Bovine Serum Albumin Interaction with Polyanionic and Polycationic Brushes: The Case Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Model
2.2. Insertion Free Energy
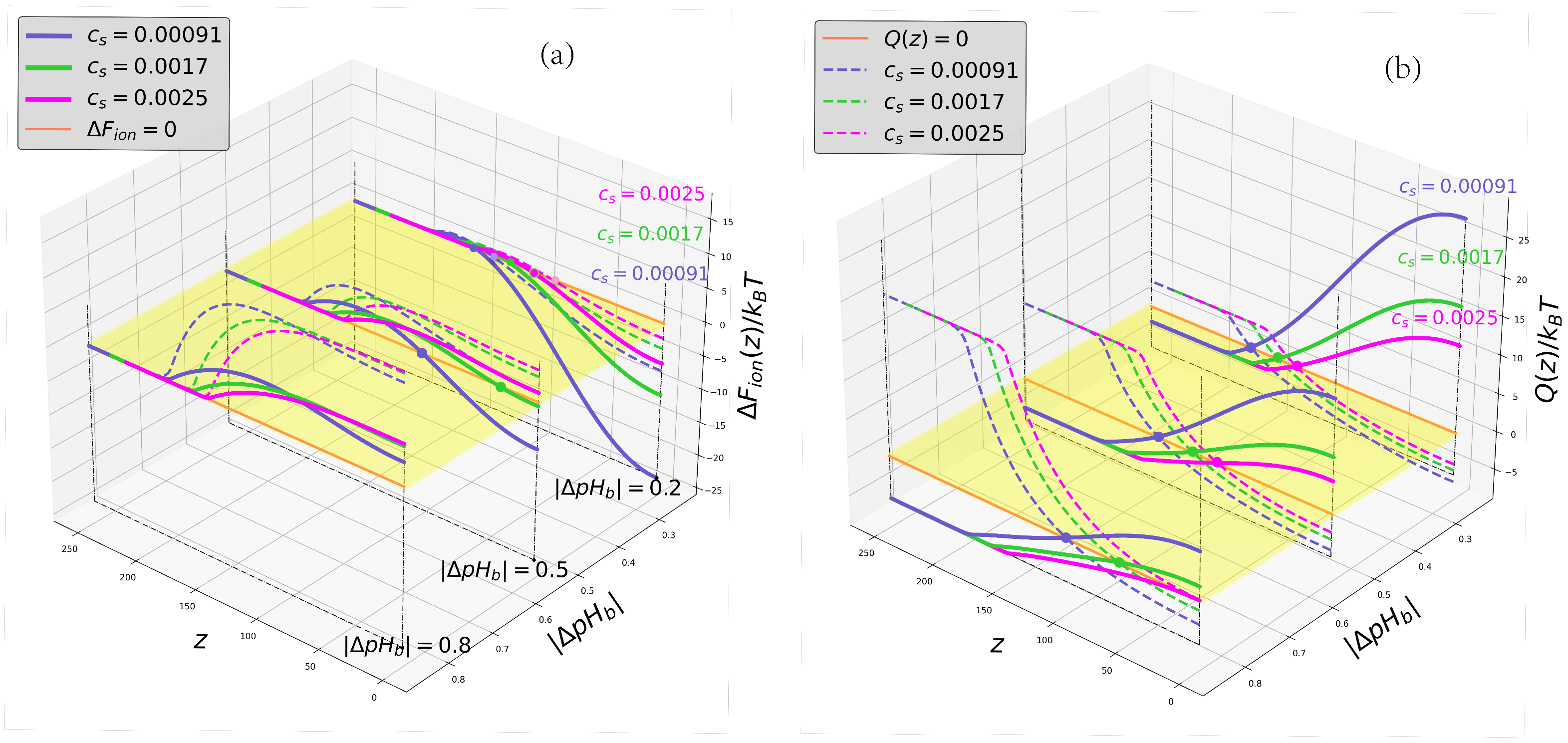
2.3. Ionic Contribution to the Free Energy
2.4. Osmotic and Non-Electrostatic Contributions to the Free Energy
2.5. The Net Insertion Free Energy
3. Methods and Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wittemann, A.; Haupt, B.; Ballauff, M. Adsorption of proteins on spherical polyelectrolyte brushes in aqueous solution. Phys. Chem. Chem. Phys. 2003, 5, 1671–1677. [Google Scholar] [CrossRef]
- Wittemann, A.; Ballauff, M. Interaction of proteins with linear polyelectrolytes and spherical polyelectrolyte brushes in aqueous solution. Phys. Chem. Chem. Phys. 2006, 8, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Henzler, K.; Welsch, N.; Ballauff, M.; Borisov, O.V. Proteins and polyelectrolytes: A charge relationship. Curr. Opimion Colloid Interface Sci. 2012, 17, 90–96. [Google Scholar] [CrossRef]
- Walkowiak, J.; Gradzielski, M.; Zauscher, S.; Ballauff, M. Interaction of Proteins with a Planar Poly(acrylic acid) Brush: Analysis by Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D). Polymers 2021, 13, 122. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Wittemann, A. A modified box model including charged polyelectrolyte brush: Self-consistent field theory. J. Phys. Chem. B 2005, 109, 4209–4214. [Google Scholar] [CrossRef]
- de Vos, W.M.; Leermakers, F.A.M.; de Keizer, A.; Cohen Stuart, M.A.; Klein, J.M. Field theoretical analysis of driving forces for the uptake of proteins by like-charge polyelectrolyte brushes: Effects of charge regulation and patchiness. Langmuir 2010, 26, 249–259. [Google Scholar] [CrossRef]
- Laktionov, M.Y.; Zhulina, E.B.; Borisov, O.V. Proteins and polyampholytes interacting with polyelectrolyte brushes and microgels: The charge reversal concept revised. Langmuir 2021, 37, 2865–2873. [Google Scholar] [CrossRef]
- Leermakers, F.A.M.; Ballauff, M.; Borisov, O.V. On the mechanisms of interaction of globular proteins with polyelectrolyte brushes. Langmuir 2007, 23, 237–247. [Google Scholar]
- Xu, X.; Angioletti-Uberti, S.; Lu, Y.; Dzubiella, J.; Ballauff, M. Interaction of Proteins with Polyelectrolytes: Comparison of Theory to Experiment. Langmuir 2019, 35, 5373–5391. [Google Scholar] [CrossRef]
- Kim, S.; Sureka, H.V.; Kayitmazer, A.B.; Wang, G.; Swan, J.W.; Olsen, B.D. Effect of protein surface charge distribution on protein-polyelectrolyte complexation. Biomacromolecules 2020, 21, 6878–6890. [Google Scholar] [CrossRef]
- Achazi, K.; Haag, R.; Ballauff, M.; Dernedde, J.; Kizhakkedathu, J.N.; Maysinger, D.; Multhaup, G. Understanding the Interaction of Polyelectrolyte Architectures with Proteins and Biosystems. Angew. Chem. Int. Edit. 2021, 60, 3882–3904. [Google Scholar] [CrossRef]
- Nie, C.A.X.; Pouyan, P.; Lauster, D.; Trimpert, J.; Kerkhoff, Y.; Szekeres, G.P.; Wallert, M.; Block, S.; Sahoo, A.K.; Dernedde, J.; et al. Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions. Angew. Chem. Int. Edit. 2021, 60, 15870–15878. [Google Scholar] [CrossRef]
- Kapelner, R.A.; Yeong, V.; Obermeyer, A.C. Molecular determinants of protein-based coacervates. Current Opin. Colloid Interface Sci. 2021, 52, 101407. [Google Scholar] [CrossRef]
- Yeong, V.; Werth, E.G.; Brown, L.M.; Obermeyer, A.C. Formation of biomolecular condensates in bacteria by tuning protein electrostatics. ACS Cent. Sci. 2020, 6, 2301–2310. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan sulfate proteoglycans and viral attachment: True receptors or adaptation bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Kabanov, V.A. Interpolyelectrolyte and block ionomer complexes for gene delivery: Physico-chemical aspects. Adv. Drug Deliv. Rev. 1998, 30, 49–60. [Google Scholar] [CrossRef]
- Schallon, A.; Synatschke, C.V.; Jerome, V.; Müller, A.H.E.; Freitag, R. Nanopartiulate Nonviral Agent for the Effective Delivery of pDNA and si RNA to Differentiated Cells and Primary Human T Lymphocytes. Biomacromolecules 2012, 13, 3463–3474. [Google Scholar] [CrossRef]
- Miyata, K.; Nishiyama, N.; Kataoka, K. Rational design of smart supramolecular assemblies for gene delivery: Chemical challenges in the creation of artificial viruses. Chem. Soc. Rev. 2012, 41, 2562–2574. [Google Scholar] [CrossRef]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.-L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.-P.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. 2012, 51, 8606–8612. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive Amphiphilic Dendrimer-Based Nanoassemblies as Robust and Versatile siRNA Delivery Systems. Angew. Chem. Int. Ed. 2014, 126, 12016–12021. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Y.; Xu, W.; Li, L. Linear-Dendritic Block Copolymer for Drug and Gene Delivery. Mater. Sci. Eng. C 2016, 62, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M.; Bysell, H.; Hansson, P. Biomacromolecules in Microgels—Opportunities and Challenges for Drug Delivery. Curr. Opin. Colloid Interface Sci. 2010, 15, 435–444. [Google Scholar] [CrossRef]
- Bysell, H.; Mansson, R.; Hansson, P.; Malmsten, M. Microgels and Microcapsules in Peptide and Protein Drug Delivery. Adv. Drug Delivery Rev. 2011, 63, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 2019, 313, 131–147. [Google Scholar] [CrossRef]
- Braatz, D.; Dimde, M.; Ma, G.; Zhong, Y.; Tully, M.; Grotzinger, C.; Zhang, Y.; Mavroskoufis, A.; Schirner, M.; Zhong, Z.; et al. Toolbox of Biodegradable Dendritic (Poly glycerol sulfate)-SS-poly(ester) Micelles for Cancer Treatment: Stability, Drug Release, and Tumor Targeting. Biomacromolecules 2021, 22, 2625–2640. [Google Scholar] [CrossRef]
- Ye, Z.; Li, L.; Dai, L.; Wang, Y.; Yang, Q.; von Klitzing, R.; Guo, X. Selective uptake of different proteins by annealed and quenched cationic spherical polyelectrolyte brushes. J. Polymer Sci. 2020, 58, 3018–3030. [Google Scholar] [CrossRef]
- Henzler, K.; Wittemann, A.; Breininger, E.; Ballauff, M.; Rosenfeldt, S. Adsorption of bovine hemoglobin onto spherical polyelectrolyte brushes monitored by small-angle X-ray scattering and Fourier transform infrared spectroscopy. Biomacromolecules 2007, 8, 3674–3681. [Google Scholar] [CrossRef]
- Rosenfeldt, S.; Wittemann, A.; Ballauff, M.; Breininger, E.; Bolze, J.; Dingenouts, N. Interaction of proteins with spherical polyelectrolyte brushes in solution as studied by small-angle x-ray scattering. Phys. Rev. E 2004, 70, 061403. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Structure and Interactions of Weakly Charged Polyelectrolyte Brushes: Self-Consistent Field Theory. J. Chem. Phys. 1997, 107, 5952–5967. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Klein Wolterink, J.; Borisov, O.V. Screening Effects in Polyelectrolyte Brush: Self-Consistent Field Theory. Macromolecules 2000, 33, 4945–4953. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Poisson-Boltzmann Theory of pH-Sensitive (Annealing) Polyelectrolyte Brush. Langmuir 2011, 27, 10615–10633. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine serum albumin interactions with metal complexes. Clujul Med. 2014, 87, 215. [Google Scholar] [CrossRef]
- Mitternacht, S. FreeSASA: An Open Source C Library for Solvent Accessible Surface Area Calculations. F1000Research 2016, 5, 189. [Google Scholar] [CrossRef]
- Tanford, C.; Swanson, S.A.; Shore, W.S. Hydrogen ion equilibria of bovine serum albumin. J. Am. Chem. 1955, 77, 6414–6421. [Google Scholar] [CrossRef]
- Laktionov, M.Y.; Shavykin, O.V.; Leermakers, F.A.M.; Zhulina, E.B.; Borisov, O.V. Colloidal particles interacting with a polymer brush: A self-consistent field theory. Phys. Chem. Chem. Phys. 2022, 24, 8463–8476. [Google Scholar] [CrossRef]
- Bohinc, K.; Bossa, G.V.; May, S. Incorporation of ion and solvent structure into mean-field modeling of the electric double layer. Adv. Colloid Interface Sci. 2017, 249, 220–233. [Google Scholar] [CrossRef]
- Jackler, G.; Wittemann, A.; Ballauff, M.; Czeslik, C. Spherical polyelectrolyte brushes as carrier particles for proteins: An investigation of the structure of adsorbed and desorbed bovine serum albumin. Spectroscopy 2004, 18, 289–299. [Google Scholar] [CrossRef]
- Wittemann, A.; Ballauff, M. Secondary structure analysis of proteins embedded in spherical polyelectrolyte brushes by FT-IR spectroscopy. Anal. Chem. 2004, 76, 2813–2819. [Google Scholar] [CrossRef]
- Han, L.; Yan, B.; Zhang, L.; Wu, M.; Wang, J.; Huang, J.; Deng, Y. Hongbo Zeng Tuning protein adsorption on charged polyelectrolyte brushes via salinity adjustment. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 37–45. [Google Scholar] [CrossRef]







| Amino Acid Name | N | pKa |
|---|---|---|
| Asp | 40 | 3.92 |
| Glu | 59 | 3.92 |
| His | 16 | 6.9 |
| Tyr | 19 | 10.35 |
| Lys | 57 | 9.8 |
| Arg | 22 | 12.0 |
| N-terminus | 1 | 7.75 |
| C-terminus | 1 | 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamatova, T.O.; Zhulina, E.B.; Borisov, O.V. Bovine Serum Albumin Interaction with Polyanionic and Polycationic Brushes: The Case Theoretical Study. Int. J. Mol. Sci. 2023, 24, 3395. https://doi.org/10.3390/ijms24043395
Salamatova TO, Zhulina EB, Borisov OV. Bovine Serum Albumin Interaction with Polyanionic and Polycationic Brushes: The Case Theoretical Study. International Journal of Molecular Sciences. 2023; 24(4):3395. https://doi.org/10.3390/ijms24043395
Chicago/Turabian StyleSalamatova, Tatiana O., Ekaterina B. Zhulina, and Oleg V. Borisov. 2023. "Bovine Serum Albumin Interaction with Polyanionic and Polycationic Brushes: The Case Theoretical Study" International Journal of Molecular Sciences 24, no. 4: 3395. https://doi.org/10.3390/ijms24043395
APA StyleSalamatova, T. O., Zhulina, E. B., & Borisov, O. V. (2023). Bovine Serum Albumin Interaction with Polyanionic and Polycationic Brushes: The Case Theoretical Study. International Journal of Molecular Sciences, 24(4), 3395. https://doi.org/10.3390/ijms24043395







