Functional Study of PgGRAS68-01 Gene Involved in the Regulation of Ginsenoside Biosynthesis in Panax ginseng
Abstract
:1. Introduction
2. Results
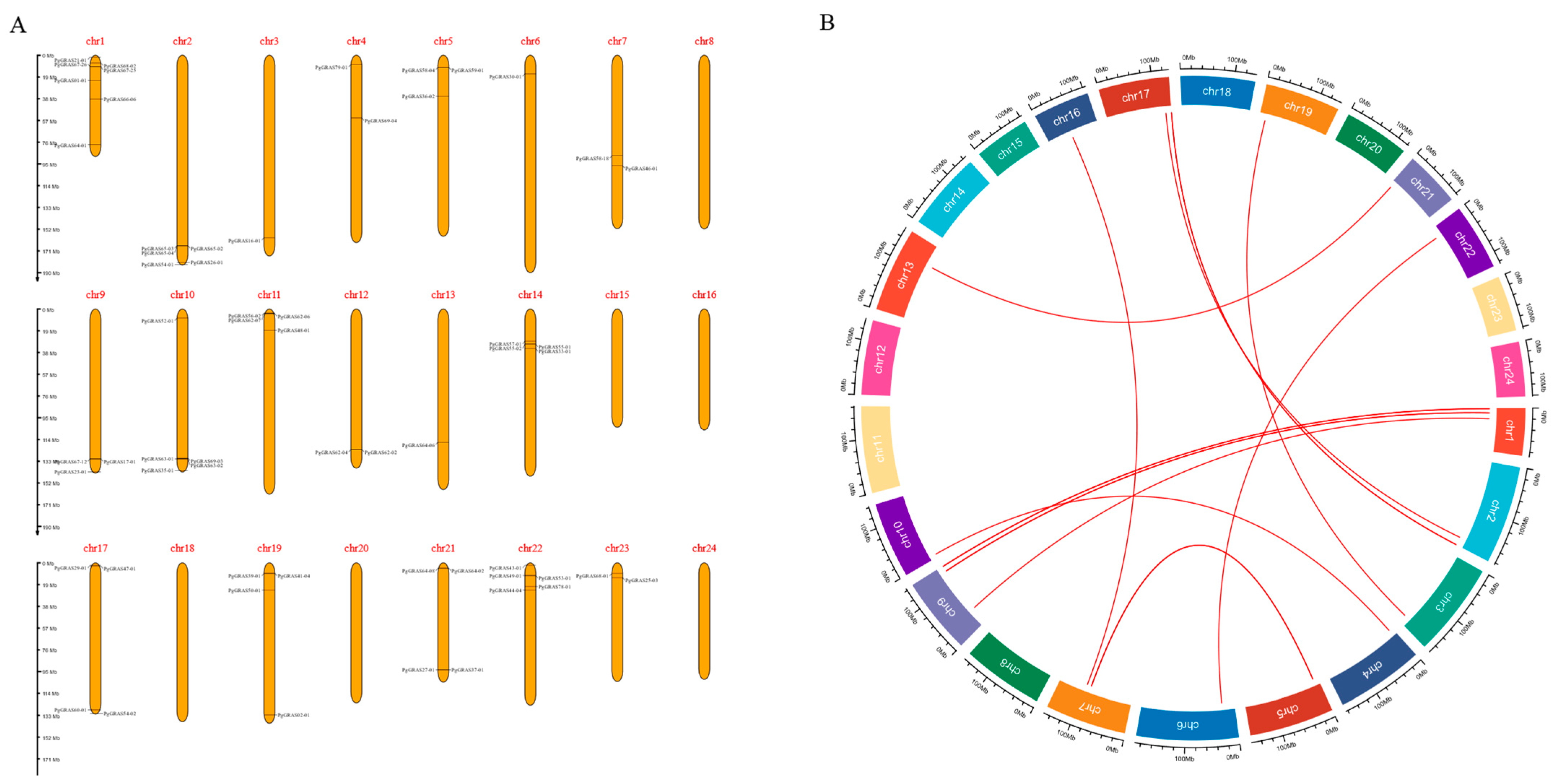
2.1. Chromosome Distribution and Gene Replication of PgGRAS Gene
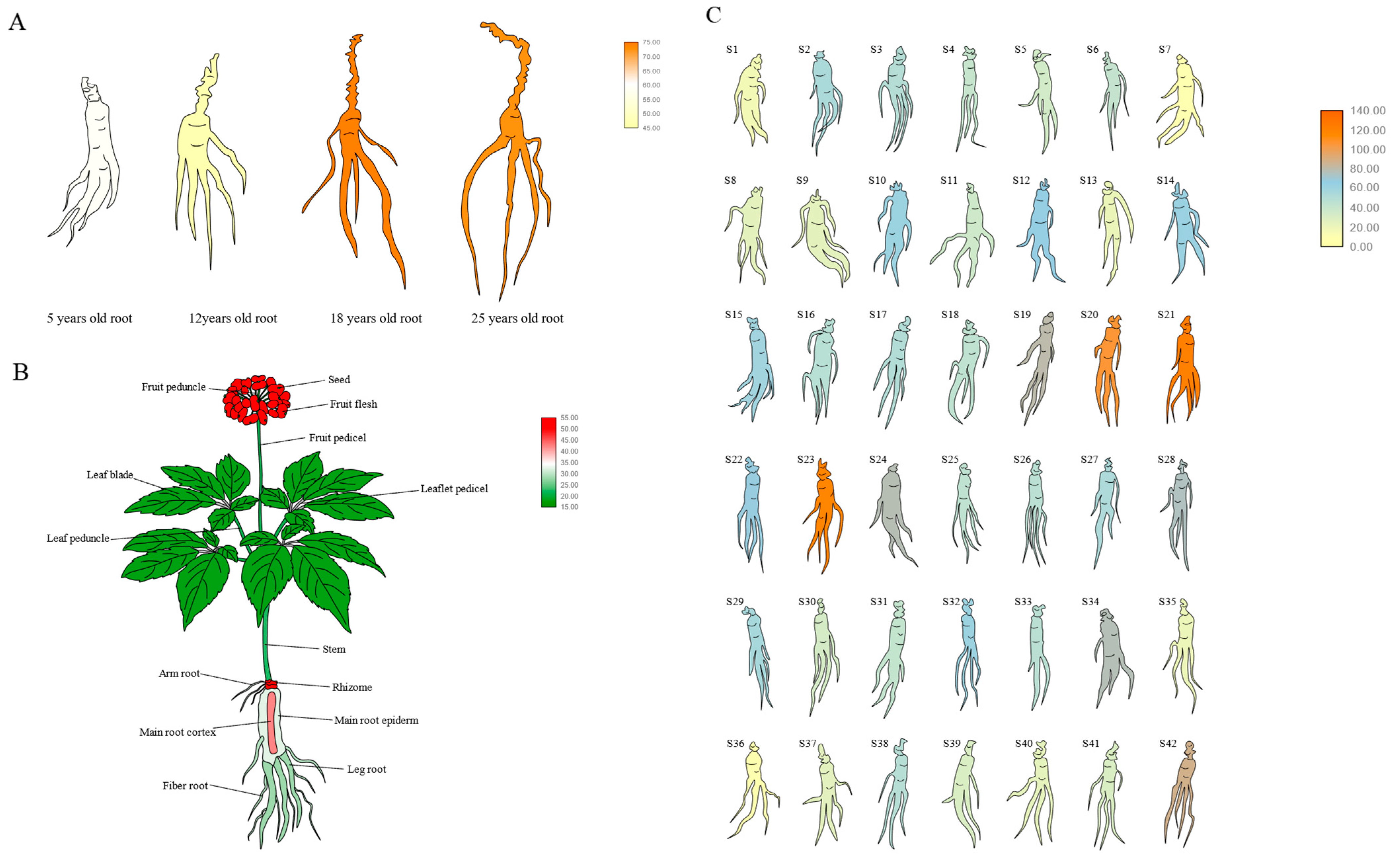
2.2. GA Treatment Affects the Synthesis of Ginsenosides
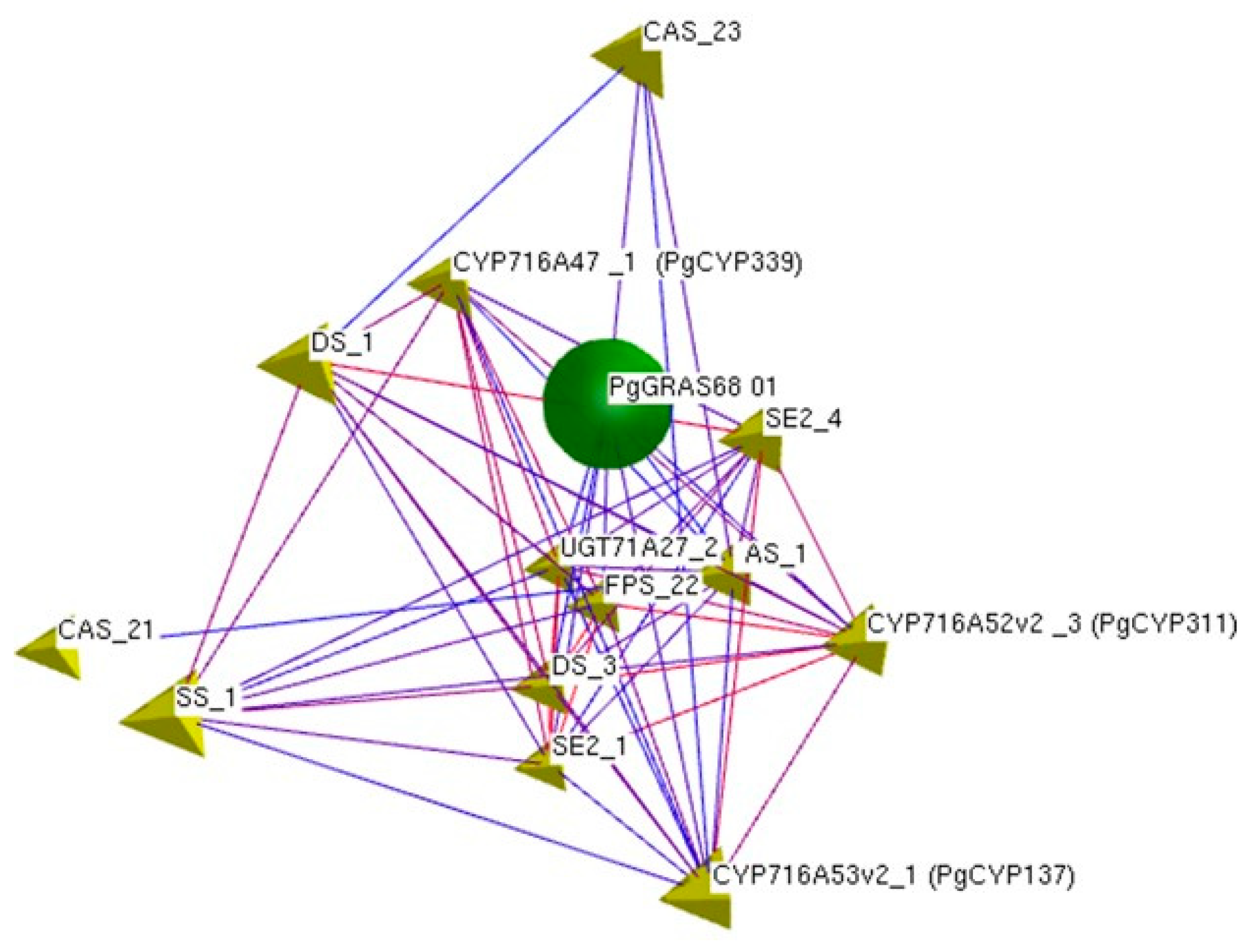
2.3. Screening of GRAS Candidate Genes Involved in Ginsenoside Biosynthesis
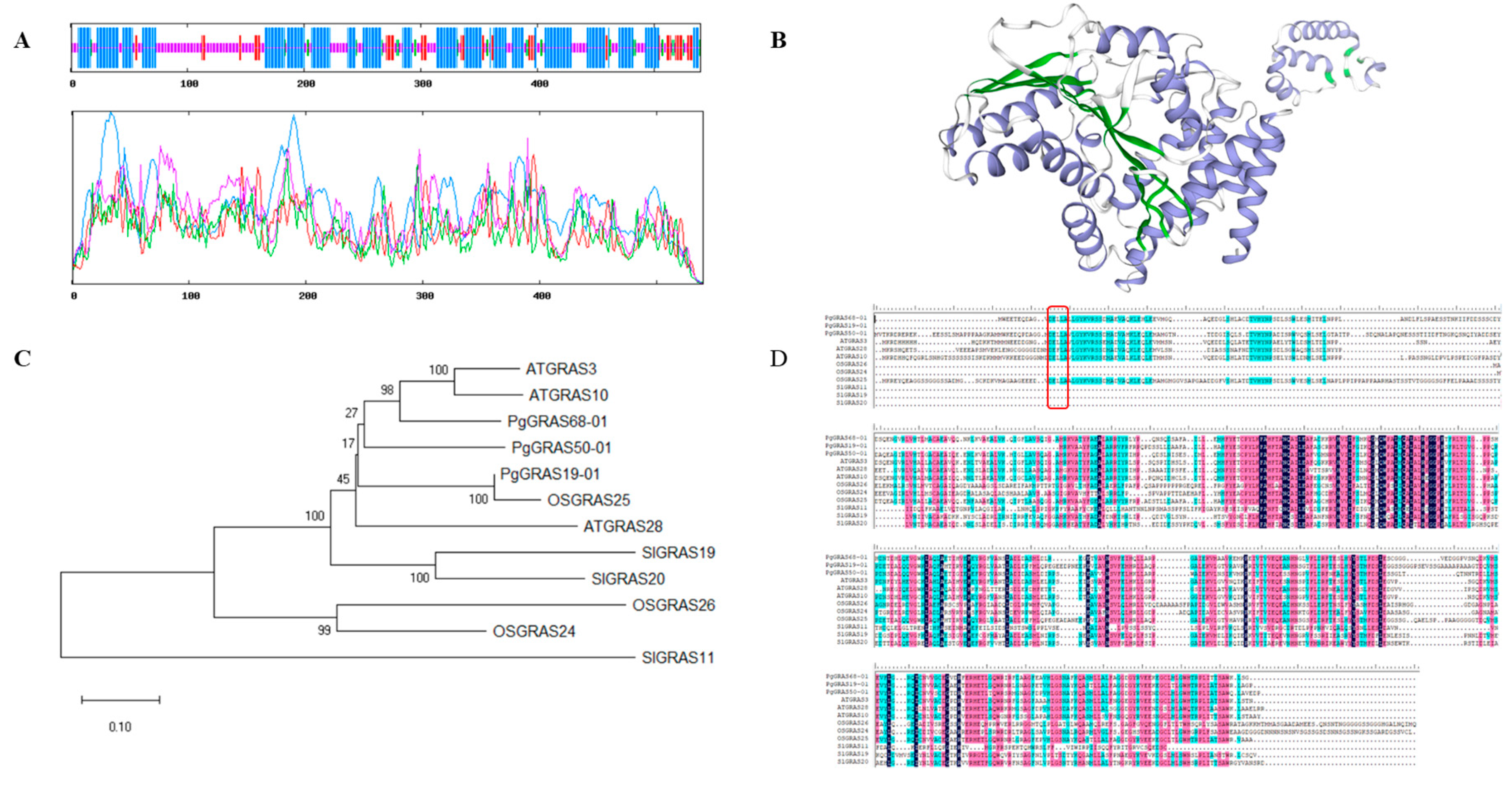
2.4. Sequence Analysis of PgGRAS68-01 Gene
2.5. Expression Pattern Analysis of PgGRAS68-01 Gene in Ginseng
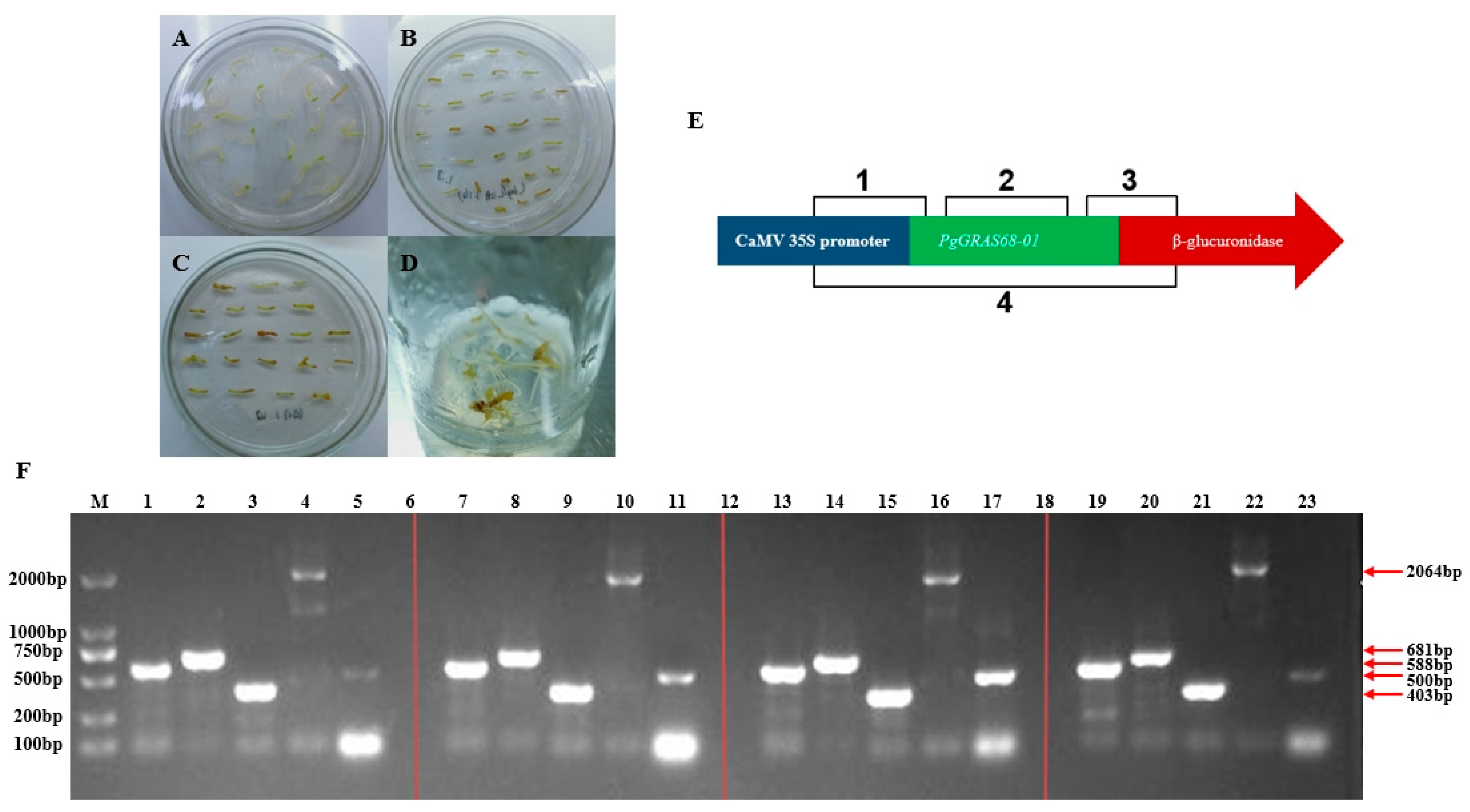
2.6. Cloning and Vector Construction of PgGRAS68-01 Gene
2.7. Genetic Transformation of Ginseng by Recombinant Plasmid
2.8. Detection of Ginsenoside Content in Positive Ginseng Hairy Roots
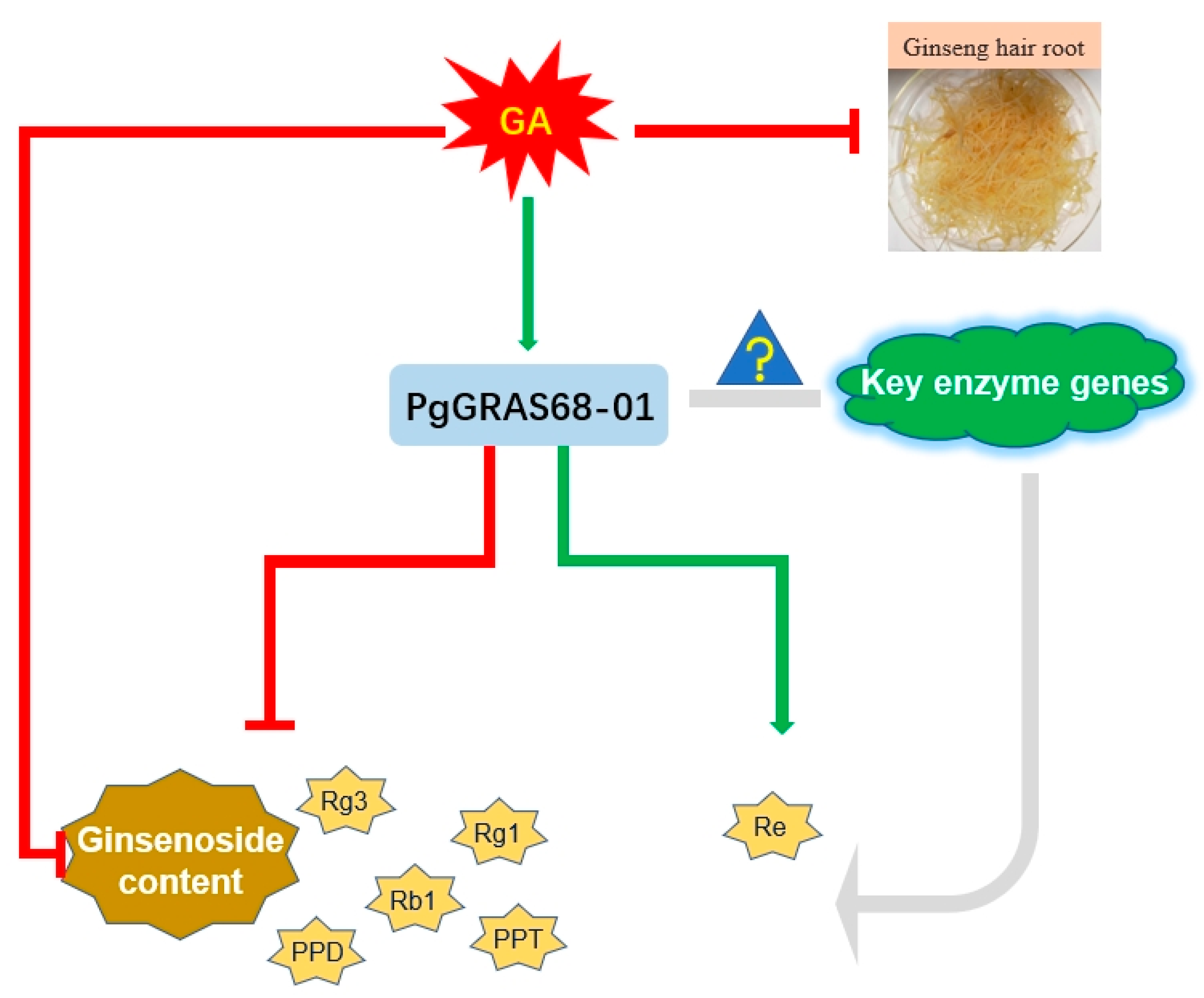
3. Discussion
4. Materials and Methods
4.1. Data and Materials
4.2. PgGRAS Gene Duplication and Chromosome Localization
4.3. Culture of Ginseng Hair Roots Induced by Gibberellin and Analysis of Saponin Content Change
4.4. Identification of PgGRAS Genes Related to Ginsenoside Synthesis
4.5. Characteristic Analysis of PgGRAS68-01 Gene
4.6. Expression Analysis of PgGRAS68-01 Gene
4.7. Cloning of PgGRAS68-01 Gene
4.8. Construction of Vector and Genetic Transformation of Ginseng
4.9. Ginsenoside Content of Positive Hair Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, M.Q.; Wang, R.F.; Zhao, S.J.; Wang, Z.T. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar]
- Lu, J.; Li, J.X.; Wang, S.H.; Yao, L.; Liang, W.X.; Wang, J.; Gao, W.Y. Advances in ginsenoside biosynthesis and metabolic regulation. Biotechnol. Appl. Bioc. 2018, 65, 514–522. [Google Scholar]
- Tian, M.; Li, L.N.; Zheng, R.R.; Yang, L.; Wang, Z.T. Advances on hormone-like activity of Panax ginseng and ginsenosides. Chin. J. Nat. Med. 2020, 18, 526–535. [Google Scholar] [CrossRef]
- Kim, Y.S.; Han, J.Y.; Lim, S.; Choi, Y.E. Ginseng metabolic engineering: Regulation of genes related to ginsenoside biosynthesis. J. Med. Plants Res. 2009, 3, 1270–1276. [Google Scholar]
- Yao, L.; Lu, J.; Wang, J.; Gao, W.Y. Advances in biosynthesis of triterpenoid saponins in medicinal plants. Chin. J. Nat. Med. 2020, 18, 417–424. [Google Scholar] [PubMed]
- Yonekura-Sakakibara, K.; Hanada, K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011, 66, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Li, P.; Lu, X. Research advances in cytochrome P450-catalysed pharmaceutical terpenoid biosynthesis in plants. J. Exp. Bot. 2019, 70, 4619–4630. [Google Scholar]
- Cenci, A.; Rouard, M. Evolutionary analyses of GRAS transcription factors in Angiosperms. Front. Plant Sci. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Pysh, L.D.; Wysocka-Diller, J.W.; Camilleri, C.; Bouchez, D.; Benfey, P.N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999, 18, 111–119. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cao, Y.P.; Shang, C.; Lie, J.K.; Wang, J.L.; Wu, Z.Y.; Ma, L.C.; Qi, T.X.; Fu, C.X.; Bai, Z.T.; et al. Genome-wide characterization of GRAS family genes in Medicago truncatula reveals their evolutionary dynamics and functional diversification. PLoS ONE 2017, 12, e0185439. [Google Scholar]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Song, S.K.; Heo, J.O.; Yu, N.I.; Lee, S.A.; Kim, M.; Kim, D.G.; Sohn, S.O.; Lim, C.E.; et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 659–670. [Google Scholar] [CrossRef]
- Khan, Y.; Xiong, Z.; Zhang, H.; Liu, S.; Yaseen, T.; Hui, T. Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation-a review. Plant Bioloyg. 2022, 24, 404–416. [Google Scholar]
- Okada, K.; Ito, T.; Fukazawa, J.; Takahashi, Y. Gibberellin induces an increase in cytosolic Ca2+ via a DELLA-independent signaling pathway. Plant Physiol. 2017, 175, 1536–1542. [Google Scholar]
- Yamamoto, Y.; Hirai, T.; Yamamoto, E.; Kawamura, M.; Sato, T.; Kitano, H.; Matsuoka, M.; Ueguchi-Tanaka, M. A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. Plant Cell. 2010, 22, 3589–3602. [Google Scholar]
- Thomas, S.G.; Rieu, I.; Steber, C.M. Gibberellin metabolism and signaling. Vitam Horm. 2005, 72, 289–338. [Google Scholar]
- Xu, H.; Liu, Q.; Yao, T.; Fu, X.D. Shedding light on integrative GA signaling. Curr. Opin. Plant Biol. 2014, 21, 89–95. [Google Scholar]
- Liu, S.S.; Chen, J.; Li, S.C.; Zeng, X.; Meng, Z.X.; Guo, S.X. Comparative transcriptome analysis of genes involved in GA-GID1-DELLA regulatory module in symbiotic and asymbiotic seed germination of Anoectochilus roxburghii (Wall.) Lind1. (Orchidaceae). Int. J. Mol. Sci. 2015, 16, 30190–30203. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, Z.J.; Liu, J.P.; Lin, S.; Wang, J.F.; Lin, W.X.; Xu, W.F. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017, 36, 557–569. [Google Scholar]
- Zhou, W.; Wang, S.; Shen, Y.; Liu, Y.; Maoz, I.; Gao, X.; Chen, C.; Liu, T.; Wang, C.; Kai, G. Overexpression of SmSCR1 promotes tanshinone accumulation and hairy root growth in Salvia miltiorrhiza. Front. Plant Sci. 2022, 13, 860033. [Google Scholar]
- Zeng, X.; Ling, H.; Chen, X.; Guo, S. Genome-wide identification, phylogeny and function analysis of GRAS gene family in Dendrobium catenatum (Orchidaceae). Gene 2019, 705, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; He, Q.; Wang, Y.; Liang, G.; Liu, T. Genome-wide identification and characterization of the GRAS transcription factors in garlic (Allium sativum L.). Front. Plant Sci. 2022, 13, 890052. [Google Scholar] [PubMed]
- Wang, N.; Wang, K.; Li, S.; Jiang, Y.; Li, L.; Zhao, M.; Jiang, Y.; Zhu, L.; Wang, Y.; Su, Y.; et al. Transcriptome-wide identification, evolutionary analysis, and GA stress response of the GRAS gene family in Panax ginseng C. A. Meyer. Plants 2020, 9, 190. [Google Scholar]
- Kumari, P.; Gahlaut, V.; Kaur, E.; Singh, S.; Kumar, S.; Jaiswal, V. Genome-wide identification of GRAS transcription factors and their potential roles in growth and development of Rose (Rosa chinensis). J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Tian, C.; Wan, P.; Sun, S.; Li, J.; Chen, M. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol. Biol. 2004, 54, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xian, Z.Q.; Kang, X.; Tang, N.; Li, Z.G. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Grimplet, J.; Agudelo-Romero, P.; Teixeira, R.T.; Martinez-Zapater, J.M.; Fortes, A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016, 7, 353. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, B.; Wei, X. Genome wide identification and expression pattern analysis of the GRAS family in quinoa. Funct. Plant Biol. 2021, 48, 948–962. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Sun, Y.; Qin, Z.; Feng, P. Genome-wide analysis and characterization of GRAS family in switchgrass. Bioengineered 2021, 12, 6096–6114. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, X.B.; Lai, D.L.; Yang, H.; Feng, L.; Li, L.; Niu, K.X.; Chen, L.; Xiang, D.B.; Ruan, J.J.; et al. Genome-wide investigation of the GRAS transcription factor family in foxtail millet (Setaria italica L.). BMC Plant Biol. 2021, 21, 508. [Google Scholar] [CrossRef]
- Fan, Y.; Yan, J.; Lai, D.; Yang, H.; Xue, G.; He, A.; Guo, T.; Chen, L.; Cheng, X.B.; Xiang, D.B.; et al. Genome-wide identification, expression analysis, and functional study of the GRAS transcription factor family and its response to abiotic stress in sorghum [Sorghum bicolor (L.) Moench]. BMC Genom. 2021, 22, 509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Wang, X.; Sun, M.; Song, R.; Mao, P.; Jia, S. Genome-wide identification of GRAS gene family and their responses to abiotic Stress in Medicago sativa. Int. J. Mol. Sci. 2021, 22, 7729. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Sun, C.; Lin, Y.; Yin, R.; Wang, Y.; Zhang, M. The spatial and temporal transcriptomic landscapes of ginseng, Panax ginseng C. A. Meyer. Sci. Rep. 2015, 5, 18283. [Google Scholar]
- Wang, Z.H.; Wang, X.F.; Lu, T.; Li, M.R.; Jiang, P.; Zhao, J.; Liu, S.T.; Fu, X.Q.; Wendel, J.F.; Van, P.Y.; et al. Reshuffling of the ancestral core-eudicot genome shaped chromatin topology and epigenetic modification in Panax. Nat. Commun. 2022, 13, 1902. [Google Scholar]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo, C.T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar]





| Time (min) | Solvent A (%) | Solvent B (%) | Velocity (mL/min) |
|---|---|---|---|
| 0–40 | 18–21 | 82–79 | 1 |
| 40–42 | 21–26 | 79–74 | 1 |
| 42–46 | 26–32 | 74–68 | 1 |
| 46–66 | 32–33.5 | 68–66.5 | 1 |
| 66–71 | 33.5–38 | 66.5–62 | 1 |
| 71–86 | 38–65 | 62–35 | 1 |
| 86–91 | 65 | 35 | 1 |
| 91–96 | 65–85 | 35–15 | 1 |
| 96–103 | 85 | 15 | 1 |
| 103–105 | 85–18 | 15–82 | 1 |
| 105–106 | 18 | 82 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, K.; Yun, Z.; Liu, W.; Zhao, M.; Wang, Y.; Hu, J.; Liu, T.; Wang, N.; Wang, Y.; et al. Functional Study of PgGRAS68-01 Gene Involved in the Regulation of Ginsenoside Biosynthesis in Panax ginseng. Int. J. Mol. Sci. 2023, 24, 3347. https://doi.org/10.3390/ijms24043347
Liu C, Wang K, Yun Z, Liu W, Zhao M, Wang Y, Hu J, Liu T, Wang N, Wang Y, et al. Functional Study of PgGRAS68-01 Gene Involved in the Regulation of Ginsenoside Biosynthesis in Panax ginseng. International Journal of Molecular Sciences. 2023; 24(4):3347. https://doi.org/10.3390/ijms24043347
Chicago/Turabian StyleLiu, Chang, Kangyu Wang, Ziyi Yun, Wenbo Liu, Mingzhu Zhao, Yanfang Wang, Jian Hu, Tao Liu, Nan Wang, Yi Wang, and et al. 2023. "Functional Study of PgGRAS68-01 Gene Involved in the Regulation of Ginsenoside Biosynthesis in Panax ginseng" International Journal of Molecular Sciences 24, no. 4: 3347. https://doi.org/10.3390/ijms24043347
APA StyleLiu, C., Wang, K., Yun, Z., Liu, W., Zhao, M., Wang, Y., Hu, J., Liu, T., Wang, N., Wang, Y., & Zhang, M. (2023). Functional Study of PgGRAS68-01 Gene Involved in the Regulation of Ginsenoside Biosynthesis in Panax ginseng. International Journal of Molecular Sciences, 24(4), 3347. https://doi.org/10.3390/ijms24043347







