Integrative mRNA and microRNA Analysis Exploring the Inducing Effect and Mechanism of Diallyl Trisulfide (DATS) on Potato against Late Blight
Abstract
1. Introduction
2. Results
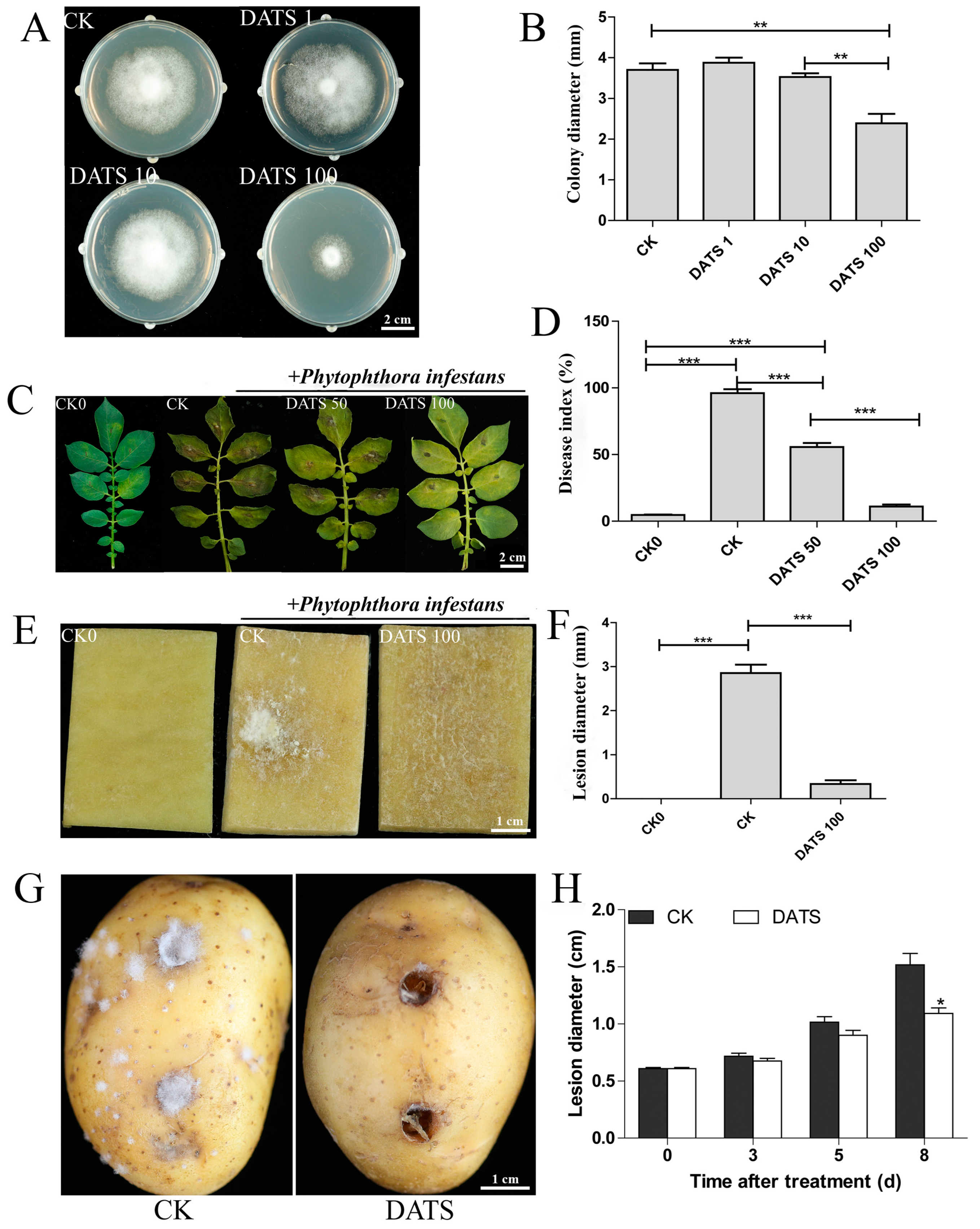
2.1. Effect of DATS on P. infestans and Potato
2.2. Effects of DATS Treatment on Antioxidant Enzyme Activity (POD, CAT, SOD) and MDA Content of Potato Tubers
2.3. Overview of mRNA Sequencing
2.3.1. The Regulatory Effects of DATS Treatment on Transcription Factors
2.3.2. The Regulatory Effects of DATS Treatment on Plant Hormone Signals
2.3.3. The Regulatory Effects of DATS Treatment on ROS
2.4. Overview of miRNA Sequencing
2.5. Regulatory Network from the Integrated Analysis of miRNA-mRNA Data
2.6. Model of DATS Regulating Potato Tuber Late Blight Resistance
3. Discussion
4. Materials and Methods
4.1. Culture Medium, Strain, Plant Material
4.2. The Effect of DATS on the Growth of P. infestans In Vitro
4.3. The Effect of DATS on the Infection of P. infestans Sporangia on the Detached Potato Leaves and Cubes
4.4. The Effect of DATS on Improving the Resistance of Potato Tubers against P. infestans
4.5. Antioxidant Enzyme Activity Detection
4.6. MRNA and miRNA Transcriptome Sequencing
4.7. Real Time-Quantitative PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and Onions: Their Cancer Prevention Properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef]
- Haas, B.J.; Kamoun, S.; Zody, M.C.; Jiang, R.H.Y.; Handsaker, R.E.; Cano, L.M.; Grabherr, M.; Kodira, C.D.; Raffaele, S.; Torto-Alalibo, T.; et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 2009, 461, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J. Pathogens which threaten food security: Phytophthora infestans, the potato late blight pathogen. Food Secur. 2021, 13, 247–253. [Google Scholar] [CrossRef]
- Nyankanga, R.O.; Olanya, O.M.; Wien, H.C.; Elbedewy, R.; Karinga, J.; Ojiambo, P.S. Development of Tuber Blight (Phytophthora infestans) on Potato Cultivars Based on In Vitro Assays and Field Evaluations. Hortscience 2008, 43, 1501–1508. [Google Scholar]
- Nyankanga, R.O.; Olanya, O.M.; Ojiambo, P.S.; Wien, H.C.; Honeycutt, C.W.; Kirk, W.W. Validation of tuber blight (Phytophthora infestans) prediction model. Crop Prot. 2011, 30, 547–553. [Google Scholar] [CrossRef]
- Gachango, E.; Kirk, W.; Schafer, R.; Wharton, P. Evaluation and comparison of biocontrol and conventional fungicides for control of postharvest potato tuber diseases. Biol. Control. 2012, 63, 115–120. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabieres, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Tec. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Perez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of Postharvest Fruit Fungal Diseases by Bacterial Antagonists: A Review. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Das, A.; Henderson, F.; Lowe, S.; Wallace, G.C.; Vandergrift, W.A.; Lindhorst, S.M.; Varma, A.K.; Infinger, L.K.; Giglio, P.; Banik, N.L.; et al. Single agent efficacy of the HDAC inhibitor DATS in preclinical models of glioblastoma. Cancer Chemoth. Pharm. 2018, 82, 945–952. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhang, F.; Zhou, L.; Kong, D.S.; Chen, L.; Lu, Y.; Zheng, S.Z. Diallyl trisulfide attenuates carbon tetrachloride-caused liver injury and fibrogenesis and reduces hepatic oxidative stress in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 445–455. [Google Scholar] [CrossRef]
- Nkrumah-Elie, Y.M.; Reuben, J.S.; Hudson, A.; Taka, E.; Badisa, R.; Ardley, T.; Israel, B.; Sadrud-Din, S.Y.; Oriaku, E.; Darling-Reed, S.F. Diallyl trisulfide as an inhibitor of benzo(a)pyrene-induced precancerous carcinogenesis in MCF-10A cells. Food Chem. Toxicol. 2012, 50, 2524–2530. [Google Scholar] [CrossRef]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Tsai, H.W.; Lin, K.J.; Wu, Z.Y.; Hu, H.Y.; Chang, Y.; Wei, H.J.; Sung, H.W. An in situ slow-releasing H2S donor depot with long-term therapeutic effects for treating ischemic diseases. Mat. Sci. Eng. C 2019, 104, 109954. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. Garlic and Gaseous Mediators. Trends Pharmacol. Sci. 2018, 39, 624–634. [Google Scholar] [CrossRef]
- Annamalai, S.; Mohanam, L.; Raja, V.; Dev, A.; Prabhu, V. Antiobesity, antioxidant and hepatoprotective effects of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomed. Pharmacother. 2017, 93, 81–87. [Google Scholar] [CrossRef]
- Liu, P.; Guo, J.; Liu, H.; Cheng, Y.; Wang, B.; Long, C.-A.; Deng, B. Diallyl trisulfide (DATS) effectively induced apoptosis of postharvest disease Penicillium expansum of citrus. Ann. Microbiol. 2009, 59, 675–679. [Google Scholar] [CrossRef]
- Gándara-Ledezma, A.; Corrales-Maldonado, C.; Rivera-Domínguez, M.; Martínez-Téllez, M.Á.; Vargas-Arispuro, I. Post-harvest control of gray mold in table grapes using volatile sulfur compounds from Allium sativum. J. Sci. Food Agric. 2014, 95, 497–503. [Google Scholar] [CrossRef]
- Li, W.R.; Zhang, Z.Q.; Yao, J.W.; Liao, K.; Zhu, L.P.; Shi, Q.S.; Huang, X.B.; Xie, X.B. Diallyl trisulfide attenuates Pseudomonas aeruginosa virulence via inhibiting quorum sensing. Int. Biodeterior. Biodegrad. 2022, 173, 105463. [Google Scholar] [CrossRef]
- Tsao, S.M.; Yin, M.C. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2001, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial Effect and the Mechanism of Diallyl Trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Su, X.; Liu, H. Diallyl Trisulfide, the Antifungal Component of Garlic Essential Oil and the Bioactivity of Its Nanoemulsions Formed by Spontaneous Emulsification. Molecules 2021, 26, 7186. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Sustainable disease management in a European context. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar]
- Mugao, L.G.; Muturi, P.W.; Gichimu, B.M.; Njoroge, E.K. In Vitro Control of Phytophthora infestans and Alternaria solani Using Crude Extracts and Essential Oils from Selected Plants. Int. J. Agron. 2020, 2020, 8845692. [Google Scholar] [CrossRef]
- Ndacnou, M.K.; Pantaleon, A.; Tchinda, J.B.S.; Mangapche, E.L.N.; Keumedjio, F.; Boyoguemo, D.B. Phytochemical study and anti-oomycete activity of Ageratum conyzoides Linnaeus. Ind. Crop. Prod. 2020, 153, 112589. [Google Scholar] [CrossRef]
- Rguez, S.; Djebali, N.; Ben Slimene, I.; Abid, G.; Hammemi, M.; Chenenaoui, S.; Bachkouel, S.; Daami-Remadi, M.; Ksouri, R.; Hamrouni-Sellami, I. Cupressus sempervirens essential oils and their major compounds successfully control postharvest grey mould disease of tomato. Ind. Crop. Prod. 2018, 123, 135–141. [Google Scholar] [CrossRef]
- Chang, M.M.; Shah, S.; Wu, M.Y.; Zhang, S.S.; Wu, G.; Yang, F.L. Effect of Diallyl Trisulfide on the Reproductive Behavior of the Grain Moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insects 2019, 11, 21. [Google Scholar] [CrossRef]
- Shah, S.; Ma, M.; Ali, A.; Kaya, M.; Li, X.G.; Wu, G.; Yang, F.L. Effects of diallyl trisulfide, an active substance from garlic essential oil, on structural chemistry of chitin in Sitotroga cerealella (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2021, 172, 104765. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Zhao, P.C.; Zhang, P.A.; Su, L.Y.; Jia, H.R.; Wei, X.K.; Fang, J.G.; Jia, H.F. Integrative transcriptomics and metabolomics data exploring the effect of chitosan on postharvest grape resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Lorenzo, O.; Piqueras, R.; Sanchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef]
- Berrocal-Lobo, M.; Molina, A. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol. Plant Microbe Interact. 2004, 17, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Du, M.M.; Zhao, J.H.; Tzeng, D.T.W.; Liu, Y.Y.; Deng, L.; Yang, T.X.; Zhai, Q.Z.; Wu, F.M.; Huang, Z.; Zhou, M.; et al. MYC2 Orchestrates a Hierarchical Transcriptional Cascade That Regulates Jasmonate-Mediated Plant Immunity in Tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. Jasmonate signaling: Toward an integrated view. Plant Physiol. 2008, 146, 1459–1468. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 2008, 20, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Serrano, M.; L’Haridon, F.; Tjamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Li, T.T.; Zhao, Y.H.; Zhang, B.H.; Li, A.Q.; Zhao, S.Z.; Hou, L.; Xia, H.; Fan, S.J.; Qiu, J.J.; et al. Integrated small RNA and mRNA expression profiles reveal miRNAs and their target genes in response to Aspergillus flavus growth in peanut seeds. Bmc Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Song, X.W.; Li, Y.; Cao, X.F.; Qi, Y.J. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Shivaprasad, P.V.; Chen, H.M.; Patel, K.; Bond, D.M.; Santos, B.A.C.M.; Baulcombe, D.C. A MicroRNA Superfamily Regulates Nucleotide Binding Site-Leucine-Rich Repeats and Other mRNAs. Plant Cell 2012, 24, 859–874. [Google Scholar] [CrossRef]
- Avrova, A.O.; Venter, E.; Birch, P.R.J.; Whisson, S.C. Profiling and quantifying differential gene transcription in Phytophthora infestans prior to and during the early stages of potato infection. Fungal Genet. Biol. 2003, 40, 4–14. [Google Scholar]
- Feng, S.; Jin, L.; Tang, S.; Jian, Y.; Li, Z. Combination of rhizosphere bacteria isolated from resistant potato plants for biocontrol of potato late blight. Pest Manag. Sci. 2021, 78, 166–176. [Google Scholar] [CrossRef]
- Zhang, S.; Khalid, A.R.; Guo, D.; Zhang, J.; Xiong, F.; Ren, M. TOR inhibitors synergistically suppress the growth and development of Phytophthora infestans, a highly destructive pathogenic oomycete. Front. Microbiol. 2021, 12, 596874. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Allen, E.; Xie, Z.X.; Gustafson, A.M.; Carrington, J.C. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Env. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]





| #ID | log2FC | Regulated | FDR | Pfam_Annotation |
|---|---|---|---|---|
| PGSC0003DMG400003568 | 1.11 | Up | 2.78 × 10−5 | Zinc knuckle |
| PGSC0003DMG400016441 | −1.23 | Down | 0.001005508 | WRKY DNA-binding domain |
| PGSC0003DMG400020206 | −1.29 | Down | 0.001670048 | WRKY DNA-binding domain |
| PGSC0003DMG400023462 | 1.11 | Up | 0.007290252 | Ubiquitin family |
| PGSC0003DMG400002052 | 1.07 | Up | 5.82 × 10−8 | Transcription initiation factor IIA |
| PGSC0003DMG400006588 | 1.52 | Up | 1.38 × 10−7 | Timeless protein C terminal region |
| Solanum_tuberosum_newGene_4204 | −1.37 | Down | 5.34 × 10−6 | TCP family transcription factor |
| PGSC0003DMG400027770 | 1.16 | Up | 0.003989554 | Homeobox domain |
| PGSC0003DMG400003846 | 1.03 | Up | 0.009284718 | RNA polymerase Rpc34 subunit |
| PGSC0003DMG400009063 | 1.19 | Up | 2.80 × 10−8 | Ribosomal proteins L26 eukaryotic |
| Solanum_tuberosum_newGene_1998 | 1.24 | Up | 0.005085243 | Regulator of chromosome condensation (RCC1) repeat |
| PGSC0003DMG400026195 | 1.38 | Up | 3.13 × 10−6 | Protein of unknown function, DUF573 |
| PGSC0003DMG400021211 | 1.96 | Up | 2.87 × 10−7 | Piwi domain |
| PGSC0003DMG400014182 | 1.01 | Up | 0.002884298 | NF-X1 type zinc finger |
| PGSC0003DMG400003055 | 1.53 | Up | 5.67 × 10−7 | Myb-like DNA-binding domain |
| PGSC0003DMG400026758 | −1.10 | Down | 0.000566068 | Myb-like DNA-binding domain |
| PGSC0003DMG400015536 | −1.13 | Down | 0.003151643 | Myb-like DNA-binding domain |
| PGSC0003DMG400005657 | 2.55 | Up | 2.74 × 10−12 | Myb-like DNA-binding domain |
| PGSC0003DMG401010883 | −1.41 | Down | 0.004240926 | Myb-like DNA-binding domain |
| PGSC0003DMG400030328 | −1.58 | Down | 1.33 × 10−11 | Myb-like DNA-binding domain |
| PGSC0003DMG400027794 | 1.13 | Up | 0.000647544 | Myb-like DNA-binding domain |
| PGSC0003DMG400000922 | −1.04 | Down | 0.000123933 | Myb-like DNA-binding domain |
| PGSC0003DMG401024549 | −1.44 | Down | 3.33 × 10−7 | Myb-like DNA-binding domain |
| Solanum_tuberosum_newGene_4889 | −1.09 | Down | 1.25 × 10−5 | MYB-CC type transfactor |
| Solanum_tuberosum_newGene_1595 | −1.52 | Down | 2.40 × 10−5 | Myb/SANT-like DNA-binding domain |
| PGSC0003DMG400020098 | 1.15 | Up | 0.000702071 | Myb/SANT-like DNA-binding domain |
| PGSC0003DMG400014811 | 1.00 | Up | 0.001674446 | HSF-type DNA-binding |
| PGSC0003DMG400025342 | −1.18 | Down | 0.000950719 | Homeobox domain |
| PGSC0003DMG400016148 | −1.51 | Down | 4.77 × 10−5 | Homeobox domain |
| PGSC0003DMG400018509 | −1.15 | Down | 0.000430995 | Homeobox domain |
| PGSC0003DMG400006132 | −1.02 | Down | 0.00568511 | GRAS domain family |
| PGSC0003DMG400025001 | 2.10 | Up | 2.60 × 10−12 | FACT complex subunit (SPT16/CDC68) |
| PGSC0003DMG400026783 | 1.73 | Up | 4.24 × 10−7 | ERCC3/RAD25/XPB C-terminal helicase |
| PGSC0003DMG400002507 | −1.22 | Down | 0.003764879 | Dof domain, zinc finger |
| PGSC0003DMG400010684 | 1.10 | Up | 0.004424802 | GATA zinc finger |
| PGSC0003DMG401010056 | −1.92 | Down | 2.81 × 10−10 | CCT motif; B-box zinc finger |
| PGSC0003DMG400000272 | 1.10 | Up | 0.007616122 | CCAAT-binding transcription factor (CBF-B/NF-YA) subunit B |
| PGSC0003DMG400016383 | 1.04 | Up | 0.007395023 | CAF1 family ribonuclease |
| PGSC0003DMG400030946 | −1.35 | Down | 0.002894626 | bZIP transcription factor |
| PGSC0003DMG400001161 | −1.29 | Down | 0.006448084 | bHLH-MYC and R2R3-MYB transcription factors N-terminal |
| PGSC0003DMG402019457 | 1.15 | Up | 0.006582071 | AUX/IAA family |
| PGSC0003DMG400006393 | −1.28 | Down | 2.26 × 10−10 | AUX/IAA family |
| PGSC0003DMG400002608 | −1.51 | Down | 0.000393116 | AUX/IAA family |
| PGSC0003DMG400019635 | −1.01 | Down | 0.004468473 | Associated with HOX |
| PGSC0003DMG400013256 | 1.45 | Up | 0.000291407 | AP2 domain |
| PGSC0003DMG400004005 | 1.09 | Up | 0.004163584 | AP2 domain |
| PGSC0003DMG401023951 | 1.13 | Up | 1.71 × 10−6 | AP2 domain |
| PGSC0003DMG401013782 | −1.31 | Up | 3.89 × 10−5 | AP2 domain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, Y.; Feng, S.; Huang, A.; Zhu, Z.; Zhang, J.; Tang, S.; Jin, L.; Ren, M.; Dong, P. Integrative mRNA and microRNA Analysis Exploring the Inducing Effect and Mechanism of Diallyl Trisulfide (DATS) on Potato against Late Blight. Int. J. Mol. Sci. 2023, 24, 3474. https://doi.org/10.3390/ijms24043474
Jian Y, Feng S, Huang A, Zhu Z, Zhang J, Tang S, Jin L, Ren M, Dong P. Integrative mRNA and microRNA Analysis Exploring the Inducing Effect and Mechanism of Diallyl Trisulfide (DATS) on Potato against Late Blight. International Journal of Molecular Sciences. 2023; 24(4):3474. https://doi.org/10.3390/ijms24043474
Chicago/Turabian StyleJian, Yongfei, Shun Feng, Airong Huang, Zhiming Zhu, Jiaomei Zhang, Shicai Tang, Liang Jin, Maozhi Ren, and Pan Dong. 2023. "Integrative mRNA and microRNA Analysis Exploring the Inducing Effect and Mechanism of Diallyl Trisulfide (DATS) on Potato against Late Blight" International Journal of Molecular Sciences 24, no. 4: 3474. https://doi.org/10.3390/ijms24043474
APA StyleJian, Y., Feng, S., Huang, A., Zhu, Z., Zhang, J., Tang, S., Jin, L., Ren, M., & Dong, P. (2023). Integrative mRNA and microRNA Analysis Exploring the Inducing Effect and Mechanism of Diallyl Trisulfide (DATS) on Potato against Late Blight. International Journal of Molecular Sciences, 24(4), 3474. https://doi.org/10.3390/ijms24043474






