Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1
Abstract
:1. Introduction
2. Results
2.1. Cashmere Quality and Yield
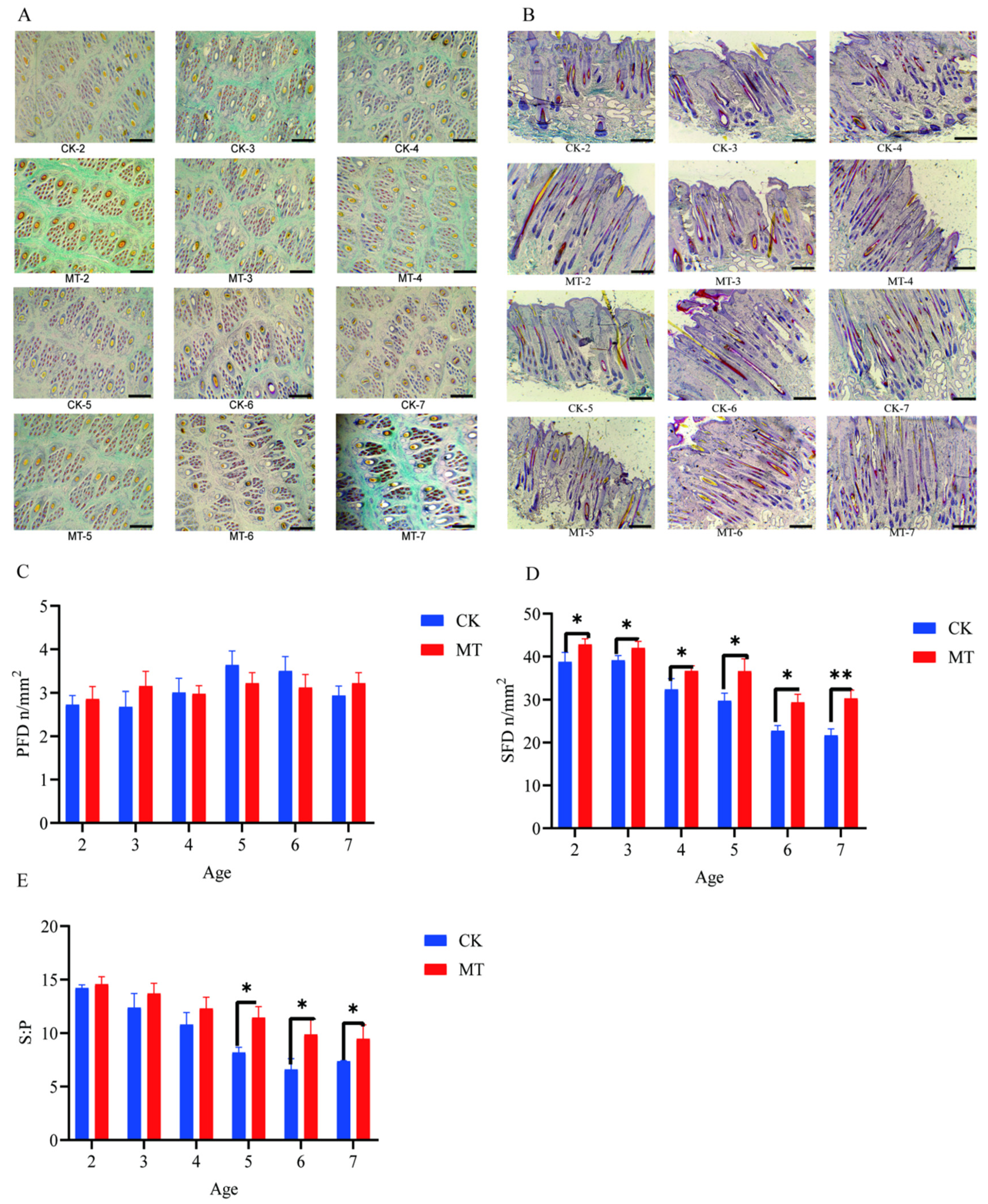
2.2. Primary and Secondary Hair Follicles
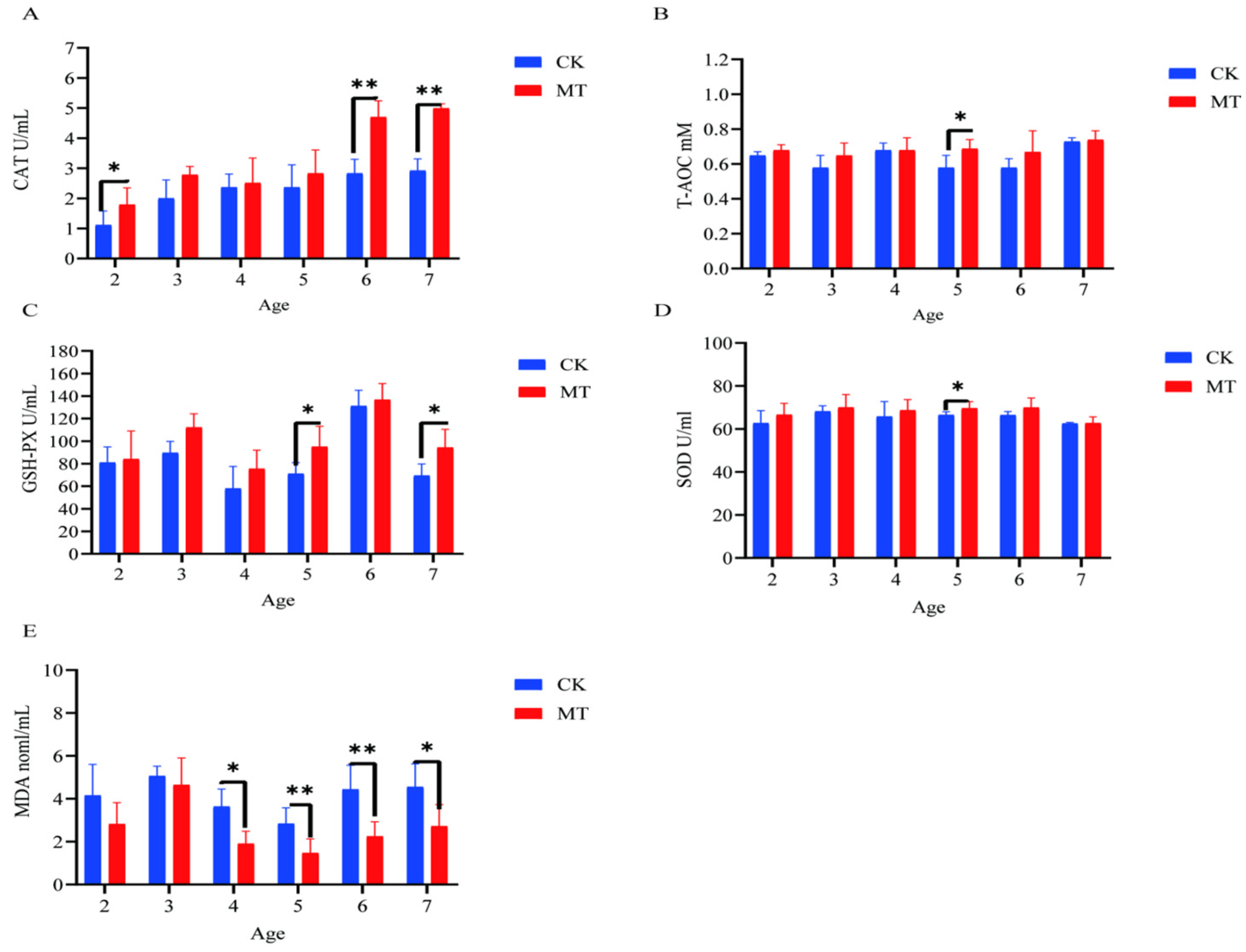
2.3. Antioxidant Capacity of Serum and Skin
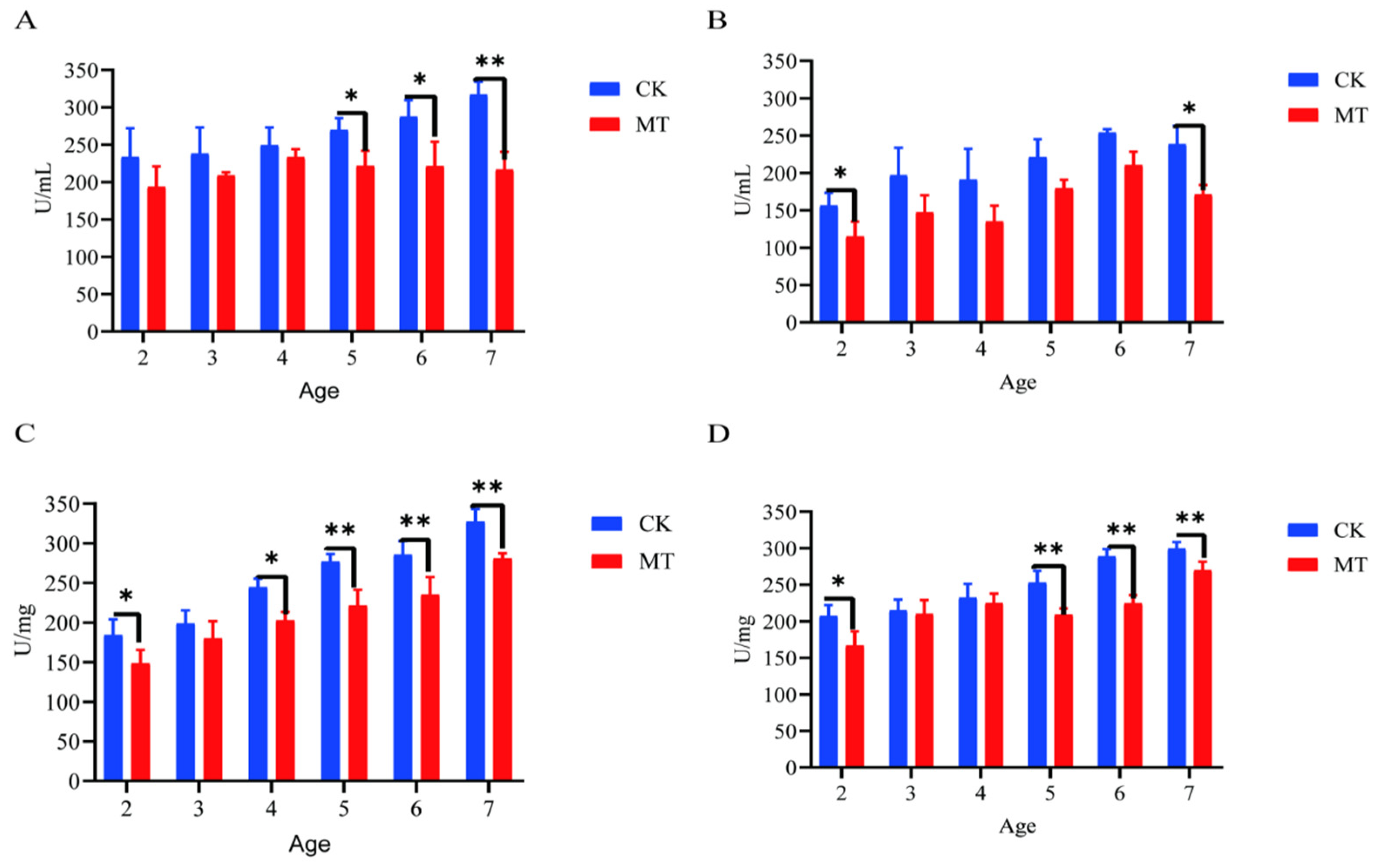
2.4. Markers of Oxidative Stress
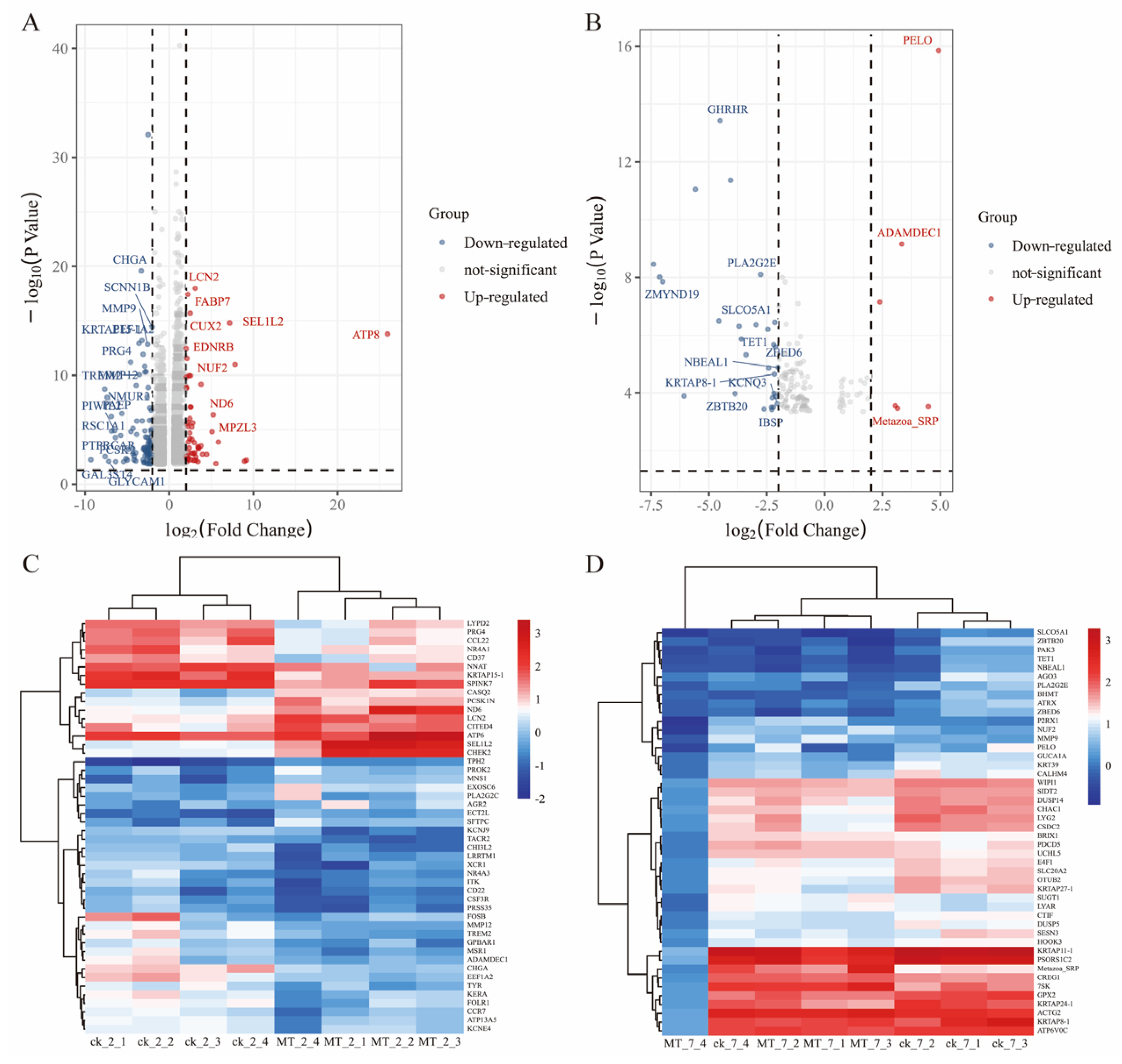
2.5. Differential mRNA Expression between MT vs. CK
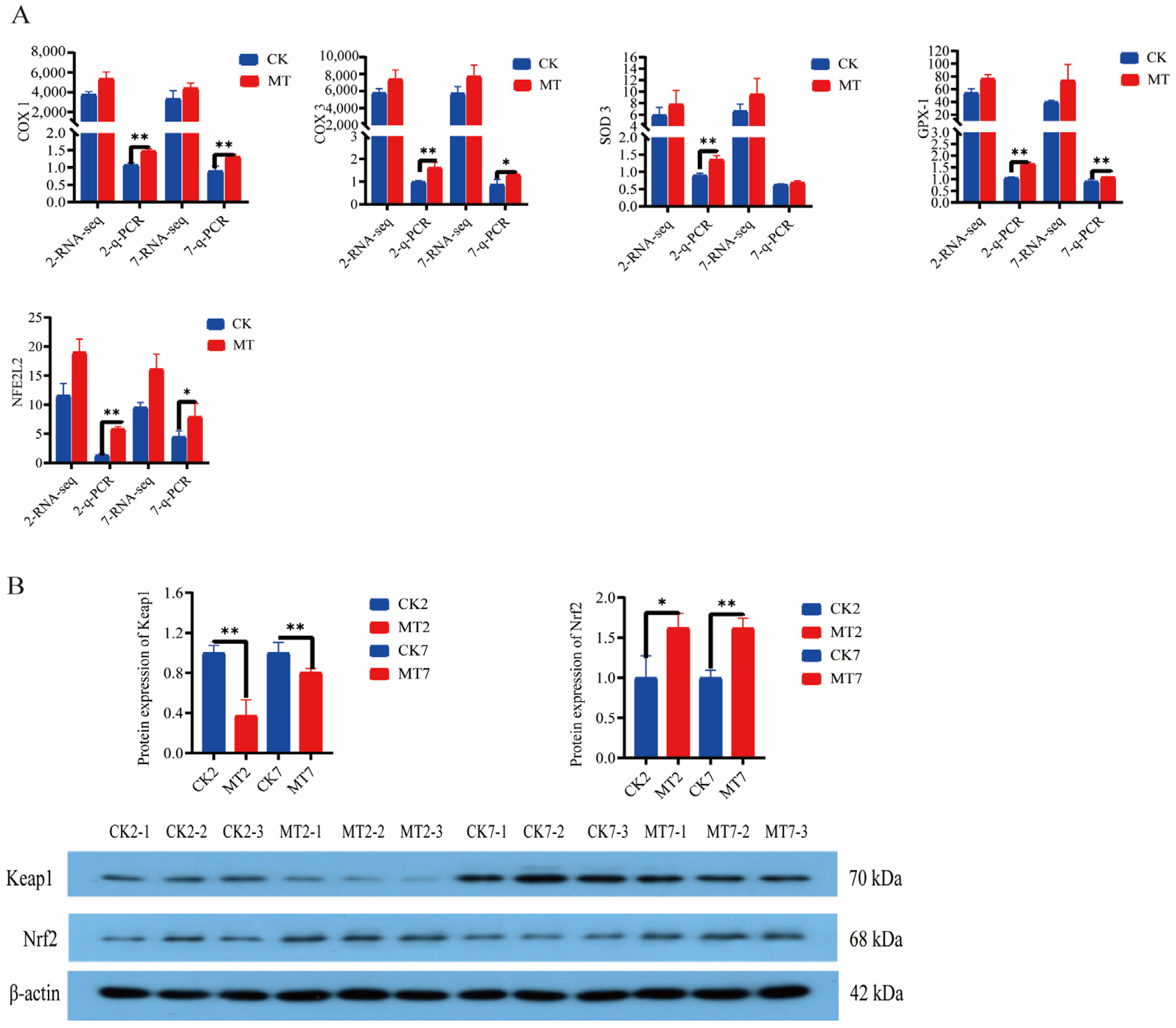
2.6. Antioxidant Gene Expression and Protein Levels
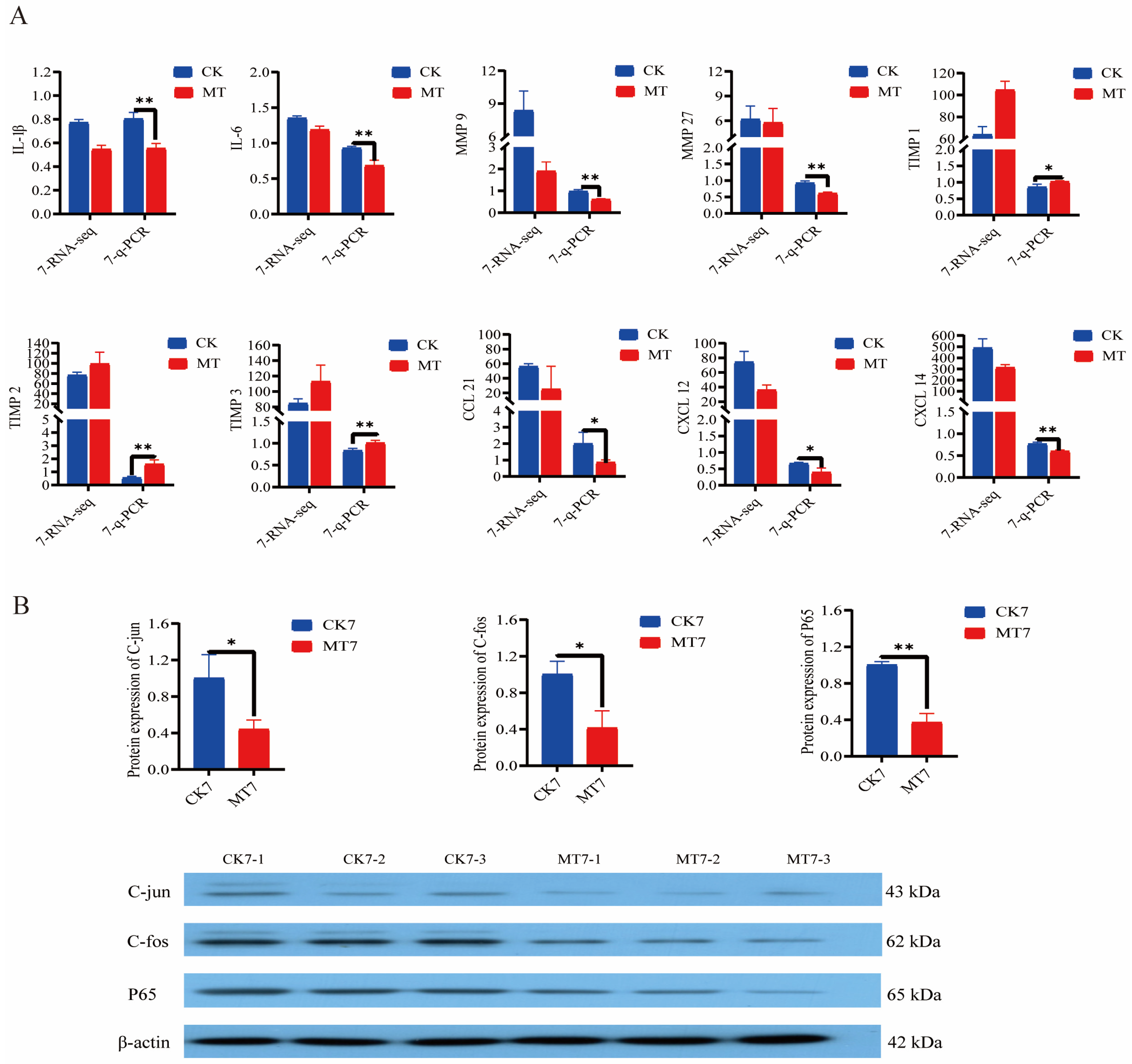
2.7. Anti-Aging Gene Expression and Protein Levels
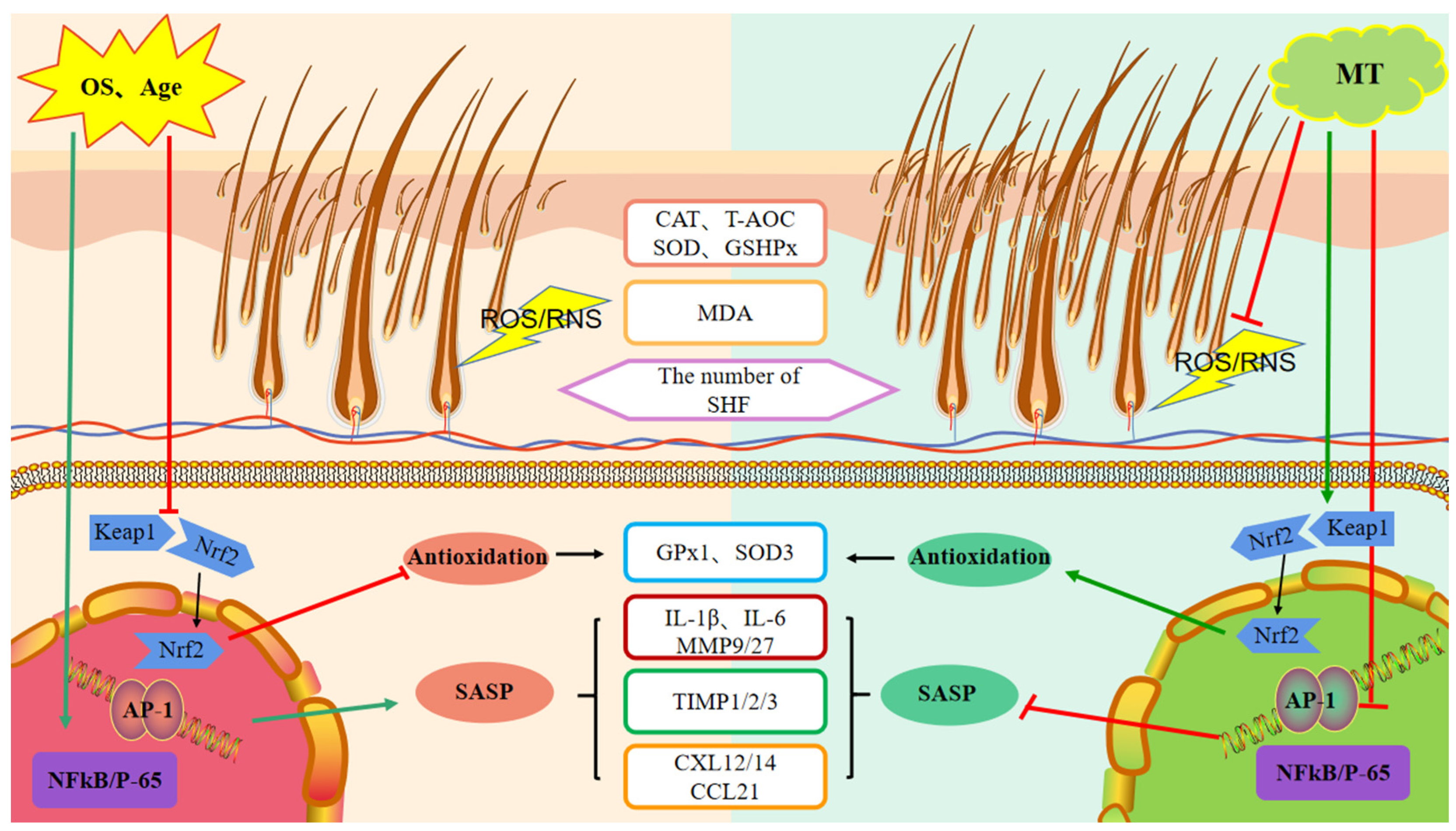
3. Discussion
4. Materials and Methods
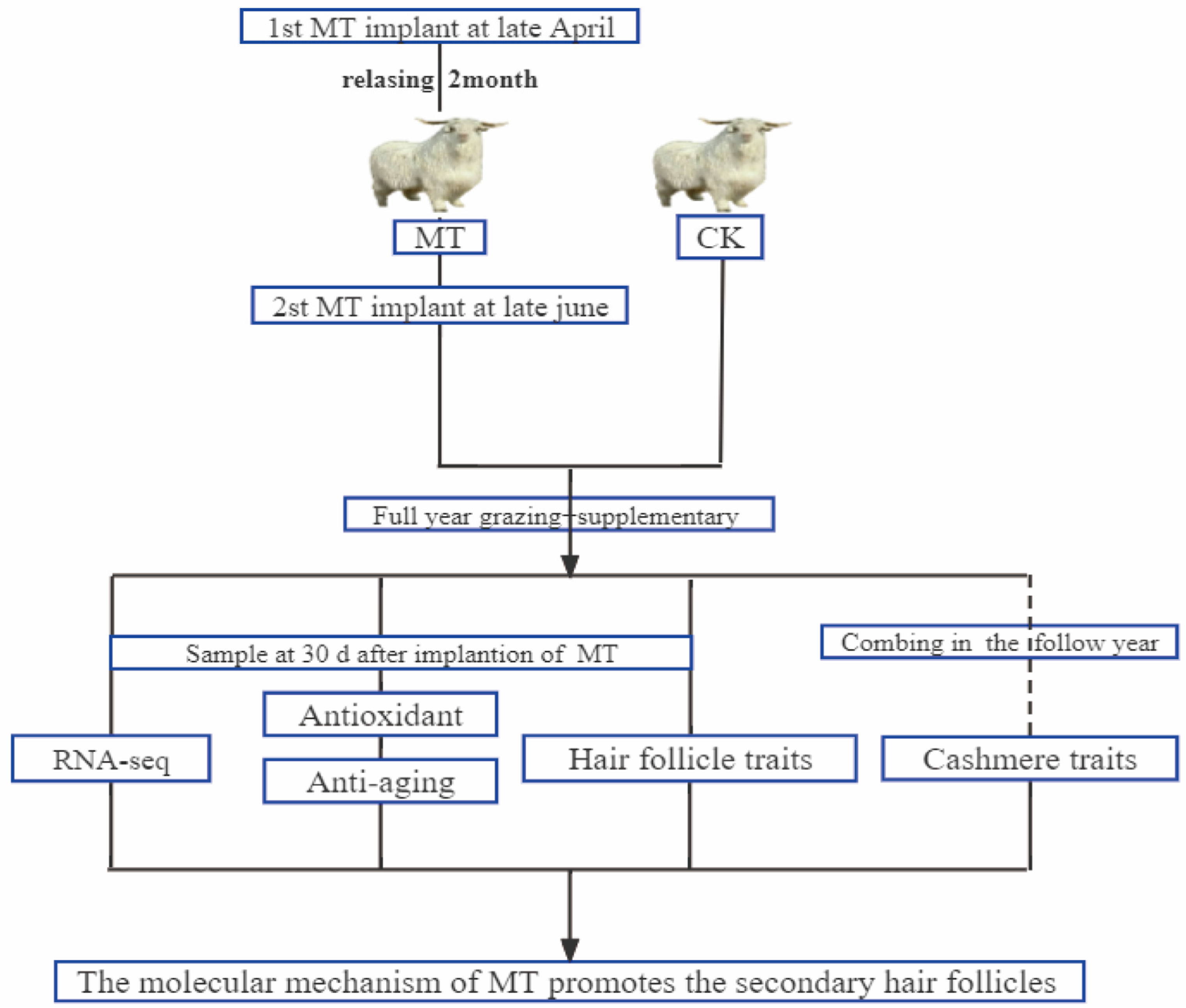
4.1. Animals and Management
4.2. Samples Collection and Processing
4.3. mRNA Transcriptome Analysis
4.4. RT-qPCR and Western Blotting
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, C.; Xu, J.; Zhang, J.; Zhang, W.; Sun, Y.; Jia, Z. Effects of melatonin implantation on cashmere growth, hormone concentrations and cashmere yield in cashmere-perennial-type Liaoning cashmere goats. Anim. Prod. Sci. 2016, 57, 60–64. [Google Scholar] [CrossRef]
- Duan, C.; Xu, J.; Sun, C.; Jia, Z.; Zhang, W. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Gong, G.; Fan, Y.; Li, W.; Yan, X.; Yan, X.; Zhang, L.; Wang, N.; Chen, O.; Zhang, Y.; Wang, R.; et al. Identification of the key genes associated with different hair types in the Inner Mongolia cashmere goat. Animals 2022, 12, 1456. [Google Scholar] [CrossRef]
- Yang, C.H.; Wu, Z.Y.; Li, Y.; Zhang, W. Effect of melatonin administration to lactating cashmere goats on milk production of dams and on hair follicle development in their offspring. Animal 2020, 14, 1241–1248. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Sun, H.Z.; Li, S.L.; Song, D.; Zhang, C.H.; Jin, J.; Antonini, M.; Zhao, C.F. Effects of photoperiod on nutrient digestibility, hair follicle activity and cashmere quality in Inner Mongolia white cashmere goats. Asian Austral. J. Anim. 2019, 32, 541. [Google Scholar] [CrossRef]
- Zhou, H.M.; Allain, D.; Li, J.Q.; Zhang, W.G.; Yu, X.C. Effects of non-genetic factors on production traits of Inner Mongolia cashmere goats in China. Small Ruminant. Res. 2003, 47, 85–89. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, N.S.; Singh, L.B. Non-genetic factors affecting growth of Beetal halfbred kids. Indian J. Anim. Sci. 2000, 70, 1165–1166. [Google Scholar] [CrossRef]
- Islam, R.; Liu, X.; Gebreselassie, G.; Abied, A.; Ma, Y. Genome-wide association analysis reveals the genetic locus for high reproduction trait in Chinese albas cashmere goat. Genes Genom. 2020, 42, 893–899. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, L.; Gong, G.; Yan, X.C.; Zhang, L.T.; Zhang, F.T.; Liu, H.F.; Lv, Q.; Wang, Z.Y.; Wang, R.J.; et al. Genome-wide association study of fleece traits in Inner Mongolia cashmere goats. Anim. Genet. 2021, 52, 375–379. [Google Scholar] [CrossRef]
- Gong, G.; Fan, Y.; Yan, X.; Li, W.; Yan, X.; Liu, H.; Zhang, L.; Su, Y.; Zhang, J.; Jiang, W.; et al. Identification of genes related to hair follicle cycle development in inner Mongolia cashmere goat by WGCNA. Front. Vet. Sci. 2022, 9, 894380. [Google Scholar] [CrossRef]
- Li, C.; Feng, C.; Ma, G.Y.; Fu, S.Y.; Chen, M.; Zhang, W.G.; Li, J.Q. Time-course RNA-seq analysis reveals stage-specific and melatonin-triggered gene expression patterns during the hair follicle growth cycle in capra hircus. BMC Genom. 2022, 23, 140. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Zhang, T.; Wang, M.; Wang, X. Roles of melatonin in goat hair follicle stem cell proliferation and pluripotency through regulating the Wnt signaling pathway. Front. Cell Dev. Biol. 2021, 9, 686805. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.; Khatib, H.; Ge, W.; Wang, S.H.; Sun, B. Melatonin promotes cashmere goat (Capra hircus) secondary hair follicle growth: A view from integrated analysis of long non-coding and coding RNAs. Cell Cycle 2018, 17, 1255–1267. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, F.; Xia, L.X.; Wang, H.X.; Ye, X.Y. Melatonin and the aging brain. Neurochem. Int. 2007, 50, 571–580. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Pierrefiche, G.; Laborit, H. Oxygen free radicals, melatonin, and aging. Exp. Gerontol. 1995, 30, 213–227. [Google Scholar] [CrossRef]
- Moi, C.S.; Xue, Z.L.; Koh, R.Y.; Ng, K.Y.; Desk, S. The roles of melatonin in Parkinson’s disease: An overview. Am. J. Physiol.-Lung C 2017, 2, 115–120. [Google Scholar] [CrossRef]
- Rose, J.; Oldfield, J.; Stormshak, F. Apparent role of melatonin and prolactin in initiating winter fur growth in mink. Gen. Comp. Endocr. 1987, 65, 212–215. [Google Scholar] [CrossRef]
- Jones, E.J.; Poole, K.C.; Sollini, J. Seasonal weight changes in laboratory ferrets. PLoS ONE 2020, 15, e0232733.15. [Google Scholar] [CrossRef]
- Nieminen, P.; Pyyknen, T.; Asikainen, J.; Mononen, J.; Mustonen, A.M. Effects of fasting and exogenous melatonin on annual rhythms in the blue fox (Alopex lagopus). Comp. Biochem. Phys. A 2004, 139, 183–197. [Google Scholar] [CrossRef]
- Feng, Y.; Gun, S. Melatonin supplement induced the hair follicle development in offspring rex rabbits. J. Anim. Physiol. Anim. N. 2021, 105, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Duan, C.; Wu, Z.; Li, Y.; Luan, Y.; Fu, X.; Zhang, C.; Zhang, W. Effects of melatonin administration to cashmere goats on cashmere production and hair follicle characteristics in two consecutive cashmere growth cycles. Domest. Anim. Endocrin. 2021, 74, 106534. [Google Scholar] [CrossRef]
- McGregor, B.A.; Kerven, C.; Toigonbaev, S. Sources of variation contributing to production and quality attributes of Kyrgyz cashmere in Osh and Naryn provinces: Implications for industry development. Small Ruminant Res. 2009, 84, 89–99. [Google Scholar] [CrossRef]
- Keva, S. Qualitative and Quantitative Characteristics of Cashmere Produced by South African Indigenous Goats; University of Pretoria: Pretoria, South Africa, 2007; Available online: http://hdl.handle.net/2263/23157 (accessed on 13 March 2007).
- McGregor, B.A. Influence of nutrition, fibre diameter and fibre length on the fibre curvature of cashmere. Aust. J. Exp. Agr. 2003, 43, 1199–1209. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Staege, S.; Brinckmann, J.; Jenkins, G.J.; Bhogal, R.; Lim, F.L.; Farjo, N.; Farjo, B. Oxidative damage control in a human (mini-) organ: Nrf2 activation protects against oxidative stress-induced hair growth inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Sikkink, S.K.; Mine, S.; Freis, O.; Danoux, L.; Tobin, D.J. Stress-sensing in the human greying hair follicle: Ataxia Telangiectasia Mutated (ATM) depletion in hair bulb melanocytes in canities-prone scalp. Sci. Rep. 2020, 10, 18711. [Google Scholar] [CrossRef]
- Sharma, M.; Palacios-Bois, J.; Schwartz, G.; Iskandar, M.; Thakur, R.; Quirion, N.P.; Nair, V. Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiat. 1989, 25, 305–319. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Yang, L. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef]
- Bishop, S.C.; Russel, A.J.F. Cashmere production from feral and imported cashmere goat kids. Anim. Sci. J. 1994, 58, 135–144. [Google Scholar] [CrossRef]
- Zhu, X.P.; Zhou, J.P.; Yang, J.; Jia, Z.H. Dietary iodine and selenium affected the mRNA expression levels of skin monodeiodinase (II, III) in Liaoning cashmere goats. Biol. Trace Elem. Res. 2013, 151, 360–364. [Google Scholar] [CrossRef]
- Bafti, M.S.; Salehi, M.; Moumen, S.M.; Ezatkhah, M. Correlations between serum mineral content and cashmere traits in Raeini goats. Anim. Prod. Sci. 2017, 57, 1665–1673. [Google Scholar] [CrossRef]
- Celi, P.; Seren, E.; Celi, R. Relationships between blood hormonal concentrations and secondary fibre shedding in young cashmere-bearing goats at their first moult. Anim. Sci. J. 2003, 77, 371–381. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, C.; Guo, Y.; Zhang, Y.; Liu, Y. Inhibition of prolactin promotes secondary skin follicle activation in cashmere goats. J. Anim. Sci. 2021, 99, skab079. [Google Scholar] [CrossRef]
- Tuncer, S.S. Some cashmere characteristics of hair goats raised in Van province. Austral. J. Vet. Sci. 2018, 50, 125–128. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Xu, X.; Xu, Q.; Qin, C.K.; Liu, G.F.; Wei, C.; Zhang, G.P.; Tian, K.C.; Fu, X.F. Effect of the FA2H gene on cashmere fineness of Jiangnan cashmere goats based on transcriptome sequencing. BMC Genom. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Klören, W.R.L.; Norton, B.W. Melatonin and fleece growth in Australian cashmere goats. Small Ruminant Res. 1995, 17, 179–185. [Google Scholar] [CrossRef]
- Mccabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Foster, A.R.; Chami, C.E.; O’Neill, C.A.; Watson, R. Osmolyte transporter expression is reduced in photoaged human skin: Implications for skin hydration in aging. Aging Cell 2020, 19, e13058. [Google Scholar] [CrossRef]
- Linton, S.; Davies, M.J.; Dean, R.T. Protein oxidation and ageing. Exp. Gerontol. 2001, 36, 1503–1518. [Google Scholar] [CrossRef]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int. J. Cosmetic Sci. 2008, 30, 313–322. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Ju, H.L.; Kim, T.G. Cellular senescence and inflammaging in the skin microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Ji, J.; Ho, B.S.Y.; Qian, G.; Xie, X.M.; Bigliardi, P.L.; Qi, M.B. Aging in hair follicle stem cells and niche microenvironment. J. Dermat. Nurses Assoc. 2017, 44, 1097–1104. [Google Scholar] [CrossRef]
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef]
- Shin, W.; Sparks, H.D.; Sinha, S.; Rahmani, W.; Biernaskie, J. Dysfunction of hair follicle mesenchymal progenitors contributes to age-associated hair loss. Dev. Cell 2020, 53, 185–198.e7. [Google Scholar] [CrossRef]
- Horsley, V.; Aliprantis, A.O.; Polak, L.; Glimcher, L.H.; Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 2008, 132, 299–310. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z. Protective role of melatonin and its metabolites in skin aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Erefolu, M.; Seyhan, M.; Gül, M.; Parlakpnar, H.; Batolu, K.; Uyumlu, B. Potent therapeutic effect of melatonin on aging skin in pinealectomized rats. J. Pineal Res. 2005, 39, 231–237. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, W.; Zhang, Y.; Zheng, Y.; Li, F.; Wang, S.; Liu, J.; Tan, S.; Yan, Z.; Wang, L.; et al. A single-cell transcriptome atlas of cashmere goat hair follicle morphogenesis. Genom. Proteom. Bioinform. 2021, 19, 437–451. [Google Scholar] [CrossRef]
- Jiao, Q.; Yin, R.H.; Zhao, S.J.; Wang, Z.Y.; Zhu, Y.B.; Wang, W.; Zheng, Y.Y.; Yin, X.B.; Guo, D.; Wang, S.Q.; et al. Identification and molecular analysis of a lncRNA-HOTAIR transcript from secondary hair follicle of cashmere goat reveal integrated regulatory network with the expression regulated potentially by its promoter methylation. Gene 2019, 688, 182–192. [Google Scholar] [CrossRef]
- Su, R.; Fan, Y.; Qiao, X.; Li, X.; Zhang, L.; Li, C.; Li, J. Transcriptomic analysis reveals critical genes for the hair follicle of Inner Mongolia cashmere goat from catagen to telogen. PLoS ONE 2018, 13, e204404. [Google Scholar] [CrossRef]
- Qiu, W.; Lei, M.; Zhou, L.; Bai, X.; Lai, X.; Yu, Y.; Tian, Y.; Lian, X.H. Hair follicle stem cell proliferation, Akt and Wnt signaling activation in TPA-induced hair regeneration. Histochem. Cell Biol. 2017, 147, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, F.; Zhao, M.; Mu, Q.; Zhao, H.Y. Skin transcriptome reveals the periodic changes in genes underlying hair follicle cycling in cashmere goats. bioRxiv 2019, 554030. [Google Scholar] [CrossRef]
- Beaudoin, G.M.J.; Sisk, J.M.; Coulombe, P.A.; Thompson, C.C. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc. Natl. A Sci. India B 2005, 102, 14653–14658. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qiu, X.; Piao, J.; Zhang, L.; Piao, J.; Zhao, F. Study on the roles of melatonin in regulating dermal fibroblast growth in Liaoning cashmere goats by transcriptome sequencing. Anim. Biotechnol. 2021, 33, 1255–1267. [Google Scholar] [CrossRef]
- Rundhaug, J.E.; Fischer, S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem. Photobiol. 2010, 84, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Tiano, H.F.; Loftin, C.D.; Akunda, J.; Lee, C.A.; Langenbach, R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002, 62, 3395–3401. [Google Scholar] [CrossRef]
- Kämpfer, H.; Bräutigam, L.; Geisslinger, G.; Pfeilschifter, J.; Frank, S. Cyclooxygenase-1-coupled prostaglandin biosynthesis constitutes an essential prerequisite for skin repair. J. Investig. Dermatol. 2003, 120, 880–890. [Google Scholar] [CrossRef]
- Zarkovic, N. Roles and functions of ROS and RNS in cellular physiology and pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Reis, R.L.; Marques, A.P. Dermal papilla cells and melanocytes response to physiological oxygen levels depends on their interactions. Cell Proliferat. 2021, 54, e13013. [Google Scholar] [CrossRef]
- Thi, M.H.N.; Shao, P.L.; Liao, J.D.; Lin, C.C.K.; Yip, H.K. Enhancement of angiogenesis and epithelialization processes in mice with burn wounds through ROS/RNS signals generated by non-thermal N2/Ar micro-plasma. Plasma Process Polym. 2014, 11, 1076–1088. [Google Scholar] [CrossRef]
- Xue, J.; Yu, C.X.; Sheng, W.J.; Zhu, W.; Luo, J.D.; Zhang, Q.; Yang, H.Y.; Cao, H.; Zhou, J.D.; Wu, J.C.; et al. The Nrf2/GCH1/BH4 axis ameliorates radiation-induced skin injury by modulating the ROS cascade. J. Investig. Dermatol. 2017, 137, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichol. 2009, 1, 6. [Google Scholar] [CrossRef]
- Jadkauskaite, L.; Coulombe, P.A.; Schäfer, M. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? Bioessays 2017, 39, 1700029. [Google Scholar] [CrossRef]
- Telorack, M.; Meyer, M.; Ingold, I. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genet. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Feng, Y.; Tian, W.; Cao, R.; Fu, K. Aucubin ameliorates the LPS-induced inflammatory response in bovine endometrial epithelial cells by inhibiting NF-κB and activating the Keap1/Nrf2 signalling pathway. Reprod. Domest. Anim. 2021, 56, 972–982. [Google Scholar] [CrossRef]
- Zhao, P.; Park, N.H.; Alam, M.B. Fuzhuan brick tea boosts melanogenesis and prevents hair graying through reduction of oxidative stress via NRF2-HO-1 signaling. Antioxidants 2022, 11, 599. [Google Scholar] [CrossRef]
- Li, L.; Hwang, E.; Ngo, H.; Lin, P.; Gao, W.; Liu, Y. Antiphotoaging effect of Prunus yeonesis blossom extract via inhibition of MAPK/AP-1 and regulation of the TGF-βI/Smad and Nrf2/ARE signaling pathways. Photochem. Photobiol. 2018, 94, 725–732. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Jena, G.B. Effect of melatonin on the expression of Nrf2 and NF-κB during cyclophosphamide-induced urinary bladder injury in rat. J. Pineal Res. 2010, 48, 324–331. [Google Scholar] [CrossRef]
- Langton, A.K.; Graham, H.K.; Griffiths, C.; Watson, R. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Piérard-Franchimont, C.; Loussouarn, G.; Panhard, S. Immunohistochemical patterns in the interfollicular caucasian scalps: Influences of age, gender, and alopecia. Biomed. Res. Int. 2013, 2013, 769489. [Google Scholar] [CrossRef] [PubMed]
- Pea, M.; Lopez-Soler, R.; Melendez, J.A. Senescence in chronic allograft nephropathy. Am. J. Physiol.-Renal. 2018, 315, F880–F889. [Google Scholar] [CrossRef]
- Jarrousse, F.; Boisnic, S.; Branchet, M.C.; Jean-Yves, B.; Gaston, B.; Lionel, G.; Bruno, B.; Yann, B.; Mahé, F. Identification of clustered cells in human hair follicle responsible for MMP-9 gelatinolytic activity: Consequences for the regulation of hair growth. Int. J. Dermatol. 2001, 40, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Lee, I.A. Effect of MMP/TIMP balancing of cynoglossus semilaevis shell extracts on skin protection. Fishes 2021, 6, 34. [Google Scholar] [CrossRef]
- Sadowski, T.; Dietrich, S.; Müller, M.; Blanka, H.; Michael, S.; Ehrhardt, P.; Markus, S.M.; Radislav, S. Matrix metalloproteinase-19 expression in normal and diseased skin: Dysregulation by epidermal proliferation. J. Investig. Dermatol. 2003, 121, 989–996. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, C.H.; Zhang, J.W. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging 2019, 11, 11391. [Google Scholar] [CrossRef]
- Lin, Y.W.; Lee, L.M.; Lee, W.J.; Chu, C.Y.; Tan, P.; Yang, Y.C.; Chen, W.Y.; Yang, S.F.; Hsiao, M.; Hsien, M. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin, and the pro-and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef]



| Target | Primers Sequences (5′-3′) | Product Size (bp) |
|---|---|---|
| GAPDH | F: ATGTTTGTGATGGGCGTGAA R: GGCGTGGACAGTGGTCATAAGT | 153 |
| MMP9 | F: TAGAGAGCACGGAGATGGGTATC R: GAAGTGGGCATCTCCCTGAAT | 97 |
| MMP27 | F: CTTGATGTTCCCAAACTACGTCTC R: GTAGATGGATTGGATTCCGTTG | 85 |
| TIMP1 | F: GTCATCAGGGCCAAGTTCGT R: GGAAGTATCCGCAGACGCTC | 169 |
| TIMP2 | F: AGGTGGACTCTGGCAACGA R: TTCAGGCTCTTCTTCTGGGTG | 274 |
| TIMP3 | F: GATCAAATCCTGCTACTACCTGCC R: CCGGATGCAAGCGTAGTGTTT | 121 |
| CCL14 | F: TTCAAGAGGACCTTACCACCCT R: ATGTAATGGCCCTTCTTGGTG | 147 |
| CCL21 | F: CTCCAGGTCCAAGGCAGTGAT R: CAGTCATCTTTAGGGTCTGCACATA | 190 |
| SOD3 | F: CACTACAACCCGATGTCCGTG R: CCGCGTTACCGTTCTCCA | 214 |
| CXCL12 | F: CTACAGATGCCCTTGCCGATT R: GTTCTTCAGCCTTGCCACGAT | 115 |
| COX1 | F: TTCTGATTCTTTGGACACCCTG R: AACCCGATTGATATTATGGCTC | 143 |
| COX3 | F: GGAAGGAGACCGTAACCACATA R: CTACGAAGAAAGTTGAACCGTAGA | 143 |
| GPX1 | F:GAAAAGTGCGAGGTCAATGGC R: ACAGCAGGGTTTCAATGTCAGG | 253 |
| IL-1β | F: TGCTGGATAGCCCATGTGTG | 75 |
| R: TGCAGAACACCACTTCTCGG | ||
| IL6 | F:TGAAGGAAAAGATCGCAGGTCTAA R: ACCTTTGCGTTCTTTACCCACT | 100 |
| CXCL14 | F:AGCATGAGGCTCCTGACCG R: TAGCGAATCTTGGGTCCCTTTC | 110 |
| NFE2L2 | F:GTAGCCACTGCTGATTTAGACGA R: CCAACTTCTTTATCCAGTGAGGG | 206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, X.; Duan, C.; Yao, L.; Qin, J.; He, L.; Zhang, W. Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1. Int. J. Mol. Sci. 2023, 24, 3403. https://doi.org/10.3390/ijms24043403
Diao X, Duan C, Yao L, Qin J, He L, Zhang W. Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1. International Journal of Molecular Sciences. 2023; 24(4):3403. https://doi.org/10.3390/ijms24043403
Chicago/Turabian StyleDiao, Xiaogao, Chunhui Duan, Lingyun Yao, Jiaxin Qin, Liwen He, and Wei Zhang. 2023. "Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1" International Journal of Molecular Sciences 24, no. 4: 3403. https://doi.org/10.3390/ijms24043403





