Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. TMB Analysis in Tumoral Specimen
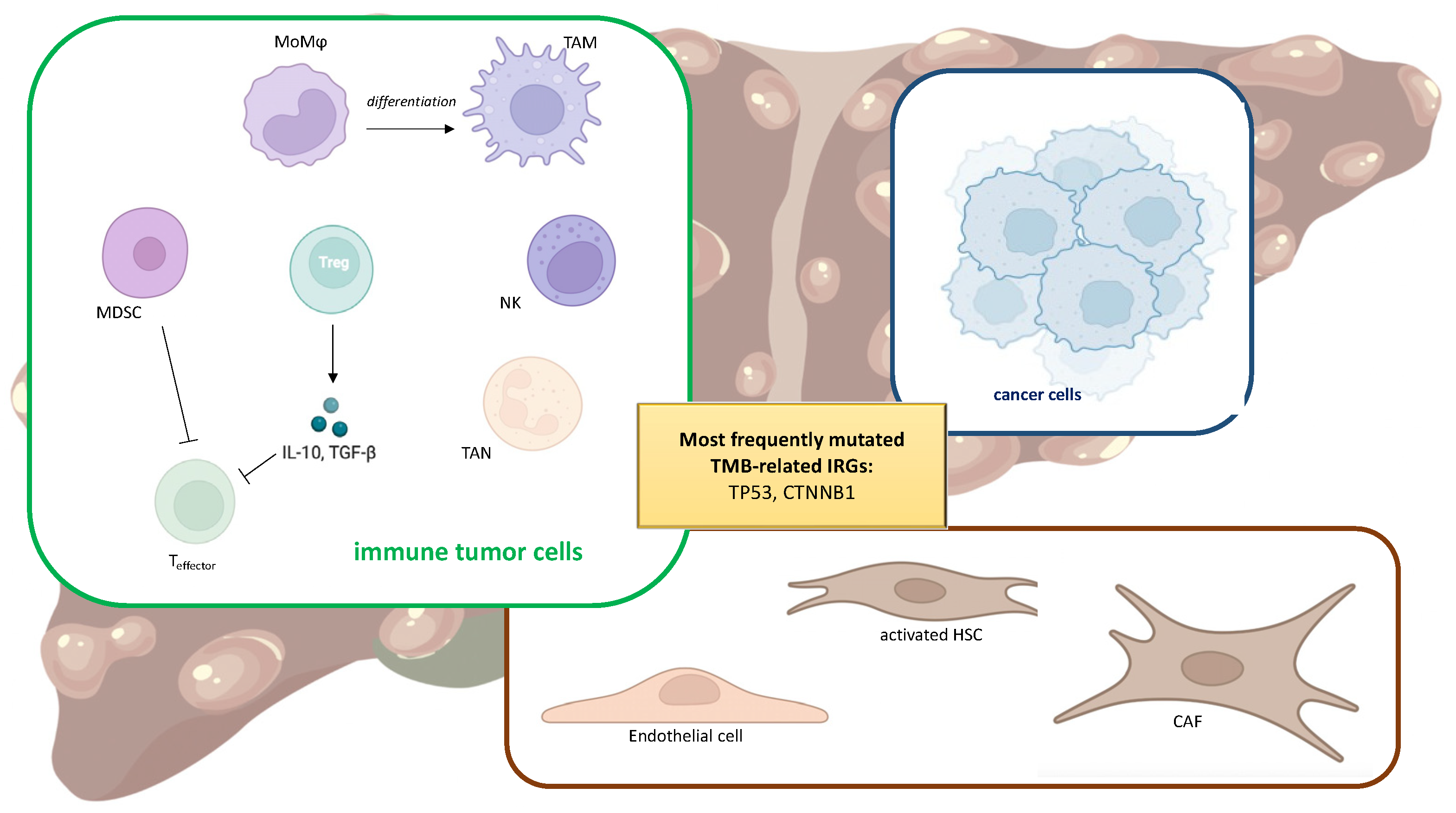
3. Tumor Immune Microenvironment
4. TMB and Immune TME
5. TMB in the Different Etiologies of HCC and Its Progression
6. TMB as Biomarker of Therapy Outcome
7. Combination of TMB and Other Specific Genetic Biomarkers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Koroki, K.; Kanzaki, H.; Kobayashi, K.; Kiyono, S.; Nakamura, M.; Kanogawa, N.; Saito, T.; Kondo, T.; Nakagawa, R.; et al. Changes in Therapeutic Options for Hepatocellular Carcinoma in Asia. Liver Int. 2022, 42, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Guo, X.G.; Wang, Z.H.; Dong, W.; He, X.D.; Liu, F.C.; Liu, H. Specific CYP450 Genotypes in the Chinese Population Affect Sorafenib Toxicity in HBV/HCV-Associated Hepatocellular Carcinoma Patients. Biomed. Environ. Sci. 2018, 31, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wen, Q.; Li, S.-F.; Zhang, Y.-F.; Gao, N.; Tian, X.; Fang, Y.; Gao, J.; Cui, M.-Z.; He, X.-P.; et al. Significant Change of Cytochrome P450s Activities in Patients with Hepatocellular Carcinoma. Oncotarget 2016, 7, 50612–50623. [Google Scholar] [CrossRef] [PubMed]
- De Martin, S.; Gabbia, D.; Albertin, G.; Sfriso, M.M.; Mescoli, C.; Albertoni, L.; Paliuri, G.; Bova, S.; Palatini, P. Differential Effect of Liver Cirrhosis on the Pregnane X Receptor-Mediated Induction of CYP3A1 and 3A2 in the Rat. Drug Metab. Dispos. 2014, 42, 1617–1626. [Google Scholar] [CrossRef]
- Floreani, M.; Gabbia, D.; Barbierato, M.; DE Martin, S.; Palatini, P. Differential Inducing Effect of Benzo[a]Pyrene on Gene Expression and Enzyme Activity of Cytochromes P450 1A1 and 1A2 in Sprague-Dawley and Wistar Rats. Drug Metab. Pharmacokinet. 2012, 27, 640–652. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Nemorosone Inhibits the Proliferation and Migration of Hepatocellular Carcinoma Cells. Life Sci. 2019, 235, 116817. [Google Scholar] [CrossRef]
- Kurma, K.; Zeybek Kuyucu, A.; Roth, G.S.; Sturm, N.; Mercey-Ressejac, M.; Abbadessa, G.; Yu, Y.; Lerat, H.; Marche, P.N.; Decaens, T.; et al. Effect of Novel AKT Inhibitor Vevorisertib as Single Agent and in Combination with Sorafenib on Hepatocellular Carcinoma in a Cirrhotic Rat Model. Int. J. Mol. Sci. 2022, 23, 16206. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. JCO 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune Checkpoint Inhibitors and Cardiovascular Toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jiang, J.; Zhan, M.; Zhang, H.; Wang, Q.-T.; Sun, S.-N.; Guo, X.-K.; Yin, H.; Wei, Y.; Liu, J.O.; et al. Targeting Neoantigens in Hepatocellular Carcinoma for Immunotherapy: A Futile Strategy? Hepatology 2021, 73, 414–421. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Qi, F.; Hu, X.; Luo, J. Exploration of the Relationships between Tumor Mutation Burden with Immune Infiltrates in Clear Cell Renal Cell Carcinoma. Ann. Transl. Med. 2019, 7, 648. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Chae, Y.K.; Costa, R.L.; Kalyan, A.; Shah, H.A.; Helenowski, I.; Rademaker, A.W.; Mahalingam, D.; Giles, F.J. Correlation of Tumor Mutational Burden and Treatment Outcomes in Patients with Colorectal Cancer. J. Gastrointest. Oncol. 2017, 8, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, J.; Chen, L. Exploration of Gene Expression Profiles and Immune Microenvironment between High and Low Tumor Mutation Burden Groups in Prostate Cancer. Int. Immunopharmacol. 2020, 86, 106709. [Google Scholar] [CrossRef]
- Park, S.E.; Park, K.; Lee, E.; Kim, J.-Y.; Ahn, J.S.; Im, Y.-H.; Lee, C.; Jung, H.; Cho, S.Y.; Park, W.-Y.; et al. Clinical Implication of Tumor Mutational Burden in Patients with HER2-Positive Refractory Metastatic Breast Cancer. OncoImmunology 2018, 7, e1466768. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Qi, F.; Wang, J.; Luo, J. Multi-omics Analysis of Tumor Mutation Burden Combined with Immune Infiltrates in Bladder Urothelial Carcinoma. J. Cell. Physiol. 2020, 235, 3849–3863. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Mukai, S.; Kanzaki, H.; Ogasawara, S.; Ishino, T.; Ogawa, K.; Nakagawa, M.; Fujiwara, K.; Unozawa, H.; Iwanaga, T.; Sakuma, T.; et al. Exploring Microsatellite Instability in Patients with Advanced Hepatocellular Carcinoma and Its Tumor Microenvironment. JGH Open 2021, 5, 1266–1274. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Chen, S.; Huang, W. Immunological Value of Prognostic Signature Based on Cancer Stem Cell Characteristics in Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2021, 9, 710207. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Xin, H.-Y.; Luo, C.-B.; Li, J.; Zhou, Z.-J.; Zou, J.-X.; Zhou, S.-L. Associations among the Mutational Landscape, Immune Microenvironment, and Prognosis in Chinese Patients with Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2021, 70, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Kim, J.T.; Cox, B.; Larson, B.K.; Kim, S.; Waters, K.M.; Vail, E.; Guindi, M. Evaluation of Tumor Mutational Burden in Small Early Hepatocellular Carcinoma and Progressed Hepatocellular Carcinoma. Hepatic Oncol. 2021, 8, HEP39. [Google Scholar] [CrossRef]
- Murugesan, K.; Sharaf, R.; Montesion, M.; Moore, J.A.; Pao, J.; Pavlick, D.C.; Frampton, G.M.; Upadhyay, V.A.; Alexander, B.M.; Miller, V.A.; et al. Genomic Profiling of Combined Hepatocellular Cholangiocarcinoma Reveals Genomics Similar to Either Hepatocellular Carcinoma or Cholangiocarcinoma. JCO Precis. Oncol. 2021, 5, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Campesato, L.F.; Barroso-Sousa, R.; Jimenez, L.; Correa, B.R.; Sabbaga, J.; Hoff, P.M.; Reis, L.F.L.; Galante, P.A.F.; Camargo, A.A. Comprehensive Cancer-Gene Panels Can Be Used to Estimate Mutational Load and Predict Clinical Benefit to PD-1 Blockade in Clinical Practice. Oncotarget 2015, 6, 34221–34227. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Frampton, G.M.; Rioth, M.J.; Yusko, E.; Xu, Y.; Guo, X.; Ennis, R.C.; Fabrizio, D.; Chalmers, Z.R.; Greenbowe, J.; et al. Targeted next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol. Res. 2016, 4, 959–967. [Google Scholar] [CrossRef]
- Roszik, J.; Haydu, L.E.; Hess, K.R.; Oba, J.; Joon, A.Y.; Siroy, A.E.; Karpinets, T.V.; Stingo, F.C.; Baladandayuthapani, V.; Tetzlaff, M.T.; et al. Novel Algorithmic Approach Predicts Tumor Mutation Load and Correlates with Immunotherapy Clinical Outcomes Using a Defined Gene Mutation Set. BMC Med. 2016, 14, 168. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Gassa, A.; Buchner, D.; Alakus, H.; Dong, Q.; Ren, N.; Liu, M.; Odenthal, M.; Stippel, D.; et al. Circulating Tumor DNA as an Emerging Liquid Biopsy Biomarker for Early Diagnosis and Therapeutic Monitoring in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2020, 16, 1551–1562. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Z.; Li, Z.; Dong, X.; Xu, H.; Lin, J.; Chen, L.; Zhang, H.; Liu, X.; Liu, J. Clonal Evolution in Long-Term Follow-up Patients with Hepatocellular Carcinoma. Int. J. Cancer 2018, 143, 2862–2870. [Google Scholar] [CrossRef] [Green Version]
- Franses, J.W.; Lim, M.; Burgoyne, A.M.; Mody, K.; Lennerz, J.; Chang, J.; Imperial, R.; Dybel, S.N.; Dinh, T.M.; Masannat, J.; et al. Profile and Predictors of Blood Tumor Mutational Burden in Advanced Hepatocellular Carcinoma. Oncologist 2022, 27, e908–e911. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.N.; Fessas, P.; Dominy, K.; Mauri, F.A.; Kaneko, T.; Parcq, P.D.; Khorashad, J.; Toniutto, P.; Goldin, R.D.; Avellini, C.; et al. Qualification of Tumour Mutational Burden by Targeted Next-Generation Sequencing as a Biomarker in Hepatocellular Carcinoma. Liver Int. 2021, 41, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Zeuli, R.; Cavalluzzo, B.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Ceccarelli, M.; Tagliamonte, M.; Buonaguro, L. High Somatic Mutation and Neoantigen Burden Do Not Correlate with Decreased Progression-Free Survival in HCC Patients Not Undergoing Immunotherapy. Cancers 2019, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tan, J.; Wu, M.; Fan, W.; Wei, J.; Zhu, B.; Guo, J.; Wang, S.; Zhou, P.; Zhang, H.; et al. High-Affinity Neoantigens Correlate with Better Prognosis and Trigger Potent Antihepatocellular Carcinoma (HCC) Activity by Activating CD39+CD8+ T Cells. Gut 2021, 70, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Yu, J.; Yang, F.; Zhang, M.; Liu, Y.; Ma, S.; Zhou, X.; Wang, J.; Han, Y. Identification and Validation a Costimulatory Molecule Gene Signature to Predict the Prognosis and Immunotherapy Response for Hepatocellular Carcinoma. Cancer Cell Int. 2022, 22, 97. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liang, J.; Long, P.; Zhu, L.; Hou, W.; Wu, X.; Luo, C. ZCCHC17 Served as a Predictive Biomarker for Prognosis and Immunotherapy in Hepatocellular Carcinoma. Front. Oncol. 2022, 11, 799566. [Google Scholar] [CrossRef]
- Li, Y.; Mo, H.; Wu, S.; Liu, X.; Tu, K. A Novel Lactate Metabolism-Related Gene Signature for Predicting Clinical Outcome and Tumor Microenvironment in Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2022, 9, 801959. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Wang, X.; Wei, P.; Wang, H.; Tan, S. Identification of the Immune Cell Infiltration Landscape in Hepatocellular Carcinoma to Predict Prognosis and Guide Immunotherapy. Front. Genet. 2021, 12, 777931. [Google Scholar] [CrossRef]
- Liu, F.; Hou, W.; Liang, J.; Zhu, L.; Luo, C. LRP1B Mutation: A Novel Independent Prognostic Factor and a Predictive Tumor Mutation Burden in Hepatocellular Carcinoma. J. Cancer 2021, 12, 4039–4048. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Q.; Huang, J.; Zhan, M.; Zhao, W.; Yang, X.; Li, Y.; Qiu, L.; Zhang, F.; Lu, L.; et al. Prognostic Analysis of Tumor Mutation Burden and Immune Infiltration in Hepatocellular Carcinoma Based on TCGA Data. Aging 2021, 13, 11257–11280. [Google Scholar] [CrossRef]
- Xie, C.; Wu, H.; Pan, T.; Zheng, X.; Yang, X.; Zhang, G.; Lian, Y.; Lin, J.; Peng, L. A Novel Panel Based on Immune Infiltration and Tumor Mutational Burden for Prognostic Prediction in Hepatocellular Carcinoma. Aging 2021, 13, 8563–8587. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhou, L.; Xu, R. Identification of Tumor Mutation Burden and Immune Infiltrates in Hepatocellular Carcinoma Based on Multi-Omics Analysis. Front. Mol. Biosci. 2021, 7, 599142. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Wang, Y.; Cao, Z.; Li, P.; Zhang, S. An Integrative Analysis Reveals the Underlying Association between CTNNB1 Mutation and Immunotherapy in Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liang, X.-L.; Liu, X.-G.; Chen, N.-P. The Landscape of PD-L1 Expression and Somatic Mutations in Hepatocellular Carcinoma. J. Gastrointest. Oncol. 2021, 12, 1132–1140. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, D.; Liu, L.; Zhou, X.; Yao, Y.; Li, Z.; Li, J.; Chen, J.; Lei, Q.; Han, X. Development and Validation of a Robust Immune-Related Risk Signature for Hepatocellular Carcinoma. Medicine 2021, 100, e24683. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, C.; Li, M.; Li, W.; Zhang, M.; Jiang, X.; Chang, Y.; Liu, L.; Wang, F.; Zhao, Q. Identification of a Prognostic and Therapeutic Immune Signature Associated with Hepatocellular Carcinoma. Cancer Cell Int. 2021, 21, 98. [Google Scholar] [CrossRef]
- Xie, F.; Bai, Y.; Yang, X.; Long, J.; Mao, J.; Lin, J.; Wang, D.; Song, Y.; Xun, Z.; Huang, H.; et al. Comprehensive Analysis of Tumour Mutation Burden and the Immune Microenvironment in Hepatocellular Carcinoma. Int. Immunopharmacol. 2020, 89, 107135. [Google Scholar] [CrossRef]
- Huo, J.; Wu, L.; Zang, Y. A Prognostic Model of 15 Immune-Related Gene Pairs Associated With Tumor Mutation Burden for Hepatocellular Carcinoma. Front. Mol. Biosci. 2020, 7, 581354. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, D.; Yao, C.; Ding, S.; Lu, Z. Development of a Predictive Immune-Related Gene Signature Associated with Hepatocellular Carcinoma Patient Prognosis. Cancer Control 2020, 27, 1073274820977114. [Google Scholar] [CrossRef]
- Floreani, A.; De Martin, S.; Secchi, M.F.; Cazzagon, N. Extrahepatic Autoimmunity in Autoimmune Liver Disease. Eur. J. Intern. Med. 2019, 59, 1–7. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Immune Surveillance by the Liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Pusole, G.; Demurtas, L.; Puzzoni, M.; Mascia, R.; Morgan, G.; Giampieri, R.; Scartozzi, M. Tumor Infiltrating Lymphocytes in Gastrointestinal Tumors: Controversies and Future Clinical Implications. Crit. Rev. Oncol./Hematol. 2017, 110, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Germano, G.; Marchesi, F.; Locatelli, M.; Biswas, S.K. Cancer-Promoting Tumor-Associated Macrophages: New Vistas and Open Questions. Eur. J. Immunol. 2011, 41, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wang, L.; Tian, H.; Wu, X.; Xiao, C.; Pan, Y.; Fan, M.; Tai, Y.; Liu, W.; Zhang, Q.; et al. CAF-Derived Exosomes Transmitted Gremlin-1 Promotes Cancer Progression and Decreases the Sensitivity of Hepatoma Cells to Sorafenib. Mol. Carcinog. 2022, 61, 764–775. [Google Scholar] [CrossRef]
- Li, X.-F.; Chen, D.-P.; Ouyang, F.-Z.; Chen, M.-M.; Wu, Y.; Kuang, D.-M.; Zheng, L. Increased Autophagy Sustains the Survival and Pro-Tumourigenic Effects of Neutrophils in Human Hepatocellular Carcinoma. J. Hepatol. 2015, 62, 131–139. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Rizvi, S.; Wang, J.; El-Khoueiry, A.B. Liver Cancer Immunity. Hepatology 2021, 73, 86–103. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 Family Revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A Rheostat for Immune Responses: The Unique Properties of PD-1 and Their Advantages for Clinical Application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T Cell Costimulatory Receptor CD28 Is a Primary Target for PD-1-Mediated Inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Aravalli, R.N. Role of Innate Immunity in the Development of Hepatocellular Carcinoma. World J. Gastroenterol. 2013, 19, 7500–7514. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction: Distinctive Features and Clinical Relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, T.; Pan, W.; Zhu, L.-Y.; Li, L.; Zheng, L. Increased Intratumoral Regulatory T Cells Are Related to Intratumoral Macrophages and Poor Prognosis in Hepatocellular Carcinoma Patients. Int. J. Cancer 2009, 125, 1640–1648. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Guo, X.; Lu, Y.; Xie, Y.; Huang, X.; Lin, J.; Tan, W.; Wang, C. Myeloid-Derived Suppressor Cells Promote Tumor Growth and Sorafenib Resistance by Inducing FGF1 Upregulation and Fibrosis. Neoplasia 2022, 28, 100788. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Jia, W.; Liang, S.; Cheng, B.; Ling, C. The Role of Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and the Value of Traditional Chinese Medicine Treatment. Front. Oncol. 2021, 11, 763519. [Google Scholar] [CrossRef]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Chen, G.; Zhang, W.; Xing, C.; Xu, X.; Xie, H.; Lu, A.; Chen, K.; Guo, H.; Ren, Z.; et al. MRC-5 Fibroblast-Conditioned Medium Influences Multiple Pathways Regulating Invasion, Migration, Proliferation, and Apoptosis in Hepatocellular Carcinoma. J. Transl. Med. 2015, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-Modified Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells Improve Hepatocellular Carcinoma Chemosensitivity through MTOR Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the Cytokines Produced by Human Neutrophils in Tumors. Semin. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Huang, X.-W.; Wang, Z.; Chen, E.-B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic Coordination of T Cell Quiescence and Activation. Nat. Rev. Immunol. 2020, 20, 55–70. [Google Scholar] [CrossRef]
- He, Y.; Jiang, Z.; Chen, C.; Wang, X. Classification of Triple-Negative Breast Cancers Based on Immunogenomic Profiling. J. Exp. Clin. Cancer Res. 2018, 37, 327. [Google Scholar] [CrossRef]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Xu, X.; Zhang, Y. Comprehensive Analysis of Genomic and Immunological Profiles in Chinese and Western Hepatocellular Carcinoma Populations. Aging 2021, 13, 11564–11594. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Deng, R.; Wang, Z.; Li, N.; Qi, Z.; Zhao, J.; Huang, W. Multi-Omics Analysis Reveals Prognostic Value of Tumor Mutation Burden in Hepatocellular Carcinoma. Cancer Cell Int. 2021, 21, 342. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, D.; He, Y.; Wei, J. Correlation Analysis of Tumor Mutation Burden of Hepatocellular Carcinoma Based on Data Mining. J. Gastrointest. Oncol. 2021, 12, 1117–1131. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Hui, V.W.-K.; Yip, T.C.-F.; Liang, L.Y.; Zhang, X.; Tse, Y.-K.; Lai, J.C.-T.; Chan, H.L.-Y.; Wong, V.W.-S. Universal HBV Vaccination Dramatically Reduces the Prevalence of HBV Infection and Incidence of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2022, 56, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Saponaro, M.; Sarcognato, S.; Guido, M.; Ferri, N.; Carrara, M.; De Martin, S. Fucus Vesiculosus and Ascophyllum Nodosum Ameliorate Liver Function by Reducing Diet-Induced Steatosis in Rats. Mar. Drugs 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.; Rossi, M.C.; Petrelli, M. Effectiveness of Ascophyllum Nodosum and Fucus Vesiculosus on Metabolic Syndrome Components: A Real-World, Observational Study. J. Diabetes Res. 2021, 2021, 3389316. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.; van Buuren, N.; Gamelin, L.; Soulette, C.; May, L.; Han, D.; Yu, M.; Choy, R.; Cheng, G.; Bhardwaj, N.; et al. Targeted Long-Read Sequencing Reveals Comprehensive Architecture, Burden, and Transcriptional Signatures from Hepatitis B Virus-Associated Integrations and Translocations in Hepatocellular Carcinoma Cell Lines. J. Virol. 2021, 95, e0029921. [Google Scholar] [CrossRef]
- Chen, X.-P.; Long, X.; Jia, W.; Wu, H.-J.; Zhao, J.; Liang, H.-F.; Laurence, A.; Zhu, J.; Dong, D.; Chen, Y.; et al. Viral Integration Drives Multifocal HCC during the Occult HBV Infection. J. Exp. Clin. Cancer Res. 2019, 38, 261. [Google Scholar] [CrossRef]
- Fan, X.; Yuan, H.; Zhao, S.; Yang, X.; Shi, R.; Wang, J.; Zhao, H. Epigenetic Age Acceleration of Early Stage Hepatocellular Carcinoma Tightly Associated with Hepatitis B Virus Load, Immunoactivation, and Improved Survival. Cancer Biol. Ther. 2020, 21, 899–906. [Google Scholar] [CrossRef]
- Gentilini, D.; Scala, S.; Gaudenzi, G.; Garagnani, P.; Capri, M.; Cescon, M.; Grazi, G.L.; Bacalini, M.G.; Pisoni, S.; Dicitore, A.; et al. Epigenome-Wide Association Study in Hepatocellular Carcinoma: Identification of Stochastic Epigenetic Mutations through an Innovative Statistical Approach. Oncotarget 2017, 8, 41890–41902. [Google Scholar] [CrossRef]
- Gao, J.; Xi, L.; Yu, R.; Xu, H.; Wu, M.; Huang, H. Differential Mutation Detection Capability Through Capture-Based Targeted Sequencing in Plasma Samples in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 596789. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated Analysis of Somatic Mutations and Focal Copy-Number Changes Identifies Key Genes and Pathways in Hepatocellular Carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Zhang, X.; Wood, L.D.; Anders, R.A.; Choti, M.A.; Pawlik, T.M.; Daniel, H.D.; Kannangai, R.; Offerhaus, G.J.A.; et al. Inactivating Mutations of the Chromatin Remodeling Gene ARID2 in Hepatocellular Carcinoma. Nat. Genet. 2011, 43, 828–829. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shi, H.; Liu, T.; Li, M.; Zhou, S.; Qiu, X.; Wang, Z.; Hu, W.; Guo, W.; Chen, X.; et al. Mutation Profile and Its Correlation with Clinicopathology in Chinese Hepatocellular Carcinoma Patients. Hepatobiliary Surg. Nutr. 2021, 10, 172–179. [Google Scholar] [CrossRef]
- Donati, B.; Pietrelli, A.; Pingitore, P.; Dongiovanni, P.; Caddeo, A.; Walker, L.; Baselli, G.; Pelusi, S.; Rosso, C.; Vanni, E.; et al. Telomerase Reverse Transcriptase Germline Mutations and Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancer Med. 2017, 6, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Pinyol, R.; Torrecilla, S.; Wang, H.; Montironi, C.; Piqué-Gili, M.; Torres-Martin, M.; Wei-Qiang, L.; Willoughby, C.E.; Ramadori, P.; Andreu-Oller, C.; et al. Molecular Characterisation of Hepatocellular Carcinoma in Patients with Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. MTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer Cell 2017, 32, 807–823.e12. [Google Scholar] [CrossRef] [PubMed]
- Conde de la Rosa, L.; Garcia-Ruiz, C.; Vallejo, C.; Baulies, A.; Nuñez, S.; Monte, M.J.; Marin, J.J.G.; Baila-Rueda, L.; Cenarro, A.; Civeira, F.; et al. STARD1 Promotes NASH-Driven HCC by Sustaining the Generation of Bile Acids through the Alternative Mitochondrial Pathway. J. Hepatol. 2021, 74, 1429–1441. [Google Scholar] [CrossRef]
- Longo, M.; Paolini, E.; Meroni, M.; Dongiovanni, P. Remodeling of Mitochondrial Plasticity: The Key Switch from NAFLD/NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4173. [Google Scholar] [CrossRef]
- Ang, C.; Klempner, S.J.; Ali, S.M.; Madison, R.; Ross, J.S.; Severson, E.A.; Fabrizio, D.; Goodman, A.; Kurzrock, R.; Suh, J.; et al. Prevalence of Established and Emerging Biomarkers of Immune Checkpoint Inhibitor Response in Advanced Hepatocellular Carcinoma. Oncotarget 2019, 10, 4018–4025. [Google Scholar] [CrossRef]
- Li, L.; Rao, X.; Wen, Z.; Ding, X.; Wang, X.; Xu, W.; Meng, C.; Yi, Y.; Guan, Y.; Chen, Y.; et al. Implications of Driver Genes Associated with a High Tumor Mutation Burden Identified Using Next-Generation Sequencing on Immunotherapy in Hepatocellular Carcinoma. Oncol. Lett. 2020, 19, 2739–2748. [Google Scholar] [CrossRef]
- Wang, X.; Li, M. Correlate Tumor Mutation Burden with Immune Signatures in Human Cancers. BMC Immunol. 2019, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Zhang, Y.; Zhang, H.; Cui, C.; Li, C.; Lu, S. Prognostic Role of Tumor Mutation Burden in Hepatocellular Carcinoma after Radical Hepatectomy. J. Surg. Oncol. 2020, 121, 1007–1014. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Liu, W.-R.; Tang, Z.; Qu, W.-F.; Fang, Y.; Jiang, X.-F.; Song, S.-S.; Wang, H.; Tao, C.-Y.; Zhou, P.-Y.; et al. Serial Circulating Tumor DNA to Predict Early Recurrence in Patients with Hepatocellular Carcinoma: A Prospective Study. Mol. Oncol. 2022, 16, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, S.; Tu, Z.; Sun, J.; Rui, T.; Zhang, X.; Huang, H.; Ling, Q.; Zheng, S. Sarcomatoid Hepatocellular Carcinoma: From Clinical Features to Cancer Genome. Cancer Med. 2021, 10, 6227–6238. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-H.; Su, T.-H.; Jeng, Y.-M.; Liang, P.-C.; Chen, D.-S.; Chen, C.-H.; Kao, J.-H. Clinical Manifestations and Outcomes of Patients with Sarcomatoid Hepatocellular Carcinoma. Hepatology 2019, 69, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tsilimigras, D.I.; Farooq, A.; Hyer, J.M.; Merath, K.; Paredes, A.Z.; Mehta, R.; Sahara, K.; Shen, F.; Pawlik, T.M. Management and Outcomes among Patients with Sarcomatoid Hepatocellular Carcinoma: A Population-Based Analysis. Cancer 2019, 125, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; Tsokos, C.G.; Umetsu, S.E.; Shain, A.H.; Kelley, R.K.; Onodera, C.; Bowman, S.; Talevich, E.; Ferrell, L.D.; Kakar, S.; et al. Genomic Profiling of Combined Hepatocellular-Cholangiocarcinoma Reveals Similar Genetics to Hepatocellular Carcinoma. J. Pathol. 2019, 248, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Kudo, M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology 2017, 92, 50–62. [Google Scholar] [CrossRef]
- Gao, Q.; Qiu, S.-J.; Fan, J.; Zhou, J.; Wang, X.-Y.; Xiao, Y.-S.; Xu, Y.; Li, Y.-W.; Tang, Z.-Y. Intratumoral Balance of Regulatory and Cytotoxic T Cells Is Associated with Prognosis of Hepatocellular Carcinoma after Resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef]
- Ho, W.J.; Danilova, L.; Lim, S.J.; Verma, R.; Xavier, S.; Leatherman, J.M.; Sztein, M.B.; Fertig, E.J.; Wang, H.; Jaffee, E.; et al. Viral Status, Immune Microenvironment and Immunological Response to Checkpoint Inhibitors in Hepatocellular Carcinoma. J. Immunother. Cancer 2020, 8, e000394. [Google Scholar] [CrossRef] [Green Version]
- Sapena, V.; Enea, M.; Torres, F.; Celsa, C.; Rios, J.; Rizzo, G.E.M.; Nahon, P.; Mariño, Z.; Tateishi, R.; Minami, T.; et al. Hepatocellular Carcinoma Recurrence after Direct-Acting Antiviral Therapy: An Individual Patient Data Meta-Analysis. Gut 2022, 71, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lin, Y.-S.; Chang, C.-W.; Chang, C.-W.; Wang, T.-E.; Wang, H.-Y.; Chen, M.-J. Impact of Direct-Acting Antiviral Therapy for Hepatitis C-Related Hepatocellular Carcinoma. PLoS ONE 2020, 15, e0233212. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019, 156, 1683–1692.e1. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.S.; Hayashi, P.H.; Burke, L.M.; McRee, A.J. Decreased Hepatocellular Carcinoma Tumor Burden with the Achievement of Hepatitis C Virus Sustained Virologic Response: Unlocking the Potential of T-Cell-Mediated Immunosurveillance. J. Hepatocell. Carcinoma 2018, 5, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.; Vendemiale, G.; Serviddio, G. Molecular Mechanisms Involved in HCC Recurrence after Direct-Acting Antiviral Therapy. Int. J. Mol. Sci. 2018, 20, 49. [Google Scholar] [CrossRef]
- FDA. FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors; FDA: Silver Spring, MD, USA, 2020.
- Xu, J.; Zhang, Y.; Jia, R.; Yue, C.; Chang, L.; Liu, R.; Zhang, G.; Zhao, C.; Zhang, Y.; Chen, C.; et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-Label, Dose Escalation and Expansion Study. Clin. Cancer Res. 2019, 25, 515–523. [Google Scholar] [CrossRef]
- Pinyol, R.; Montal, R.; Bassaganyas, L.; Sia, D.; Takayama, T.; Chau, G.-Y.; Mazzaferro, V.; Roayaie, S.; Lee, H.C.; Kokudo, N.; et al. Molecular Predictors of Prevention of Recurrence in HCC with Sorafenib as Adjuvant Treatment and Prognostic Factors in the Phase 3 STORM Trial. Gut 2019, 68, 1065–1075. [Google Scholar] [CrossRef]
- Lyu, N.; Wang, X.; Li, J.-B.; Lai, J.-F.; Chen, Q.-F.; Li, S.-L.; Deng, H.-J.; He, M.; Mu, L.-W.; Zhao, M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). JCO 2022, 40, 468–480. [Google Scholar] [CrossRef]
- Xue, M.; Wu, Y.; Zhu, B.; Zou, X.; Fan, W.; Li, J. Advanced Hepatocellular Carcinoma Treated by Transcatheter Arterial Chemoembolization with Drug-Eluting Beads plus Lenvatinib versus Sorafenib, a Propensity Score Matching Retrospective Study. Am. J. Cancer Res. 2021, 11, 6107–6118. [Google Scholar]
- Wang, Y.; Lu, L.-C.; Guan, Y.; Ho, M.-C.; Lu, S.; Spahn, J.; Hsu, C.-H. Atezolizumab plus Bevacizumab Combination Enables an Unresectable Hepatocellular Carcinoma Resectable and Links Immune Exclusion and Tumor Dedifferentiation to Acquired Resistance. Exp. Hematol. Oncol. 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Venniyoor, A. Synergism between Anti-Angiogenic and Immune Checkpoint Inhibitor Drugs: A Hypothesis. Med. Hypotheses 2021, 146, 110399. [Google Scholar] [CrossRef]
- Huang, T.; Yan, T.; Chen, G.; Zhang, C. Development and Validation of a Gene Mutation-Associated Nomogram for Hepatocellular Carcinoma Patients From Four Countries. Front. Genet. 2021, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Takeda, H.; Nishijima, N.; Orito, E.; Joko, K.; Uchida, Y.; Izumi, N.; Nishio, K.; Osaki, Y. Targeted DNA and RNA Sequencing of Fine-Needle Biopsy FFPE Specimens in Patients with Unresectable Hepatocellular Carcinoma Treated with Sorafenib. Oncotarget 2015, 6, 21636–21644. [Google Scholar] [CrossRef]
- Ou, Q.; Yu, Y.; Li, A.; Chen, J.; Yu, T.; Xu, X.; Xie, X.; Chen, Y.; Lin, D.; Zeng, Q.; et al. Association of Survival and Genomic Mutation Signature with Immunotherapy in Patients with Hepatocellular Carcinoma. Ann. Transl. Med. 2020, 8, 230. [Google Scholar] [CrossRef]
- Spahn, S.; Roessler, D.; Pompilia, R.; Gabernet, G.; Gladstone, B.P.; Horger, M.; Biskup, S.; Feldhahn, M.; Nahnsen, S.; Hilke, F.J.; et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers 2020, 12, 3830. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, J.; Chen, X.; Jiang, Y.; Zhao, H. Efficacy of Cabozantinib and Nivolumab in Treating Hepatocellular Carcinoma with RET Amplification, High Tumor Mutational Burden, and PD-L1 Expression. Oncologist 2020, 25, 470–474. [Google Scholar] [CrossRef]
- Zheng, J.; Shao, M.; Yang, W.; Ren, J.; Chen, X.; Yang, H. Benefits of Combination Therapy with Immune Checkpoint Inhibitors and Predictive Role of Tumour Mutation Burden in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Int. Immunopharmacol. 2022, 112, 109244. [Google Scholar] [CrossRef]
- Wei, J.; Fang, D.L.; Zhou, W.; He, Y.F. N6-Methyladenosine (M6A) Regulatory Gene Divides Hepatocellular Carcinoma into Three Subtypes. J. Gastrointest. Oncol. 2021, 12, 1860–1872. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, Y.; Chen, S.; Zhu, Y.; Li, S.; Shen, F.; Guo, Y.; Sun, T.; Chen, X.; Jiang, J.; et al. Immunological Significance of Prognostic DNA Methylation Sites in Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 683240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Shui, Y.; Li, P.; Tian, Z.; Duan, S.; Wei, Q. Implications of M6A-Associated SnRNAs in the Prognosis and Immunotherapeutic Responses of Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 1001506. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Tian, Y.; Luo, J.; Shao, G.; Zheng, J. An N6-Methyladenosine-Associated LncRNA Signature for Predicting Clinical Outcome and Therapeutic Responses in Hepatocellular Carcinoma. Ann. Transl. Med. 2022, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Z.; Wang, X.; You, L.; Li, W.; Zheng, C.; Zhang, J.; Wang, L.; Kong, X.; Gao, Y.; et al. Comprehensive Analysis of Nine M7G-Related LncRNAs as Prognosis Factors in Tumor Immune Microenvironment of Hepatocellular Carcinoma and Experimental Validation. Front. Genet. 2022, 13, 929035. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, R. Analysis of the Role of M6A and LncRNAs in Prognosis and Immunotherapy of Hepatocellular Carcinoma. Heliyon 2022, 8, e10612. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, Y.; Ye, R.; Yue, H.; Zhang, H.; Huang, T.; Liu, M.; Zheng, Y.; Wang, Y.; Zhou, Y.; et al. Development and Validation of an ECM-Related Prognostic Signature to Predict the Immune Landscape of Human Hepatocellular Carcinoma. BMC Cancer 2022, 22, 1036. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Liu, X.; Jiang, J. A Novel Necroptosis-Related LncRNA Signature Predicts the Prognosis and Immune Microenvironment of Hepatocellular Carcinoma. Front. Genet. 2022, 13, 985191. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, L.; Wang, S.; Zhang, Q.; Li, L. Comprehensive Analysis of M5C-Related LncRNAs in the Prognosis and Immune Landscape of Hepatocellular Carcinoma. Front. Genet. 2022, 13, 990594. [Google Scholar] [CrossRef]
- Huang, E.-M.; Ma, N.; Ma, T.; Zhou, J.-Y.; Yang, W.-S.; Liu, C.-X.; Hou, Z.-H.; Chen, S.; Zong, Z.; Zeng, B.; et al. Cuproptosis-Related Long Non-Coding RNAs Model That Effectively Predicts Prognosis in Hepatocellular Carcinoma. World J. Gastrointest. Oncol. 2022, 14, 1981–2003. [Google Scholar] [CrossRef]
- Qi, F.; Du, X.; Zhao, Z.; Zhang, D.; Huang, M.; Bai, Y.; Yang, B.; Qin, W.; Xia, J. Tumor Mutation Burden-Associated LINC00638/MiR-4732-3p/ULBP1 Axis Promotes Immune Escape via PD-L1 in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 729340. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Hu, Y.; Huang, W. Prognostic Role of CeRNA Network in Immune Infiltration of Hepatocellular Carcinoma. Front. Genet. 2021, 12, 739975. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, D.; Zhuang, L.; Zhang, J.; Wu, J. Identification of an Epithelial-Mesenchymal Transition-Related Long Non-Coding RNA Prognostic Signature to Determine the Prognosis and Drug Treatment of Hepatocellular Carcinoma Patients. Front. Med. 2022, 9, 850343. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, G.; Si, W.; Li, G.; Chai, J.; Liu, Y.; Liu, J. Identification of a Glycolysis-Related Gene Signature for Predicting Prognosis in Patients with Hepatocellular Carcinoma. BMC Cancer 2022, 22, 142. [Google Scholar] [CrossRef]
- Chen, J.; Tao, Q.; Lang, Z.; Gao, Y.; Jin, Y.; Li, X.; Wang, Y.; Zhang, Y.; Yu, S.; Lv, B.; et al. Signature Construction and Molecular Subtype Identification Based on Pyroptosis-Related Genes for Better Prediction of Prognosis in Hepatocellular Carcinoma. Oxidative Med. Cell. Longev. 2022, 2022, 4494713. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.-Q.; Chen, J.; Li, Z.-B.; Chen, J.-X.; Lu, Q.-Q.; Han, Y.-S.; Dai, W.; Xie, C.; Li, J.-C. Identifying Prognostic Significance of RCL1 and Four-Gene Signature as Novel Potential Biomarkers in HCC Patients. J. Oncol. 2021, 2021, 5574150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.; Li, W. A Comprehensive Prognostic and Immunological Analysis of a New Three-Gene Signature in Hepatocellular Carcinoma. Stem Cells Int. 2021, 2021, 5546032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Sun, L.; Guo, W.; Hu, X. The Effect of Ferroptosis-Related Genes on Prognosis and Tumor Mutational Burden in Hepatocellular Carcinoma. J. Oncol. 2021, 2021, 7391560. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, Y.; Zhou, Z.; Guo, Y.; Huang, X.; Westover, K.D.; Zhang, Z.; Chen, B.; Hua, Y.; Li, S.; et al. Intergrated Analysis of ELMO1, Serves as a Link between Tumour Mutation Burden and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. eBioMedicine 2019, 46, 105–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, T.; Huang, P. Integrated Analysis of Tumor Mutation Burden and Immune Infiltrates in Hepatocellular Carcinoma. Diagnostics 2022, 12, 1918. [Google Scholar] [CrossRef]

| Study | Mutation Pathways | Most Frequently Mutated Genes Associated with High TMB | Population and Database |
|---|---|---|---|
| Hu et al. [23] | High TMB (>10) is associated with increased PDL1 expression, CD3+ T-cell infiltration, and high numbers of CD68+ TAMs and CD66b+ TANs. High-TMB patients display recurrence and poor overall survival after curative resection. | TP53, TSC1, CTNNB1 | 182 Chinese HCC patients (91.2% HBV-related etiology) |
| Hu et al. [35] | Low-risk cluster of patients, assessed by six costimulatory molecule gene (CMGs) signature, had a lower TMB, low frequency rate of TP53 mutation, higher immunophenoscore (IPS), IPS-CTLA4, IPS-PD1/PD-L1/PD-L2, and IPS-PD1/PD-L1/PD-L2+CTLA compared to the high-risk cluster. | TP53 | 50 normal samples and 374 HCC samples The Cancer Genome Atlas (TCGA) |
| Liu et al. [36] | The high expression of ZCCHC17 is related to AFP, histologic grade, tumor status, vascular invasion, and pathological stage. A high expression of ZCCHC17 was associated with high TMB and microsatellite instability. | TP53 | 374 HCC patients TCGA LIHC (hepatocellular carcinoma) project |
| Li et al. [37] | Probability of genetic mutations, overall survival and median survival in the high-LMRGS group were significantly shorter than in the low-LMRGS group. In the high-LMRGS group, the immune microenvironment presented more inhibitory immune cell infiltration (follicular helper T cells and regulatory T cells). | TP53, TTN, CTNNB1 | TCGA-HCC dataset used as the training cohort, ICGC-LIRI-JP dataset as validation set |
| Yang et al. [38] | High immune cell infiltration score was characterized by enhanced activation of immune-related signaling pathways and a significantly higher TMB. Immune cell infiltration score could predict patient responses to immunotherapy independently from TMB. | n.a. | 571 HCC patients The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) cohorts |
| Liu et al. [34] | CD39+PD-1intCD8+ TILs displayed an effector phenotype and stronger antitumor activity in high-high-affinity neoantigens (HAN) versus low-HAN group. | TP53, CTNNB1, ARID1A | 56 patients with HCC in The First Affiliated Hospital of Sun Yatsen University |
| Mauriello et al. [33] | In cancer patients undergoing immunotherapy, a stronger correlation between TMB, number of predicted neoantigens, and survival was observed. | n.a. | 115 Hepatocellular carcinoma (HCC) patients available from The Cancer Genome Atlas (TCGA) |
| Liu et al. [39] | Low-density lipoprotein (LDL) receptor-related protein 1B (LRP1B) mutation was associated with a higher TMB and higher expression of HHLA2. The prognosis of HCC patients with LRP1B mutation was poor. | LRP1B, TP53, TTN, MUC16, AHNAK2, OBSCN, FLG, PCLO, HMCN1, USH2A, CSMD3, XIRP2, RYR2 | 361 HCC patients from TCGA 399 cases from International Cancer Genome Consortium (ICGC) |
| Liu et al. [40] | Higher infiltrating abundance in the high-TMB group correlated with worse OS and hazard risk for high-TMB patients in HCC. CD8+ T cells and B cells were associated with improved survival outcomes. High TMB indicated good HCC prognosis and promoted tumor immune infiltration. | TP53, TTN, CTNNB1, MUC16 | 376 HCC patients from The Cancer Genome Atlas (TCGA) cohort |
| Xie et al. [41] | The prognosis of the high-TMB group was worse than that of the low-TMB group (cutoff TMB limit = 4.9). | TP53, TTN, MUC16, CTNNB1, PCLO | 374 LIHC patients were downloaded from the TCGA database through the GDC data portal 203 HCC patients from Japan were also downloaded from ICGC |
| Yin et al. [42] | CECR7, GABRA3, IL7R, and TRIM16L mutations were associated with TMB and immune infiltration, and promoted antitumor immunity in HCC. | TP53, TTN, CTNNB1, MUC16 | 374 HCC samples and 50 matched normal samples from GDC portal |
| Mo et al. [43] | CTNNB1 was one of the frequently mutated genes in HCC and highly associated with survival and TMB. CTNNB1 mutation was significantly associated with a better prognosis. | TP53, TTN, CTNNB1, MUC16 | 260 patients from LIRI-JP, 369 from LICA-FR, and 394 from LINC-JP in ICGC database |
| Xu et al. [44] | PD-L1 positive patients had more vascular invasion and advanced CCLC stage. PD-L1 positive patients exhibited a lower TMB compared to the PD-L1 negative group. The most frequent driver gene mutations included TP53, CTNNB1, KMT2D, AXIN1, ALK, and NOTCH1. | TP53, CTNNB1, KMT2D, AXIN1 | 32 patients with primary HCC who were admitted to Hospital of Guangdong Medical University |
| Liu et al. [45] | Identification of a specific gene expression signature useful to predict prognosis and stratify patients with different sensitivities to immunotherapy. TMB was higher in the high-risk group than in the low-risk group. | n.a. | 597 HCC patients from The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) |
| Peng et al. [46] | Identification of an immune signature included seven differentially expressed IRGs (BIRC5, CACYBP, NR0B1, RAET1E, S100A8, SPINK5, and SPP1) to predict HCC patients’ survival and immunotherapy response. The high-risk group had significantly higher TMB than the low-risk group. The high-risk group had higher TMB, and immunotherapy might be more effective in the high-risk group. | TP53, CTNNB1, TTN, MUC16 | 372 TCGA-HCC samples were used 242 data sets downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo/ accessed on 20 January 2023) database and 232 patients’ data from LIRI-JP of International Cancer Genome Consortium (ICGC) database |
| Xie et al. [47] | Higher TMB was associated with worse prognosis in HCC patients. Less CD8+ T-cell enrichment was found in patients with higher TMB. The poor prognosis was in accordance with higher TMB and more activated NK cells. | TP53, CTNNB1, TTN, MUC16 | LIHC cohort were collected from The Cancer Genome Atlas (TCGA) database GSE14520 dataset; LIRI cohort were acquired from the International Cancer Genome Consortium (ICGC) database |
| Huo et al. [48] | HCC patients with high TMB had a poor prognosis, and displayed higher proportion of CD8+ T lymphocyte infiltration compared to the low-TMB group. | TP53, TTN, CTNNB1, MUC16, PCLO | 801 HCC patients from The Cancer Genome Atlas Liver Hepatocellular Carcinoma (TCGA-LIHC)1 and International Cancer Genome Consortium (ICGC), LIRI-JP) |
| NCT Number | Status | Outcome Measures | Study Population | Type of Study | Number of Enrolled Patients |
|---|---|---|---|---|---|
| NCT03236935 | Active, not recruiting | Maximum tolerated dose (MTD); dose-limiting toxicities (DLTs) and other adverse events; recommended interventional phase 2 dose (RP2D) of L-NMMA in combination with pembrolizumab; antitumor activity; plasma concentrations of L-NMMA when combined with pembrolizumab | 18 years and older | Interventional phase 1b study | 12 |
| NCT04042805 | Recruiting | TMB performed by NSG in association with ORR and survival after treatment with Sintilimab (PD-1 antibody) combined with Lenvatinib(TKI) | 18 to 100 years | Interventional single-arm, single-center, unrandomized, open-label phase II study | 36 |
| NCT04484636 | Recruiting | Distribution of mutations in HCC Evaluation of relative frequency of targetable mutations (incl. TMB and MSI status) per disease group Number of patients receiving therapies in accordance with their genomic profiles | 18 years and older | Prospective, multicenter, observational cohort study with biobanking | 200 |
| NCT05240404 | Recruiting | Evaluation of TMB in patients undergoing adjuvant toripalimab therapy after curative-intent ablation for HCC recurrence | 18 to 75 years | Interventional phase 2 | 116 |
| NCT04523493 | Recruiting | Evaluation of the correlation between TMB and therapy efficacy in advanced HCC patients undergoing toripalimab combined with lenvatinib vs. lenvatinib | 18 to 75 years | Prospective, randomized, placebo-controlled, double-blind, multicenter phase III registration clinical study | 519 |
| NCT04605731 | Recruiting | Evaluation of TMB, response, and survival outcomes in patients treated with durvalumab and tremelimumab after radioembolization | 18 years and older | Interventional phase 1b | 32 |
| NCT04723004 | Active, not recruiting | Correlation between TMB and the efficacy of toripalimab combined with bevacizumab in advanced HCC | 18 to 75 years | Prospective, randomized, open-label, parallel-group, active controlled, multi-center phase III | 326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbia, D.; De Martin, S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3441. https://doi.org/10.3390/ijms24043441
Gabbia D, De Martin S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(4):3441. https://doi.org/10.3390/ijms24043441
Chicago/Turabian StyleGabbia, Daniela, and Sara De Martin. 2023. "Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 4: 3441. https://doi.org/10.3390/ijms24043441
APA StyleGabbia, D., & De Martin, S. (2023). Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. International Journal of Molecular Sciences, 24(4), 3441. https://doi.org/10.3390/ijms24043441







