Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mucilage Polysaccharides Fractionation
2.2. Molecular Dimensions
2.3. Monosaccharide Composition and Content
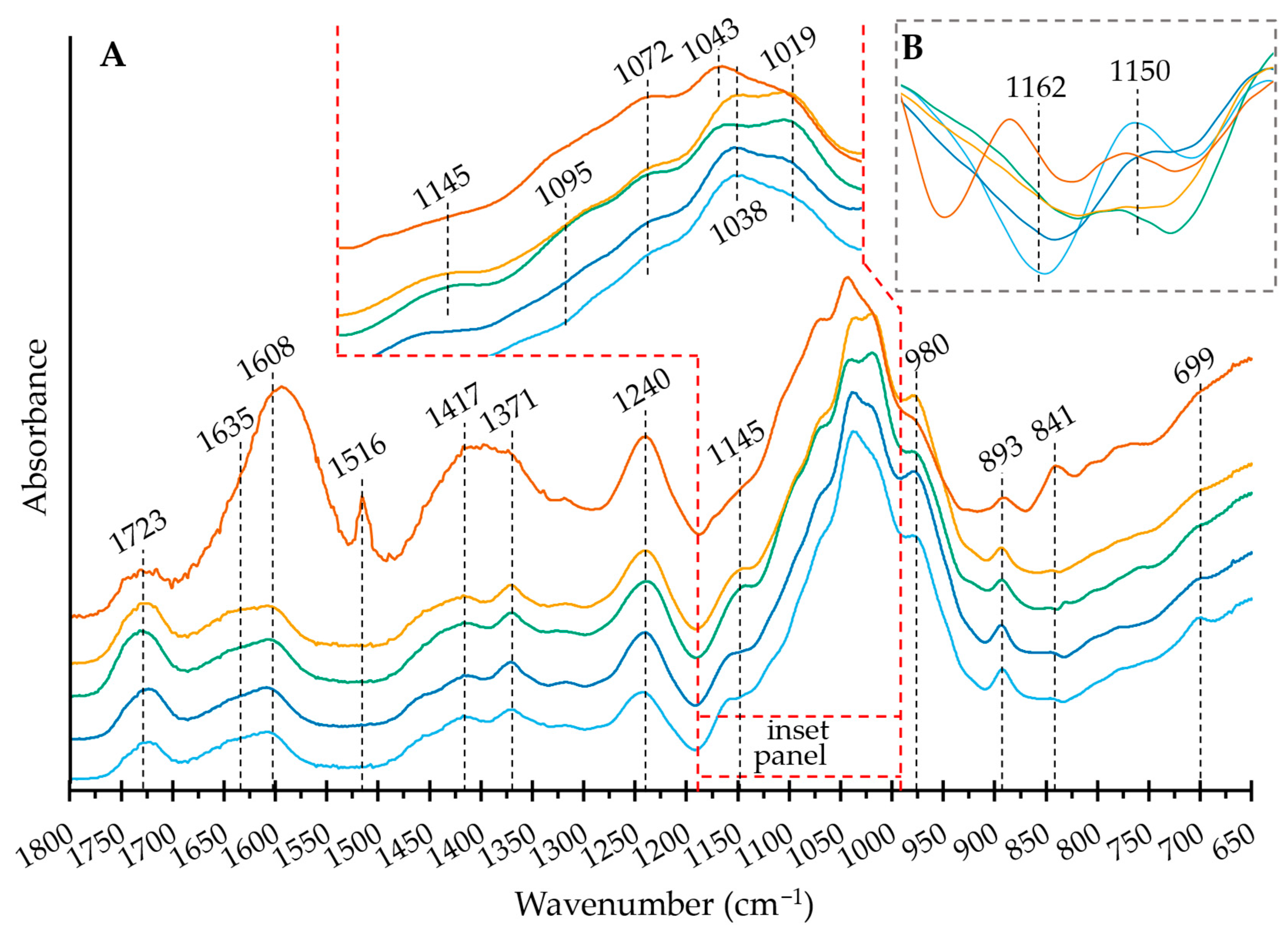
2.4. Structural Features: Fourier-Transform Infrared Spectroscopy (FT IR) Spectroscopy—SEC-UV/VIS
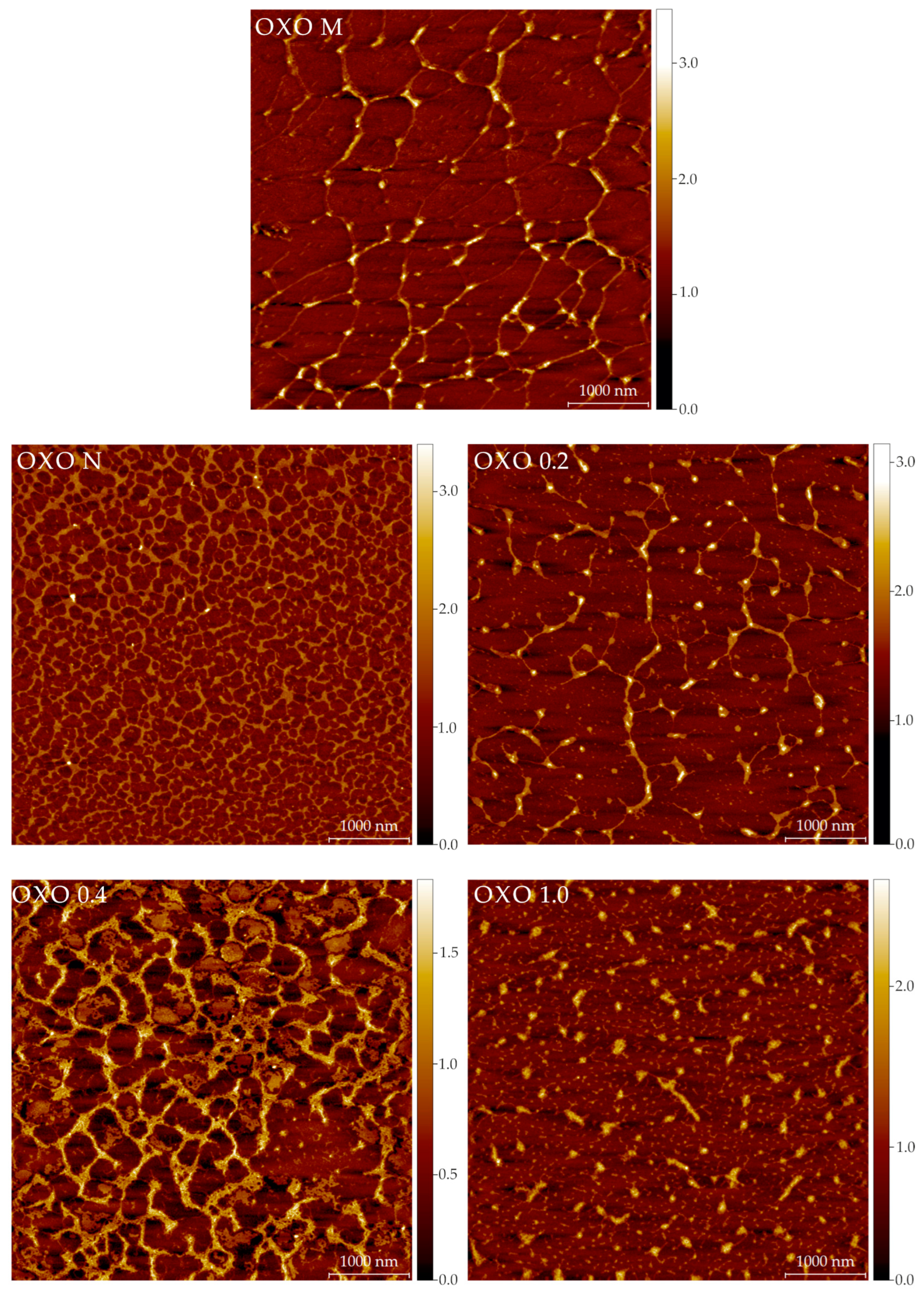
2.5. Structural Features: Atomic Force Microscopy (AFM) Imaging
2.6. Prebiotic Potential
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals, Carbohydrate Standards and Intestinal Bacterial Species
3.3. Mucilage Extraction
3.4. Mucilage Fractionation by Preparative Ion Exchange Chromatography
3.5. Preparative Size Exclusion Chromatography
3.6. Analytical Size Exclusion Chromatography
3.7. Methanolysis—Trifluoroacetic Acid Hydrolysis
3.8. Monosaccharide Determination by High Performance Anion Exchange Chromatography—Pulsed Amperometric Detection (HPAEC-PAD)
3.9. Total Carbohydrate Content
3.10. FT IR Spectroscopy
3.11. Atomic Force Microscopy Imaging
Image Analysis
3.12. Evaluation of the Prebiotic Potential
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

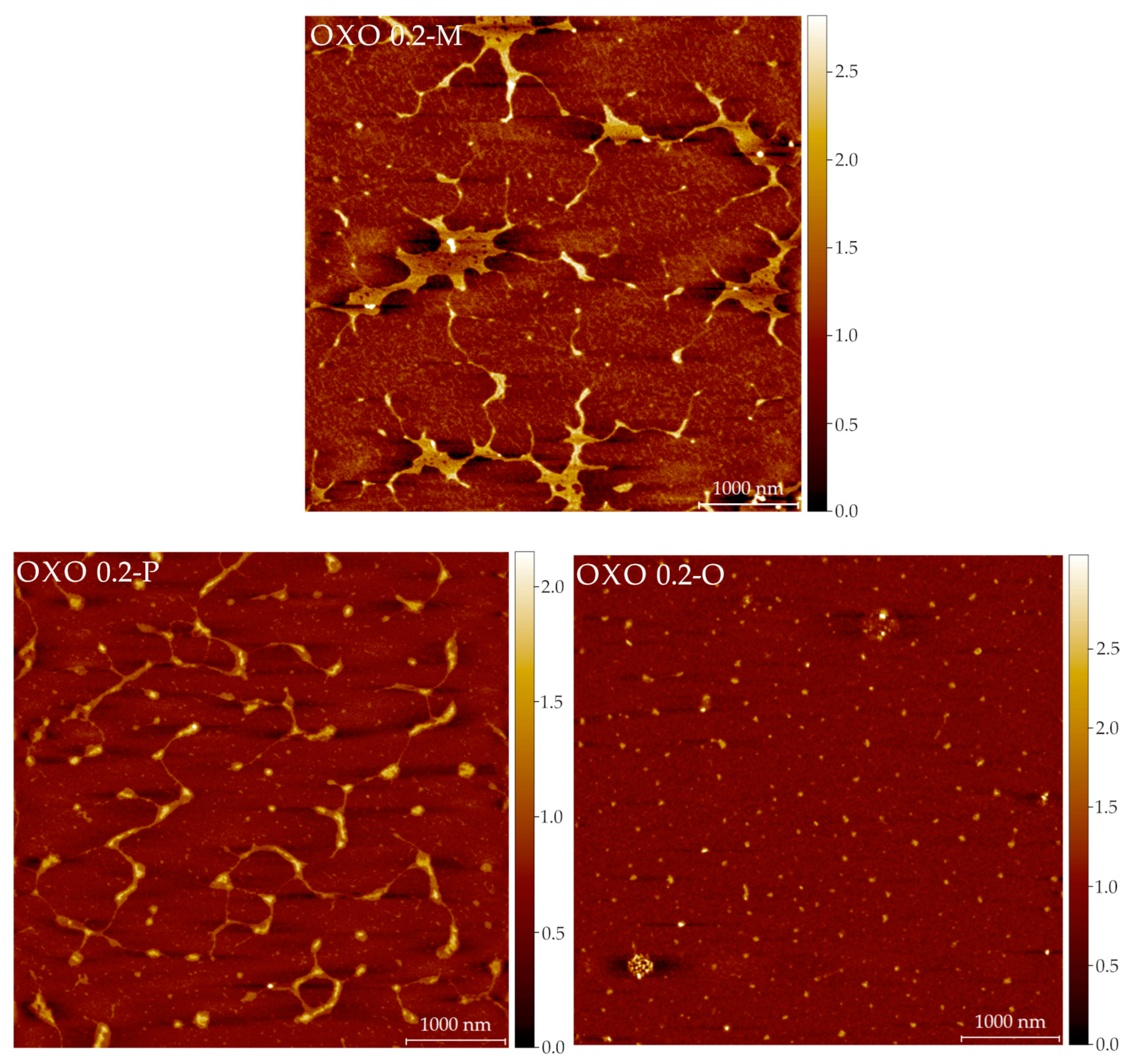
| Sample | Fibers | |||
| Fiber Length | Skeleton Length | Mean Height | Elongation | |
| (nm) | (nm) | (nm) | ||
| OXO M | 546 ± 27.8 | 761 ± 67.7 | 0.33 ± 0.0 | 0.59 ± 0.1 |
| OXO N | 365 ± 7.6 | 554 ± 30.1 | 0.44 ± 0.0 | 0.47 ± 0.0 |
| OXO 0.2 | 436 ± 0.2 | 564 ± 21.6 | 0.40 ± 0.0 | 0.52 ± 0.0 |
| OXO 0.4 | 438 ± 11.6 | 773 ± 39.7 | 0.41 ± 0.0 | 0.43 ± 0.0 |
| OXO 1.0 | 423 ± 20.6 | 1062 ± 157.9 | 0.45 ± 0.0 | 0.48 ± 0.0 |
| Aggregates | ||||
| Mean Height | Perimeter | Area | Volume | |
| (nm) | (nm) | (nm2) | (nm3) | |
| OXO M | 0.80 ± 0.0 | 195.4 ± 2.7 | 3353 ± 3598 | 5752 ± 1713 |
| OXO N | n.a. | n.a. | n.a. | n.a. |
| OXO 0.2 | 0.42 ± 0.0 | 208.7 ± 2.2 | 3948 ± 103 | 3382 ± 336 |
| OXO 0.4 | n.a | n.a | n.a | n.a |
| OXO 1.0 | 0.49 ± 0.0 | 188.5 ± 2.5 | 3503 ± 3738 | 2785 ± 335 |
References
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than Just a Coating: Ecological Importance, Taxonomic Occurrence and Phylogenetic Relationships of Seed Coat Mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Gregory, M.; Baas, P. A Survey of Mucilage Cells in Vegetative Organs of the Dicotyledons. Isr. J. Bot. 1989, 38, 125–174. [Google Scholar] [CrossRef]
- Galloway, A.F.; Knox, P.; Krause, K. Sticky Mucilages and Exudates of Plants: Putative Microenvironmental Design Elements with Biotechnological Value. New Phytol. 2020, 225, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, C.L.; van Wyk, A.E. Structure of Mucilaginous Epidermal Cell Walls in Passerina (Thymelaeaceae). Bot. J. Linn. Soc. 1999, 129, 223–238. [Google Scholar] [CrossRef]
- Mastroberti, A.A.; de Araujo Mariath, J.E. Development of Mucilage Cells of Araucaria angustifolia (Araucariaceae). Protoplasma 2008, 232, 233–245. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mohamed, A.H. Strategies for Drought Tolerance in Xerophytes. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 269–293. ISBN 9789811521560. [Google Scholar]
- Zhang, R.; Chen, Y.; Yang, L.; Xie, Y.; Yan, W.; He, Z. Study on the Relationship between Mucilage Cells and Low Temperature Tolerance of Sedum Aizoon L. IOP Conf. Ser. Earth Environ. Sci. 2020, 461, 012055. [Google Scholar] [CrossRef]
- Orozco-Segovia, A.; Márquez-Guzmán, J.; Sánchez-Coronado, M.E.; Gamboa de Buen, A.; Baskin, J.M.; Baskin, C.C. Seed Anatomy and Water Uptake in Relation to Seed Dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae). Ann. Bot. 2007, 99, 581–592. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Fahn, A. The Mucilage Cells of Opuntia ficus-indica (L.) Mill.-Development, Ultrastructure, and Mucilage Secretion. Bot. Gaz. 1981, 142, 206–213. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Mayer, A.M. Calcium Oxalate Crystals in Opuntia ficus indica (L.) Mill.: Development and Relation to Mucilage Cells—A Stereological Analysis. Protoplasma 1981, 109, 271–283. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; Trejo, C.; Arroyo-Peña, V.B.; Sánchez Urdaneta, A.B.; Balois Morales, R. Diversity of Unavailable Polysaccharides and Dietary Fiber in Domesticated Nopalito and Cactus Pear Fruit (Opuntia Spp.). Chem. Biodivers. 2012, 9, 1599–1610. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical Composition and Phenolic Compounds Profile of Cladodes from Opuntia Spp. Cultivars with Different Domestication Gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- López-Palacios, C.; Peña-Valdivia, C.B.; Reyes-Agüero, J.A.; Rodríguez-Hernández, A.I. Effects of Domestication on Structural Polysaccharides and Dietary Fiber in Nopalitos (Opuntia Spp.). Genet. Resour. Crop Evol. 2012, 59, 1015–1026. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Loeppert, R.; Viernstein, H.; Praznik, W. The Effect of Cladode Drying Techniques on the Prebiotic Potential and Molecular Characteristics of the Mucilage Extracted from Opuntia ficus-indica and Opuntia joconostle. Sci. Pharm. 2020, 88, 43. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Viernstein, H.; Loeppert, R.; Praznik, W. Prebiotic Potential and Chemical Characterization of the Poly and Oligosaccharides Present in the Mucilage of Opuntia ficus-indica and Opuntia joconostle. Food Chem. 2021, 362, 130167. [Google Scholar] [CrossRef]
- Guevara-Arauza, J.C.; de Jesús Ornelas-Paz, J.; Pimentel-González, D.J.; Rosales Mendoza, S.; Soria Guerra, R.E.; Paz Maldonado, L.M.T. Prebiotic Effect of Mucilage and Pectic-Derived Oligosaccharides from Nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Kacčuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Okra Pectin Contains an Unusual Substitution of Its Rhamnosyl Residues with Acetyl and Alpha-Linked Galactosyl Groups. Carbohydr. Res. 2009, 344, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Proctor, A. Determination of Pectin Degree of Esterification by Diffuse Reflectance Fourier Transform Infrared Spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and Quantitative Assessment of Water Sorption in Natural Fibres Using ATR-FTIR Spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacio, S.; Aitkenhead, M.; Escudero, A.; Montserrat-Martí, G.; Maestro, M.; Robertson, A.H.J. Gypsophile Chemistry Unveiled: Fourier Transform Infrared (FTIR) Spectroscopy Provides New Insight into Plant Adaptations to Gypsum Soils. PLoS ONE 2014, 9, e107285. [Google Scholar] [CrossRef] [PubMed]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef] [PubMed]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the Contribution of ATR-FTIR Spectroscopy to Characterize Plant Cell Wall Polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Sene, C.F.B.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy (An Investigation of Five Higher Plant Cell Walls and Their Components). Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [CrossRef]
- Ravcheev, D.A.; Godzik, A.; Osterman, A.L.; Rodionov, D.A. Polysaccharides Utilization in Human Gut Bacterium Bacteroides Thetaiotaomicron: Comparative Genomics Reconstruction of Metabolic and Regulatory Networks. BMC Genom. 2013, 14, 873. [Google Scholar] [CrossRef]
- Centanni, M.; Hutchison, J.C.; Carnachan, S.M.; Daines, A.M.; Kelly, W.J.; Tannock, G.W.; Sims, I.M. Differential Growth of Bowel Commensal Bacteroides Species on Plant Xylans of Differing Structural Complexity. Carbohydr. Polym. 2017, 157, 1374–1382. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Hazra, A. Using the Confidence Interval Confidently. J. Thorac. Dis. 2017, 9, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menges, F. Spectragryph—Optical Spectroscopy Software. 2022. Version 1.2.16.1. Available online: http://www.effemm2.de/spectragryph/ (accessed on 17 October 2022).
- Cybulska, J.; Halaj, M.; Cepák, V.; Lukavský, J.; Capek, P. Nanostructure Features of Microalgae Biopolymer. Starch-Stärke 2016, 68, 629–636. [Google Scholar] [CrossRef]
- Khalighi, A.; Behdani, R.; Kouhestani, S.; Khalighi, A.; Behdani, R.; Kouhestani, S. Probiotics: A Comprehensive Review of Their Classification, Mode of Action and Role in Human Nutrition; IntechOpen: London, UK, 2016; ISBN 978-953-51-2476-4. [Google Scholar]

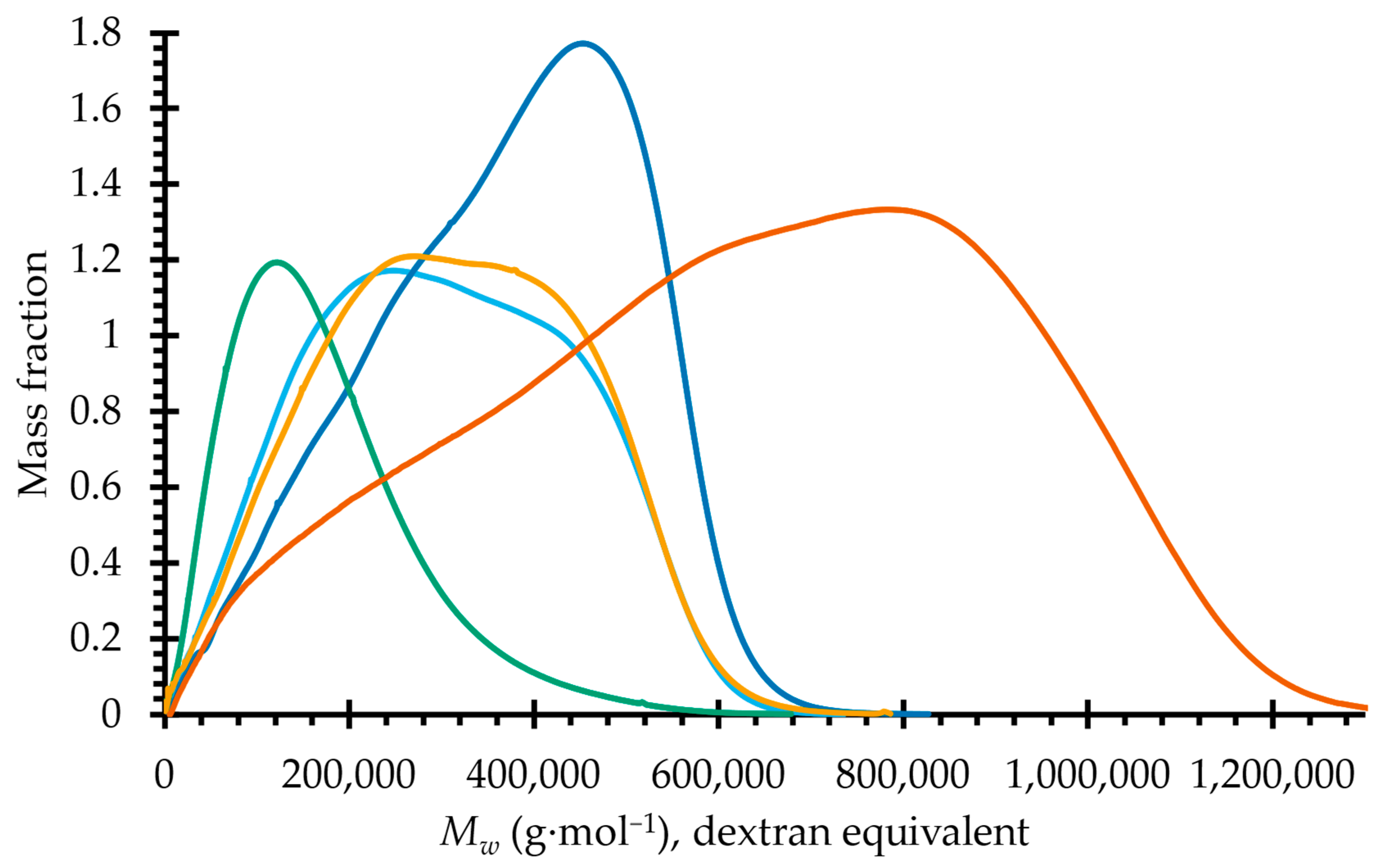

| Sample | Yield | Ð3 | Distribution | ||
|---|---|---|---|---|---|
| %w/w | (g∙mol−1) | (g∙mol−1) | (g∙mol−1) | ||
| OXO M [14] | 100.0 | 4.5 × 105 | 1.3 × 105 | 3.40 | 5.5 × 103–1.3 × 106 |
| OXO N | 8.8 | 2.1 × 105 | 7.9 × 104 | 2.68 | 2.5 × 103–7.4 × 105 |
| OXO 0.2 | 44.8 | 2.8 × 105 | 1.1 × 105 | 2.41 | 3.0 × 103–8.3 × 105 |
| OXO 0.4 | 36.8 | 1.1 × 105 | 3.9 × 104 | 2.86 | 9.3 × 102–6.8 × 103 |
| OXO 1.0 | 6.4 | 2.2 × 105 | 5.7 × 104 | 3.77 | 1.2 × 103–7.9 × 105 |
| Sample | Rha | Ara | Xyl | Gal | Fuc | Glc | GalA | Carbohydrate Content |
|---|---|---|---|---|---|---|---|---|
| (%mol) 1 | (%mol) 1 | (%mol) 1 | (%mol) 1 | (%mol) 1 | (%mol) 1 | (%mol) 1 | (%) | |
| OXO M [14] | 3.4 ± 0.2 | 28.4 ± 0.5 | 16.7 ± 1.7 | 33.6 ± 0.2 | n.d. | 2.3 ± 0.5 | 15.5 ± 1.7 | 81.2 ± 0.1 |
| OXO N | 4.3 ± 0.1 | 18.6 ± 0.6 | 10.6 ± 0.3 | 62.7 ± 2.8 | 1.8 ± 0.0 | 1.9 ± 0.5 | n.d. | 93.5 ± 0.1 |
| OXO 0.2 | 8.3 ± 0.2 | 33.6 ± 0.9 | 20.1 ± 0.4 | 27.1 ± 1.9 | 0.8 ± 0.1 | 0.7 ± 0.1 | 9.6 ± 0.1 | 91.8 ± 0.1 |
| OXO 0.4 | 8.3 ± 0.5 | 26.5 ± 1.0 | 13.4 ± 0.2 | 25.3 ± 3.2 | 1.4 ± 0.4 | 0.5 ± 0.2 | 24.6 ± 0.1 | 92.1 ± 0.1 |
| OXO 1.0 | 8.3 ± 0.4 | 23.0 ± 3.0 | 11.9 ± 0.5 | 20.5 ± 1.2 | 0.6 ± 0.4 | 1.1 ± 0.4 | 34.6 ± 0.6 | 91.3 ± 0.1 |
| OXO M 2 | 7.9 | 28.8 | 16.1 | 29.2 | 1.1 | 0.8 | 16.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Rubio, J.M.; Riva, A.; Cybulska, J.; Zdunek, A.; Berry, D.; Loeppert, R.; Viernstein, H.; Praznik, W.; Maghuly, F. Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential. Int. J. Mol. Sci. 2023, 24, 3208. https://doi.org/10.3390/ijms24043208
Cruz-Rubio JM, Riva A, Cybulska J, Zdunek A, Berry D, Loeppert R, Viernstein H, Praznik W, Maghuly F. Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential. International Journal of Molecular Sciences. 2023; 24(4):3208. https://doi.org/10.3390/ijms24043208
Chicago/Turabian StyleCruz-Rubio, José Manuel, Alessandra Riva, Justyna Cybulska, Artur Zdunek, David Berry, Renate Loeppert, Helmut Viernstein, Werner Praznik, and Fatemeh Maghuly. 2023. "Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential" International Journal of Molecular Sciences 24, no. 4: 3208. https://doi.org/10.3390/ijms24043208
APA StyleCruz-Rubio, J. M., Riva, A., Cybulska, J., Zdunek, A., Berry, D., Loeppert, R., Viernstein, H., Praznik, W., & Maghuly, F. (2023). Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential. International Journal of Molecular Sciences, 24(4), 3208. https://doi.org/10.3390/ijms24043208










