Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications
Abstract
1. Introduction
2. Quercetin
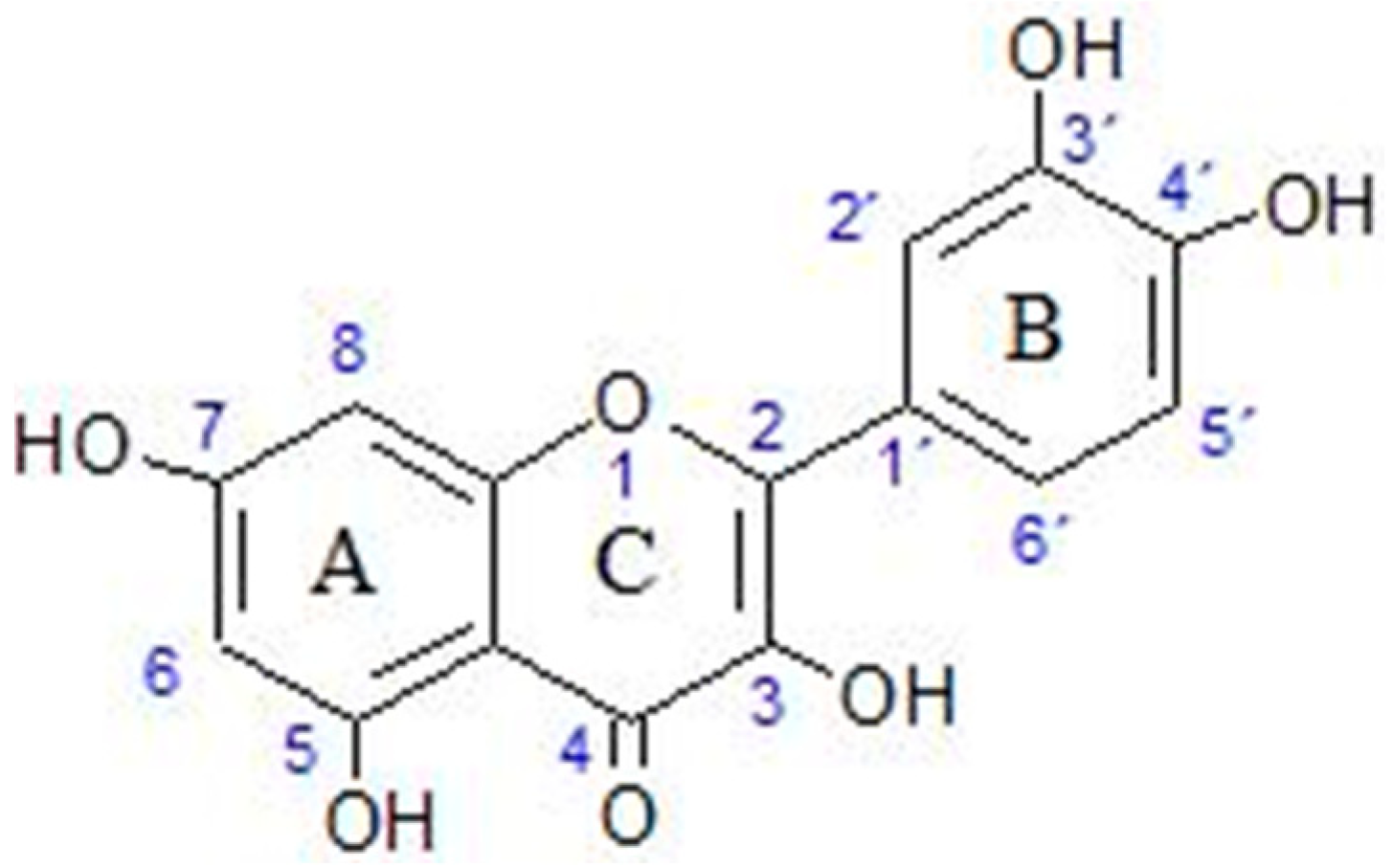
2.1. Basic Properties of Quercetin
2.2. Derivatives and Metabolites of Quercetin
2.3. Beneficial Effects of Quercetin on Health
- Chelates metal ions, preventing the metal ions from catalyzing oxidation reactions;
- Inhibits lipid peroxidation by donating hydrogen ions to α-tocopherol radicals, regenerating this primary oxidant;
- Elevates the level of glutathione (another important molecule in reducing oxidative stress).
2.4. Effect of Quercetin on Signalling Pathways
2.5. Contraindications, Possible Toxicity and Dangers of Quercetin Supplements
- Antibiotics (quercetin may reduce effectiveness);
- Anticoagulants (quercetin may enhance the effect of these blood thinners);
- Chemotherapy (may increase the effect of doxorubicin or decrease the effect of cisplatin—doctors in disagreement as to whether antioxidants are good or bad during chemotherapy);
- Corticosteroids (quercetin may prolong the circulation time of these drugs);
- Cyclosporine (quercetin may interfere with absorption);
- Digoxin (quercetin may increase the risks of this API);
- Fluoroquinolones (quercetin may reduce the effectiveness of API);
- Medications changed by the liver (quercetin may alter the metabolism of APIs by the liver) [52].
2.6. Difficulties in Quercetin Administration as a Therapeutic API
3. Dosage Forms and Means of Administration
4. Chitosan and Chitosan Nanoparticles
4.1. Chitosan
- It is biodegradable and biocompatible [75].
- It is soluble in weak acids, such as acetic acid or lactic acid, at pH 6.5.
- It can interact with some important anionic pharmaceutically active products, for example, nucleic acids.
- It can easily form nanoparticles by ionic interaction with negatively charged substances such as tripolyphosphate (ionic gelation method) [76].
- Nanoparticles can be made under mild conditions with no organic solvents [7].
- Its carrying ability increases with charge, which can be changed with pH [79].
- It has a chelating ability, which could inactivate free-radical-producing metal ions.
- Chitosan can be modified to optimise beneficial properties according to the needs of the application, for example, giving better aqueous solubility. This can be done by changing the average molecular weight, degree of deacetylation, cross-linking, adding covalently bound functional groups and moieties, coordinated anions or polyanions, etc. [7].
- Sulfonation can turn cationic chitosan anionic. This can make the modified chitosan have water-soluble qualities, better paste fluidity, a high water-reducing ratio, and anticoagulant properties [94].
- An increase in cross-linking can be achieved with anions, dextran, sulphate, glyoxal, genipin, tripolyphosphate, formaldehyde and glutaraldehyde. More or stronger cross-linking gives mechanical strength to particles, slows down drug release and prevents burst release [86]. Highly cross-linked particles also show less swelling, less inside water penetration and less outside drug diffusion [7].
- In-situ gelling properties can be improved by thiolation [77].
- Better mucoadhesion can be achieved by the trimethylation of primary amino groups and PEG-ylation. Thiol groups added to chitosan can interact with the cysteine-rich region of mucous glycol protein. Mucoadhesion can also be improved by the formation of complexes with multivalent drugs, excipients and multivalent inorganic ions [7,77,79].
- Trimethyl chitosan is soluble at all pHs [7].
- Trimethyl chitosan enhances permeation by opening tight junctions and increasing paracellular transport [79].
- Modifications can come at the cost of reproducibility [72].
4.2. Chitosan Nanoparticles
- Less toxicity compared with other materials used for making nanoparticles;
- Enhanced biocompatibility;
- Has a mucoadhesive character;
- Stability;
- Can be used to deliver a wide variety of drugs;
- Can be produced using very mild conditions;
- Site-specific drug targeting;
- Increased therapeutic index of the drug;
- Frequent, expensive, and unpleasant dosing is reduced;
- Can be engineered to reduce the drug in a controlled way.
- Low mechanical resistance;
- Difficult to control pore size;
- Possible contraction;
- Difficult electrospinning for pure chitosan;
- Preparation by cross-linking can affect the intrinsic properties of chitosan;
- Low solubility in neutral and alkaline pH;
- Method of preparation depends on the drug to be delivered [7].
5. Studies of Chitosan Nanoparticles Carrying Quercetin
5.1. Cancer Treatment
5.1.1. Breast Cancer Treatment
5.1.2. Lung Cancer Resistance
5.1.3. Liver Cancer
5.1.4. Colorectal Cancer
5.2. Dermal Administration for Skin Problems
5.2.1. UVB Protection
5.2.2. Wound Healing
5.3. Ocular Diseases
5.4. General Antioxidant
5.5. Other Diseases
6. Applications of Quercetin-Loaded Chitosan Nanoparticles
- Tissue engineering;
- Cancer therapy;
- Antioxidants;
- Drug delivery systems;
- Enzyme immobilization support;
- Encapsulation of biologically active compounds;
- Water treatment;
- Antimicrobial agents;
- Agriculture [126].
7. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Hamad, D.; Hamad, N.; Abdel-Rahman, A.A.-H.; Fouda, A.; Wei, Y.; Guibal, E.; El-Etrawy, A.-A. Functionalization of magnetic chitosan microparticles for high-performance removal of chromate from aqueous solutions and tannery effluent. Chem. Eng. J. 2022, 428, 131773. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized biobased composite for metal decontamination—Insight on uranium and application to water samples collected from wells in mining areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdel-Rahman, A.A.-H.; Hawata, M.A.; El Araby, R.; Guibal, E.; Fouda, A.; Wei, Y.; Hamad, N.A. Functionalization of magnetic chitosan microparticles—Comparison of trione and trithione grafting for enhanced silver sorption and application to metal recovery from waste X-ray photographic films. J. Environ. Chem. Eng. 2022, 10, 107939. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Kası̧kcı, M.B.; Bağdatlıoğlu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Gross, M.; Pfeiffer, M.; Martini, M.; Campbell, D.; Slavin, J.; Potter, J. The quantitation of metabolites of quercetin flavonols in human urine. Cancer Epidemiol. Prev. Biomark. 1996, 5, 711–720. [Google Scholar]
- Konishi, Y. Transepithelial transport of microbial metabolites of quercetin in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2005, 53, 601–607. [Google Scholar] [CrossRef]
- Oršolić, N.; Jembrek, M.J.; Terzić, S. Honey and quercetin reduce ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food Agric. Immunol. 2017, 28, 812–833. [Google Scholar] [CrossRef]
- Moon, J.H.; Nakata, R.; Oshima, S.; Inakuma, T.; Terao, J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R461–R467. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-β-D glucuronide an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- SokÓŁ-Łętowska, A.; Osmianski, J.; Wojdylo, A. Antioxidant activity of phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Oh, W.Y.; Ambigaipalan, P.; Shahidi, F. Preparation of quercetin esters and their antioxidant activity. J. Agric. Food Chem. 2019, 67, 10653–10659. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.-L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B 2014, 123, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Lawson, M.; Drostinova, L.; Lauro, L.; Poprac, P.; Brezova, V.; Michalik, M.; Lukes, V.; Valko, M. Protective role of quercetin against copper(II)-induced oxidative stress: A spectroscopic, theoretical and DNA damage study. Food Chem. Toxicol. 2017, 110, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Babenkova, I.V.; Osipov, A.N.; Teselkin, Y.O. The effect of dihydroquercetin on catalytic activity of Iron (II) ions in the Fenton reaction. Bull. Exp. Biol. Med. 2018, 165, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Yu, B.; Cho, S.; Her, E.; Park, D. The inhibition by quercetin and ganhuangenin on oxidatively modified low density lipoprotein. Phytother. Res. 1998, 12, 340–345. [Google Scholar] [CrossRef]
- Mbikay, M.; Sirois, F.; Simoes, S.; Mayne, J.; Chretien, M. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio 2014, 4, 755–762. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Odbayar, T.O.; Kimura, T.; Tsushida, T.; Ide, T. Isoenzyme-specific up-regulation of glutathione transferase and aldo-keto reductase mRNA expression by dietary quercetin in rat liver. Mol. Cell. Biochem. 2009, 325, 121–130. [Google Scholar] [CrossRef]
- Granado-Serrano, B.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathionerelated defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef]
- Dell’Albani, P.; Di Marco, B.; Grasso, S.; Rocco, C.; Foti, M.C. Quercetin derivatives as potent inducers of selective cytotoxicity in glioma cells. Eur. J. Pharm. Sci. 2017, 101, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Si, X.; Liu, X.; Deng, X.; Niu, X.; Jin, Y.; Wang, D.; Wang, J. Quercetin protects rats from catheter-related Staphylococcus aureus infections by inhibiting coagulase activity. J. Cell. Mol. Med. 2019, 23, 4808–4818. [Google Scholar] [CrossRef]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.T.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Read, M.A. Flavonoids: Naturally occurring antiinflammatory agents. Am. J. Clin. Pathol. 1995, 147, 235–237. [Google Scholar]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938. [Google Scholar] [CrossRef]
- Adewole, S.O.; Caxton-Martins, E.A.; Ojewole, J.A. Protective effect of quercetin on the morphology of pancreatic beta-cells of streptozotocin-treated diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2006, 4, 64–74. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In-vitro and In-vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxid. Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Jo, S.H.; Kim, I.S.; Dash, R.; Choi, D.K. Potential therapeutic targets of quercetin and its derivatives: Its role in the therapy of cognitive impairment. J. Clin. Med. 2019, 8, 1789. [Google Scholar] [CrossRef]
- Olson, J. Clinical Pharmacology Made Ridiculously Simple, 4th ed.; MedMaster, Inc.: Miami, FL, USA, 2015; p. 132. [Google Scholar]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New insights into quercetin nanoformulations for topical delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high-performance liquid chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for In-vivo tyrosine kinase inhibition. Clin. Cancer. Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef]
- Crebelli, R.; Aquilina, G.; Falcone, E.; Carere, A. Urinary and faecal mutagenicity in Sprague-Dawley rats dosed with the food mutagens quercetin and rutin. Food Chem. Toxicol. 1987, 25, 9–15. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of In-vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Mount Sinai. Health Library. Available online: https://www.mountsinai.org/health-library/supplement/quercetin (accessed on 25 November 2022).
- RxList. Consumer_Quercetin. Cunha, J.P. (Ed.) Available online: https://www.rxlist.com/consumer_quercetin/drugs-condition.htm (accessed on 25 November 2022).
- Boots, A.W.; Li, H.; Schins, R.P.; Duffin, R.; Heemskerk, J.W.; Bast, A.; Haenen, G.R. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Kunsági-Máté, S.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Molecular displacement of warfarin from human serum albumin by flavonoid aglycones. J. Lumin. 2013, 142, 122–127. [Google Scholar] [CrossRef]
- Poór, M.; Boda, G.; Needs, P.W.; Kroon, P.A.; Lemli, B.; Bencsik, T. Interaction of quercetin and its metabolites with warfarin: Displacement of warfarin from serum albumin and inhibition of CYP2C9 enzyme. Biomed. Pharmacother. 2017, 88, 574–581. [Google Scholar] [CrossRef]
- Poór, M.; Boda, G.; Kunsági-Máté, S.; Needs, P.W.; Kroon, P.A.; Lemli, B. Fluorescence spectroscopic evaluation of the interactions of quercetin, isorhamnetin, and quercetin-3′-sulfate with different albumins. J. Lumin. 2018, 194, 156–163. [Google Scholar] [CrossRef]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef]
- Wang, S.Y.; Duan, K.M.; Li, Y.; Mei, Y.; Sheng, H.; Liu, H.; Mei, X.; Ouyang, W.; Zhou, H.H.; Liu, Z.Q. Effect of quercetin on P-glycoprotein transport ability in Chinese healthy subjects. Eur. J. Clin. Nutr. 2013, 67, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, R.; Temesfői, V.; Sali, N.; Poór, M.W.; Needs, P.A.; Kroon, P.; Kőszegi, T. A One-Step Extraction and Luminescence Assay for Quantifying Glucose and ATP Levels in Cultured HepG2 Cells. Int. J. Mol. Sci. 2018, 19, 2670. [Google Scholar] [CrossRef]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef]
- Raman, R. Healthline Nitrition. Available online: https://www.healthline.com/nutrition/quercetin#what-it-is (accessed on 25 November 2022).
- Shunatona, B. Byrdie. Available online: https://www.byrdie.com/quercetin-5101124 (accessed on 25 November 2022).
- Maramaldi, G.; Togni, S.; Pagin, I.; Giacomelli, L.; Cattaneo, R.; Eggenhöffner, R.; Burastero, S.E. Soothing and anti-itch effect of quercetin phytosome in human subjects: A single-blind study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 55–62. [Google Scholar] [CrossRef]
- Sharma, A.R.; Kundu, S.K.; Nam, J.S.; Sharma, G.; Priya Doss, C.G.; Lee, S.S.; Chakraborty, C. Next generation delivery system for proteins and genes of therapeutic purpose: Why and how? BioMed Res. Int. 2014, 2014, 327950. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Nam, J.S.; Doss, G.P.C.; Lee, S.S.; Chakraborty, C. Nanoparticle based insulin delivery system: The next generation efficient therapy for Type 1 diabetes. J. Nanobiotechnol. 2015, 13, 74. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Edison, T.N.J.I.; Lewis Oscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. The Good, the Bad and the Ugly of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; di Bari, M.T.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef]
- Lim, C.; Lee, D.W.; Israelachvili, J.N.; Jho, Y.S.; Hwang, D.S. Contact time- and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces. Carbohydr. Polym. 2015, 117, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular interactions of chitosan: Degree of acetylation and molecular weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef]
- Kumar, A.; Vimal, A.; Kumar, A. Why chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, W.M.; Xu, G.; Li, X.Y.; Bai, X.F.; Wei, P.; Yu, C.; Du, Y.G. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol. Res. 2009, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Gogoi, P.; Maji, T.K. Chitosan: A promising biopolymer in drug delivery applications. J. Mol. Genet. Med. 2015, 4, 899–910. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Chen, L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017, 24, 108–113. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of chitosan and its derivatives for controlled drug release application—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modififications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 158, 11627. [Google Scholar] [CrossRef]
- Ma, Y.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in-vivo activity of chitosan and its micro- and nanoparticles as microbial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, P.; Gou, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Dunnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Burr, S.J.; Williams, P.A.; Ratcliffe, I. Synthesis of cationic alkylated chitosans and an investigation of their rheological properties and interaction with anionic surfactant. Carbohydr. Polym. 2018, 201, 615–623. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef]
- Jesus, S.; Marques, A.P.; Duarte, A.; Soares, E.; Costa, J.P.; Colaço, M.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; et al. Chitosan Nanoparticles: Shedding Light on Immunotoxicity and Hemocompatibility. Front. Bioeng. Biotechnol. 2020, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, E.; Gollapudi, B.; Spencer, P. Genetic toxicity and carcinogenicity studies of glutaraldehyde—A review. Mutat. Res. Rev. Mutat. Res. 2005, 589, 136–151. [Google Scholar] [CrossRef]
- Fürst, W.; Banerjee, A. Release of gutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in-vitro and in-vivo Toxicity. Ann. Thorac. Surg. 2005, 79, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in-vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Malhaire, H.; Gimel, J.C.; Roger, E.; Benoît, J.P.; Lagarce, F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv. Drug Deliv. Rev. 2016, 106, 320–336. [Google Scholar] [CrossRef]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer particle shape independently influences binding and internalization by macrophages. J. Control Release 2010, 147, 408–412. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in-vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- de Oliveira Pedro, R.; Hoffmann, S.; Pereira, S.; Goycoolea, F.M.; Schmitt, C.C.; Neumann, M.G. Self-assembled amphiphilic chitosan nanoparticles for quercetin delivery to breast cancer cells. Eur. J. Pharm. Biopharm. 2018, 131, 203–210. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Sherif, N.M.; Hassan, N.S.; Althobaiti, F.; Hanafy, N.A.N.; Sahyon, H.A. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int. J. Biol. Macromol. 2021, 185, 134–152. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111442. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Tang, C.; Yin, C. Dual stimulus-responsive chitosan-based nanoparticles co-delivering doxorubicin and quercetin for cancer therapy. Mater. Lett. 2021, 305, 130826. [Google Scholar] [CrossRef]
- Patil, P.; Killedar, S. Formulation and characterization of gallic acid and quercetin chitosan nanoparticles for sustained release in treating colorectal cancer. J. Drug Deliv. Sci. Technol. 2021, 63, 102523. [Google Scholar] [CrossRef]
- Choudhary, A.; Kant, V.; Jangir, B.L.; Joshi, V.G. Quercetin loaded chitosan tripolyphosphate nanoparticles accelerated cutaneous wound healing in Wistar rat. Eur. J. Pharmacol. 2020, 880, 173172. [Google Scholar] [CrossRef]
- Lan, Q.; Di, D.; Wang, S.; Zhao, Q.; Gao, Y.; Chang, D.; Jiang, T. Chitosan-N-acetylcysteine modified HP-β-CD inclusion complex as a potential ocular delivery system for anti-cataract drug: Quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101407. [Google Scholar] [CrossRef]
- Moon, H.; Lertpatipanpong, P.; Hong, Y.; Kim, C.T.; Baek, S.J. Nano-encapsulated quercetin by soluble soybean polysaccharide/chitosan enhances anti-cancer, anti-inflammation, and anti-oxidant activities. J. Funct. Foods 2021, 87, 104756. [Google Scholar] [CrossRef]
- Wang, Y.; Zi, X.Y.; Su, J.; Zhang, H.X.; Zhang, X.R.; Zhu, H.Y.; Li, J.X.; Yin, M.; Yang, F.; Hu, Y.P. Cuprous oxide nanoparticles selectively induce apoptosis of tumor cells. Int. J. Nanomed. 2012, 7, 2641–2652. [Google Scholar] [CrossRef]
- Hanagata, N.; Zhuang, F.; Connolly, S.; Li, J.; Ogawa, N.; Xu, M. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano 2011, 5, 9326–9338. [Google Scholar] [CrossRef]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar]
- Yan, N.; Shi, Y. Mechanisms of apoptosis through structural biology. Annu. Rev. Cell. Dev. Biol. 2005, 21, 35–56. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Histochem. Acta 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Chen, K.; Liao, S.; Guo, S.; Zheng, X.; Wang, B.; Duan, Z.; Zhang, H.; Gong, Q.; Luo, K. Multistimuli-responsive PEGylated polymeric bioconjugate-based nano-aggregate for cancer therapy. Chem. Eng. J. 2020, 391, 123543. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zhang, X.; Li, Z.; Zhang, Q.; Wang, W.; Pan, D.; Zheng, X.; Gu, Z.; Zhang, H.; et al. Tumor microenvironment-responsive PEGylated heparin-pyropheophorbide-a nanoconjugates for photodynamic therapy. Carbohydr. Polym. 2021, 255, 117490. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, V.; Sharma, P.K. Pharmaceutical microemulsion: Formulation, characterization and drug deliveries across skin. Int. J. Drug Dev. Res. 2014, 6, 1–21. [Google Scholar]
- Yang, R.; Shim, W.S.; Cui, F.D.; Cheng, G.; Han, X.; Jin, Q.R.; Kim, D.D.; Chung, S.J.; Shim, C.K. Enhanced electrostatic interaction between chitosan-modified PLGA nanoparticle and tumor. Int. J. Pharm. 2009, 371, 142–147. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar] [CrossRef]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschlager, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin surface pH, stratum corneum hydration, transepidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef]
- Tang, S.C.; Liao, P.Y.; Hung, S.J.; Ge, J.S.; Chen, S.M.; Lai, J.C.; Hsiao, Y.P.; Yang, J.H. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 2017, 86, 238–248. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J. Fabrication of chitosan-based functional nanocomposite films: Effect of quercetin-loaded chitosan nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Valencia, M.S.; da Silva Júnior, M.F.; Júnior, F.H.; de Oliveira Veras, B.; de Oliveira Borba, E.F.; da Silva, T.G.; Xavier, V.L.; de Souza, M.P.; das Graças Carneiro-da-Cunha, M. Bioactivity and cytotoxicity of quercetin-loaded, lecithin-chitosan nanoparticles. Agric. Biotechnol. 2021, 31, 101879. [Google Scholar] [CrossRef]

| Abnormal or Disease State | Signalling Molecules or Enzyme Activity Reduced or Increased by Quercetin | Effect | Reference |
|---|---|---|---|
| Cancer | p53↑, p21↑, Caspase-3↑ | Cell cycle arrest, Reduced angiogenesis and cell proliferation | [45] |
| Extrinsic pathways P13K/Akt↓, NFkB↓, FasL↑ Intrinsic pathways p53↑, Bax↑, Bcl-2↓, ↑FasL↑, p38 MAPK↑ | Apoptosis | ||
| MMP-9↓ | Prevention of cell migration (metastasis) | ||
| p53↑, Bax↑, Bcl-2↓, ↑FasL↑, p38 MAPK↑ | Reduction of tumour growth | ||
| Acne | TLR-2↓, DNA gyrase↓ | Inhibition of nucleic acid synthesis of bacteria | [45] |
| Psoriasis | GSH↑, SOD↑ IL1β↓, TNFα↓ | Suppresses inflammation | [45] |
| Hyper pigmentation | Tyrosinase↓ | Reduces melanogenesis | [46] |
| Diabetes | α-glucosidase and α-amylase | [40] |
| Disease Targeted | Administration Route | Type of Nanoparticle | Preparation Method | Comments | References |
|---|---|---|---|---|---|
| Breast Cancer | Intravenous injection | Hydrophilic and hydrophobic moieties attached to chitosan so that they are amphiphilic and release best at pH 5.0 | Self-assembly | Amphiphilic chitosan | [104] |
| Breast Cancer | Intraperitoneal injection | CuO NPs coated with chitosan and TPP | CuO NPs coated with chitosan using ionic gelation with TPP | Nanoparticles contain CuO | [105] |
| Lung Cancer | Intraperitoneal injection | Chitosan with TPP and targeting antibody | Ionic gelation | Paclitaxel-resistant lung cancer cells | [106] |
| Liver Cancer | Intravenous injection | pH and redox responsive with trimethyl chitosan, disulphide bridges and PEG | Self-assembly, sonication but see original paper for details | Co-delivery of quercetin with doxorubicin, which act synergistically | [107] |
| Colorectal Cancer | Oral | Lipid core of glyceryl mono oleate with chitosan shell | o/w nanoemulsion with sonication, high-pressure homogenisation and lyophilisation | Co-delivery of quercetin with gallic acid | [108] |
| UVB damage to skin | dermal | Chitosan with TPP | Ionic gelation | In-vitro and in-vivo studies | [1] |
| Wound healing | dermal | Chitosan with TPP | Ionic gelation | Wister rat model | [109] |
| Cataract prevention | Topical application to cornea | Cyclodextrin inclusion complex coated with chitosan-N-acetyl-l-cysteine complex | Solvent evaporation method | Improvement of permeability into lens due to thiol groups of cysteine and perhaps chitosan. Evidence for prevention of cataract is weak | [110] |
| General antioxidant (cancer prevention, anti-inflammation) | Oral as liquid dosage form | Soybean polysaccharide (SSPS)/chitosan | pH-driven encapsulation method, with quercetin added to SSPS at pH 12.0 and then adjusted to pH 7 before adding chitosan | Chitosan is the minor ingredient that makes SSPS nanoparticles more stable | [111] |
| HepG-2 | MCF-7 | CaCO-2 | WI38 | |
|---|---|---|---|---|
| CuO NPs | 38.79 ± 2.8 | 55.65 ± 3.4 | 66.67 ± 3.7 | 93.13 ± 6.91 |
| CuO-ChNPs_Q | 26.08 ± 2.3 | 46.89 ± 2.9 | 54.29 ± 3.4 | 215.6 ± 24.7 |
| Free quercetin | 103.9 ± 13.7 | 118.55 ± 22.5 | 69.34 ± 4.6 | 454.5 ± 48.1 |
| Doxorubicin | 4.50 ± 0.2 | 4.17 ± 0.2 | 12.49 ± 1.1 | 6.72 ± 0.5 |
| Treatment | Lining Epithelium | Tumour Mass | Neoplastic Cells |
|---|---|---|---|
| CONTROL healthy rats untreated | Slight expression of caspase-3 and PCNA | ||
| DMBA-induced rats | Overexpression of PCNA | Slight expression of caspase-3 | Low expression of caspase-3 |
| CuO NPs on DMBA-induced rats | Increase in caspase-3 Decrease in PCNA | ||
| Free quercetin on DMBA-induced rats | Increase in caspase-3 Decrease in PCNA | ||
| CuO-ChNPs-Q on DMBA-induced rats | Marked decrease in PCNA | Marked increase in caspase-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, M.K. Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications. Int. J. Mol. Sci. 2023, 24, 3293. https://doi.org/10.3390/ijms24043293
Lawson MK. Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications. International Journal of Molecular Sciences. 2023; 24(4):3293. https://doi.org/10.3390/ijms24043293
Chicago/Turabian StyleLawson, Michael Kenneth. 2023. "Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications" International Journal of Molecular Sciences 24, no. 4: 3293. https://doi.org/10.3390/ijms24043293
APA StyleLawson, M. K. (2023). Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications. International Journal of Molecular Sciences, 24(4), 3293. https://doi.org/10.3390/ijms24043293






