Inhibition of Canonical Transient Receptor Potential Channels 4/5 with Highly Selective and Potent Small-Molecule HC-070 Alleviates Mechanical Hypersensitivity in Rat Models of Visceral and Neuropathic Pain
Abstract
:1. Introduction
2. Results
2.1. Assessment of Primary Pharmacology of HC-070 Using Manual Patch-Clamp
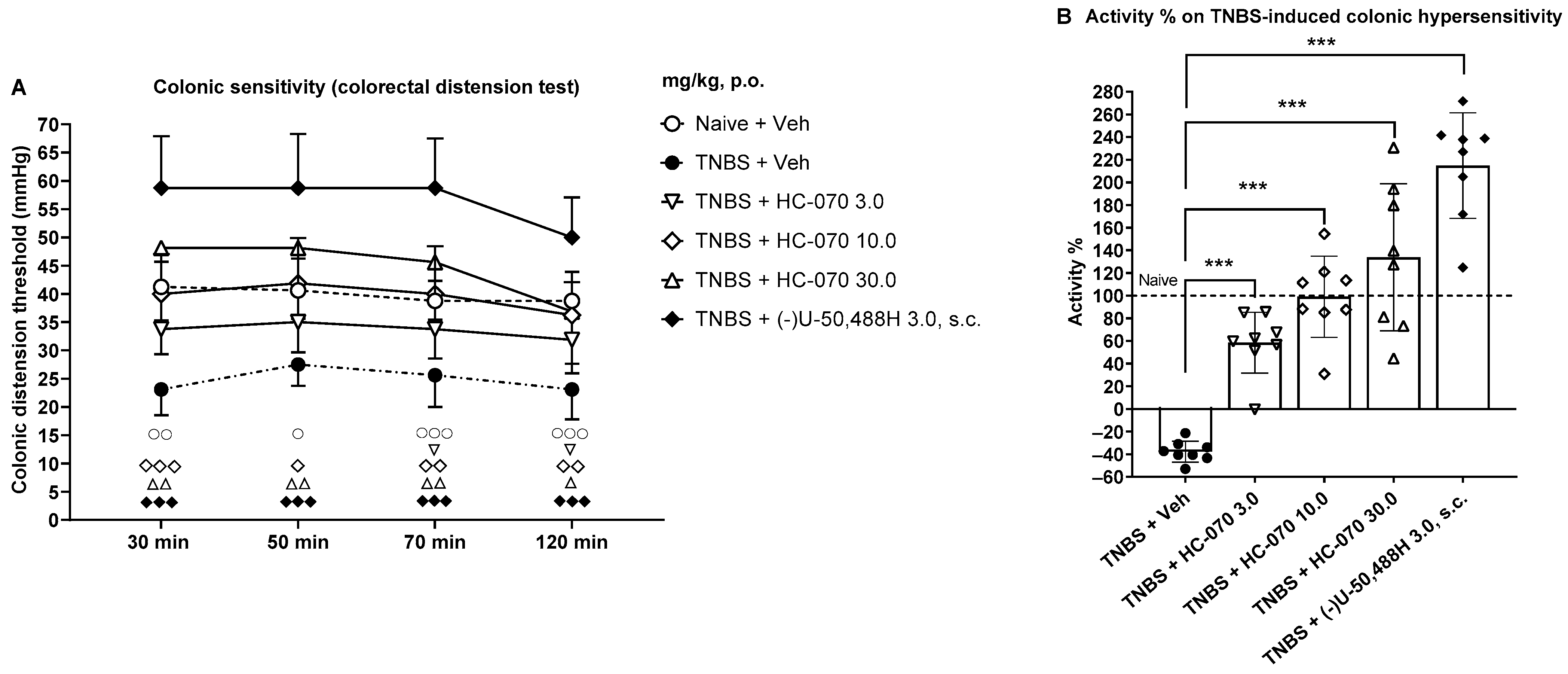
2.2. Anti-Hypersensitivity Effect of TRPC4/5 Antagonist HC-070 in the Model of Trinitrobenzene Sulfonic Acid-Induced Colonic Hypersensitivity in Male Rats
2.3. Colonic Anti-Hypersensitivity Effect of TRPC4/5 Antagonist HC-070 in the Model of Partial Restraint Stress in Female Rats
2.4. Mechanical Anti-Hypersensitivity Effect of TRPC4/5 Antagonist HC-070 in the Model of Chronic Constriction Injury in Male Rats
2.5. HC-070 Pharmacokinetics in Rat Indicates Ready Access to the Brain
3. Discussion
4. Materials and Methods
4.1. Patch-Clamp Recordings to Assess HC-070 Potency on Human TRPC4
4.2. Experimental Animals
4.3. Assessment of Colonic Hypersensitivity in the Rat Model of Trinitrobenzene Sulfonic Acid-Induced Colonic Hypersensitivity
4.4. Assessment of Colonic Hypersensitivity in the Model of Partial Restraint Stress in Female Rats
4.5. Measurement of the Visceral Pain Threshold Using the Colonic Distension Test in Rat TNBS and PRS Models
4.6. Assessment of Mechanical Hypersensitivity Using the Paw Pressure or Randall and Selitto Test in the Model of Peripheral Mononeuropathy in Male Rats
4.7. Pharmacokinetics and Bioanalytical
4.8. Plasma Protein Binding and Brain Homogenate Binding
4.9. Drugs
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamm, M.A. Rapid changes in epidemiology of inflammatory bowel disease. Lancet 2017, 390, 2741–2742. [Google Scholar] [PubMed]
- Ng, S.; Shi, H.Y.; Hamidi, N.; Underwood, F.; Tang, W.; Benchimol, E.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, J.; Ak, M.; Müller-Mottet, S.; Scharl, S.; Biedermann, L.; Fournier, N.; Frei, P.; Pittet, V.; Scharl, M.; Fried, M. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS ONE 2016, 11, e0156666. [Google Scholar]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar]
- Eijsbouts, C.; Zheng, T.; Kennedy, N.A.; Bonfiglio, F.; Anderson, C.A.; Moutsianas, L.; Holliday, J.; Shi, J.; Shringarpure, S.; 23 and Me Research Team. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021, 53, 1543–1552. [Google Scholar]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef]
- Brierley, S.M.; Linden, D.R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627. [Google Scholar]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Swallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Buniel, M.; Wisnoskey, B.; Glazebrook, P.A.; Schilling, W.P.; Kunze, D.L. Distribution of TRPC channels in the visceral sensory pathway. Novartis Found. Symp. 2004, 258, 236–243. [Google Scholar]
- Sadler, K.E.; Moehring, F.; Shiers, S.I.; Laskowski, L.J.; Mikesell, A.R.; Plautz, Z.R.; Brezinski, A.N.; Mecca, C.M.; Dussor, G.; Price, T.J.; et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 2021, 13, eabd7702. [Google Scholar] [CrossRef]
- Tavares-Ferreira, D.; Shiers, S.; Ray, P.R.; Wangzhou, A.; Jeevakumar, V.; Sankaranarayanan, I.; Cervantes, A.M.; Reese, J.C.; Chamessian, A.; Copits, B.A.; et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 2022, 14, eabj8186. [Google Scholar] [CrossRef]
- Zimmermann, K.; Lennerz, J.K.; Hein, A.; Link, A.S.; Kaczmarek, J.S.; Delling, M.; Uysal, S.; Pfeifer, J.D.; Riccio, A.; Clapham, D.E. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 18114–18119. [Google Scholar]
- Corder, G.; Ahanonu, B.; Grewe, B.F.; Wang, D.; Schnitzer, M.J.; Scherrer, G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 2019, 363, 276–281. [Google Scholar] [CrossRef]
- Fowler, M.A.; Sidiropoulou, K.; Ozkan, E.D.; Phillips, C.W.; Cooper, D.C. Corticolimbic Expression of TRPC4 and TRPC5 Channels in the Rodent Brain. PLoS ONE 2007, 2, e573. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.; Silva, R.; Pinto-Ribeiro, F.; Pêgo, J.M.; Bessa, J.M.; Pertovaara, A.; Sousa, N.; Almeida, A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp. Neurol. 2008, 213, 48–56. [Google Scholar] [CrossRef]
- Riccio, A.; Medhurst, A.D.; Mattei, C.; Kelsell, R.E.; Calver, A.R.; Randall, A.D.; Benham, C.D.; Pangalos, M.N. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Mol. Brain Res. 2002, 109, 95–104. [Google Scholar] [CrossRef]
- Riccio, A.; Li, Y.; Moon, J.; Kim, K.-S.; Smith, K.S.; Rudolph, U.; Gapon, S.; Yao, G.L.; Tsvetkov, E.; Rodig, S.J.; et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 2009, 137, 761–772. [Google Scholar] [CrossRef]
- Riccio, A.; Li, Y.; Tsvetkov, E.; Gapon, S.; Yao, G.L.; Smith, K.S.; Engin, E.; Rudolph, U.; Bolshakov, V.Y.; Clapham, D.E. Decreased anxiety-aike behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 Channels. J. Neurosci. 2014, 34, 3653–3667. [Google Scholar] [CrossRef]
- Wei, H.; Sagalajev, B.; Yüzer, M.A.; Koivisto, A.; Pertovaara, A. Regulation of neuropathic pain behavior by amygdaloid TRPC4/5 channels. Neurosci. Lett. 2015, 608, 12–17. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Z.; Lei, J.; You, H.J.; Pertovaara, A. Reduced mechanical hypersensitivity by inhibition of the amygdala in experimental neuropathy: Sexually dimorphic contribution of spinal neurotransmitter receptors. Brain Res. 2022, 17, 148128. [Google Scholar] [CrossRef]
- Bernal, L.; Sotelo-Hitschfeld, P.; König, C.; Sinica, V.; Wyatt, A.; Winter, Z.; Hein, A.; Touska, F.; Reinhardt, S.; Tragl, A.; et al. Odontoblast TRPC5 channels signal cold pain in teeth. Sci. Adv. 2021, 7, eabf5567. [Google Scholar] [CrossRef]
- Faber, E.S.L.; Sedlak, P.; Vidovic, M.; Sah, P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience 2006, 137, 781–794. [Google Scholar] [CrossRef]
- Gomis, A.; Soriano, S.; Belmonte, C.; Viana, F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J. Physiol. 2008, 586, 5633–5649. [Google Scholar] [CrossRef]
- Just, S.; Chenard, B.L.; Ceci, A.; Strassmaier, T.; Chong, J.A.; Blair, N.T.; Gallaschun, R.J.; del Camino, D.; Cantin, S.; D’Amours, M.; et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE 2018, 13, e0191225. [Google Scholar] [CrossRef]
- Rubaiy, H. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019, 176, 832–846. [Google Scholar] [CrossRef]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.-B.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a Novel Potent Antagonist That Selectively Modulates Native TRPC4/C5 Ion Channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, L.P.; Ma, F.; Nesemeier, R.; Ruiz, J.C.; Ostertag, E.M.; Crawford, J.S.; Babinski, K.; Marcinkiewicz, M.M. A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 2014, 262, 165–175. [Google Scholar] [CrossRef]
- Griffin, C.S.; Thornbury, K.D.; Hollywood, M.A.; Sergeant, G.P. Muscarinic receptor-induced contractions of the detrusor are impaired in TRPC4 deficient mice. Sci. Rep. 2018, 8, 9264. [Google Scholar] [CrossRef]
- Zhou, Y.; Castonguay, P.; Sidhom, E.H.; Clark, A.R.; Dvela-Levitt, M.; Kim, S.; Sieber, J.; Wieder, N.; Jung, J.Y.; Andreeva, S.; et al. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 2017, 358, 1332–1336. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Bauer, C.C.; Pickles, I.B.; Hosseini-Farahabadi, S.; Balgi, A.D.; Choi, K.; Linley, D.M.; Beech, D.J.; Roberge, M.; Bon, R.S. Nonselective TRPC channel inhibition and suppression of aminoglycoside-induced premature termination codon readthrough by the small molecule AC1903. J. Biol. Chem. 2022, 298, 101546. [Google Scholar] [CrossRef]
- Diop, L.; Raymond, F.; Fargeau, H.; Petoux, F.; Chovet, M.; Doherty, A.M. Pregabalin (CI-1008) Inhibits the Trinitrobenzene Sulfonic Acid-Induced Chronic Colonic Allodynia in the Rat. J. Pharmacol. Exp. Ther. 2002, 302, 1013–1022. [Google Scholar] [CrossRef]
- Al–Chaer, E.D.; Kawasaki, M.; Pasricha, P.J. A New Model of Chronic Visceral Hypersensitivity in Adult Rats Induced by Colon Irritation During Postnatal Development. Gastroenterology 2000, 119, 1276–1285. [Google Scholar] [CrossRef]
- Bradesi, S.; Eutamene, H.; Garcia-Villar, R.; Fioramonti, J.; Buéno, L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol. Motil. 2002, 14, 75–82. [Google Scholar] [CrossRef]
- Kim, J.; Ko, J.; Hong, C.; So, I. Structure–Function Relationship and Physiological Roles of Transient Receptor Potential Canonical (TRPC) 4 and 5 Channels. Cells 2019, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Kollewe, A.; Schwarz, Y.; Oleinikov, K.; Raza, A.; Haupt, A.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Ectors, F.; Bildl, W. Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes. Neuron 2022, 110, 1–14. [Google Scholar] [CrossRef]
- Ray, P.; Torck, A.; Quigley, L.; Wangzhou, A.; Neiman, M.; Rao, C.; Lam, T.; Kim, J.-Y.; Kim, T.H.; Zhang, M.Q.; et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018, 159, 1325–1345. [Google Scholar] [CrossRef]
- Shen, B.; Wong, C.-O.; Lau, O.-C.; Woo, T.; Bai, S.; Huang, Y.; Yao, X. Plasma membrane mechanical stress activates TRPC5 channels. PLoS ONE 2015, 10, e0122227. [Google Scholar] [CrossRef]
- Drossman, D.A.; Creed, F.H.; Olden, K.W.; Svedlund, J.; Toner, B.B.; Whitehead, W.E. Psychosocial aspects of the functional gastrointestinal disorders. Gut 1999, 45 (Suppl. 2), II25–II30. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.J.; Camilleri, M.; Zinsmeister, A.R.; Hanson, R.B. Psychosensory Modulation of Colonic Sensation in the Human Transverse and Sigmoid Colon. Gastroenterology 1995, 109, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Gebhart, G.F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994, 107, 271–293. [Google Scholar] [CrossRef]
- Ness, T.J.; Metcalf, A.M.; Gebhart, G.F. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 1990, 43, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 1973, 14, 125–132. [Google Scholar] [CrossRef]
- Boesmans, W.; Owsianik, G.; Tack, J.; Voets, T.; Berghe, P.V. TRP channels in neurogastroenterology: Opportunities for therapeutic intervention. Br. J. Pharmacol. 2011, 162, 18–37. [Google Scholar] [CrossRef]
- Hockley, J.R.F.; Taylor, T.S.; Callejo, G.; Wilbrey, A.L.; Gutteridge, A.; Bach, K.; Winchester, W.J.; Bulmer, D.C.; McMurray, G.; Smith, E.S.J. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019, 68, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. 1957, 111, 409–419. [Google Scholar]
- Diop, L.; Rivière, P.J.; Pascaud, X.; Dassaud, M.; Junien, J.L. Role of vagal afferents in the antinociception produced by morphine and U-50,488H in the colonic pain reflex in rats. Eur. J. Pharmacol. 1994, 257, 181–187. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Su, X.; Joshi, S.; Ozaki, N.; Sengupta, J.N. Peripheral opioid modulation of visceral pain. Ann. N. Y. Acad. Sci. 2000, 909, 41–50. [Google Scholar] [CrossRef]
- Meier, M.L.; Stämpfli, P.; Vrana, A.; Humphreys, B.K.; Seifritz, E.; Hotz-Boendermaker, S. Neural Correlates of Fear of Movement in Patients with Chronic Low Back Pain vs. Pain-Free Individuals. Front. Hum. Neurosci. 2016, 10, 386. [Google Scholar]
- Meier, M.L.; Stämpfli, P.; Humphreys, B.K.; Vrana, A.; Seifritz, E.; Schweinhardt, P. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain. Pain Rep. 2017, 2, e601. [Google Scholar] [CrossRef]
- Meier, M.L.; Vrana, A.; Humphreys, B.K.; Seifritz, E.; Stämpfli, P.; Schweinhardt, P. Pain-Related Fear—Dissociable Neural Sources of Different Fear Constructs. eNeuro 2019, 5. [Google Scholar] [CrossRef]
- Traub, R.J.; Silva, E.; Gebhart, G.F.; Solodkin, A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci. Lett. 1996, 215, 165–168. [Google Scholar]
- Chowdhury, G.M.; Fujioka, T.; Nakamura, S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res. Bull. 2000, 52, 171–182. [Google Scholar]
- Crock, L.W.; Kolber, B.J.; Morgan, C.D.; Sadler, K.A.; Vogt, S.K.; Bruchas, M.R.; Gereau, R.W., 4th. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J. Neurosci. 2012, 32, 14217–14226. [Google Scholar]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Yang, D.; Jacobson, A.; Meerschaert, K.A.; Sifakis, J.J.; Wu, M.; Chen, X.; Yang, T.; Zhou, Y.; Anekal, P.V.; Rucker, R.A.; et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 2022, 185, 4190–4205. [Google Scholar] [CrossRef]
- Amores-Bonet, L.; Kleene, R.; Theis, T.; Schachner, M. Interactions between the Polysialylated Neural Cell Adhesion Molecule and the Transient Receptor Potential Canonical Channels 1, 4, and 5 Induce Entry of Ca2+ into Neurons. Int. J. Mol. Sci. 2022, 23, 10027. [Google Scholar] [CrossRef]
- King, E.A.; Davis, J.W.; Degner, J.F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019, 15, e1008489. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalava, N.; Kaskinoro, J.; Chapman, H.; Morales, M.; Metsänkylä, H.; Heinonen, S.-M.; Koivisto, A.-P. Inhibition of Canonical Transient Receptor Potential Channels 4/5 with Highly Selective and Potent Small-Molecule HC-070 Alleviates Mechanical Hypersensitivity in Rat Models of Visceral and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 3350. https://doi.org/10.3390/ijms24043350
Jalava N, Kaskinoro J, Chapman H, Morales M, Metsänkylä H, Heinonen S-M, Koivisto A-P. Inhibition of Canonical Transient Receptor Potential Channels 4/5 with Highly Selective and Potent Small-Molecule HC-070 Alleviates Mechanical Hypersensitivity in Rat Models of Visceral and Neuropathic Pain. International Journal of Molecular Sciences. 2023; 24(4):3350. https://doi.org/10.3390/ijms24043350
Chicago/Turabian StyleJalava, Niina, Janne Kaskinoro, Hugh Chapman, Miguel Morales, Hanna Metsänkylä, Satu-Maarit Heinonen, and Ari-Pekka Koivisto. 2023. "Inhibition of Canonical Transient Receptor Potential Channels 4/5 with Highly Selective and Potent Small-Molecule HC-070 Alleviates Mechanical Hypersensitivity in Rat Models of Visceral and Neuropathic Pain" International Journal of Molecular Sciences 24, no. 4: 3350. https://doi.org/10.3390/ijms24043350





