Cell State Transitions and Phenotypic Heterogeneity in Luminal Breast Cancer Implicating MicroRNAs as Potential Regulators
Abstract
1. Introduction
2. Results
2.1. Cell Surface Expression of Stem-Cell-Associated Markers in Breast Cancer
2.2. ALDH1-Expressing Stem-like Cells with High Self-Renewal Potential
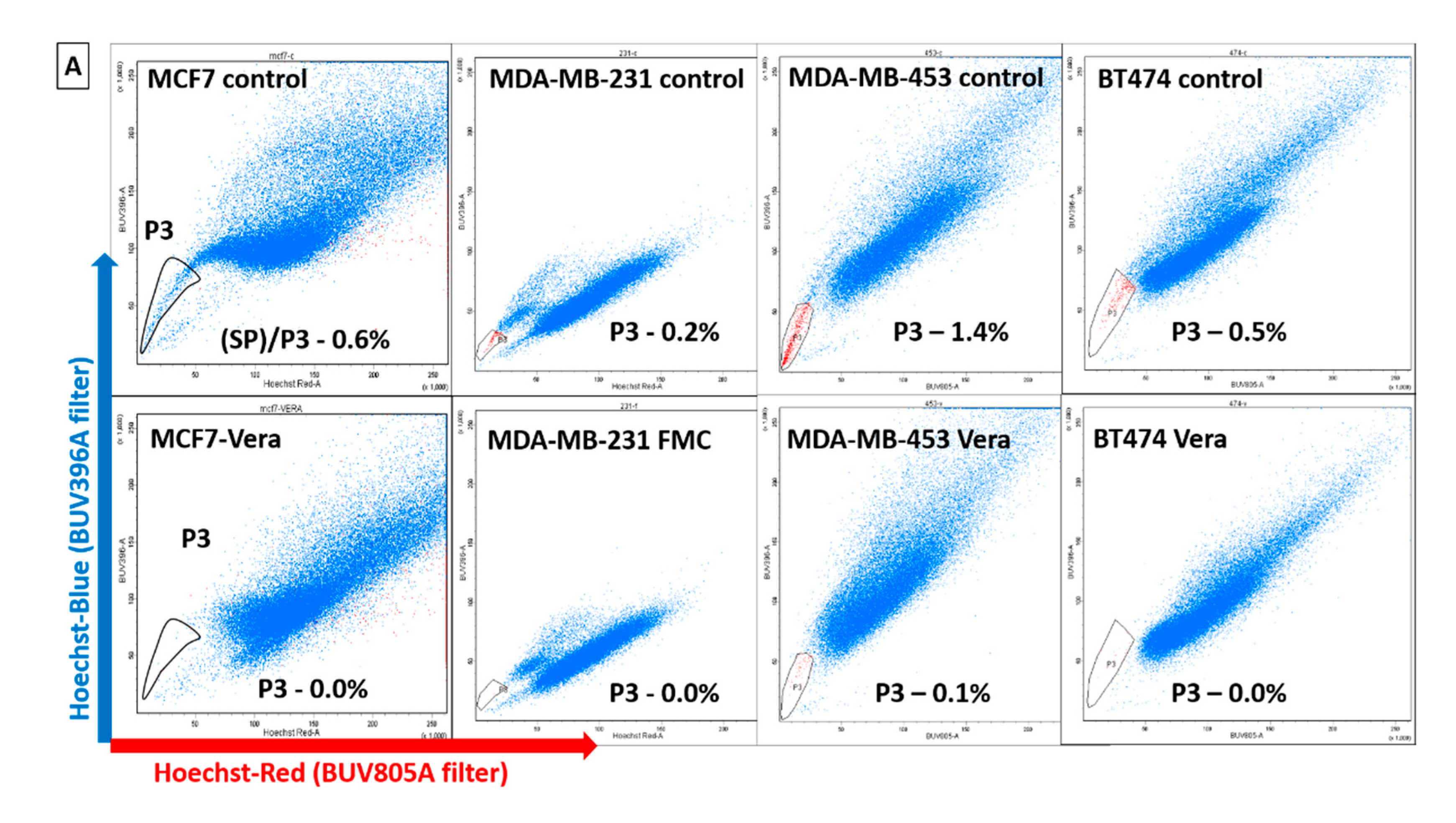
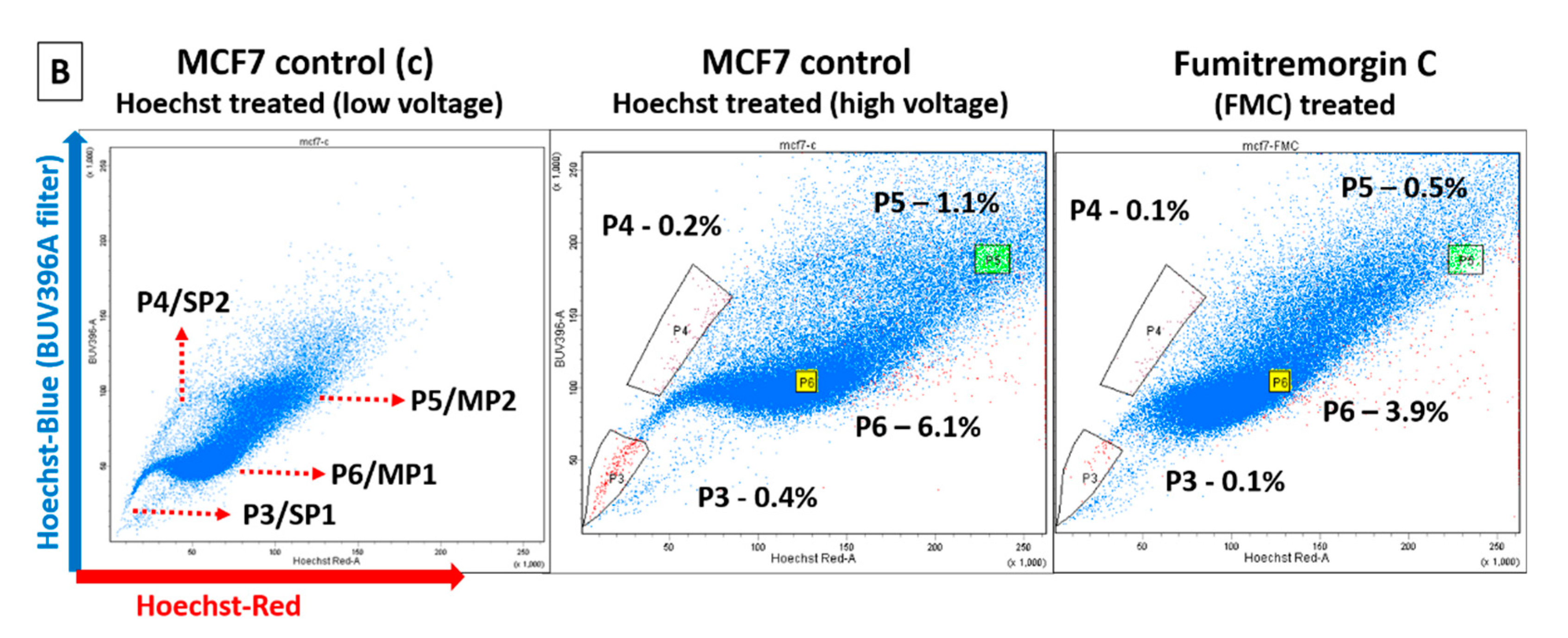
2.3. Identification of Dye-Effluxing Stem-Cell-Enriched Fractions in Breast Cancer
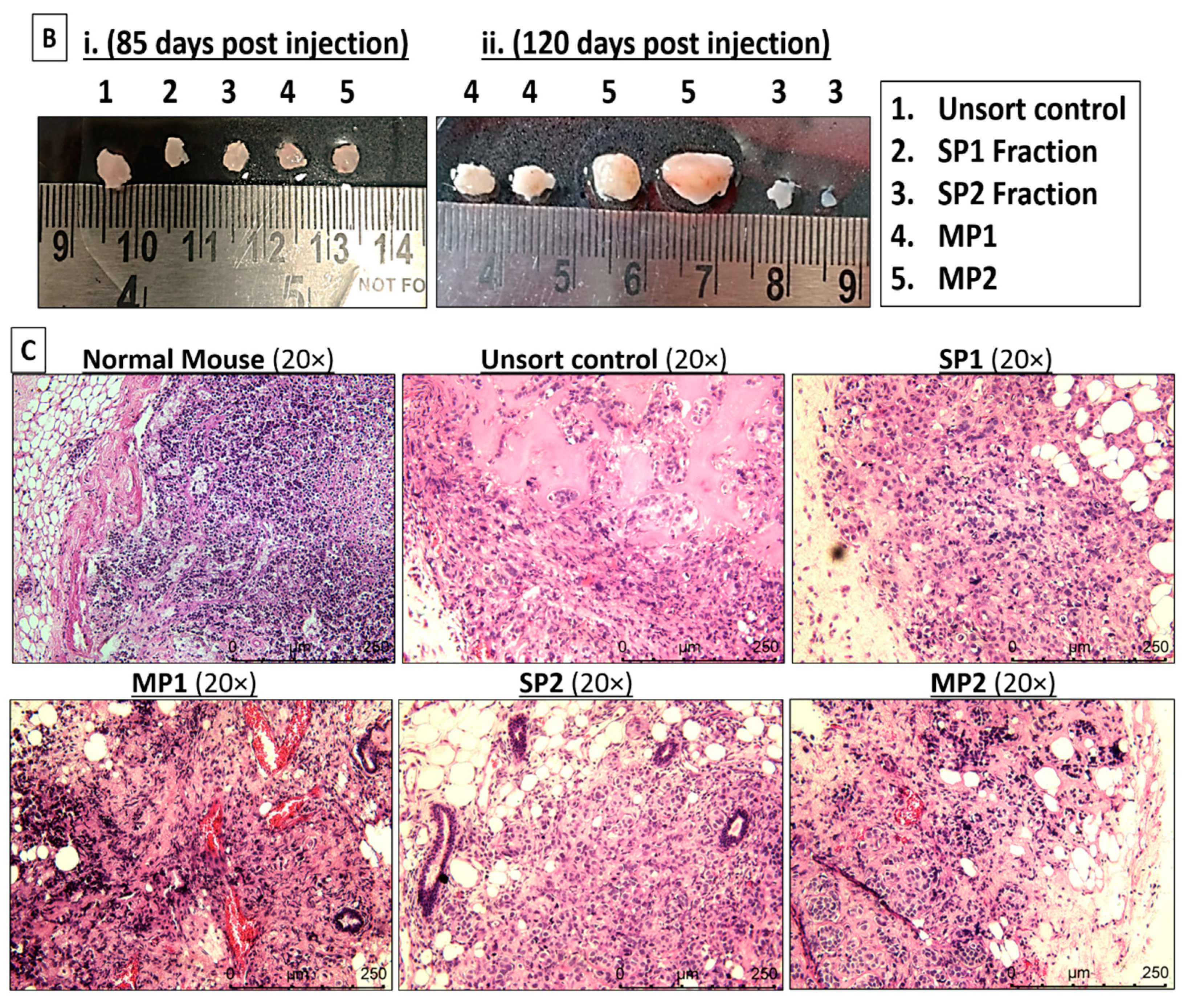
2.4. Orthotopic Breast Tumor Regeneration Potential in NSG Mice
2.5. Histopathological Analysis of Xenografts in NSG Mice
2.6. Expression Analysis of ER/PR/HER2 Receptor Proteins on Xenograft Tumors
2.7. Scoring for ER Gene Expression (ERness Score) and Tumor Aggressiveness
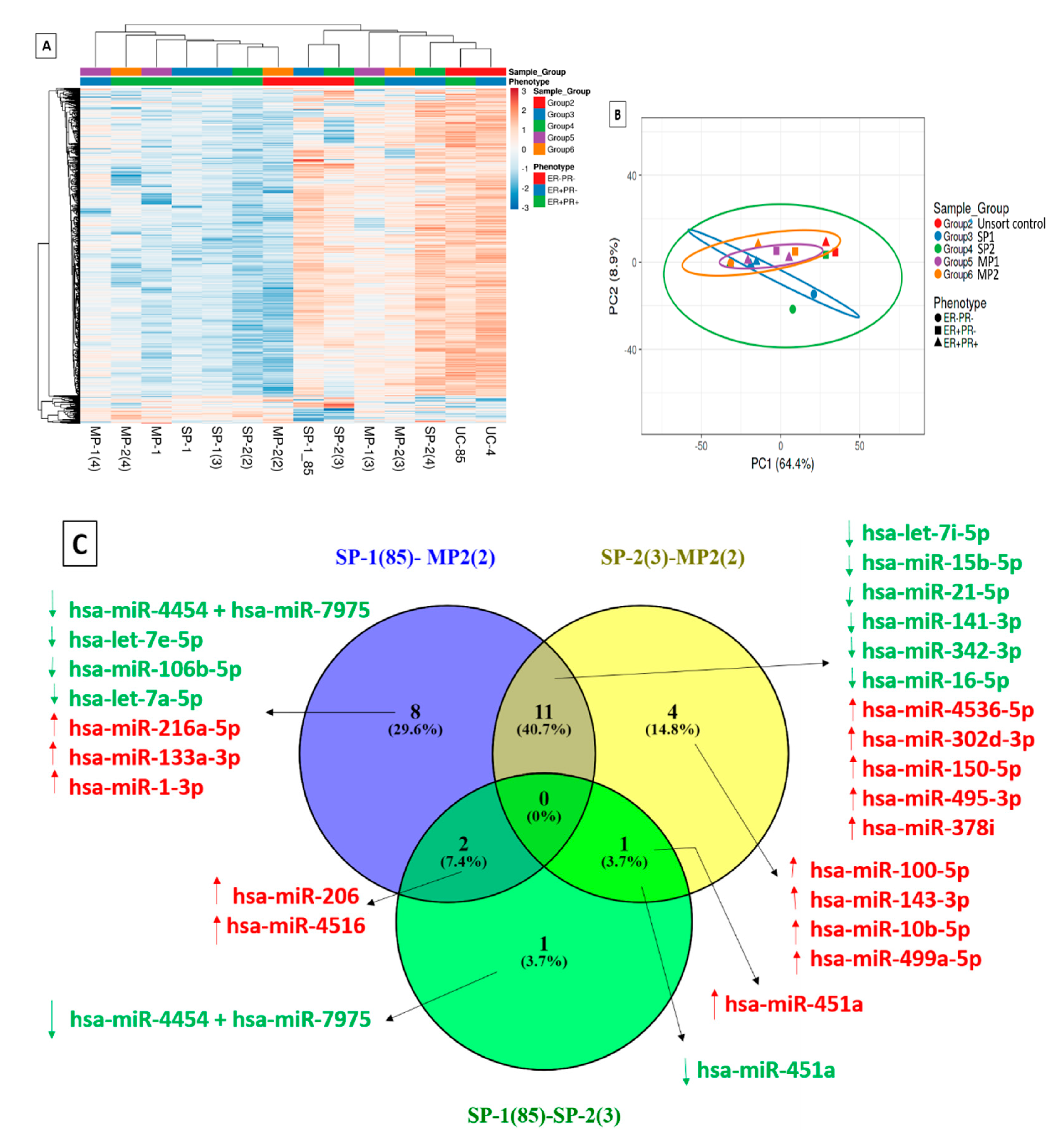
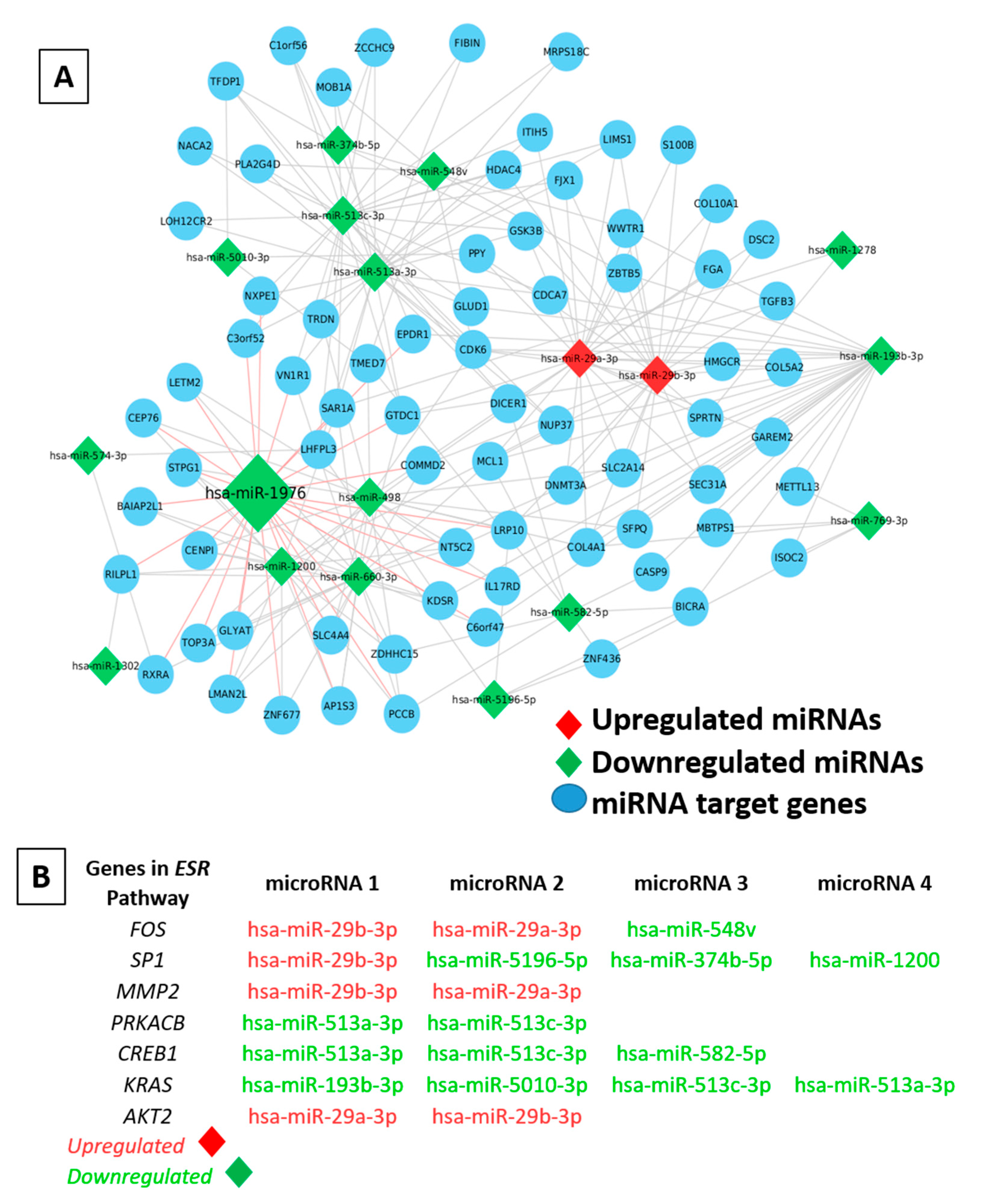
2.8. Differential Expression of MicroRNAs in Multiple Tumorigenic Fractions
2.9. Determining the Role of miRNA–mRNA Interactions in Tumor Plasticity
3. Discussion
4. Materials and Methods
4.1. Breast Cancer Cell Lines and Monolayer Culture
4.2. Flow-Cytometry-Based Immunophenotypic Profiling for Stemness Markers
4.3. ALDEFLUOR Assay for Aldehyde Dehydrogenase (ALDH) Enzyme Activity
4.4. Side Population Assay to Identify the Hoechst33342 Dye Effluxing Fractions
4.5. In Vivo Tumorigenic Assay in NOD/SCID Gamma (NSG) Mice
4.6. Immunohistochemistry Staining for Hormone Receptors on Xenograft Tumors
4.7. RNA Preparation and Derivation of ERness Score and Aggression Score
4.8. NanoString miRNA Expression Array
4.9. Target Prediction, miRNA–mRNA Network Mapping, and Functional Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Wicha, M.S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2813–2820. [Google Scholar] [CrossRef]
- Pece, S.; Tosoni, D.; Confalonieri, S.; Mazzarol, G.; Vecchi, M.; Ronzoni, S.; Bernard, L.; Viale, G.; Pelicci, P.G.; Di Fiore, P.P. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010, 140, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kordon, E.C.; Smith, G.H. An entire functional mammary gland may comprise the progeny from a single cell. Development 1998, 125, 1921–1930. [Google Scholar] [CrossRef]
- Richard, V.; Davey, M.G.; Annuk, H.; Miller, N.; Dwyer, R.M.; Lowery, A.; Kerin, M.J. MicroRNAs in molecular classification and pathogenesis of breast tumors. Cancers 2021, 13, 5332. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Pusztai, L.J.T.L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011, 378, 1812–1823. [Google Scholar]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef]
- Stierer, M.; Rosen, H.; Weber, R.; Hanak, H.; Spona, J.; Tüchler, H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann. Surg. 1993, 218, 13. [Google Scholar] [CrossRef]
- Porras, L.; Ismail, H.; Mader, S. Positive Regulation of Estrogen Receptor Alpha in Breast Tumorigenesis. Cells 2021, 10, 2966. [Google Scholar] [CrossRef]
- Caciolla, J.; Bisi, A.; Belluti, F.; Rampa, A.; Gobbi, S.J.M. Reconsidering Aromatase for Breast Cancer Treatment: New Roles for an Old Target. Molecules 2020, 25, 5351. [Google Scholar] [CrossRef]
- Shea, M.P.; O’Leary, K.A.; Fakhraldeen, S.A.; Goffin, V.; Friedl, A.; Wisinski, K.B.; Alexander, C.M.; Schuler, L.A.J.C.r. Antiestrogen therapy increases plasticity and cancer stemness of prolactin-induced ERα+ mammary carcinomas. Cancer Res. 2018, 78, 1672–1684. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006, 66, 1883–1890; discussion 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Kumar, T.R.S.; Pillai, R.M. Transitional dynamics of cancer stem cells in invasion and metastasis. Transl. Oncol. 2021, 14, 100909. [Google Scholar] [CrossRef]
- Easwaran, H.; Tsai, H.-C.; Baylin, S.B. Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell 2014, 54, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.A.; Jackson, H.W.; Beristain, A.G.; Di Grappa, M.A.; Mote, P.A.; Clarke, C.L.; Stingl, J.; Waterhouse, P.D.; Khokha, R.J.N. Progesterone induces adult mammary stem cell expansion. Nature 2010, 465, 803–807. [Google Scholar]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef]
- Richard, V.; Sebastian, P.; Nair, M.G.; Nair, S.N.; Malieckal, T.T.; Santhosh Kumar, T.R.; Pillai, M.R. Multiple drug-resistant, tumorigenic stem-like cells in oral cancer. Cancer Lett. 2013, 338, 300–316. [Google Scholar] [CrossRef]
- Goodell, M.A.; McKinney-Freeman, S.; Camargo, F.D. Isolation and characterization of side population cells. Methods Mol. Biol. 2005, 290, 343–352. [Google Scholar] [CrossRef]
- Achuthan, S.; Santhoshkumar, T.R.; Prabhakar, J.; Nair, S.A.; Pillai, M.R. Drug-induced Senescence Generates Chemoresistant Stem-like Cells with Low Reactive Oxygen Species. J. Biol. Chem. 2011, 286, 37813–37829. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Pillai, M.R. The stem cell code in oral epithelial tumorigenesis: ‘The cancer stem cell shift hypothesis’. Biochim. Biophys. Acta BBA Rev. Cancer 2010, 1806, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Leccia, F.; Del Vecchio, L.; Mariotti, E.; Di Noto, R.; Morel, A.-P.; Puisieux, A.; Salvatore, F.; Ansieau, S. ABCG2, a novel antigen to sort luminal progenitors of BRCA1- breast cancer cells. Mol. Cancer 2014, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Zhou, J.; Claypool, K.; Tang, D.G. Side Population Is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005, 65, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar]
- Zhang, Y.; Eades, G.; Yao, Y.; Li, Q.; Zhou, Q. Estrogen receptor α signaling regulates breast tumor-initiating cells by down-regulating miR-140 which targets the transcription factor SOX2. J. Biol. Chem. 2012, 287, 41514–41522. [Google Scholar]
- Song, S.J.; Poliseno, L.; Song, M.S.; Ala, U.; Webster, K.; Ng, C.; Beringer, G.; Brikbak, N.J.; Yuan, X.; Cantley, L.C.; et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013, 154, 311–324. [Google Scholar] [CrossRef]
- Richard, V.; Raju, R.; Paul, A.M.; Girijadevi, R.; Santhosh Kumar, T.R.; Pillai, M.R. Analysis of microRNA-mRNA interactions in stem cell-enriched fraction of oral squamous cell carcinoma. Oncol. Res. 2018, 26, 17–26. [Google Scholar] [CrossRef]
- Mulrane, L.; McGee, S.F.; Gallagher, W.M.; O’Connor, D.P. miRNA dysregulation in breast cancer. Cancer Res. 2013, 73, 6554–6562. [Google Scholar] [CrossRef]
- Howard, E.W.; Yang, X. microRNA Regulation in Estrogen Receptor-Positive Breast Cancer and Endocrine Therapy. Biol. Proced. Online 2018, 20, 17. [Google Scholar] [CrossRef]
- Nair, M.G.; Desai, K.; Prabhu, J.S.; Hari, P.; Remacle, J.; Sridhar, T.J. Data on alteration of hormone and growth factor receptor profiles over progressive passages of breast cancer cell lines representing different clinical subtypes. Data Brief 2016, 8, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Korlimarla, A.; Desai, K.; Alexander, A.; Raghavan, R.; Anupama, C.; Dendukuri, N.; Manjunath, S.; Correa, M.; Raman, N.; et al. A Majority of Low (1–10%) ER Positive Breast Cancers Behave Like Hormone Receptor Negative Tumors. J. Cancer 2014, 5, 156–165. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Deyarmin, B.; Kane, J.L.; Valente, A.L.; van Laar, R.; Gallagher, C.; Shriver, C.D.; Ellsworth, R.E. Effect of ASCO/CAP Guidelines for Determining ER Status on Molecular Subtype. Ann. Surg. Oncol. 2013, 20, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Gruvberger, S.; Ringnér, M.; Chen, Y.; Panavally, S.; Saal, L.H.; Borg, A.; Fernö, M.; Peterson, C.; Meltzer, P.S. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001, 61, 5979–5984. [Google Scholar] [PubMed]
- Prabhu, J.S.; Korlimarla, A.; Anupama, C.E.; Alexander, A.; Raghavan, R.; Kaul, R.; Desai, K.; Rajarajan, S.; Manjunath, S.; Correa, M.; et al. Dissecting the Biological Heterogeneity within Hormone Receptor Positive HER2 Negative Breast Cancer by Gene Expression Markers Identifies Indolent Tumors within Late Stage Disease. Transl. Oncol. 2017, 10, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hughes, C.J.; Ford, H.L. Cellular Plasticity in Breast Cancer Progression and Therapy. Front. Mol. Biosci. 2020, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Barinoff, J.; du Bois, O.; Hils, R.; Fisseler-Eckhoff, A.; Harter, P.; Heitz, J.; Willenbrock, K.; Traut, A.; du Bois, A. Differences in the receptor status between primary and recurrent breast cancer--the frequency of and the reasons for discordance. Oncology 2013, 84, 319–325. [Google Scholar] [CrossRef]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, identification and isolation methods, regulating mechanisms, cellular origin, and beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef]
- Sarrio, D.; Franklin, C.K.; Mackay, A.; Reis-Filho, J.S.; Isacke, C.M. Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells 2012, 30, 292–303. [Google Scholar] [CrossRef]
- Tanei, T.; Morimoto, K.; Shimazu, K.; Kim, S.J.; Tanji, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Croker, A.K.; Allan, A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44⁺ human breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [PubMed]
- Singh, R.; Yadav, V.; Saini, N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Sci. Rep. 2015, 5, 17454. [Google Scholar] [CrossRef]
- McAnena, P.; Tanriverdi, K.; Curran, C.; Gilligan, K.; Freedman, J.E.; Brown, J.A.L.; Kerin, M.J. Circulating microRNAs miR-331 and miR-195 differentiate local luminal a from metastatic breast cancer. BMC Cancer 2019, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Xiong, Y.; Ge, Y.Y.; Yun, J.P.; Zhuang, S.M. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology 2009, 50, 113–121. [Google Scholar]
- Wu, Y.; Shi, W.; Tang, T.; Wang, Y.; Yin, X.; Chen, Y.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis. 2019, 10, 176. [Google Scholar] [CrossRef]
- Zhang, B.; Shetti, D.; Fan, C.; Wei, K. miR-29b-3p promotes progression of MDA-MB-231 triple-negative breast cancer cells through downregulating TRAF3. Biol. Res. 2019, 52, 38. [Google Scholar] [CrossRef]
- Luo, C.W.; Hou, M.F.; Huang, C.W.; Wu, C.C.; Ou-Yang, F.; Li, Q.L.; Wu, C.C.; Pan, M.R. The CDK6-c-Jun-Sp1-MMP-2 axis as a biomarker and therapeutic target for triple-negative breast cancer. Am. J. Cancer Res. 2020, 10, 4325–4341. [Google Scholar] [PubMed]
- Cochrane, D.R.; Cittelly, D.M.; Howe, E.N.; Spoelstra, N.S.; McKinsey, E.L.; LaPara, K.; Elias, A.; Yee, D.; Richer, J.K. MicroRNAs link estrogen receptor alpha status and Dicer levels in breast cancer. Horm. Cancer 2010, 1, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Korlimarla, A.; Prabhu, J.S.; Anupama, C.E.; Remacle, J.; Wahi, K.; Sridhar, T.S. Separate quality-control measures are necessary for estimation of RNA and methylated DNA from formalin-fixed, paraffin-embedded specimens by quantitative PCR. J. Mol. Diagn. JMD 2014, 16, 253–260. [Google Scholar] [CrossRef] [PubMed]



| No | Sample IDs | Phenotype | Tumor Content | Grade | ER | PR | HER2 | Subtype | LVI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | 1 | MCF7 cells (+control) | ER+PR+ | P7-15 | -- | + | + | − | ER+PR+HER2− | − |
| Group 2 | 2 | UC-85 | ER+PR+ | 90% | 3 | + | + | − | ER+PR+HER2− | − |
| 3 | UC(4)- 120 days | ER+PR− | 60% | 2 | + | − | − | ER+PR−HER2− | − | |
| Group 3 | 4 | SP-1 85 | ER−PR− | 15% | 2 | − | − | − | ER−PR−HER2− | − |
| 5 | SP-1-90 days | ER+PR+ | 35% | 2 | + | + | − | ER+PR+HER2− | − | |
| 6 | SP-1 (3)- 120 days | ER+PR+ | 70% | 3 | + | + | − | ER+PR+HER2− | − | |
| Group 4 | 7 | SP-2 (2)- 90 days | ER+PR+ | 90% | 2 | + | + | − | ER+PR+HER2− | − |
| 8 | SP-2 (3)- 120 days | ER−PR− | 20% | 2 | − | − | − | ER−PR−HER2− | − | |
| 9 | SP-2 (4)- 120 days | ER+PR− | 35% | 2 | + | − | − | ER+PR−HER2− | − | |
| Group 5 | 10 | MP-1- 85 days | ER+PR+ | 70% | 2 | + | + | − | ER+PR+HER2− | − |
| 11 | MP-1 (3)- 120 days | ER+PR+ | 80% | 3 | + | + | − | ER+PR+HER2− | − | |
| 12 | MP-1 (4)- 120 days | ER+PR− | 90% | 3 | + | − | − | ER+PR−HER2− | − | |
| Group 6 | 13 | MP-2 (2)- 90 days | ER−PR− | 70% | 2 | − | − | − | ER−PR−HER2− | − |
| 14 | MP-2 (3)- 120 days | ER+PR− | 85% | 3 | + | − | − | ER+PR−HER2− | − | |
| 15 | MP-2 (4)- 120 days | ER+PR+ | 90% | 3 | + | + | − | ER+PR+HER2− | − |
| No | Sample | Protein Phenotype | ERness Score (ESR1-GATA-TFF1) | Aggression Score (ANLN-BCL2) | |
|---|---|---|---|---|---|
| Group 1 | 1 | MCF7 | ER+PR+HER2− | 0.997853409 | 0.805446153 |
| Group 2 | 2 | UC-85 | ER+PR+HER2− | 0.996277819 | 0.541930166 |
| 3 | UC-4 | ER+PR−HER2− | 0.990851261 | 0.51834784 | |
| Group 3 | 4 | SP1-85 | ER−PR−HER2− | 0.999199652 | 0.575028151 |
| 5 | SP1 | ER+PR+HER2− | 0.995963034 | 0.820470204 | |
| 6 | SP1-3 | ER+PR+HER2− | 0.999009728 | 0.723504152 | |
| Group 4 | 7 | SP2-2 | ER+PR+HER2− | 0.998176405 | 0.785293087 |
| 8 | SP2-3 | ER−PR−HER2− | 0.99677685 | 0.463903111 | |
| Group 5 | 9 | MP-1 | ER+PR+HER2− | 0.992496709 | 0.580040679 |
| 10 | MP1-3 | ER+PR+HER2− | 0.996628041 | 0.774492501 | |
| 11 | MP1-4 | ER+PR−HER2− | 0.99793024 | 0.863435597 | |
| Group 6 | 12 | MP2-2 | ER−PR−HER2− | 0.998328341 | 0.726683827 |
| 13 | MP2-3 | ER+PR−HER2− | 0.995958468 | 0.786116171 | |
| 14 | MP2-4 | ER+PR+HER2− | 0.998531337 | 0.502156808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, V.; Nair, M.G.; Jaikumar, V.S.; Jones, S.; Prabhu, J.S.; Kerin, M.J. Cell State Transitions and Phenotypic Heterogeneity in Luminal Breast Cancer Implicating MicroRNAs as Potential Regulators. Int. J. Mol. Sci. 2023, 24, 3497. https://doi.org/10.3390/ijms24043497
Richard V, Nair MG, Jaikumar VS, Jones S, Prabhu JS, Kerin MJ. Cell State Transitions and Phenotypic Heterogeneity in Luminal Breast Cancer Implicating MicroRNAs as Potential Regulators. International Journal of Molecular Sciences. 2023; 24(4):3497. https://doi.org/10.3390/ijms24043497
Chicago/Turabian StyleRichard, Vinitha, Madhumathy G. Nair, Vishnu S. Jaikumar, Sara Jones, Jyothi S. Prabhu, and Michael J. Kerin. 2023. "Cell State Transitions and Phenotypic Heterogeneity in Luminal Breast Cancer Implicating MicroRNAs as Potential Regulators" International Journal of Molecular Sciences 24, no. 4: 3497. https://doi.org/10.3390/ijms24043497
APA StyleRichard, V., Nair, M. G., Jaikumar, V. S., Jones, S., Prabhu, J. S., & Kerin, M. J. (2023). Cell State Transitions and Phenotypic Heterogeneity in Luminal Breast Cancer Implicating MicroRNAs as Potential Regulators. International Journal of Molecular Sciences, 24(4), 3497. https://doi.org/10.3390/ijms24043497






