Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase
Abstract
1. Introduction
2. Results
2.1. Evidence for Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ
2.2. Protein Complex between the Purified SOD1 and YWHAE or YWHAZ
2.3. Impacts of SOD1 Mutations and Oxidative Stress on Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ
2.4. Impacts of Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ on SOD1 Activity
2.5. Impacts of Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ on Their Protein Stability
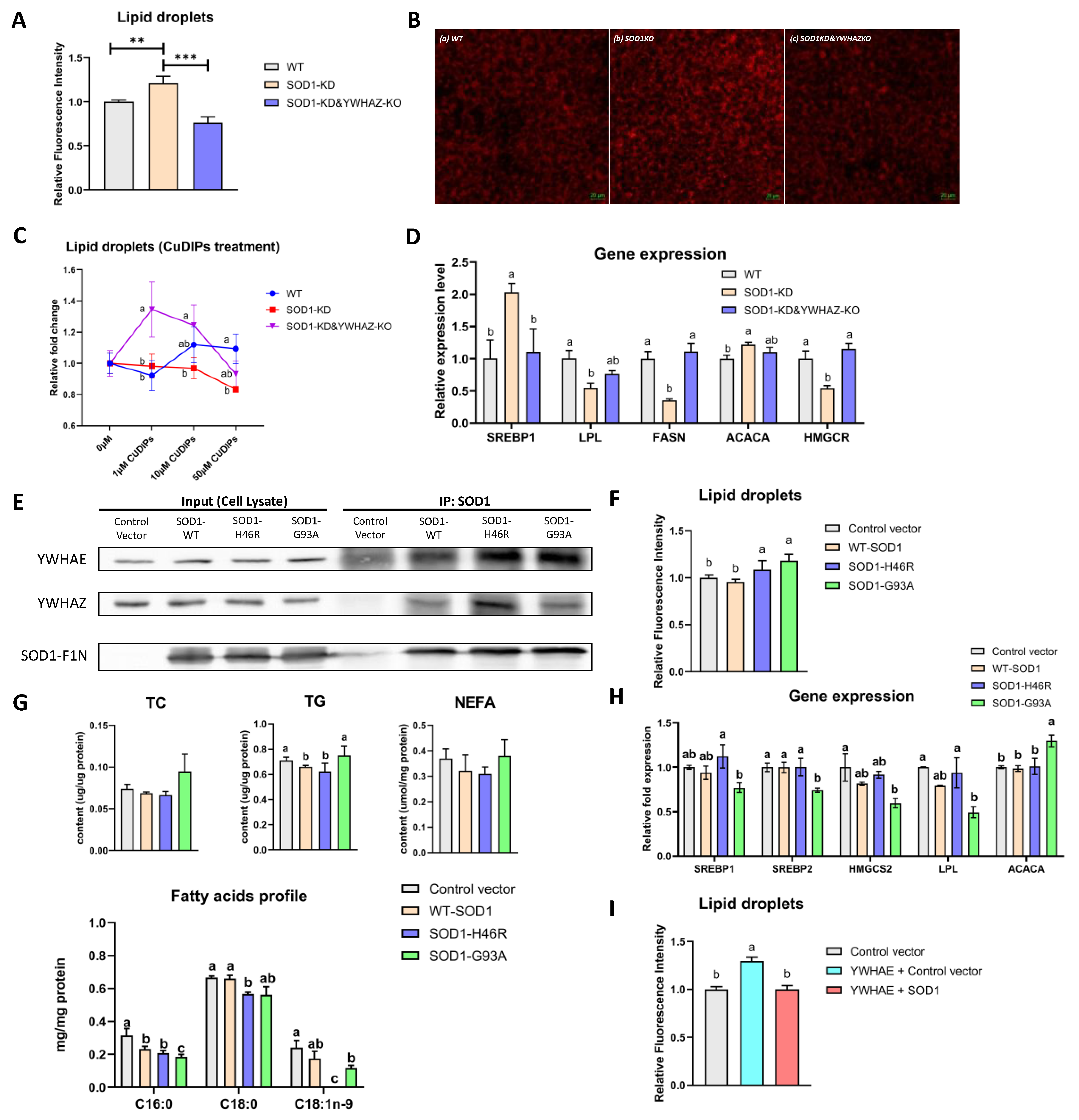
2.6. Impacts of Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ on Lipid Metabolism and Relative Gene Expression
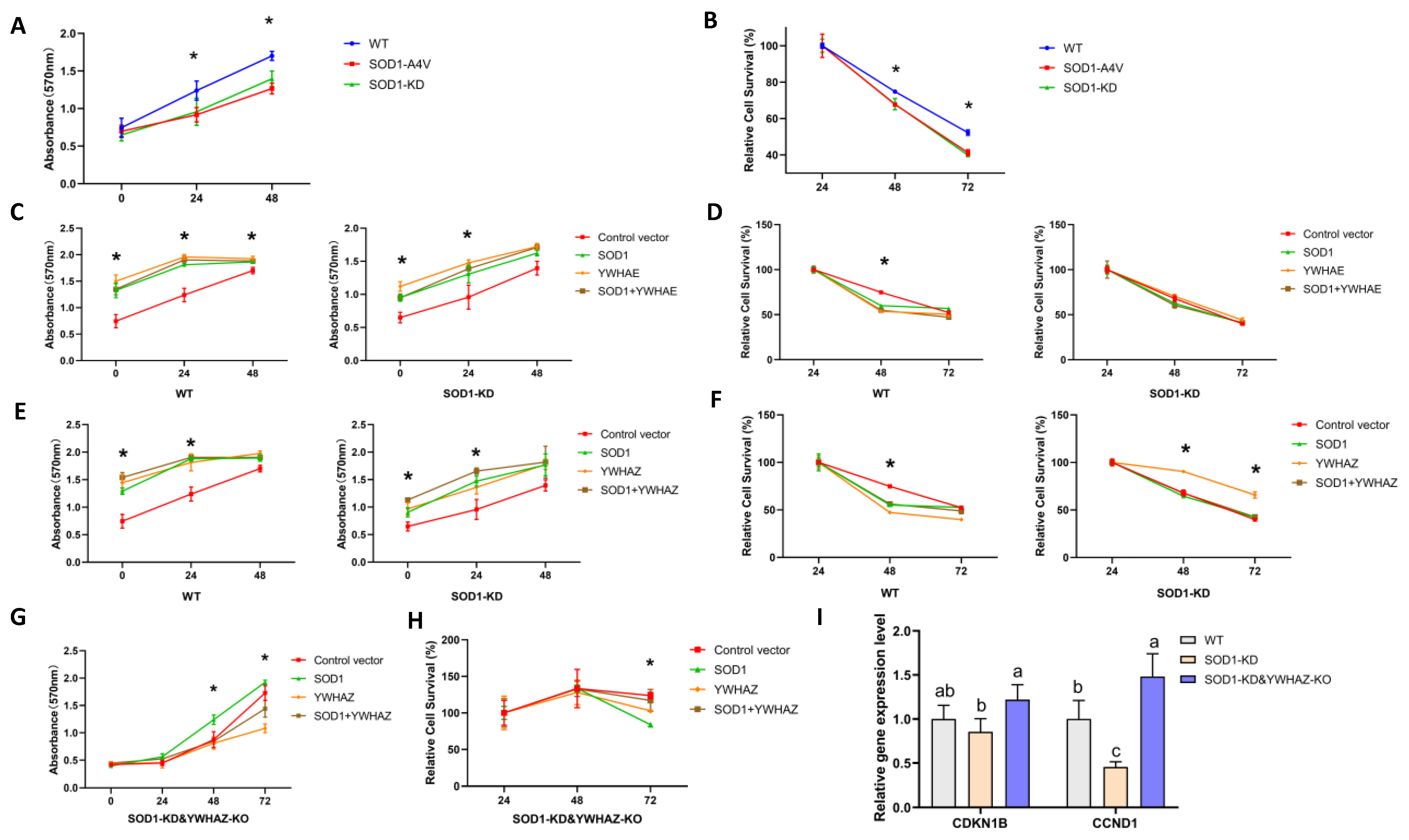
2.7. Impacts of Protein–Protein Interactions between SOD1 and YWHAE or YWHAZ on Cell Growth and Survival
2.8. Expression Correlation between SOD1 and YWHAE or YWHAZ Protein
3. Discussion
4. Materials and Methods
4.1. Cells Culture, Animals, Plasmids, and Protein Expression and Purification
4.2. Protein Complementation Assay (PCA)
4.3. Protein Complex Formation and Pull-Down Assay
4.4. Mutagenesis of the SOD1 Gene and Analyses of the Disrupted PPIs
4.5. Measurements of SOD1 and SOD2 Activities
4.6. CRISPR/Cas9 Editing Genome SOD1 and YWHAZ in HEK293T Cells
4.7. Co-Immunoprecipitation
4.8. Protein Degradation Assay
4.9. Staining of Lipid Droplets and Detection of Lipid Profiles
4.10. Cell Growth and Survival
4.11. Quantitative Real-Time PCR (qPCR)
4.12. Western Blotting
4.13. Correlation Analysis of the Protein Expression Levels
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, L.Y.; Slot, J.W.; Geuze, H.J.; Crapo, J.D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J. Cell Biol. 1988, 107, 2169–2179. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. Engl. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, L.; Viera, L.; Suswam, E.; Li, Y.; Li, X.; Estevez, A.G.; King, P.H. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J. Neurosci. 2007, 27, 7929–7938. [Google Scholar] [CrossRef]
- Li, X.; Lu, L.; Bush, D.J.; Zhang, X.; Zheng, L.; Suswam, E.A.; King, P.H. Mutant copper-zinc superoxide dismutase associated with amyotrophic lateral sclerosis binds to adenine/uridine-rich stability elements in the vascular endothelial growth factor 3’-untranslated region. J. Neurochem. 2009, 108, 1032–1044. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Nie, Z.; Qiu, S.; Huang, X.; Li, X.; Cui, Y.; Liu, C.; Liu, C. SAXS Examinations of the Redox-Dependent Formation of a DNA-SOD1 Complex. Int. J. Mol. Sci. 2022, 23, 12673. [Google Scholar] [CrossRef] [PubMed]
- Casareno, R.L.; Waggoner, D.; Gitlin, J.D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Biol. Chem. 1998, 273, 23625–23628. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.A.; Wright, G.S.A.; Antonyuk, S.V.; Garratt, R.C.; Hasnain, S.S. Molecular recognition and maturation of SOD1 by its evolutionarily destabilised cognate chaperone hCCS. PLoS Biol. 2019, 17, e3000141. [Google Scholar] [CrossRef]
- Hamilton, R.T.; Bhattacharya, A.; Walsh, M.E.; Shi, Y.; Wei, R.; Zhang, Y.; Rodriguez, K.A.; Buffenstein, R.; Chaudhuri, A.R.; Van Remmen, H. Elevated Protein Carbonylation, and Misfolding in Sciatic Nerve from db/db and Sod1−/− Mice: Plausible Link between Oxidative Stress and Demyelination. PLoS ONE 2013, 8, e65725. [Google Scholar] [CrossRef] [PubMed]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Li, X.; Weng, H.; Reece, E.A.; Yang, P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: Substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am. J. Obstet. Gynecol. 2011, 205, 84.e1–84.e6. [Google Scholar] [CrossRef]
- Li, J.; Song, M.; Moh, S.; Kim, H.; Kim, D.H. Cytoplasmic Restriction of Mutated SOD1 Impairs the DNA Repair Process in Spinal Cord Neurons. Cells 2019, 8, 1502. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.H.; McClung, J.P.; Aregullin, M.; Roneker, C.A. Mice deficient in Cu,Zn-superoxide dismutase are resistant to acetaminophen toxicity. Biochem. J. 2006, 399, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gillen, E.A.; van der Meulen, M.C.; Lei, X.G. Knockouts of Se-glutathione peroxidase-1 and Cu,Zn superoxide dismutase exert different impacts on femoral mechanical performance of growing mice. Mol. Nutr. Food Res. 2008, 52, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Vatamaniuk, M.Z.; Roneker, C.A.; Pepper, M.P.; Hu, L.G.; Simmons, R.A.; Lei, X.G. Knockouts of SOD1 and GPX1 Exert Different Impacts on Murine Islet Function and Pancreatic Integrity. Antioxid. Redox Signal. 2011, 14, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Z.; Lei, X.G. Knockout of SOD1 alters murine hepatic glycolysis, gluconeogenesis, and lipogenesis. Free. Radic. Biol. Med. 2012, 53, 1689–1696. [Google Scholar] [CrossRef]
- Wang, S.K. Impact of Copper, Zinc Superoxide Dismutase Knockout on Lipid Metabolism in Mice. Ph.D. Thesis, Faculty of the Graduate School, Cornell University, Ithaca, NY, USA, 2009. [Google Scholar]
- Dougherty, M.K.; Morrison, D.K. Unlocking the code of 14-3-3. J. Cell Sci. 2004, 117, 1875–1884. [Google Scholar] [CrossRef]
- Fu, H.; Subramanian, R.R.; Masters, S.C. 14-3-3 Proteins: Structure, Function, and Regulation. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 617–647. [Google Scholar] [CrossRef] [PubMed]
- Foote, M.; Zhou, Y. 14-3-3 proteins in neurological disorders. Int. J. Biochem. Mol. Biol. 2012, 3, 152–164. [Google Scholar]
- Liang, R.; Chen, X.Q.; Bai, Q.X.; Wang, Z.; Zhang, T.; Yang, L.; Dong, B.X.; Gao, G.X.; Gu, H.T.; Zhu, H.F. Increased 14-3-3zeta expression in the multidrug-resistant leukemia cell line HL-60/VCR as compared to the parental line mediates cell growth and apoptosis in part through modification of gene expression. Acta. Haematol. 2014, 132, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Weerasekara, V.K.; Panek, D.J.; Broadbent, D.G.; Mortenson, J.B.; Mathis, A.D.; Logan, G.N.; Prince, J.T.; Thomson, D.M.; Thompson, J.W.; Andersen, J.L. Metabolic-Stress-Induced Rearrangement of the 14-3-3ζ Interactome Promotes Autophagy via a ULK1- and AMPK-Regulated 14-3-3ζ Interaction with Phosphorylated Atg9. Mol. Cell. Biol. 2014, 34, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Komatsu, S.; Ichikawa, D.; Nagata, H.; Hirajima, S.; Takeshita, H.; Kawaguchi, T.; Arita, T.; Konishi, H.; Kashimoto, K.; et al. Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br. J. Cancer 2013, 108, 1324–1331. [Google Scholar] [CrossRef]
- Matta, A.; Siu, K.M.; Ralhan, R. 14-3-3 zeta as novel molecular target for cancer therapy. Expert Opin. Ther. Targets 2012, 16, 515–523. [Google Scholar] [CrossRef]
- Lim, G.E.; Albrecht, T.; Piske, M.; Sarai, K.; Lee, J.T.C.; Ramshaw, H.S.; Sinha, S.; Guthridge, M.A.; Acker-Palmer, A.; Lopez, A.F.; et al. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat. Commun. 2015, 6, 7671. [Google Scholar] [CrossRef]
- Lim, G.E.; Piske, M.; Lulo, J.E.; Ramshaw, H.S.; Lopez, A.F.; Johnson, J.D. Ywhaz/14-3-3ζ Deletion Improves Glucose Tolerance Through a GLP-1-Dependent Mechanism. Endocrinology 2016, 157, 2649–2659. [Google Scholar] [CrossRef]
- Craparo, A.; Freund, R.; Gustafson, T.A. 14-3-3 (ϵ) Interacts with the Insulin-like Growth Factor I Receptor and Insulin Receptor Substrate I in a Phosphoserine-dependent Manner. J. Biol. Chem. 1997, 272, 11663–11669. [Google Scholar] [CrossRef]
- Malty, R.H.; Aoki, H.; Kumar, A.; Phanse, S.; Amin, S.; Zhang, Q.; Minic, Z.; Goebels, F.; Musso, G.; Wu, Z.; et al. A Map of Human Mitochondrial Protein Interactions Linked to Neurodegeneration Reveals New Mechanisms of Redox Homeostasis and NF-kappaB Signaling. Cell Syst. 2017, 5, 564–577.e12. [Google Scholar] [CrossRef] [PubMed]
- Marcon, E.; Ni, Z.; Pu, S.; Turinsky, A.L.; Trimble, S.S.; Olsen, J.B.; Silverman-Gavrila, R.; Silverman-Gavrila, L.; Phanse, S.; Guo, H.; et al. Human-chromatin-related protein interactions identify a demethylase complex required for chromosome segregation. Cell Rep. 2014, 8, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Selbach, M. Quantitative affinity purification mass spectrometry: A versatile technology to study protein–protein interactions. Front. Genet. 2015, 6, 237. [Google Scholar] [CrossRef]
- Ratovitski, T.; Corson, L.B.; Strain, J.; Wong, P.; Cleveland, D.W.; Culotta, V.C.; Borchelt, D.R. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum. Mol. Genet. 1999, 8, 1451–1460. [Google Scholar] [CrossRef]
- Borchelt, D.R.; Lee, M.K.; Slunt, H.S.; Guarnieri, M.; Xu, Z.S.; Wong, P.C.; Brown, R.H., Jr.; Price, D.L.; Sisodia, S.S.; Cleveland, D.W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA 1994, 91, 8292–8296. [Google Scholar] [CrossRef]
- Sau, D.; De Biasi, S.; Vitellaro-Zuccarello, L.; Riso, P.; Guarnieri, S.; Porrini, M.; Simeoni, S.; Crippa, V.; Onesto, E.; Palazzolo, I.; et al. Mutation of SOD1 in ALS: A gain of a loss of function. Hum. Mol. Genet. 2007, 16, 1604–1618. [Google Scholar] [CrossRef] [PubMed]
- Saccon, R.A.; Bunton-Stasyshyn, R.K.; Fisher, E.M.; Fratta, P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 2013, 136, 2342–2358. [Google Scholar] [CrossRef]
- Nedd, S.; Redler, R.L.; Proctor, E.A.; Dokholyan, N.V.; Alexandrova, A.N. Cu,Zn-superoxide dismutase without Zn is folded but catalytically inactive. J. Mol. Biol. 2014, 426, 4112–4124. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Hu, C.D. An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques 2010, 49, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Hentati, A.; Tainer, J.A.; Iqbal, Z.; Cayabyab, A.; Hung, W.Y.; Getzoff, E.D.; Hu, P.; Herzfeldt, B.; Roos, R.P.; et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 1993, 261, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Banci, L.; Bertini, I.; Cabelli, D.E.; Hallewell, R.A.; Tung, J.W.; Viezzoli, M.S. A characterization of copper/zinc superoxide dismutase mutants at position 124. Zinc-deficient proteins. Eur. J. Biochem. 1991, 196, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H.; Benzinger, A. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 2006, 16, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Vella, D.; Zoppis, I.; Mauri, G.; Mauri, P.; Di Silvestre, D. From protein–protein interactions to protein co-expression networks: A new perspective to evaluate large-scale proteomic data. EURASIP J. Bioinform. Syst. Biol. 2017, 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.J.; Socciarelli, F.; Vacanti, N.M.; Haugen, M.H.; Zhu, Y.; Siavelis, I.; Fernandez-Woodbridge, A.; Aure, M.R.; Sennblad, B.; Vesterlund, M.; et al. Breast cancer quantitative proteome and proteogenomic landscape. Nat. Commun. 2019, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yun, J.W.; Lei, X.G. Glutathione peroxidase mimic ebselen improves glucose-stimulated insulin secretion in murine islets. Antioxid. Redox Signal. 2014, 20, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Zhao, Z.; Yan, X.; Vatamaniuk, M.Z.; Lei, X.G. Glutathione peroxidase-1 inhibits transcription of regenerating islet-derived protein-2 in pancreatic islets. Free. Radic. Biol. Med. 2019, 134, 385–393. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2021. [Google Scholar]
- Bryant, P.; Pozzati, G.; Elofsson, A. Improved prediction of protein–protein interactions using AlphaFold2. Nat. Commun. 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Wu, D.; Lee, N.; Shibata, E.; Dutta, A. 14-3-3 proteins play a role in the cell cycle by shielding cdt2 from ubiquitin-mediated degradation. Mol. Cell Biol. 2014, 34, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Kabuta, T.; Suzuki, Y.; Wada, K. Degradation of Amyotrophic Lateral Sclerosis-linked Mutant Cu,Zn-Superoxide Dismutase Proteins by Macroautophagy and the Proteasome. J. Biol. Chem. 2006, 281, 30524–30533. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Ma, W. WRINKLED1 as a novel 14-3-3 client: Function of 14-3-3 proteins in plant lipid metabolism. Plant Signal. Behav. 2018, 13, e1482176. [Google Scholar] [CrossRef]
- Obsilova, V.; Vecer, J.; Herman, P.; Pabianova, A.; Sulc, M.; Teisinger, J.; Boura, E.; Obsil, T. 14-3-3 Protein Interacts with Nuclear Localization Sequence of Forkhead Transcription Factor FoxO4. Biochemistry 2005, 44, 11608–11617. [Google Scholar] [CrossRef] [PubMed]
- Huai, J.; Zhang, Z. Structural Properties and Interaction Partners of Familial ALS-Associated SOD1 Mutants. Front. Neurol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, X.; Li, S.; Zhu, Y.; Chen, S.; Li, P.; Luo, C.; Huang, Y.; Li, X.; Hu, X.; et al. Interactions between plasma copper concentrations and SOD1 gene polymorphism for impaired glucose regulation and type 2 diabetes. Redox Biol. 2019, 24, 101172. [Google Scholar] [CrossRef]
- Saremi, L.; Taghvaei, S.; Feizy, F.; Ghaffari, M.E.; Babaniamansour, S.; Saltanatpour, Z. Association study between superoxide Dismutases gene polymorphisms and development of diabetic retinopathy and cataract in Iranian patients with type two diabetes mellitus. J. Diabetes Metab. Disord. 2021, 20, 627–634. [Google Scholar] [CrossRef]
- Vats, P.; Sagar, N.; Singh, T.P.; Banerjee, M. Association of Superoxide dismutases (SOD1 and SOD2) and Glutathione peroxidase 1 (GPx1) gene polymorphisms with type 2 diabetes mellitus. Free. Radic. Res. 2015, 49, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Guerrero, C.; Parra-Carriedo, A.; Ruiz-de-Santiago, D.; Galicia-Castillo, O.; Buenrostro-Jauregui, M.; Diaz-Gutierrez, C. Genetic polymorphisms of antioxidant enzymes CAT and SOD affect the outcome of clinical, biochemical, and anthropometric variables in people with obesity under a dietary intervention. Genes Nutr. 2018, 13, 1. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, H.; Kim, J.E.; Park, K.S.; Sung, J.J.; Kim, S.H.; Lee, K.W. Amyotrophic lateral sclerosis is associated with hypolipidemia at the presymptomatic stage in mice. PLoS ONE 2011, 6, e17985. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.C.; Treleaven, C.M.; Fidler, J.A.; Tamsett, T.J.; Bao, C.; Searles, M.; Taksir, T.V.; Misra, K.; Sidman, R.L.; Cheng, S.H.; et al. Metabolic signatures of amyotrophic lateral sclerosis reveal insights into disease pathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10812–10817. [Google Scholar] [CrossRef] [PubMed]
- Smittkamp, S.E.; Morris, J.K.; Bomhoff, G.L.; Chertoff, M.E.; Geiger, P.C.; Stanford, J.A. SOD1-G93A mice exhibit muscle-fiber-type-specific decreases in glucose uptake in the absence of whole-body changes in metabolism. Neurodegener Dis. 2014, 13, 29–37. [Google Scholar] [CrossRef]
- Wong, N.K.Y.; He, B.P.; Strong, M.J. Characterization of Neuronal Intermediate Filament Protein Expression in Cervical Spinal Motor Neurons in Sporadic Amyotrophic Lateral Sclerosis (ALS). J. Neuropathol. Exp. Neurol. 2000, 59, 972–982. [Google Scholar] [CrossRef]
- Ge, W.-W.; Volkening, K.; Leystra-Lantz, C.; Jaffe, H.; Strong, M.J. 14-3-3 protein binds to the low molecular weight neurofilament (NFL) mRNA 3’ UTR. Mol. Cell. Neurosci. 2007, 34, 80–87. [Google Scholar] [CrossRef]
- Miao, L.; Teng, J.; Lin, J.; Liao, X.; Chen, J. 14-3-3 proteins interact with neurofilament protein-L and regulate dynamic assembly of neurofilaments. J. Cell Sci. 2013, 126, 427–436. [Google Scholar] [CrossRef]
- Neal, C.L.; Yao, J.; Yang, W.; Zhou, X.; Nguyen, N.T.; Lu, J.; Danes, C.G.; Guo, H.; Lan, K.-H.; Ensor, J.; et al. 14-3-3ζ Overexpression Defines High Risk for Breast Cancer Recurrence and Promotes Cancer Cell Survival. Cancer Res. 2009, 69, 3425–3432. [Google Scholar] [CrossRef]
- Hong, L.; Chen, W.; Xing, A.; Wu, D.; Wang, S. Inhibition of Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Zeta (YWHAZ) Overcomes Drug Resistance and Tumorigenicity in Ovarian Cancer. Cell. Physiol. Biochem. 2018, 49, 53–64. [Google Scholar] [CrossRef]
- Leal, M.F. Clinical implication of 14-3-3 epsilon expression in gastric cancer. World J. Gastroenterol. 2012, 18, 1531. [Google Scholar] [CrossRef]
- Park, J.-H.; Jang, H.R.; Lee, I.Y.; Oh, H.K.; Choi, E.-J.; Rhim, H.; Kang, S. Amyotrophic lateral sclerosis-related mutant superoxide dismutase 1 aggregates inhibit 14-3-3-mediated cell survival by sequestration into the JUNQ compartment. Hum. Mol. Genet. 2017, 26, 3615–3629. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front. Cell Dev. Biol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Gan, Y.; Ye, F.; He, X.-X. The role of YWHAZ in cancer: A maze of opportunities and challenges. J. Cancer 2020, 11, 2252–2264. [Google Scholar] [CrossRef]
- Leal, M.F.; Ribeiro, H.F.; Rey, J.A.; Pinto, G.R.; Smith, M.C.; Moreira-Nunes, C.A.; Assumpção, P.P.; Lamarão, L.M.; Calcagno, D.Q.; Montenegro, R.C.; et al. YWHAE silencing induces cell proliferation, invasion and migration through the up-regulation of CDC25B and MYC in gastric cancer cells: New insights about YWHAE role in the tumor development and metastasis process. Oncotarget 2016, 7, 85393–85410. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Gargano, M.; Cao, J.; Bronson, R.T.; Heimler, I.; Hutz, R.J. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J. Biol. Chem. 1998, 273, 7765–7769. [Google Scholar] [CrossRef]
- Rodriguez, E.; Han, Y.; Lei, X.G. Cloning, sequencing, and expression of an Escherichia coli acid phosphatase/phytase gene (appA2) isolated from pig colon. Biochem. Biophys. Res. Commun. 1999, 257, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Detection of Protein–protein Interactions Using the GST Fusion Protein Pulldown Technique. CSH Protoc. 2006, 2006, pdb-prot3757. [Google Scholar] [CrossRef]
- Chen, L.L.; Huang, J.Q.; Wu, Y.Y.; Chen, L.B.; Li, S.P.; Zhang, X.; Wu, S.; Ren, F.Z.; Lei, X.G. Loss of Selenov predisposes mice to extra fat accumulation and attenuated energy expenditure. Redox Biol. 2021, 45, 102048. [Google Scholar] [CrossRef]
- Ghneim, H.K.; Al-Sheikh, Y.A.; Alshebly, M.M.; Aboul-Soud, M.A. Superoxide dismutase activity and gene expression levels in Saudi women with recurrent miscarriage. Mol. Med. Rep. 2016, 13, 2606–2612. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Adikusuma, F.; Pfitzner, C.; Thomas, P.Q. Versatile single-step-assembly CRISPR/Cas9 vectors for dual gRNA expression. PLoS ONE 2017, 12, e0187236. [Google Scholar] [CrossRef] [PubMed]
- Armon, C. SOD1 A4V familial ALS in North America: Can understanding the past lead to a better future? Neurology 2009, 72, 1628–1629. [Google Scholar] [CrossRef] [PubMed]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.W.; Lloyd, M.E.; Engle, S.M.; Rubenstein, E.M. Cycloheximide Chase Analysis of Protein Degradation in Saccharomyces cerevisiae. J. Vis. Exp. 2016, 110, e53975. [Google Scholar]
- Kim, J.; Barcus, M.; Magnuson, A.; Tao, L.; Lei, X.G. Supplemental defatted microalgae affects egg and tissue fatty acid composition differently in laying hens fed diets containing corn and flaxseed oil. J. Appl. Poult. Res. 2016, 25, 528–538. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; He, W.; Zhao, P.; Ye, C. MicroRNA-433 targets AKT3 and inhibits cell proliferation and viability in breast cancer. Oncol. Lett. 2018, 15, 3998–4004. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cheng, W.; Fu, Y.X.; Porres, J.M.; Ross, D.A.; Lei, X.G. Selenium-dependent cellular glutathione peroxidase protects mice against a pro-oxidant-induced oxidation of NADPH, NADH, lipids, and protein. FASEB J. 1999, 13, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
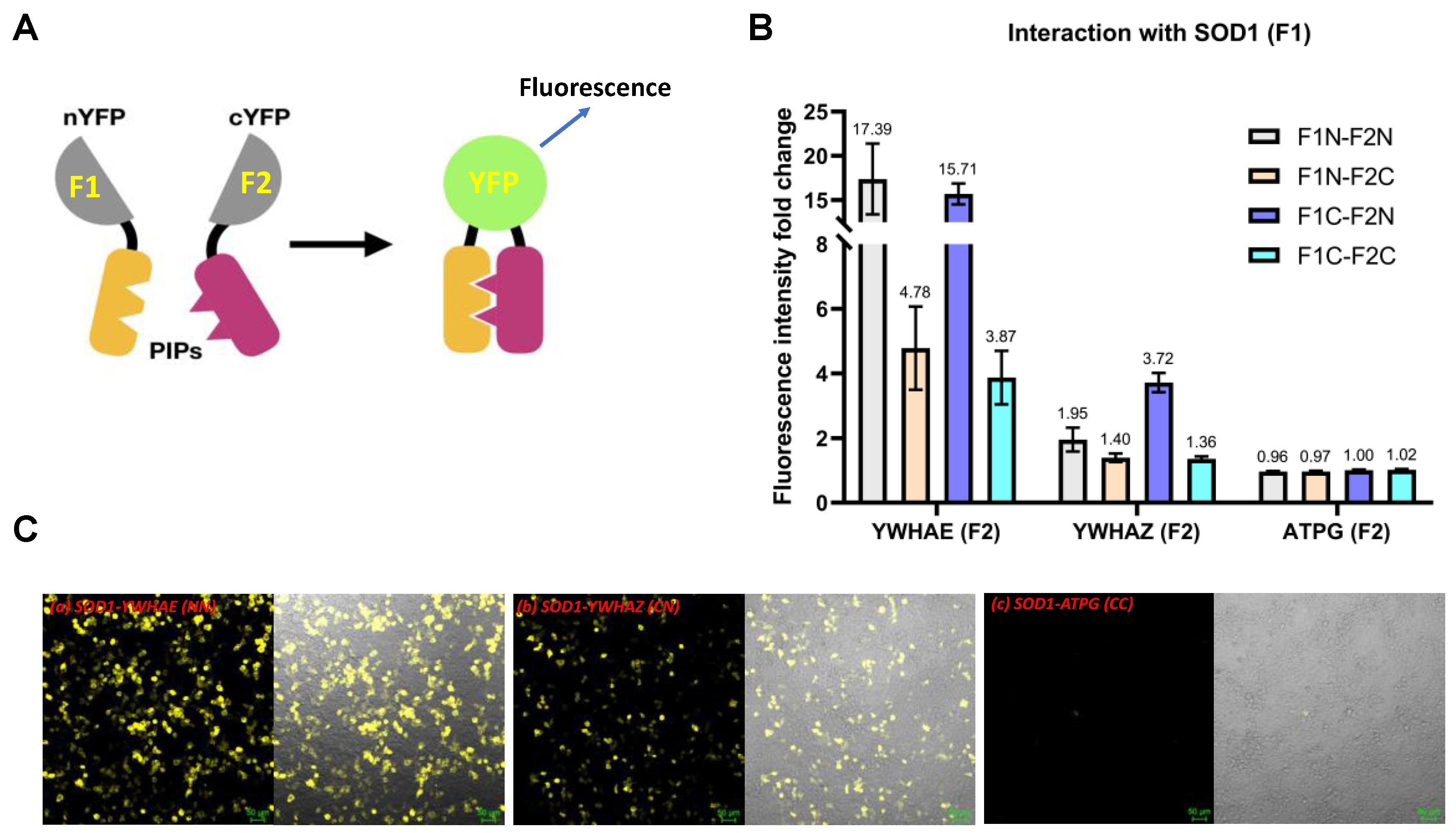
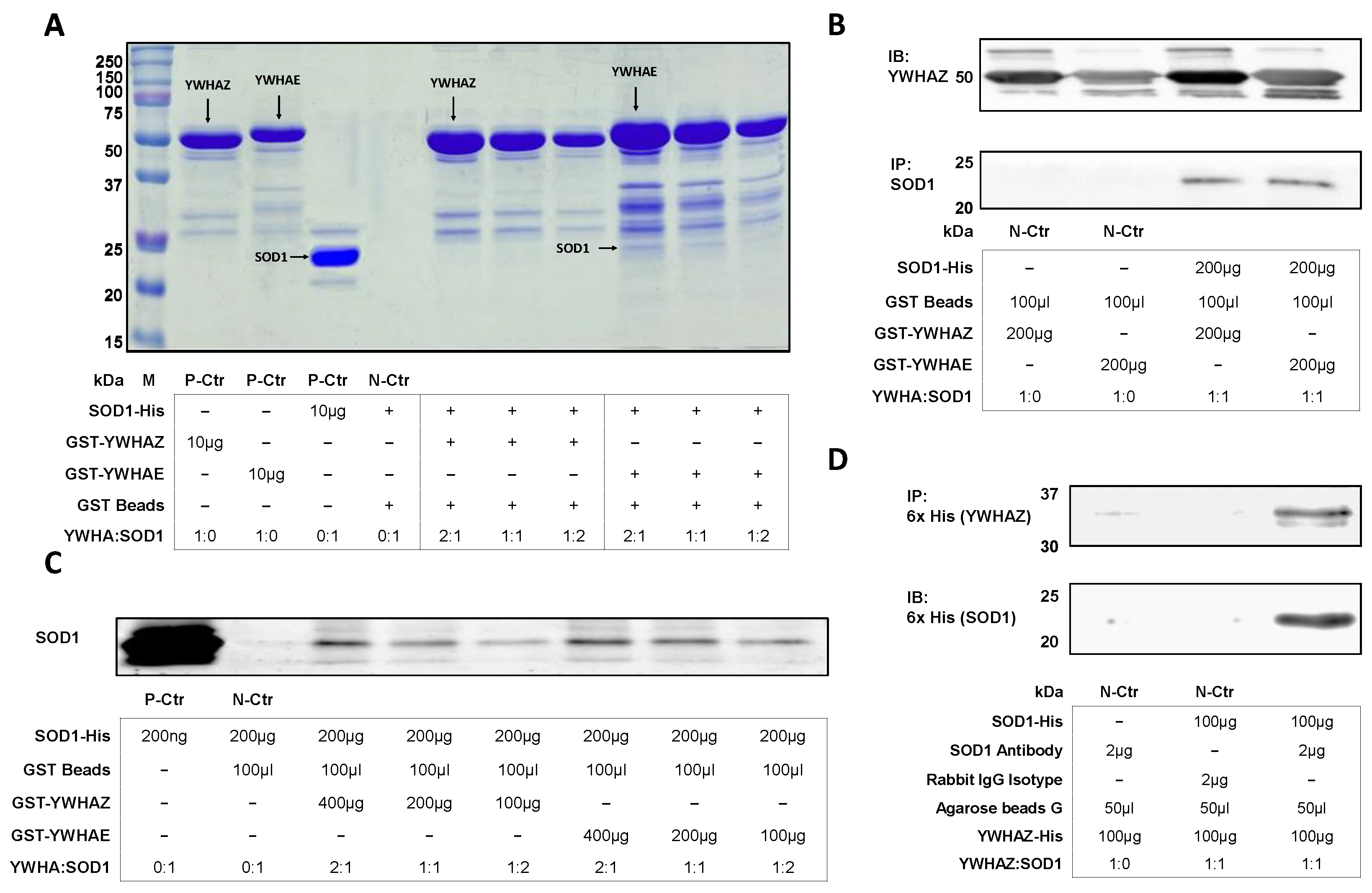
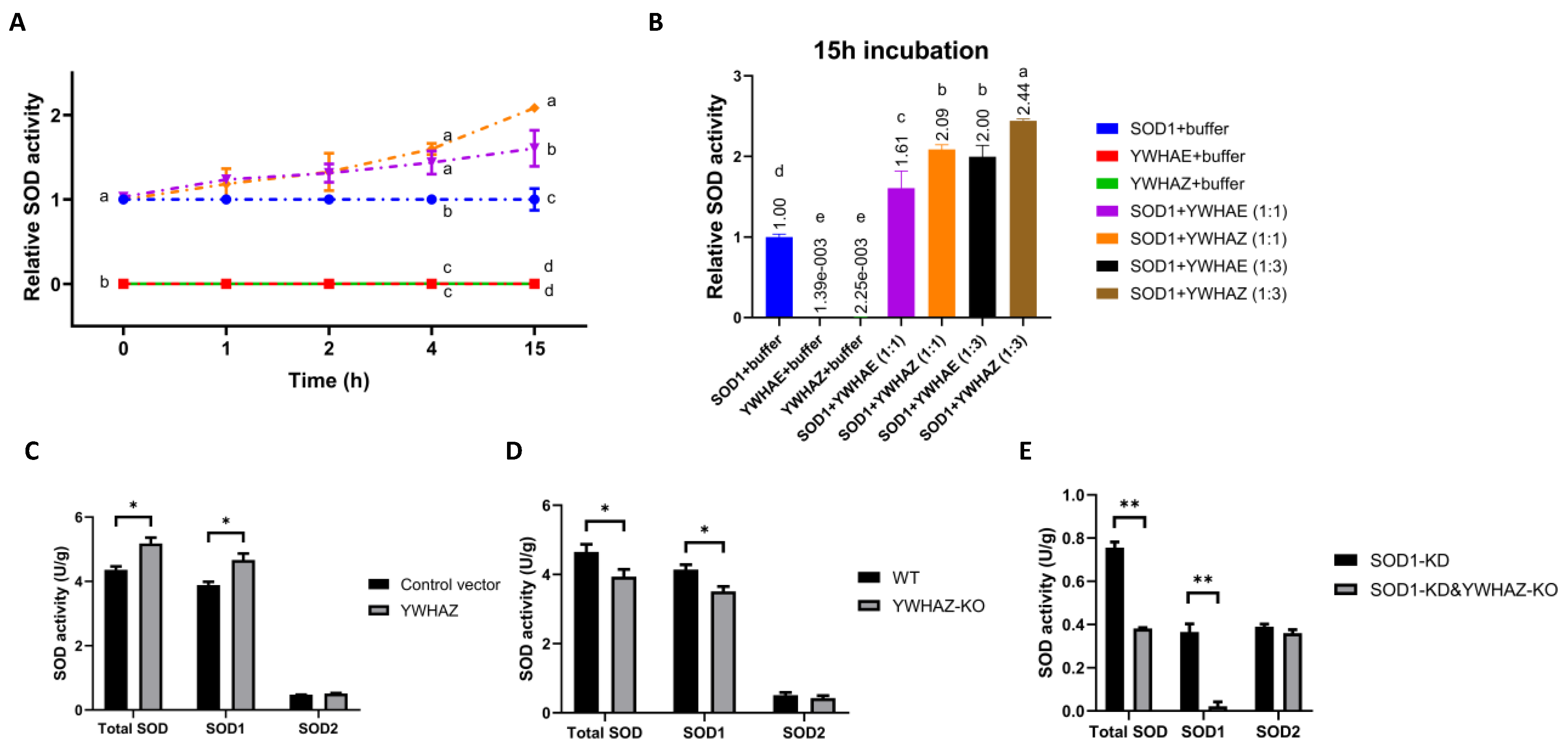
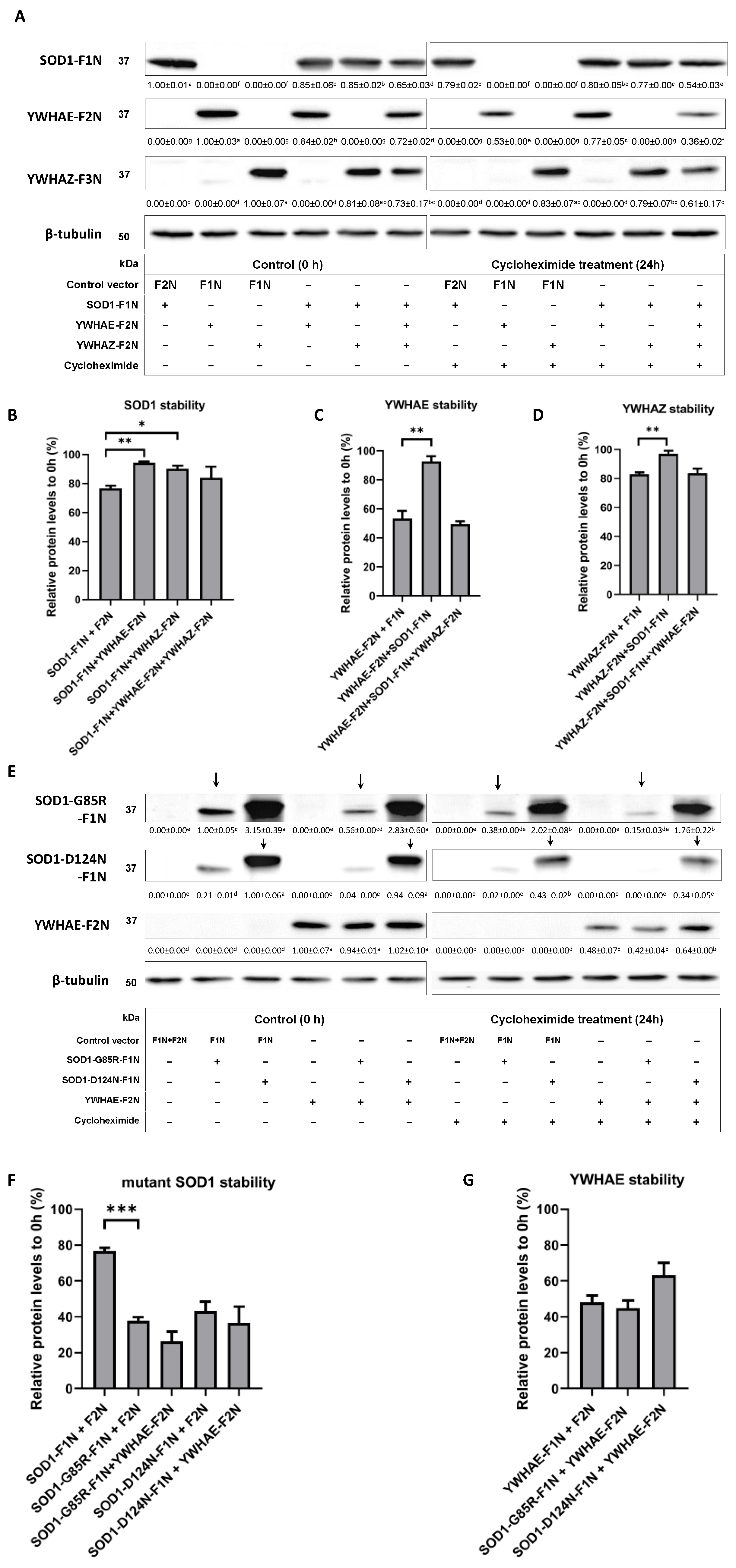

| Candidates | SOD1 | SOD1-Mutants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | H46R | G85R | G93A | D124N | ||||||
| NN | CN | NN | CN | NN | CN | NN | CN | NN | CN | |
| Fold change (To negative control) | ||||||||||
| YWHAE | 6.53 | 14.7 | 2.99 | 3.86 * | 1.50 * | 4.25 * | 2.47 * | 1.60 ** | 1.60 * | 1.54 ** |
| YWHAZ | 2.59 | 3.52 | 1.32 * | 1.72 ** | 1.01 * | 2.04 ** | 1.06 * | 1.50 ** | 1.08 * | 2.27 ** |
| Relative percentage (%, to wild type) | ||||||||||
| YWHAE | 100 | 100 | 36.0 | 20.9 | 8.99 | 23.8 | 26.6 | 4.35 | 10.8 | 3.98 |
| YWHAZ | 100 | 100 | 20.0 | 28.7 | 0.33 | 41.4 | 3.67 | 19.7 | 5.02 | 50.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Lei, X.-G. Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase. Int. J. Mol. Sci. 2023, 24, 3230. https://doi.org/10.3390/ijms24043230
Sun Z, Lei X-G. Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase. International Journal of Molecular Sciences. 2023; 24(4):3230. https://doi.org/10.3390/ijms24043230
Chicago/Turabian StyleSun, Ziqiao, and Xin-Gen Lei. 2023. "Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase" International Journal of Molecular Sciences 24, no. 4: 3230. https://doi.org/10.3390/ijms24043230
APA StyleSun, Z., & Lei, X.-G. (2023). Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase. International Journal of Molecular Sciences, 24(4), 3230. https://doi.org/10.3390/ijms24043230







