Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting
Abstract
1. Introduction
2. Overview of Nasal Anatomy
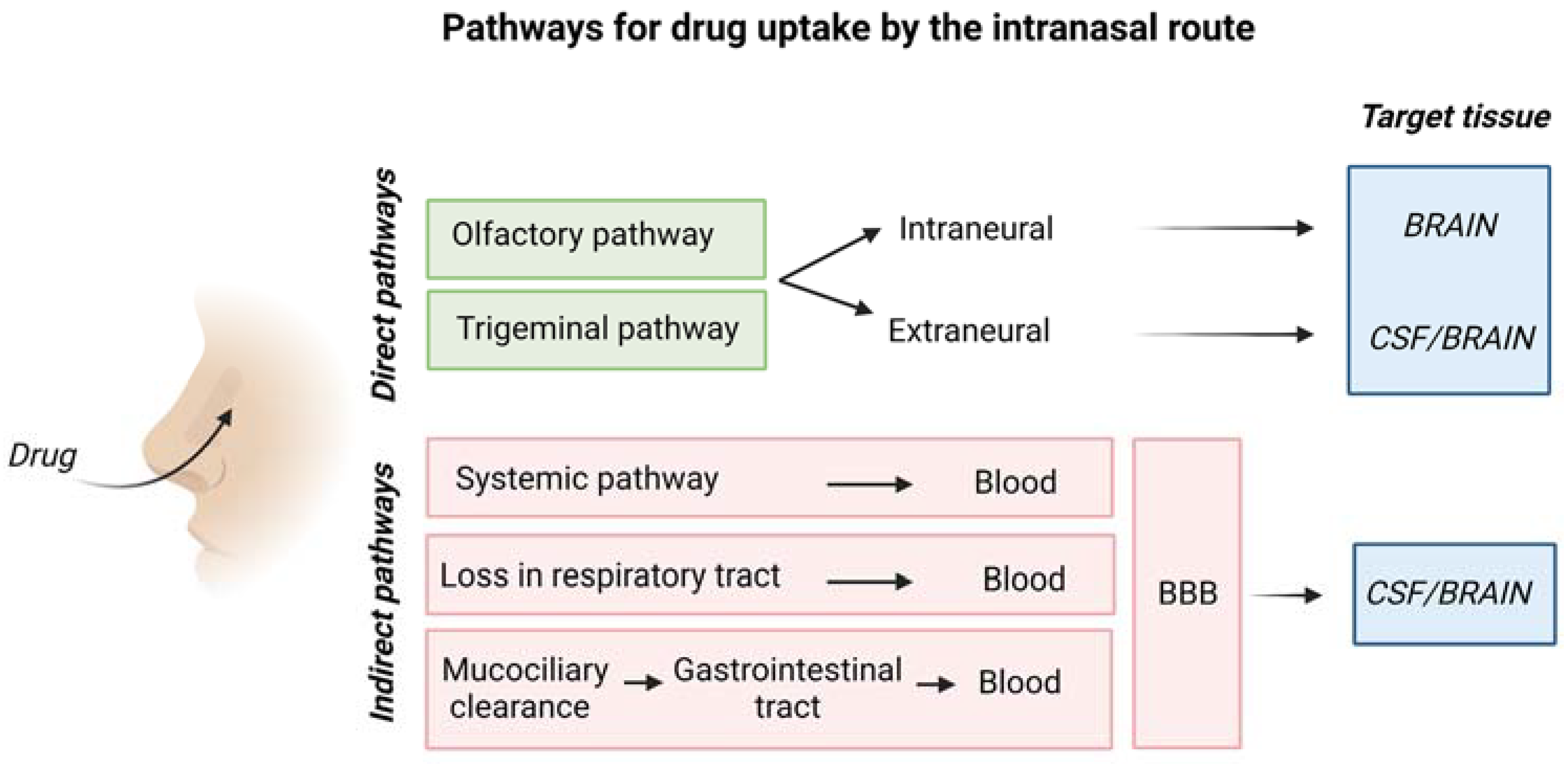
3. Mechanism for Drug Delivery to the Brain through the Intranasal Route
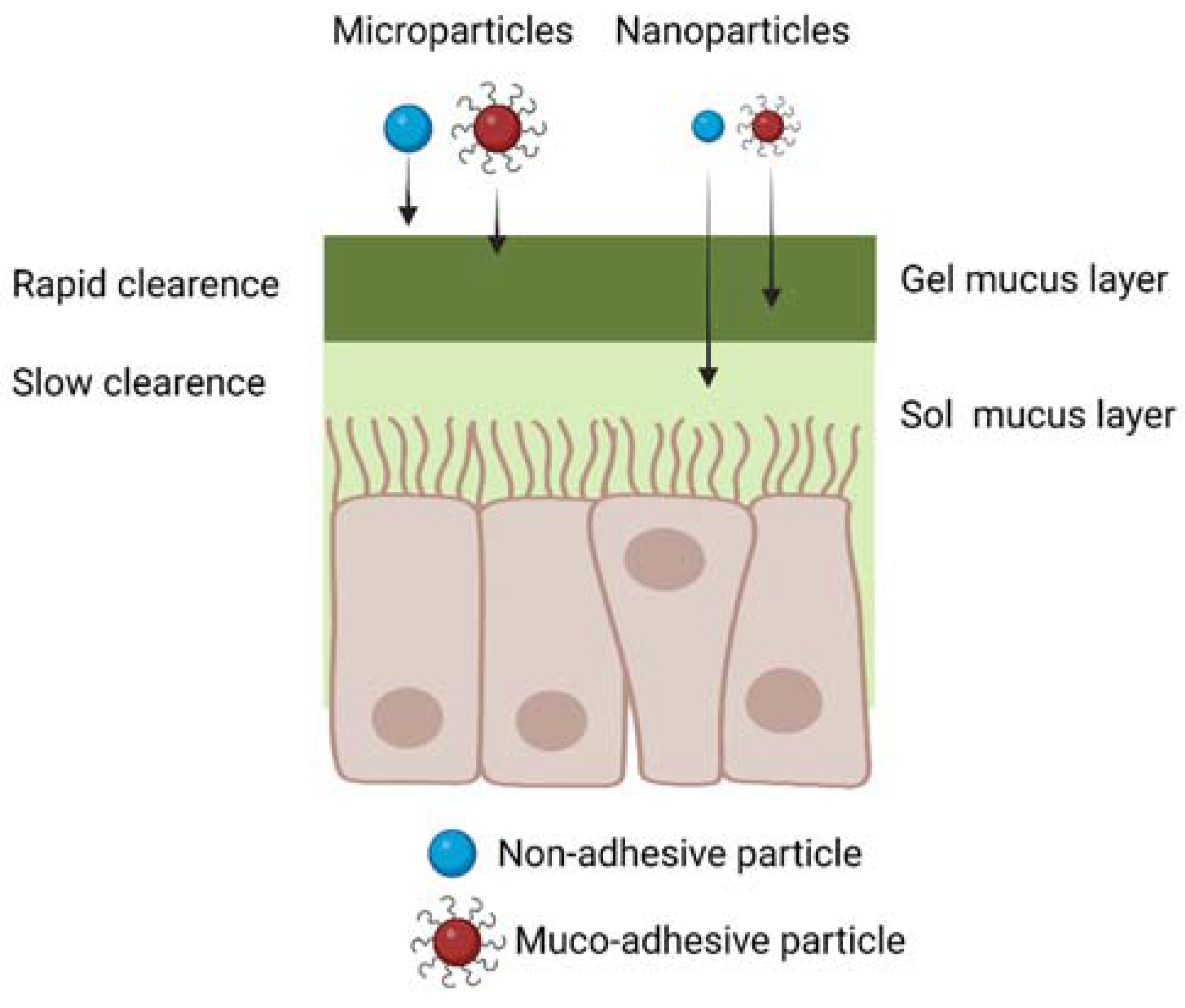
3.1. Mucus Layer: A First Barrier to Intranasal Drug Delivery
3.2. Transport of Drugs from Nasal Epithelium to the Brain
3.2.1. Transport across the Olfactory and Respiratory Epithelial Barriers
3.2.2. Transport from the Nasal Mucosa to the Sites at Brain Entry
3.2.3. Transport from the Initial Brain Entry Sites to Other Brain Areas
3.3. Synthesis of the Main Features of Intranasal Drug Transport to the Brain
3.4. Prevalent Transport Mechanisms for Hydrophilic Drugs by the Intranasal Route
4. Penetration Enhancers in IN Drug Delivery
5. Biomaterials-Based Vehicles for IN Drug Delivery
5.1. Microparticles and Nanoparticles for IN Drug Delivery
- Muco-adhesive polymers such as chitosan or polymer containing thiol groups (thiomers);
- Molecules for adsorption endocytosis by the epithelial layer (e.g., lectins, cell-penetrating peptides, such as penetratin, Tat peptide, etc.);
- Molecules for ligand-mediated endocytosis (e.g., lactoferrin);
- Mucus-penetrating (non-adhesive) polymers (e.g., PEG).
5.2. Hydrogels for IN Drug Delivery
5.3. Nanocarrier-Loaded Hydrogels for IN Delivery
6. Discussion
- Therapeutic concentrations of drugs to the brain within a short time from the administration (~30 min), with prompt therapeutic benefits for the patients.
- Improved drug bioavailability avoiding hepatic first pass metabolism.
- Reduced side effects due to the lack of accumulation into non-target tissues, such as the liver, or the possibility to avoid gastroprotective drugs (needed for orally administered drugs in the treatment of chronic diseases).
- Patient-compliant treatment exploiting a minimally invasive route.
- Reduction of therapy costs due to enhanced effectiveness including brain-targeting ability.
- Avoidance of local and systemic toxicity, which is frequently associated with long-term treatment of chronic diseases.
- Improvement of patient’s quality of life, by an effective drug administration route.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ADNP | activity-dependent neuroprotective protein |
| AUC | area under the drug |
| B% | Bioavailability |
| BBB | blood brain barrier |
| bFGF | basic Fibroblast growth factor |
| C-CPE | Clostridium perfringens |
| CNS | central nervous system |
| CPP | Cell penetrating peptide |
| CS | Chitosan |
| CSF | blood-cerebrospinal fluid |
| DTE % | drug targeting efficiency |
| DTP % | direct transport percentage |
| EDTA | Ethylenediaminetetraacetic acid |
| GCS | glycol chitosan |
| GMS | Glyceryl monostearate |
| GNLs | Gelatin nanostructured lipid carriers |
| IGF-1 | insulin-like growth factor 1 |
| IGF-I | Insulin-like Growth Factor-I |
| IN | Intranasal |
| INF-β1b | interferon-β1b |
| Lf | lactoferrin |
| LPS | lipopolysaccharide |
| MAG | Magnolia officinalis |
| Mal-PEG-PCL | maleimide poly(ethylene glycol)-co-poly(ε-caprolactone) copolymer |
| Me-PEG-PCL | Methoxy poly(ethylene glycol)-co-poly(ε-caprolactone) copolymer |
| NP | nanoparticle |
| PEG | poly(ethylene glycol) |
| PHEA | poly(2-hydroxyethyl acrylate) |
| PLGA | poly(D,L-lactic acid-co-glycolic acid) copolymer |
| POZ | poly(2-ethyl-2-oxazoline) |
| PPS−PEG | poly(propylene sulphide)-polyethylene glycol |
| PVP | poly(N-vinyl pyrrolidone) |
| RB% | relative bioavailability (RB%) |
| RDTE% | relative drug targeting efficiency |
| RDTP% | relative direct transport percentage |
| SBE-β-CD | sulfobutyl-ether-β-cyclodextrin |
| SP | Neuropeptide substance P |
| STL | Soranum tuberosum lectin |
| TEER | transepithelial electrical resistance |
| TPP | tripolyphosphate |
| VIP | Vasoactive intestinal peptide |
| WGA | Wheat germ agglutinin |
References
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A. V Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]
- Pond, S.M.; Tozer, T.N. First-pass elimination Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Goldstein, B.J. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, K.; Ebsen, M. Anatomy. Mech. Vent. Clin. Appl. Pathophysiol. 2008, 8, 69–85. [Google Scholar] [CrossRef]
- Chari, S.; Sridhar, K.; Walenga, R.; Kleinstreuer, C. Computational analysis of a 3D mucociliary clearance model predicting nasal drug uptake. J. Aerosol Sci. 2021, 155, 105757. [Google Scholar] [CrossRef]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; William Andrew Publishing: Oxford, UK, 2017; pp. 279–301. [Google Scholar] [CrossRef]
- Duan, X.; Mao, S. New strategies to improve the intranasal absorption of insulin. Drug Discov. Today 2010, 15, 416–427. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.E.; Fletcher, D.F.; Brodbelt, A.R.; Stoodley, M.A. Arterial Pulsation-driven Cerebrospinal Fluid Flow in the Perivascular Space: A Computational Model. Comput. Methods Biomech. Biomed. Eng. 2010, 6, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Schley, D.; Carare-Nnadi, R.; Please, C.P.; Perry, V.H.; Weller, R.O. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006, 238, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Hadaczek, P.; Yamashita, Y.; Mirek, H.; Tamas, L.; Bohn, M.C.; Noble, C.; Park, J.W.; Bankiewicz, K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 2006, 14, 69–78. [Google Scholar] [CrossRef]
- Scranton, R.A.; Fletcher, L.; Sprague, S.; Jimenez, D.F.; Digicaylioglu, M. The Rostral Migratory Stream Plays a Key Role in Intranasal Delivery of Drugs into the CNS. PLoS ONE 2011, 6, e18711. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Venkata Satyanarayana Reddy Karri, V. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Miyamoto, M.; Natsume, H.; Iwata, S.; Ohtake, K.; Yamaguchi, M.; Kobayashi, D.; Sugibayashi, K.; Yamashina, M.; Morimoto, Y. Improved nasal absorption of drugs using poly-l-arginine: Effects of concentration and molecular weight of poly-l-arginine on the nasal absorption of fluorescein isothiocyanate–dextran in rats. Eur. J. Pharm. Biopharm. 2001, 52, 21–30. [Google Scholar] [CrossRef]
- Merkus, F.W.H.M.; Schipper, N.G.M.; Hermens, W.A.J.J.; Romeijn, S.G.; Verhoef, J.C. Absorption enhancers in nasal drug delivery: Efficacy and safety. J. Control. Release 1993, 24, 201–208. [Google Scholar] [CrossRef]
- Corazza, E.; Abruzzo, A.; Giordani, B.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Pio Di Cagno, M.; Luppi, B. Human Lactobacillus Biosurfactants as Natural Excipients for Nasal Drug Delivery of Hydrocortisone. Pharmaceutics 2022, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.H.; Quay, S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005, 2, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Rabinowicz, A.L.; Carrazana, E.; Maggio, E.T. Improvement of Intranasal Drug Delivery with Intravail® Alkylsaccharide Excipient as a Mucosal Absorption Enhancer Aiding in the Treatment of Conditions of the Central Nervous System. Drugs R D 2021, 21, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Natsume, H.; Satoh, I.; Ohtake, K.; Yamaguchi, M.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. Effect of poly-L-arginine on the nasal absorption of FITC-dextran of different molecular weights and recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rats. Int. J. Pharm. 2001, 226, 127–138. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Coucke, D.; Schotsaert, M.; Libert, C.; Pringels, E.; Vervaet, C.; Foreman, P.; Saelens, X.; Remon, J.P. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine 2009, 27, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Watari, A.; Hashimoto, E.; Yonemitsu, M.; Kiyono, H.; Yagi, K.; Kondoh, M.; Kunisawa, J. C-Terminal Clostridium perfringens Enterotoxin-Mediated Antigen Delivery for Nasal Pneumococcal Vaccine. PLoS ONE 2015, 10, e0126352. [Google Scholar] [CrossRef]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target specific tight junction modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Song, K.H.; Kim, S.B.; Shim, C.K.; Chung, S.J.; Kim, D.D.; Rhee, S.K.; Choi, G.J.; Kim, C.H.; Kim, K. Paracellular permeation-enhancing effect of AT1002 C-terminal amidation in nasal delivery. Drug Des. Devel. Ther. 2015, 9, 1815–1823. [Google Scholar] [CrossRef]
- Sosnik, A.; Das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 12, 2030–2075. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Interindividual variability in nasal filtration as a function of nasal cavity geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Köllner, S.; Dünnhaupt, S.; Waldner, C.; Hauptstein, S.; Pereira De Sousa, I.; Bernkop-Schnürch, A. Mucus permeating thiomer nanoparticles. Eur. J. Pharm. Biopharm. 2015, 97, 265–272. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Filippov, S.K.; Maji, S.; Glassner, M.; Cegłowski, M.; Hoogenboom, R.; King, S.; Lau, W.M.; Khutoryanskiy, V.V. Mucus-penetrating nanoparticles based on chitosan grafted with various non-ionic polymers: Synthesis, structural characterisation and diffusion studies. J. Colloid Interface Sci. 2022, 626, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef]
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol. Med. 2021, 27, 436–450. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech 2015, 16, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.N.; Semina, I.I.; Salakhov, I.A.; Moustafine, R.I.; Khutoryanskiy, V.V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102432. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, I.; Pandit, J.; Emad, N.A.; Bano, S.; Dar, K.I.; Rizvi, M.M.A.; Ansari, M.D.; Aqil, M.; Sultana, Y. Brain targeted delivery of carmustine using chitosan coated nanoparticles via nasal route for glioblastoma treatment. Int. J. Biol. Macromol. 2022, 221, 435–445. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Jin, R.R.; Yang, W.; Xiang, Q.; Yu, W.Z.; Lin, Q.; Tian, F.R.; Mao, K.L.; Lv, C.Z.; Wáng, Y.X.J.; et al. Using Gelatin Nanoparticle Mediated Intranasal Delivery of Neuropeptide Substance P to Enhance Neuro-Recovery in Hemiparkinsonian Rats. PLoS ONE 2016, 11, 148848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Li, X.; Lu, C.T.; Lin, M.; Chen, L.J.; Xiang, Q.; Zhang, M.; Jin, R.R.; Jiang, X.; Shen, X.T.; et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine 2013, 10, 755–764. [Google Scholar] [CrossRef]
- Gulati, N.; Nagaich, U.; Saraf, S.A. Intranasal Delivery of Chitosan Nanoparticles for Migraine Therapy. Sci. Pharm. 2013, 81, 843–854. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; De Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef]
- Annu; Baboota, S.; Ali, J. In vitro appraisals and ex vivo permeation prospect of chitosan nanoparticles designed for schizophrenia to intensify nasal delivery. Polym. Bull. 2022, 79, 2263–2285. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Kesharwani, P.; Khanna, K.; Karwasra, R.; Sharma, N.; Rawat, S.; Sharma, D.; Ahmad, F.J.; Jain, G.K.; et al. Intranasal delivery of Naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int. J. Pharm. 2021, 599, 120428. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Chauhan, I.; Zafar, A.; Verma, M.; Noorulla, K.M.; Tura, A.J.; Alruwaili, N.K.; Haji, M.J.; Puri, D.; Gobena, W.G.; et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: Formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J. Drug Deliv. Sci. Technol. 2021, 61, 102164. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J.; et al. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, Q.; Zhu, Z.; Hao, Y.; Han, F.; Hong, J.; Zheng, W.; Ma, S.; Yang, L.; Cheng, G. High-efficiency brain-targeted intranasal delivery of BDNF mediated by engineered exosomes to promote remyelination. Biomater. Sci. 2022, 10, 5707–5718. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Noorulla, K.M.; Yasir, M.; Muzaffar, F.; Roshan, S.; Ghoneim, M.M.; Almurshedi, A.S.; Tura, A.J.; Alshehri, S.; Gebissa, T.; Mekit, S.; et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In-Vivo preclinical evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102939. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Zaki, N.M.; Mortada, N.D.; Awad, G.A.S.; ElHady, S.S.A. Rapid-onset intranasal delivery of metoclopramide hydrochloride Part II: Safety of various absorption enhancers and pharmacokinetic evaluation. Int. J. Pharm. 2006, 327, 97–103. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Das, S.K.; Sharma, S.; Kuotsu, K. A Review on Designing Poly (Lactic-co-glycolic Acid) Nanoparticles as Drug Delivery Systems. Pharm. Nanotechnol. 2021, 9, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Herman, S.; Fishel, I.; Offen, D. Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cells 2021, 39, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hurkat, P.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Thiolated Polymers: Pharmaceutical Tool in Nasal Drug Delivery of Proteins and Peptides. Int. J. Pept. Res. Ther. 2019, 25, 15–26. [Google Scholar] [CrossRef]
- Wu, A.M.; Lisowska, E.; Duk, M.; Yang, Z. Lectins as tools in glycoconjugate research. Glycoconj. J. 2009, 26, 899–913. [Google Scholar] [CrossRef]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef]
- Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG-PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials 2006, 27, 3482–3490. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2012, 20, 174–184. [Google Scholar] [CrossRef]
- Wan, X.M.; Chen, Y.P.; Xu, W.R.; Yang, W.J.; Wen, L.P. Identification of nose-to-brain homing peptide through phage display. Peptides 2009, 30, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.; Nayak, U.; Udupa, N. Smart Polymers in Nasal Drug Delivery. Indian J. Pharm. Sci. 2015, 77, 367. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Protopapa, C.; Siamidi, A.; Pavlou, P.; Vlachou, M. Excipients Used for Modified Nasal Drug Delivery: A Mini-Review of the Recent Advances. Materials 2022, 15, 6547. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.R.; Aditya, N.; Patil, S.; Cherian, L. Nasal in-situ gels for delivery of rasagiline mesylate: Improvement in bioavailability and brain localization. Drug Deliv. 2015, 22, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, Y.; Liu, Y.; Ma, R.; Luo, J.; Hong, H.; Chen, X.; Wang, S.; Liu, C.; Zhang, Y.; et al. Rational Design of Thermosensitive Hydrogel to Deliver Nanocrystals with Intranasal Administration for Brain Targeting in Parkinson’s Disease. Research 2021, 2021, 9812523. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Z.; Liu, M.; Tao, Y.; Li, Z.; Wu, Z.; Gui, S. Facile nose-to-brain delivery of rotigotine-loaded polymer micelles thermosensitive hydrogels: In vitro characterization and in vivo behavior study. Int. J. Pharm. 2020, 577, 119046. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Hu, J.; Wang, C.; Wan, D.; Li, Q.; Jiang, Q.; Du, L.; Jin, Y. Nasal Delivery of Cinnarizine Thermo- and Ion-Sensitive In Situ Hydrogels for Treatment of Microwave-Induced Brain Injury. Gels 2022, 8, 108. [Google Scholar] [CrossRef]
- Qi, X.J.; Xu, D.; Tian, M.L.; Zhou, J.F.; Wang, Q.S.; Cui, Y.L. Thermosensitive hydrogel designed for improving the antidepressant activities of genipin via intranasal delivery. Mater. Des. 2021, 206, 109816. [Google Scholar] [CrossRef]
- Zhong, M.; Kou, H.; Zhao, P.; Zheng, W.; Xu, H.; Zhang, X.; Lan, W.; Guo, C.; Wang, T.; Guo, F.; et al. Nasal Delivery of D-Penicillamine Hydrogel Upregulates a Disintegrin and Metalloprotease 10 Expression via Melatonin Receptor 1 in Alzheimer’s Disease Models. Front. Aging Neurosci. 2021, 13, 191. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Lyu, X.; Yuan, Y.; Wang, G.; Zhao, B. Chitosan-based thermosensitive hydrogel for nasal delivery of exenatide: Effect of magnesium chloride. Int. J. Pharm. 2018, 553, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Shaghlil, L.; Alshishani, A.; Sa’aleek, A.A.; Abdalkader, H.; Al-ebini, Y. Formulation and evaluation of nasal insert for nose-to-brain drug delivery of rivastigmine tartrate. J. Drug Deliv. Sci. Technol. 2022, 76, 103736. [Google Scholar] [CrossRef]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, W.J.; Zhu, J.X.; Zhu, N.; Zhang, H.M.; Wang, X.; Zhang, J.; Wang, Q.Q. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. Int. J. Clin. Exp. Med. 2015, 8, 17590. [Google Scholar]
- Jose, S.; Ansa, C.R.; Cinu, T.A.; Chacko, A.J.; Aleykutty, N.A.; Ferreira, S.V.; Souto, E.B. Thermo-sensitive gels containing lorazepam microspheres for intranasal brain targeting. Int. J. Pharm. 2013, 441, 516–526. [Google Scholar] [CrossRef]
- Perez, A.P.; Mundiña-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Increased brain radioactivity by intranasal 32P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int. J. Nanomed. 2012, 7, 1373. [Google Scholar] [CrossRef]
- Cunha, S.; Swedrowska, M.; Bellahnid, Y.; Xu, Z.; Sousa Lobo, J.M.; Forbes, B.; Silva, A.C. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: Characterisation, biocompatibility, and drug deposition studies. Int. J. Pharm. 2022, 620, 121720. [Google Scholar] [CrossRef]
- Ribeiro, T.d.C.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.d.A.; Planeta, C.d.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.u.R.; Sohail, M.; Khan, S.A.; Minhas, M.U.; Mahmood, A.; Shah, S.A.; Mohsin, S. Chitosan/guar gum-based thermoreversible hydrogels loaded with pullulan nanoparticles for enhanced nose-to-brain drug delivery. Int. J. Biol. Macromol. 2022, 215, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Andrée, L.; Oude Egberink, R.; Dodemont, J.; Hassani Besheli, N.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Gelatin Nanoparticles for Complexation and Enhanced Cellular Delivery of mRNA. Nanomaterials 2022, 12, 3423. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Pai, S.S.; Tilton, R.D.; Przybycien, T.M. Poly(ethylene glycol)-Modified Proteins: Implications for Poly(lactide-co-glycolide)-Based Microsphere Delivery. AAPS J. 2009, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Rath, G.; Goyal, A.K. In situ nasal gel drug delivery: A novel approach for brain targeting through the mucosal membrane. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1167–1176. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rajpar, S.F.; Foulds, I.S.; Abdullah, A.; Maheshwari, M. Severe adverse cutaneous reaction to insulin due to cresol sensitivity. Contact Dermat. 2006, 55, 119–120. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. J. Alzheimers Dis. 2015, 45, 1269–1270. [Google Scholar] [CrossRef]
- Rosenbloom, M.H.; Barclay, T.R.; Pyle, M.; Owens, B.L.; Cagan, A.B.; Anderson, C.P.; Frey, W.H.; Hanson, L.R. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 2014, 28, 1185–1189. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Frey, W.H.; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Chamanza, R.; Wright, J.A. A Review of the Comparative Anatomy, Histology, Physiology and Pathology of the Nasal Cavity of Rats, Mice, Dogs and Non-human Primates. Relevance to Inhalation Toxicology and Human Health Risk Assessment. J. Comp. Pathol. 2015, 153, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.; Vanbever, R. Preclinical models for pulmonary drug delivery. Expert Opin. Drug Deliv. 2009, 6, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-time quantitative monitoring of in vitro nasal drug delivery by a nasal epithelial mucosa-on-a-chip model. Expert Opin. Drug Deliv. 2021, 18, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Fucarino, A.; Burgio, S.; Intili, G.; Manna, O.M.; Pitruzzella, A.; Brucato, V.; La Carrubba, V.; Carfì Pavia, F. A dynamic air–liquid interface system for in vitro mimicking of the nasal mucosa. Biotechnol. Bioeng. 2022, 119, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
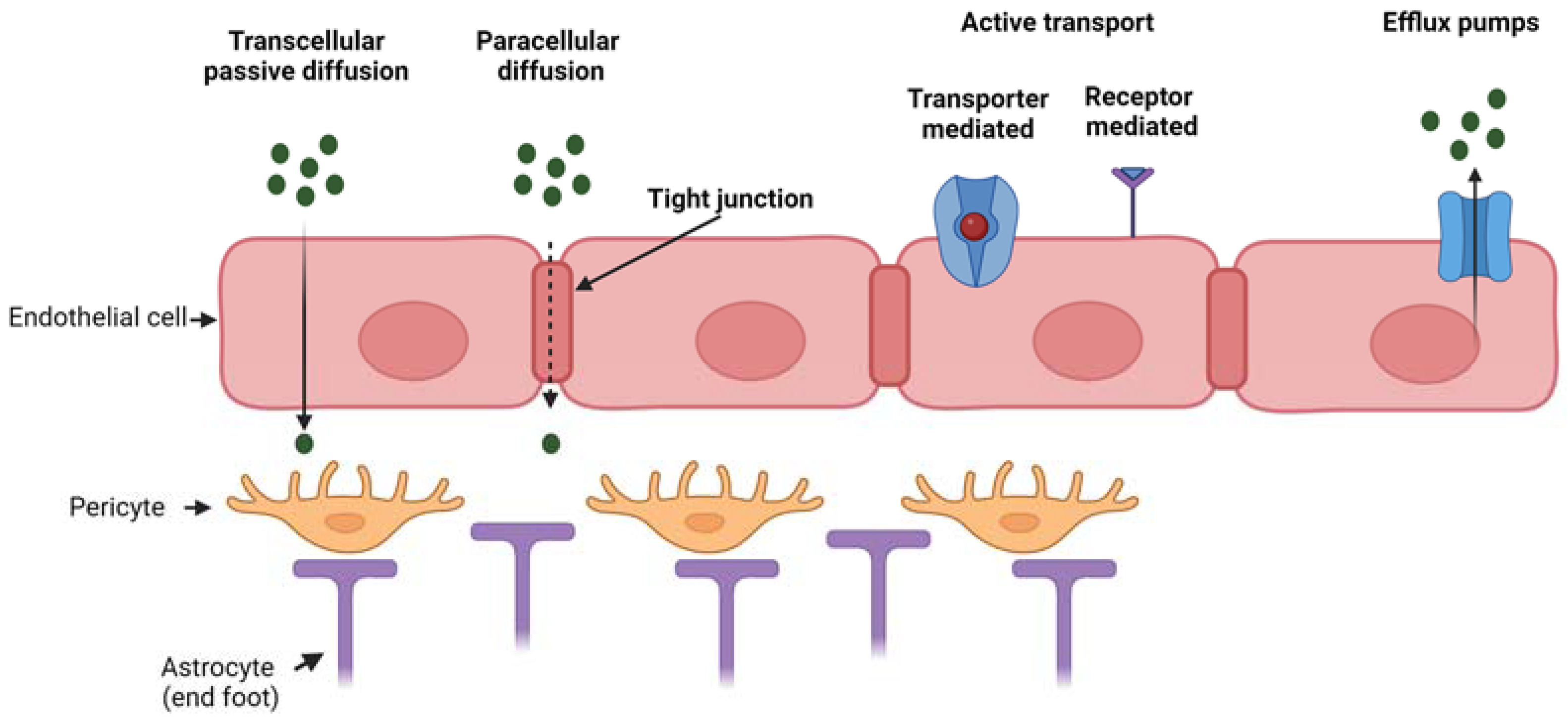
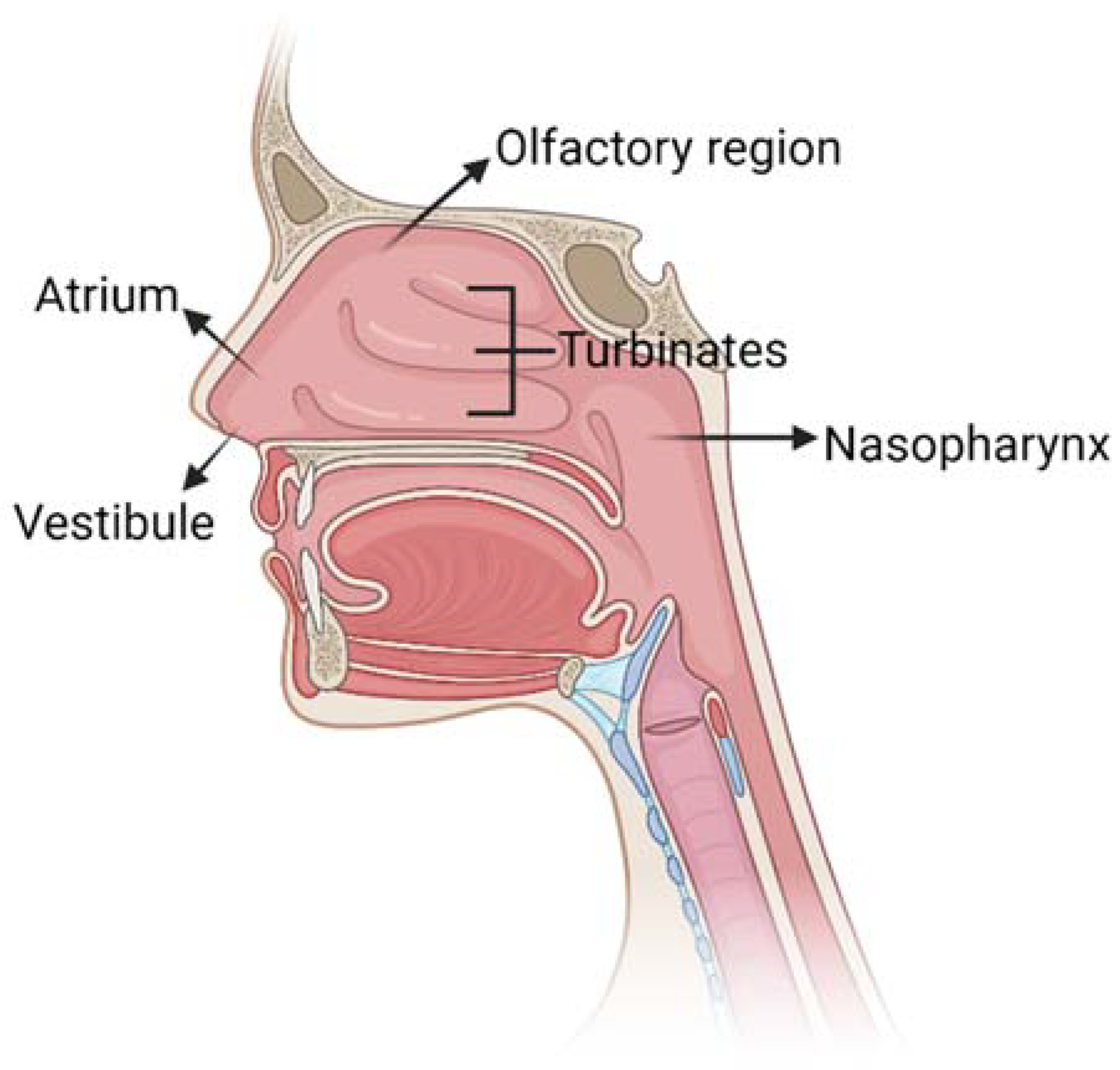
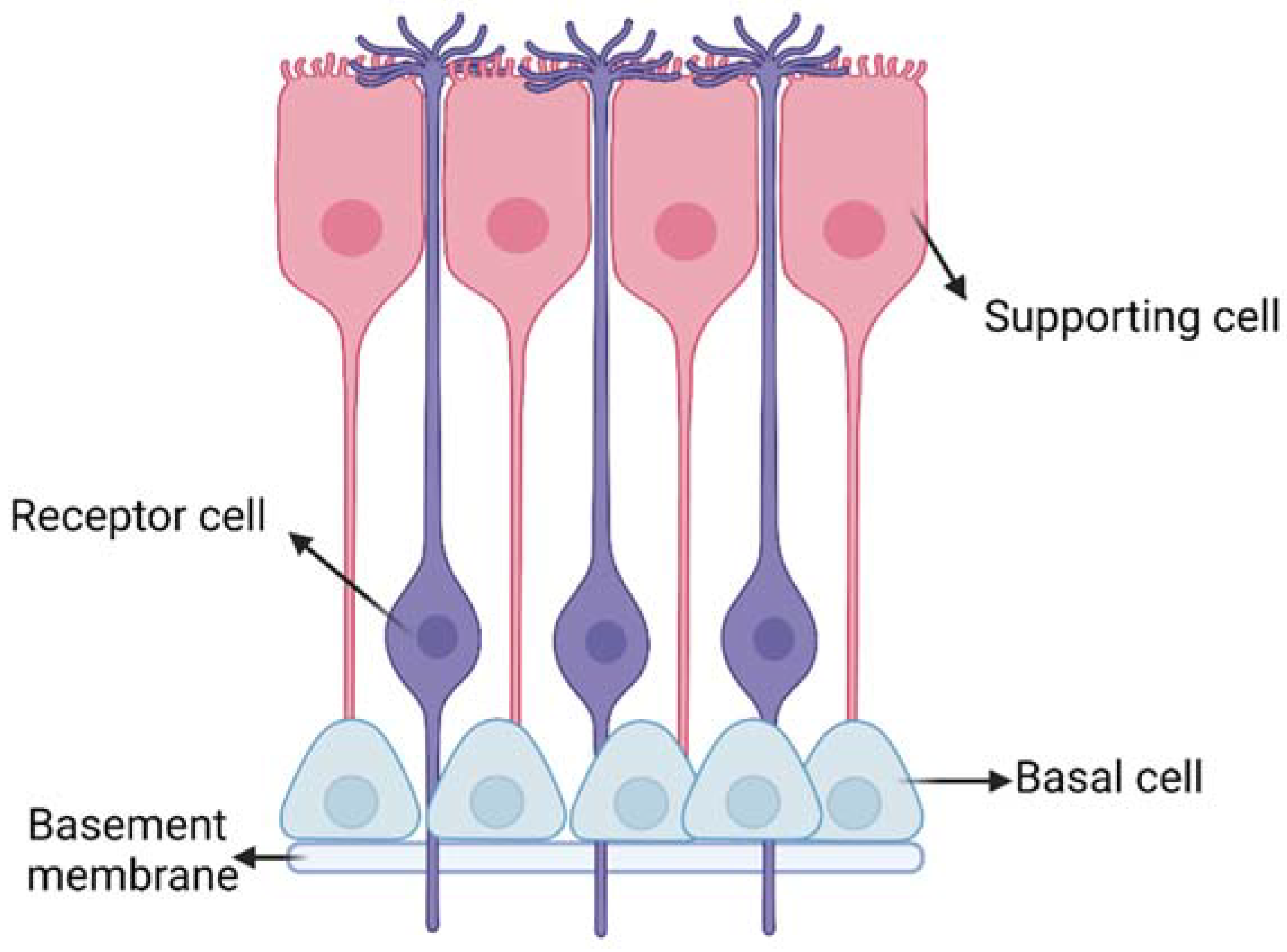
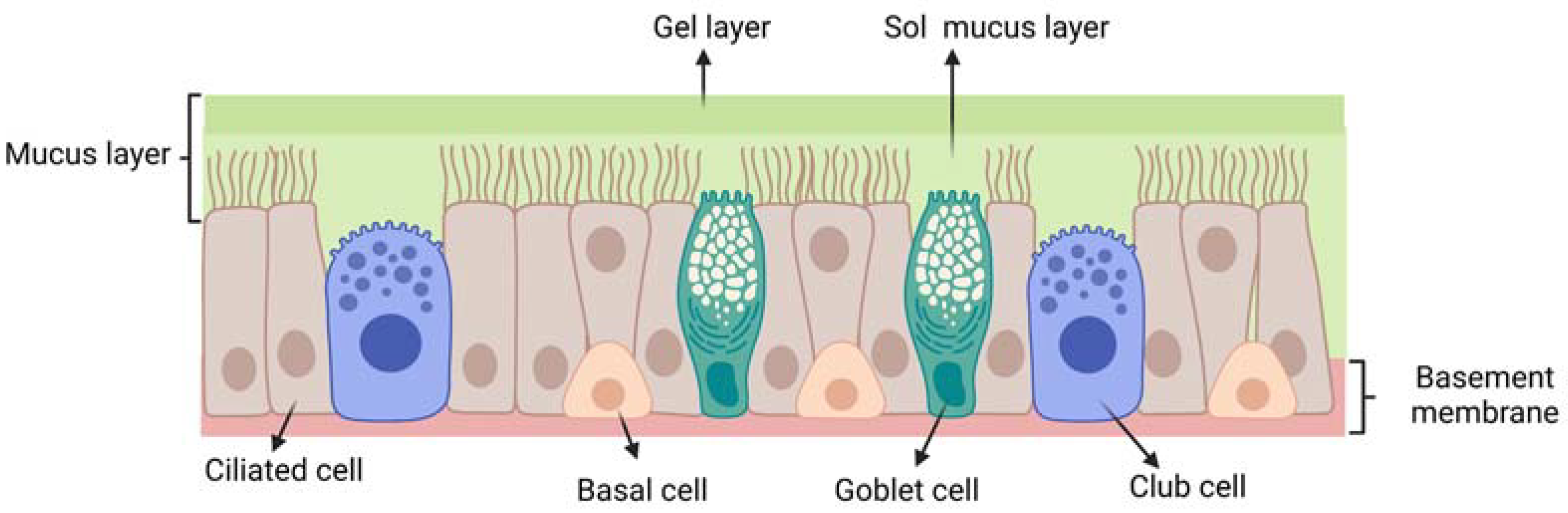

| Nasal Administration | |
|---|---|
| Advantages | Disadvantages |
| Rapid drug absorption | Possible irritation to the nasal mucosa especially for repeated administrations |
| Enhanced pharmacokinetics profile | Nasal cavity has smaller absorption surface area compared to the gastrointestinal tract |
| Drug degradation is limited with respect to oral administration | Risk for local side effects, e.g., irreversible damage to cilia. |
| Avoidance of first pass metabolism | Surfactants or penetration enhancers may elicit cytotoxic effects on nasal epithelial cells. |
| Direct brain targeting avoiding BBB crossing and possible systemic side effects of drugs | Possible partial loss of drug dose in the respiratory and gastrointestinal tracts during administration |
| Non-invasive and painless approach | Limited capacity of the nasal cavity (23 cm3 in humans receiving approximately 400 µL formulation) [10] |
| Patient compliance | Rapid muco-ciliary clearance |
| Material | Surface Moieties | Drug | Size (nm) | Main Tests | Brain Targeting Yield * | Ref. |
|---|---|---|---|---|---|---|
| Nanoparticles based on synthetic polymers | ||||||
| Methoxy poly(ethylene glycol)-co-poly(ε-caprolactone) copolymer (Me-PEG-PCL, 15 kDa) and maleimide PEG-PCL copolymer (Mal-PEG-PCL, 18 kDa) | Lactoferrin (thiolated) (Lf) | Coumarin-6 (C6) or NAP (NAPVSIPQ), an 8-amino acid neuropeptide fragment Derived from the activity- dependent neuroprotective protein (ADNP) family | C6 based: from 73.2 ± 4.2 nm to 89.0 ± 5.7 nm With Lf and NAP-based: from 76.2 ± 6.5 nm to 88.4 ± 7.8 nm | Alzheimer’s disease mouse model obtained by intracerebroventricular co-injection of pre-aggregated Aβ1–40 and a small amount of ibotenic acid | AUCbrain/AUCblood for: Lf NPs = 2.69–3.51; NPs = 1.28–1.92 | [51] |
| Methoxy PEG-b-poly(D,L-lactic acid-co- glycolic acid) copolymer (Me-PEG-PLGA) and maleimide PEG-b-PLGA (Mal-PEG-PLGA) | Soranum tuberosum lectin (STL) | Basic fibroblast growth factor (bFGF) | Non-functionalized: 104.8 nm Surface functionalized: 118.7 nm | As above | - | [26] |
| PEG-PLA | Wheat germ agglutinin (WGA) | Vasoactive intestinal peptide (VIP) | Non-functionalized: 90–100 nm Surface functionalized: 100–120 nm | Nasal biodistribution in male Sprague Dawley rats and Kunming mice (loading fluorescent probe 6-coumarin into the nanoparticles) | RB% for: WGA-NPs = 566–774; NPs = 357–474 | [52] |
| PLGA (LA:GA 50:50) | Poloxamer 407 | Diazepam (lipophilic drug to treat epilepsy) | From 148 ± 0.5 to 337 ± 1.8 nm; optimal size: 183.2 nm | Sprague Dawley rats using radiolabelled drug to detect biodistribution | DTE% = 258; DTP% = 61.3 | [53] |
| Inter-polyelectrolyte complexes of Eudragit® EPO (EPO) and anionic Eudragit® L100-55 (L100-55) and PEGylated L100-55 | - | Haloperidol (model psychoactive drug causing catalepsy in laboratory animals) | For EPO/L100-55: from 120 to140 nm For EPO/PEGylated L100-55: from 110 to 570 nm | Ex vivo retention in sheep nasal mucosa. In vivo retention studies in male Wistar rats | - | [54] |
| PLGA nanoparticles coated with chitosan | Chitosan | Carmustine (antitumor drug) | From 208 to 421 nm depending on formulation parameters | Glioblastoma treatment: ex vivo retention studies using goat nasal mucosa | DTE% = 687 ± 32; DTP% = 94 ± 3 | [55] |
| PLGA | Chitosan | Meloxicam (Alzheimer’s drug) | 142 ± 12.8 nm | Alzheimer’s disease treatment: no animal studies | - | [56] |
| Nanoparticles based on natural polymer | ||||||
| Gelatin nanostructured lipid carriers: gelatin, Poloxamer 188-grafted heparin, trehalose, cholesterol, glyceraldehyde crosslinker | Trehalose, cholesterol | Neuropeptide substance P (SP) | 166.00 ± 1.32 nm (blank) 172.00 ± 1.52 nm (with SP) | In vivo trials in rats with 6-hydroxydopamine-induced hemi-parkinsonism | - | [57] |
| Gelatin nanostructured lipid carriers (gelatin core) (GNLs) | Poloxamer shell | Basic fibroblast growth factor (bFGF) | 143 ± 1.14 nm | Parkinson’s disease treatment: in vivo trials in hemiparkinsonian rats | - | [58] |
| Chitosan (CS) crosslinked with tripolyphosphate (TPP) anions | - | Sumatriptan succinate, an antimigraine drug | 306.8 ± 3.9 nm | Migraine therapy: animal tests not reported | - | [59] |
| Chitosan, glycol CS (GCS) and corresponding thiomer-based materials. TPP or sulfobutyl-ether-β-cyclodextrin (SBE-β-CD) crosslinking agents | Chitosan and thiomers | Dopamine | 372 ± 81 nm for selected formulation (containing GCS and SBE-β-CD) | Parkinson’s disease treatment: experiments in rats | - | [60] |
| Chitosan grafted with PEG, poly(2-hydroxyethyl acrylate) (PHEA), poly(2-ethyl-2-oxazoline) (POZ) and poly(N-vinyl pyrrolidone) (PVP) | PEG, PHEA, POZ, PVP | Unmodified chitosan: 152 ± 13 nm PEG-chitosan: 137 ± 23 nm PHEA-chitosan: 142 ± 11 nm POZ-chitosan: 145 ± 21 nm PVP-chitosan: 130 ± 19 nm | No targeted disease: ex vivo penetration in sheep nasal mucosa | - | [48] | |
| Chitosan (CS) crosslinked with TPP ions | - | Lurasidone hydrochloride, an antipsychotic drug | 154.8 ± 4.5 nm | Schizophrenia treatment: ex vivo study of permeation in goat nasal mucosa | - | [61] |
| Nanoparticles based on lipids | ||||||
| Solid lipid nanoparticles: glyceryl monostearate, Pluronic 127 and Tween 80 | - | Naloxone | 190.2 nm | Opioid management: in vivo toxicity, biodistribution and pharmacokinetics studies in Sprague Dawley rats and New Zealand rabbits | AUC0-t = 17.75 ± 1.08 | [62] |
| Solid lipid nanoparticles: glyceryl dibehenate (i.e., Compritol® 888 ATO) and Tween 80 and Poloxamer 188 | Buspirone | 218.60 ± 9.18 nm | Anxiolytic treatment: in vivo pharmacokinetic, biodistribution and brain targeting studies in albino Wistar rats | DTE% = 883; DTP% = 87 | [63] | |
| Solid lipid nanoparticles: phosphatidylcholine and Poloxamer 188 | Chitosan | Meloxicam (Alzheimer’s disease drug) | 94.8 ± 7.4 nm | Alzheimer’s disease treatment: no animal studies | - | [56] |
| Liposomes | Cell-penetrating peptide (CPP) | Rivastigmine | Unmodified liposome: 166.3 ± 17.4 nm Liposome/CPP: 178.9 11 ± nm | Alzheimer’s disease treatment: in vivo pharmacokinetic and nasal toxicity studies in male Sprague Dawley rats | - | [64] |
| Exosomes | - | Curcumin or signal transducer and activator of transcription 3 Stat3 inhibitor anti-inflammatory agents | 135.9–205.3 nm | Brain inflammatory diseases in C57BL/6j mouse models: a lipopolysaccharide (LPS)-induced brain inflammation model; autoimmune encephalomyelitis disease model and GL26 brain tumour model | [65] | |
| Micelles: Micellar core made of poly(propylene sulfide)–polyethylene glycol (PPS−PEG) Outer nano-shell layer based on mesenchymal stem cell-derived exosomes | Penetratin and rabies virus glycoprotein (RVG29) peptides | Curcumin and microRNA 133b | From 135.9 to 194.9 nm | Parkinson’s disease mouse model obtained by injection of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) | - | [66] |
| Exosome | Rabies virus glycoprotein (RVG) peptide binding to neuronal acetylcholine receptor (nAchR) | Brain-derived neurotrophic factor | Around 100 nm | Multiple sclerosis: demyelination mouse model of C57BL/6 mouse model (cuprizone feeding) | - | [67] |
| Nanostructured lipid carriers: glyceryl distearate (Precirol ATO 5) or Dynasan 114 and Miglyol chosen to form lipid matrix | Chitosan | Human insulin-like growth factor 1 (IGF-1) | Precirol-based: from 72.1 ± 8.55 to 294.50 ± 22.06 nm Dynasal-based: from 127.87 ± 35.03 nm to 267.40 ± 2.12 nm | Neuroprotective and neurorestorative therapy in neurodegenerative diseases. In vivo toxicity and accumulation studies in C57 mice | - | [68] |
| Nanostructured lipid carriers: glyceryl monostearate (GMS) and oleic acid mixture and Tween 80 | Chitosan | Buspirone (anxiolytic agent) | 190.98 ± 4.72 nm | Anxiolytic treatment: in vivo pharmacokinetic and neuropharmacokinetic studies in albino Wistar rats | DTE% = 1462; DTP% = 93; RB% = 217 ± 13; B% = 306 ± 19 | [69] |
| Material | Muco-Adhesive Components | Drug | Main Tests | Brain Targeting Yield * | Ref. |
|---|---|---|---|---|---|
| Synthetic hydrogels | |||||
| Poloxamer 407, Poloxamer 188 | Carbapol 934P (CP) or chitosan | Rasagiline, an anti-Parkinson’s drug | Parkinson’s disease treatment: in vivo biodistribution in male Wistar rats and pharmacokinetic studies in female New Zealand white rabbits | Fold increase in B% for: CP-gel= 4.35; Chitosan-gel = 6.05 | [89] |
| Poly(nisopropylacrylamide) (PNIPAM) and gelatin methacryloyl | - | Hydroxylated biphenol derived from the “Houpo” herb (Magnolia officinalis) known as magnolol (MAG) | Parkinson’s disease treatment: in vivo pharmacokinetics studies in male Sprague Dawley rats | DTE% = 810; DTP% = 88 | [90] |
| Pluronic 407 and Pluronic 188 micelles | - | Rotigotine (dopamine agonists for Parkinson’s disease treatment) | Parkinson’s disease treatment: in vivo pharmacokinetics studies in male Sprague Dawley rats | B% = 84.6; DTE% = 201–327; DTP% = 49–69 | [91] |
| Poloxamer 407, deacetylated gellan gum and sulfobutyl-cyclodextrin | - | Cinnarizine (Ca2+ channel blocker) | In vivo pharmacokinetics studies and distribution in male Wistar rats subjected to microwave-induced brain injury | DTE% = 116 | [92] |
| Pluronic 407, Pluronic 188 and PEG 8000 | - | Genipin (antidepressant-like potential) | Evaluation of antidepressant effects in male Institute of Cancer Research mouse model of reserpine-induced depression and pharmacokinetics studies in male Sprague Dawley rats | RB% fold increase = 2.13 | [93] |
| Natural hydrogels | |||||
| Chitosan with β-glycerophosphate | - | D-penicillamine, a water-soluble metal chelator | Alzheimer’s disease: in vivo studies in APPswe/PS1d9 double-transgenic mice and C57BL/6 mice | - | [94] |
| Chitosan with β-glycerophosphate | - | Exenatide (therapy for the treatment of type 2 diabetes) | Treatment of type 2 diabetes: in vivo biodistribution and pharmacokinetics in male SD rats, in vivo pharmacodynamics studies in male SD obesity rat model | RB% = 11.9 ± 0.89 | [95] |
| Gelatin and hydroxypropyl methylcellulose (HPMC) | - | Rivastigmine tartrate (semisynthetic drug aganist moderately severe Alzheimer’s and Parkinson’s diseases) | Alzheimer’s disease and Parkinson’s disease treatment: no animal study | - | [96] |
| Gellan gum | Functionalization of gellan gum with primary amino groups | - | No targeted disease: ex vivo studies on adhesive properties using porcine small intestine mucus | - | [97] |
| Hydrogel Composition | Incorporated Nanocarriers | Drug | Treated Pathology | Reference |
|---|---|---|---|---|
| Chitosan or Carbopol 974 NFTM in Poloxamer | Poly(amidoamine) dendrimers | siRNA | Parkinson’s disease, Alzheimer’s disease and brain tumors | [100] |
| Poloxamer 407 and Poloxamer 188 | Solid lipid nanoparticles | Quetiapine fumarate | Schizophrenia | [98] |
| Poloxamer 127 and Poloxamer 68 | Chitosan microspheres | Lorazepam (benzodiazepine derivative for the treatment of status epilepticus) | Status epilepticus | [99] |
| Poloxamer 407 and HPMC | Nanostructured lipid carriers | Rivastigmine (acetylcholinesterase inhibitor) | Alzheimer’s disease | [101] |
| Poloxamer 407 and chitosan | Silica nanoparticles | Curcumin | Alzheimer’s disease | [102] |
| Poloxamer 127/ Poloxamer 68, chitosan and guar gum | Pullulan nanoparticles | Eletriptan hydrobromide (antimigrane drug) | Antimigraine effect | [103] |
| Chitosan | Lipid nanoparticles | Temozolomide (chemotherapeutic) | Melanoma and glioma | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcello, E.; Chiono, V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. https://doi.org/10.3390/ijms24043390
Marcello E, Chiono V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. International Journal of Molecular Sciences. 2023; 24(4):3390. https://doi.org/10.3390/ijms24043390
Chicago/Turabian StyleMarcello, Elena, and Valeria Chiono. 2023. "Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting" International Journal of Molecular Sciences 24, no. 4: 3390. https://doi.org/10.3390/ijms24043390
APA StyleMarcello, E., & Chiono, V. (2023). Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. International Journal of Molecular Sciences, 24(4), 3390. https://doi.org/10.3390/ijms24043390





