltration of Tumors by Macrophages, MDSCs, Neutrophils, and Tregs
4.3.3. Identification of Vascular Markers in Tumors
4.3.4. Reproduction-Associated Markers and Proxies
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM-An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. [Google Scholar] [CrossRef]
- Godlewska, U.; Bulanda, E.; Wypych, T.P. Bile acids in immunity: Bidirectional mediators between the host and the microbiota. Front. Immunol. 2022, 13, 949033. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, S.; Goepp, M.; Pollock, J.; Robb, C.T.; Smyth, D.J.; Zhou, Y.; Andrews, R.; Tyrrell, V.; Gkikas, K.; Adima, A.; et al. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci. Adv. 2021, 7, eabd7954. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Jung, D.; Stakenborg, M.; Jang, H.; Gu, G.J.; Jeong, M.R.; Suh, S.Y.; Kim, H.J.; Kwon, Y.H.; Sung, T.S.; et al. Prostaglandin E2 receptor PTGER4-expressing macrophages promote intestinal epithelial barrier regeneration upon inflammation. Gut 2021, 70, 2249. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef]
- Jara-Gutiérrez, Á.; Baladrón, V. The Role of Prostaglandins in Different Types of Cancer. Cells 2021, 10, 1487. [Google Scholar] [CrossRef]
- Bonham, L.W.; Sirkis, D.W.; Yokoyama, J.S. The Transcriptional Landscape of Microglial Genes in Aging and Neurodegenerative Disease. Front. Immunol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Mohri, I.; Taniike, M.; Taniguchi, H.; Kanekiyo, T.; Aritake, K.; Inui, T.; Fukumoto, N.; Eguchi, N.; Kushi, A.; Sasai, H.; et al. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Mohri, I.; Okabe-Arahori, H.; Aritake, K.; Wada, K.; Kanekiyo, T.; Narumiya, S.; Nakayama, M.; Ozono, K.; Urade, Y.; et al. Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J. Neurosci. 2007, 27, 4303–4312. [Google Scholar] [CrossRef]
- Kong, D.; Shen, Y.; Liu, G.; Zuo, S.; Ji, Y.; Lu, A.; Nakamura, M.; Lazarus, M.; Stratakis, C.A.; Breyer, R.M.; et al. PKA regulatory IIα subunit is essential for PGD2-mediated resolution of inflammation. J. Exp. Med. 2016, 213, 2209–2226. [Google Scholar] [CrossRef] [PubMed]
- Sartain, S.E.; Turner, N.A.; Moake, J.L. Brain microvascular endothelial cells exhibit lower activation of the alternative complement pathway than glomerular microvascular endothelial cells. J. Biol. Chem. 2018, 293, 7195–7208. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, E.; Possenti, R.; Coco, S.; Rizzi, L.; Meanti, R.; Molteni, L.; Locatelli, V.; Torsello, A. TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling. Int. J. Mol. Sci. 2019, 21, 130. [Google Scholar] [CrossRef]
- Pekna, M.; Stokowska, A.; Pekny, M. Targeting Complement C3a Receptor to Improve Outcome After Ischemic Brain Injury. Neurochem. Res. 2021, 46, 2626–2637. [Google Scholar] [CrossRef]
- Gong, B.; Guo, D.; Zheng, C.; Ma, Z.; Zhang, J.; Qu, Y.; Li, X.; Li, G.; Zhang, L.; Wang, Y. Complement C3a activates astrocytes to promote medulloblastoma progression through TNF-α. J. Neuroinflammation 2022, 19, 159. [Google Scholar] [CrossRef]
- Tajima, T.; Murata, T.; Aritake, K.; Urade, Y.; Hirai, H.; Nakamura, M.; Ozaki, H.; Hori, M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J. Pharmacol. Exp. Ther. 2008, 326, 493–501. [Google Scholar] [CrossRef]
- Laudati, E.; Currò, D.; Navarra, P.; Lisi, L. Blockade of CCR5 receptor prevents M2 microglia phenotype in a microglia-glioma paradigm. Neurochem. Int. 2017, 108, 100–108. [Google Scholar] [CrossRef]
- Novak, M.; Koprivnikar Krajnc, M.; Hrastar, B.; Breznik, B.; Majc, B.; Mlinar, M.; Rotter, A.; Porčnik, A.; Mlakar, J.; Stare, K.; et al. CCR5-Mediated Signaling Is Involved in Invasion of Glioblastoma Cells in Its Microenvironment. Int. J. Mol. Sci. 2020, 21, 4199. [Google Scholar] [CrossRef]
- Kranjc, M.K.; Novak, M.; Pestell, R.G.; Lah, T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol. Oncol. 2019, 53, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J. Chemokine Regulation of Angiogenesis During Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef]
- Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Matsushima, K.; Mukaida, N.; Kondo, T. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J. Clin. Investig. 2012, 122, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Guo, L.; Gao, M.; Li, J.; Xiang, S. Research Trends and Regulation of CCL5 in Prostate Cancer. Onco Targets Ther. 2021, 14, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, Y.C.; Mahalingam, J.; Huang, C.T.; Chen, T.W.; Kang, C.W.; Peng, H.M.; Chu, Y.Y.; Chiang, J.M.; Dutta, A.; et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012, 72, 1092–1102. [Google Scholar] [CrossRef]
- Kaczynski, P.; Baryla, M.; Goryszewska, E.; Bauersachs, S.; Waclawik, A. Prostaglandin F2α promotes embryo implantation and development in the pig. Reproduction 2018, 156, 405–419. [Google Scholar] [CrossRef]
- Sabaner, M.C.; Duman, R.; Vurmaz, A.; Ertekin, T. Effects of topical prostaglandin drops on angiogenesis in an in ovo chick chorioallantoic membrane model. Cutan. Ocul. Toxicol. 2021, 40, 54–60. [Google Scholar] [CrossRef]
- Catalano, R.D.; Wilson, M.R.; Boddy, S.C.; Jabbour, H.N. Comprehensive expression analysis of prostanoid enzymes and receptors in the human endometrium across the menstrual cycle. Mol. Hum. Reprod. 2011, 17, 182–192. [Google Scholar] [CrossRef]
- Su, E.J.; Ernst, L.; Abdallah, N.; Chatterton, R.; Xin, H.; Monsivais, D.; Coon, J.; Bulun, S.E. Estrogen receptor-β and fetoplacental endothelial prostanoid biosynthesis: A link to clinically demonstrated fetal growth restriction. J. Clin. Endocrinol. Metab. 2011, 96, E1558–E1567. [Google Scholar] [CrossRef]
- Byrns, M.C. Role of aldo-keto reductase enzymes in mediating the timing of parturition. Front. Pharmacol. 2011, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, A.J.; Adeniji, A.O.; Chen, M.; Byrns, M.C.; Jin, Y.; Christianson, D.W.; Marnett, L.J.; Penning, T.M. Development of Potent and Selective Indomethacin Analogues for the Inhibition of AKR1C3 (Type 5 17β-Hydroxysteroid Dehydrogenase/Prostaglandin F Synthase) in Castrate-Resistant Prostate Cancer. J. Med. Chem. 2013, 56, 2429–2446. [Google Scholar] [CrossRef] [PubMed]
- Baryla, M.; Kaczynski, P.; Goryszewska, E.; Riley, S.C.; Waclawik, A. Prostaglandin F2α stimulates adhesion, migration, invasion and proliferation of the human trophoblast cell line HTR-8/SVneo. Placenta 2019, 77, 19–29. [Google Scholar] [CrossRef]
- Janabi, N.; Chabrier, S.; Tardieu, M. Endogenous nitric oxide activates prostaglandin F2 alpha production in human microglial cells but not in astrocytes: A study of interactions between eicosanoids, nitric oxide, and superoxide anion (O2−) regulatory pathways. J. Immunol. 1996, 157, 2129–2135. [Google Scholar] [CrossRef]
- Maan, Z.N.; Hu, M.S.; Rennert, R.; Barrera, J.A.; Duscher, D.; Januszyk, M.; Whittam, A.; Padmanabhan, J.; Vial, N.; Ho, N.; et al. Endothelial CXCL12 regulates neovascularization during tissue repair and tumor progression. bioRxiv 2020. [Google Scholar] [CrossRef]
- Spencer, H.L.; Jover, E.; Cathery, W.; Avolio, E.; Rodriguez-Arabaolaza, I.; Thomas, A.C.; Alvino, V.V.; Sala-Newby, G.; Dang, Z.; Fagnano, M.; et al. Role of TPBG (Trophoblast Glycoprotein) Antigen in Human Pericyte Migratory and Angiogenic Activity. Arter. Thromb. Vasc. Biol. 2019, 39, 1113–1124. [Google Scholar] [CrossRef]
- McGinn, O.J.; Marinov, G.; Sawan, S.; Stern, P.L. CXCL12 receptor preference, signal transduction, biological response and the expression of 5T4 oncofoetal glycoprotein. J. Cell Sci. 2012, 125, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Tesio, M.; Golan, K.; Corso, S.; Giordano, S.; Schajnovitz, A.; Vagima, Y.; Shivtiel, S.; Kalinkovich, A.; Caione, L.; Gammaitoni, L.; et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 2011, 117, 419–428. [Google Scholar] [CrossRef]
- Cheng, Y.; Peng, L.; Deng, X.; Li, T.; Guo, H.; Xu, C.; Fang, T.; Liu, X.; Sun, B.; Chen, L. Prostaglandin F2α protects against pericyte apoptosis by inhibiting the PI3K/Akt/GSK3β/β-catenin signaling pathway. Ann. Transl. Med. 2021, 9, 1021. [Google Scholar] [CrossRef]
- Peng, L.; Sun, B.; Liu, M.; Huang, J.; Liu, Y.; Xie, Z.; He, J.; Chen, L.; Wang, D.; Zhu, Y.; et al. Plasma metabolic profile reveals PGF2α protecting against non-proliferative diabetic retinopathy in patients with type 2 diabetes. Biochem. Biophys. Res. Commun. 2018, 496, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Li, D.-J.; Li, M.-Q. CXCL12 in normal and pathological pregnancies: A review. Am. J. Reprod. Immunol. 2020, 84, e13280. [Google Scholar] [CrossRef]
- Roboon, J.; Hattori, T.; Ishii, H.; Takarada-Iemata, M.; Le, T.M.; Shiraishi, Y.; Ozaki, N.; Yamamoto, Y.; Sugawara, A.; Okamoto, H.; et al. Deletion of CD38 Suppresses Glial Activation and Neuroinflammation in a Mouse Model of Demyelination. Front. Cell. Neurosci. 2019, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Vaisitti, T.; Aydin, S.; Rossi, D.; Cottino, F.; Bergui, L.; D’Arena, G.; Bonello, L.; Horenstein, A.L.; Brennan, P.; Pepper, C.; et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010, 24, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Dong, J.; Dong, C.; Tian, C.; Serrero, G. Identification of Prostaglandin F2 Receptor Negative Regulator (PTGFRN) as an internalizable target in cancer cells for antibody-drug conjugate development. PLoS ONE 2021, 16, e0246197. [Google Scholar] [CrossRef]
- Aguila, B.; Morris, A.B.; Spina, R.; Bar, E.; Schraner, J.; Vinkler, R.; Sohn, J.W.; Welford, S.M. The Ig superfamily protein PTGFRN coordinates survival signaling in glioblastoma multiforme. Cancer Lett. 2019, 462, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mala, U.; Baral, T.K.; Somasundaram, K. Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene. BMC Cancer 2022, 22, 642. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-M.; Chen, Q.-D.; Wei, G.-N.; Wei, J.; Yin, J.-X.; He, J.-H.; Ge, X.; Shi, Z.-M. Hypoxia-Induced miR-137 Inhibition Increased Glioblastoma Multiforme Growth and Chemoresistance through LRP6. Front. Oncol. 2021, 10, 611699. [Google Scholar] [CrossRef]
- Abbattista, M.R.; Ashoorzadeh, A.; Guise, C.P.; Mowday, A.M.; Mittra, R.; Silva, S.; Hicks, K.O.; Bull, M.R.; Jackson-Patel, V.; Lin, X.; et al. Restoring Tumour Selectivity of the Bioreductive Prodrug PR-104 by Developing an Analogue Resistant to Aerobic Metabolism by Human Aldo-Keto Reductase 1C3. Pharmaceuticals 2021, 14, 1231. [Google Scholar] [CrossRef]
- Guise, C.P.; Abbattista, M.R.; Singleton, R.S.; Holford, S.D.; Connolly, J.; Dachs, G.U.; Fox, S.B.; Pollock, R.; Harvey, J.; Guilford, P.; et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010, 70, 1573–1584. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Peng, J.; Dou, Y.; Sun, J.; Ma, C.; Wang, Q.; Zhang, L.; Luo, X.; Kong, B.; Zhang, Y.; et al. Macrophage ERα promoted invasion of endometrial cancer cell by mTOR/KIF5B-mediated epithelial to mesenchymal transition. Immunol. Cell Biol. 2019, 97, 563–576. [Google Scholar] [CrossRef]
- Kumar, P.; Dhar, P. Preliminary Evidence of the role of estrogen and tamoxifen-induced regulation of complement proteins in rat hippocampus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Motta, E.; Nanvuma, C.; Kuhrt, L.D.; Yuan, Y.; Xia, P.; Lubas, M.; Zhu, S.; Schnauss, M.; Qazi, N.; et al. Microglia/macrophage-derived human CCL18 promotes glioma progression via CCR8-ACP5 axis analyzed in humanized slice model. Cell Rep. 2022, 39, 110670. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Chen, N.; Alieva, M.; van der Most, T.; Klazen, J.A.Z.; Vollmann-Zwerenz, A.; Hau, P.; Vrisekoop, N. Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy. Cells 2022, 11, 2196. [Google Scholar] [CrossRef]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef] [PubMed]
- Loynes Catherine, A.; Lee Jou, A.; Robertson Anne, L.; Steel Michael, J.G.; Ellett, F.; Feng, Y.; Levy Bruce, D.; Whyte Moira, K.B.; Renshaw Stephen, A. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4, eaar8320. [Google Scholar] [CrossRef]
- Lamy, J.; Nogues, P.; Combes-Soia, L.; Tsikis, G.; Labas, V.; Mermillod, P.; Druart, X.; Saint-Dizier, M. Identification by proteomics of oviductal sperm-interacting proteins. Reproduction 2018, 155, 457–466. [Google Scholar] [CrossRef]
- Borgmann, J.; Tüttelmann, F.; Dworniczak, B.; Röpke, A.; Song, H.-W.; Kliesch, S.; Wilkinson, M.F.; Laurentino, S.; Gromoll, J. The human RHOX gene cluster: Target genes and functional analysis of gene variants in infertile men. Hum. Mol. Genet. 2016, 25, 4898–4910. [Google Scholar] [CrossRef] [PubMed]
- Ezz, M.A.; Marey, M.A.; Elweza, A.E.; Kawai, T.; Heppelmann, M.; Pfarrer, C.; Balboula, A.Z.; Montaser, A.; Imakawa, K.; Zaabel, S.M.; et al. TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro. PLoS ONE 2019, 14, e0214516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Guo, Z.; Chen, X.; Liu, K.; Li, H.; Jia, W.; Wang, C.; Luo, F.; Ji, X.; Zhang, T.; et al. Excessive HSP70/TLR2 activation leads to remodeling of the tumor immune microenvironment to resist chemotherapy sensitivity of mFOLFOX in colorectal cancer. Clin. Immunol. 2022, 245, 109157. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.S.; Marey, M.A.; Hambruch, N.; Hayakawa, H.; Shimizu, T.; Hussien, H.A.; Abdel-Razek, A.-R.K.; Pfarrer, C.; Miyamoto, A. Sperm Binding to Oviduct Epithelial Cells Enhances TGFΒ1 and IL10 Expressions in Epithelial Cells as Well as Neutrophils In Vitro: Prostaglandin E2 As a Main Regulator of Anti-Inflammatory Response in the Bovine Oviduct. PLoS ONE 2016, 11, e0162309. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Sharkey, D.J.; Green, E.S.; Chan, H.Y.; Matias, R.A.; Moldenhauer, L.M.; Robertson, S.A. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun. Biol. 2021, 4, 572. [Google Scholar] [CrossRef]
- Mayoral Andrade, G.; Vásquez Martínez, G.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Zenteno, E.; Pérez-Campos Mayoral, E.; Martínez Cruz, M.; Martínez Cruz, R.; Matias-Cervantes, C.A.; Meraz Cruz, N.; et al. Molecules and Prostaglandins Related to Embryo Tolerance. Front. Immunol. 2020, 11, 555414. [Google Scholar] [CrossRef]
- Murphy, B.; Merant, C.; Schook, M.; Cook, F.; Brooks, S.; Horohov, D.; Fitzgerald, B. Equine Neutrophils Respond to PGE2 by Activating Expression of Core Circadian Clock Genes. Open Vet. Sci. J. 2008, 2, 96–103. [Google Scholar] [CrossRef]
- Suyama, K.; Silagi, E.S.; Choi, H.; Sakabe, K.; Mochida, J.; Shapiro, I.M.; Risbud, M.V. Circadian factors BMAL1 and RORα control HIF-1α transcriptional activity in nucleus pulposus cells: Implications in maintenance of intervertebral disc health. Oncotarget 2016, 7, 23056–23071. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Engström, C.; Fagman, J.B.; Smedh, U.; Lundholm, K.; Iresjö, B.M. Reduced tumor growth in EP2 knockout mice is related to signaling pathways favoring an increased local anti-tumor immunity in the tumor stroma. Oncol. Rep. 2022, 47, 118. [Google Scholar] [CrossRef]
- Reading, J.L.; Vaes, B.; Hull, C.; Sabbah, S.; Hayday, T.; Wang, N.S.; DiPiero, A.; Lehman, N.A.; Taggart, J.M.; Carty, F.; et al. Suppression of IL-7-dependent Effector T-cell Expansion by Multipotent Adult Progenitor Cells and PGE2. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Chu, Y.Y.; Narumiya, S.; Chi, J.Y.; Furuyashiki, T.; Aoki, T.; Wang, S.M.; Chang, W.C.; Wang, J.M. CCAAT/enhancer-binding protein delta/miR135a/thrombospondin 1 axis mediates PGE2-induced angiogenesis in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1356–1368. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017, 1, 3–9. [Google Scholar] [CrossRef]
- Memon, N.; Griffin, I.J.; Lee, C.W.; Herdt, A.; Weinberger, B.I.; Hegyi, T.; Carayannopoulos, M.O.; Aleksunes, L.M.; Guo, G.L. Developmental regulation of the gut-liver (FGF19-CYP7A1) axis in neonates. J. Matern Fetal Neonatal Med. 2020, 33, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zheng, L.M.; Sun, D.K.; Li, X.F.; Xu, P.; Tian, L.Q. miR-204 functions as a tumor suppressor gene, at least partly by suppressing CYP27A1 in glioblastoma. Oncol. Lett. 2018, 16, 1439–1448. [Google Scholar] [CrossRef]
- Perri, P.; Ponzoni, M.; Corrias, M.V.; Ceccherini, I.; Candiani, S.; Bachetti, T. A Focus on Regulatory Networks Linking MicroRNAs, Transcription Factors and Target Genes in Neuroblastoma. Cancers 2021, 13, 5528. [Google Scholar] [CrossRef]
- He, S.; Ma, L.; Baek, A.E.; Vardanyan, A.; Vembar, V.; Chen, J.J.; Nelson, A.T.; Burdette, J.E.; Nelson, E.R. Host CYP27A1 expression is essential for ovarian cancer progression. Endocr. Relat. Cancer 2019, 26, 659–675. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.Y.; Xing, Y.; Wang, X.Y.; Wang, Y. The oncogenic roles of 27-hydroxycholesterol in glioblastoma. Oncol. Lett. 2019, 18, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, Z.; Ren, H.; Peng, X.; Wu, A.; Ma, D.; Liu, G.; Liu, L. Twenty Metabolic Genes Based Signature Predicts Survival of Glioma Patients. J. Cancer 2020, 11, 441–449. [Google Scholar] [CrossRef]
- Baptissart, M.; Vega, A.; Martinot, E.; Pommier, A.J.; Houten, S.M.; Marceau, G.; Haze, A.d.; Baron, S.; Schoonjans, K.; Lobaccaro, J.-M.A.; et al. Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 2014, 60, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Martinot, E.; Sèdes, L.; Baptissart, M.; Holota, H.; Rouaisnel, B.; Damon-Soubeyrand, C.; De Haze, A.; Saru, J.-P.; Thibault-Carpentier, C.; Keime, C.; et al. The Bile Acid Nuclear Receptor FXRα Is a Critical Regulator of Mouse Germ Cell Fate. Stem Cell Rep. 2017, 9, 315–328. [Google Scholar] [CrossRef]
- Nagy, R.A.; Hollema, H.; Andrei, D.; Jurdzinski, A.; Kuipers, F.; Hoek, A.; Tietge, U.J.F. The Origin of Follicular Bile Acids in the Human Ovary. Am. J. Pathol. 2019, 189, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Zhao, S.; Tian, C.; Wang, P.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget 2016, 7, 83951–83963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad.Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Giotti, B.; Kaluzova, M.; Herting, C.; Pinero, G.; Vallcorba, M.P.; Cristea, S.; Ross, J.L.; Ackley, J.; Maximov, V.; et al. A paracrine circuit of IL-1β/IL-1R1 between myeloid and tumor cells drives glioblastoma progression. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Nozawa, K.; Fujishiro, M.; Kawasaki, M.; Takamori, K.; Ogawa, H.; Sekigawa, I.; Takasaki, Y. Estrogen inhibits apoptosis and promotes CC motif chemokine ligand 13 expression on synovial fibroblasts in rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2012, 34, 852–857. [Google Scholar] [CrossRef]
- Roychaudhuri, R.; Hergrueter, A.H.; Polverino, F.; Laucho-Contreras, M.E.; Gupta, K.; Borregaard, N.; Owen, C.A. ADAM9 Is a Novel Product of Polymorphonuclear Neutrophils: Regulation of Expression and Contributions to Extracellular Matrix Protein Degradation during Acute Lung Injury. J. Immunol. 2014, 193, 2469. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Rendón-Huerta, E.P.; Ortiz-Navarrete, V.; Montaño, L.F. CD38 and Regulation of the Immune Response Cells in Cancer. J. Oncol. 2021, 2021, 6630295. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; You, X.; Leimert, K.; Popowycz, K.; Fang, X.; Wood, S.L.; Slater, D.M.; Sun, Q.; Gu, H.; et al. PGF2α modulates the output of chemokines and pro-inflammatory cytokines in myometrial cells from term pregnant women through divergent signaling pathways. Mol. Hum. Reprod. 2015, 21, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, R57–R67. [Google Scholar] [CrossRef]
- Xu, C.; You, X.; Liu, W.; Sun, Q.; Ding, X.; Huang, Y.; Ni, X. Prostaglandin F2α regulates the expression of uterine activation proteins via multiple signalling pathways. Reproduction 2015, 149, 139–146. [Google Scholar] [CrossRef]
- Tirumurugaan, K.G.; Kang, B.N.; Panettieri, R.A.; Foster, D.N.; Walseth, T.F.; Kannan, M.S. Regulation of the cd38 promoter in human airway smooth muscle cells by TNF-alpha and dexamethasone. Respir. Res. 2008, 9, 26. [Google Scholar] [CrossRef]
- Lombardi, M.Y.; Assem, M. Glioblastoma Genomics: A Very Complicated Story. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications Copyright: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Carrasco-García, E.; Saceda, M.; Martínez-Lacaci, I. Role of Receptor Tyrosine Kinases and Their Ligands in Glioblastoma. Cells 2014, 3, 199–235. [Google Scholar] [CrossRef]
- Fan, Q.-W.; Cheng, C.; Knight, Z.A.; Haas-Kogan, D.; Stokoe, D.; James, C.D.; McCormick, F.; Shokat, K.M.; Weiss, W.A. EGFR Signals to mTOR Through PKC and Independently of Akt in Glioma. Sci. Signal. 2009, 2, ra4. [Google Scholar] [CrossRef]
- Aldaz, P.; Otaegi-Ugartemendia, M.; Saenz-Antoñanzas, A.; Garcia-Puga, M.; Moreno-Valladares, M.; Flores, J.M.; Gerovska, D.; Arauzo-Bravo, M.J.; Samprón, N.; Matheu, A.; et al. SOX9 promotes tumor progression through the axis BMI1-p21CIP. Sci. Rep. 2020, 10, 357. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; van Roermund, C.W.T.; Preece, M.A.; Koster, J.; Ebberink, M.S.; Waterham, H.R.; Wanders, R.J.A. A novel case of ACOX2 deficiency leads to recognition of a third human peroxisomal acyl-CoA oxidase. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hu, H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021, 9, 651317. [Google Scholar] [CrossRef]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M.; Wang, T. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, D.; Chen, Y.-J.; Xie, X.; Shi, Y.; Tabar, V.; Brennan, C.W.; Bale, T.A.; Jayewickreme, C.D.; Laks, D.R.; et al. Cell Lineage-Based Stratification for Glioblastoma. Cancer Cell 2020, 38, 366–379.e368. [Google Scholar] [CrossRef]
- Shai, R.; Shi, T.; Kremen, T.J.; Horvath, S.; Liau, L.M.; Cloughesy, T.F.; Mischel, P.S.; Nelson, S.F. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 2003, 22, 4918–4923. [Google Scholar] [CrossRef]
- Stevanovic, M.; Kovacevic-Grujicic, N.; Mojsin, M.; Milivojevic, M.; Drakulic, D. SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation. World J. Stem Cells 2021, 13, 1417–1445. [Google Scholar] [CrossRef] [PubMed]
- Rajender, S.; Rajani, V.; Gupta, N.J.; Chakravarty, B.; Singh, L.; Thangaraj, K. SRY-negative 46,XX male with normal genitals, complete masculinization and infertility. Mol. Hum. Reprod. 2006, 12, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, S.; Fleck, A.K.; Kirchberg, I.; Hucke, S.; Liebmann, M.; Klotz, L.; Kuhlmann, T. Activation of FXR pathway does not alter glial cell function. J. Neuroinflammation 2017, 14, 66. [Google Scholar] [CrossRef]
- Bennett, J.A.; Mesfin, F.B.; Andersen, T.T.; Gierthy, J.F.; Jacobson, H.I. A peptide derived from α-fetoprotein prevents the growth of estrogen-dependent human breast cancers sensitive and resistant to tamoxifen. Proc. Natl. Acad. Sci. 2002, 99, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Q.; Liu, K.; Dong, X.; Zhu, M.; Li, M. Alpha-Fetoprotein Binding Mucin and Scavenger Receptors: An Available Bio-Target for Treating Cancer. Front. Oncol. 2021, 11, 625936. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Xing, F.Q.; Sui, H.; Wang, Y.L.; Mai, X.F.; Luo, Z.Q.; Chen, X.Q.; Chen, G.H.; Kong, Z.J. CA 125 expression in cervical and vaginal secretions in women in normal reproductive period. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 173–175. [Google Scholar] [PubMed]
- Reinartz, S.; Pfisterer, J.; du Bois, A.; Jackisch, C.; Baumann, K.H.; Wagner, U. Suppressive activity rather than frequency of FoxP3(+) regulatory T cells is essential for CA-125-specific T-cell activation after abagovomab treatment. Hum. Immunol. 2010, 71, 36–44. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, G.; Bai, Y.; Tao, Y.; Wang, L.; Yang, L.; Wu, H.; Huang, F.; Shi, H.; Wu, X. Natural bear bile powder suppresses neuroinflammation in lipopolysaccharide-treated mice via regulating TGR5/AKT/NF-κB signaling pathway. J. Ethnopharmacol. 2022, 289, 115063. [Google Scholar] [CrossRef]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 2021, 12, 411–425. [Google Scholar] [CrossRef]
- Vonderohe, C.; Guthrie, G.; Stoll, B.; Chacko, S.; Dawson, H.; Burrin, D.G. Tissue-specific mechanisms of bile acid homeostasis and activation of FXR-FGF19 signaling in preterm and term neonatal pigs. Am. J. Physiol. Gastrointest Liver Physiol. 2022, 322, G117–G133. [Google Scholar] [CrossRef]
- Pandak, W.M.; Kakiyama, G. The acidic pathway of bile acid synthesis: Not just an alternative pathway(☆). Liver Res. 2019, 3, 88–98. [Google Scholar] [CrossRef]
- Gravendeel, L.A.M.; Kouwenhoven, M.C.M.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.C.; Kloosterhof, N.K.; et al. Intrinsic Gene Expression Profiles of Gliomas Are a Better Predictor of Survival than Histology. Cancer Res. 2009, 69, 9065. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401. [Google Scholar] [CrossRef]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.W.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro-Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, K.; Sun, Y.; Cao, Y.; Yang, J. Transcriptional mechanism of IRF8 and PU.1 governs microglial activation in neurodegenerative condition. Protein Cell 2019, 10, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Han, S.; Li, J.; Wang, H.; Liu, J.; Li, N.; Yang, X.; Shi, J.; Han, J.; Li, Y.; et al. IRF8 is the target of SIRT1 for the inflammation response in macrophages. Innate Immun 2017, 23, 188–195. [Google Scholar] [CrossRef]
- Yang, B.H.; Hagemann, S.; Mamareli, P.; Lauer, U.; Hoffmann, U.; Beckstette, M.; Föhse, L.; Prinz, I.; Pezoldt, J.; Suerbaum, S.; et al. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016, 9, 444–457. [Google Scholar] [CrossRef]
- Alvisi, G.; Brummelman, J.; Puccio, S.; Mazza, E.M.; Tomada, E.P.; Losurdo, A.; Zanon, V.; Peano, C.; Colombo, F.S.; Scarpa, A.; et al. IRF4 instructs effector Treg differentiation and immune suppression in human cancer. J. Clin. Investig. 2020, 130, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ji, N.; Gu, C.; Yao, J.; Ding, M.; Zhou, D.; Huang, M.; Zhang, M. IRF4 is Correlated with the Conversion to a Th17-Like Phenotype in Regulatory T Cells from the Malignant Pleural Effusion. Int. J. Gen Med. 2021, 14, 6009–6019. [Google Scholar] [CrossRef] [PubMed]
- Cretney, E.; Xin, A.; Shi, W.; Minnich, M.; Masson, F.; Miasari, M.; Belz, G.T.; Smyth, G.K.; Busslinger, M.; Nutt, S.L.; et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011, 12, 304–311. [Google Scholar] [CrossRef]
- Tripathi, A.; Singh Rawat, B.; Addya, S.; Surjit, M.; Tailor, P.; Vrati, S.; Banerjee, A. Lack of Interferon (IFN) Regulatory Factor 8 Associated with Restricted IFN-γ Response Augmented Japanese Encephalitis Virus Replication in the Mouse Brain. J. Virol. 2021, 95, e0040621. [Google Scholar] [CrossRef]
- Wang, S.; Ren, D.; Kaushik, A.-L.; Matherat, G.; Lécluse, Y.; Filipp, D.; Vainchenker, W.; Raslova, H.; Plo, I.; Godin, I. Lyl-1 regulates primitive macrophages and microglia development. bioRxiv 2021. [Google Scholar] [CrossRef]
- Minten, C.; Terry, R.; Deffrasnes, C.; King, N.J.C.; Campbell, I.L. IFN Regulatory Factor 8 Is a Key Constitutive Determinant of the Morphological and Molecular Properties of Microglia in the CNS. PLoS ONE 2012, 7, e49851. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, S.; Johnson, S.; Rosin, V.; Lee, J.; Colomb, E.; Witt, R.; Jaworski, A.; Weiss, S.J.; Si, M.S. Tissue-specific angiogenic and invasive properties of human neonatal thymus and bone MSCs: Role of SLIT3-ROBO1. Stem Cells Transl. Med. 2020, 9, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-x.; Geng, J.-g. Over-expression of Slit2 induces vessel formation and changes blood vessel permeability in mouse brain. Acta Pharmacol. Sin. 2011, 32, 1327–1336. [Google Scholar] [CrossRef]
- Yoshida, H.; Taguchi, H.; Kitahara, T.; Takema, Y.; Visscher, M.O.; Schweizer, J.; Langbein, L. Keratins of the human occipital hair medulla: Androgenic regulation of in vitro hair keratin K37 expression. Br. J. Dermatol. 2013, 169, 218–221. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharpe, M.A.; Baskin, D.S.; Johnson, R.D.; Baskin, A.M. Acquisition of Immune Privilege in GBM Tumors: Role of Prostaglandins and Bile Salts. Int. J. Mol. Sci. 2023, 24, 3198. https://doi.org/10.3390/ijms24043198
Sharpe MA, Baskin DS, Johnson RD, Baskin AM. Acquisition of Immune Privilege in GBM Tumors: Role of Prostaglandins and Bile Salts. International Journal of Molecular Sciences. 2023; 24(4):3198. https://doi.org/10.3390/ijms24043198
Chicago/Turabian StyleSharpe, Martyn A., David S. Baskin, Ryan D. Johnson, and Alexandra M. Baskin. 2023. "Acquisition of Immune Privilege in GBM Tumors: Role of Prostaglandins and Bile Salts" International Journal of Molecular Sciences 24, no. 4: 3198. https://doi.org/10.3390/ijms24043198





