MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate
Abstract
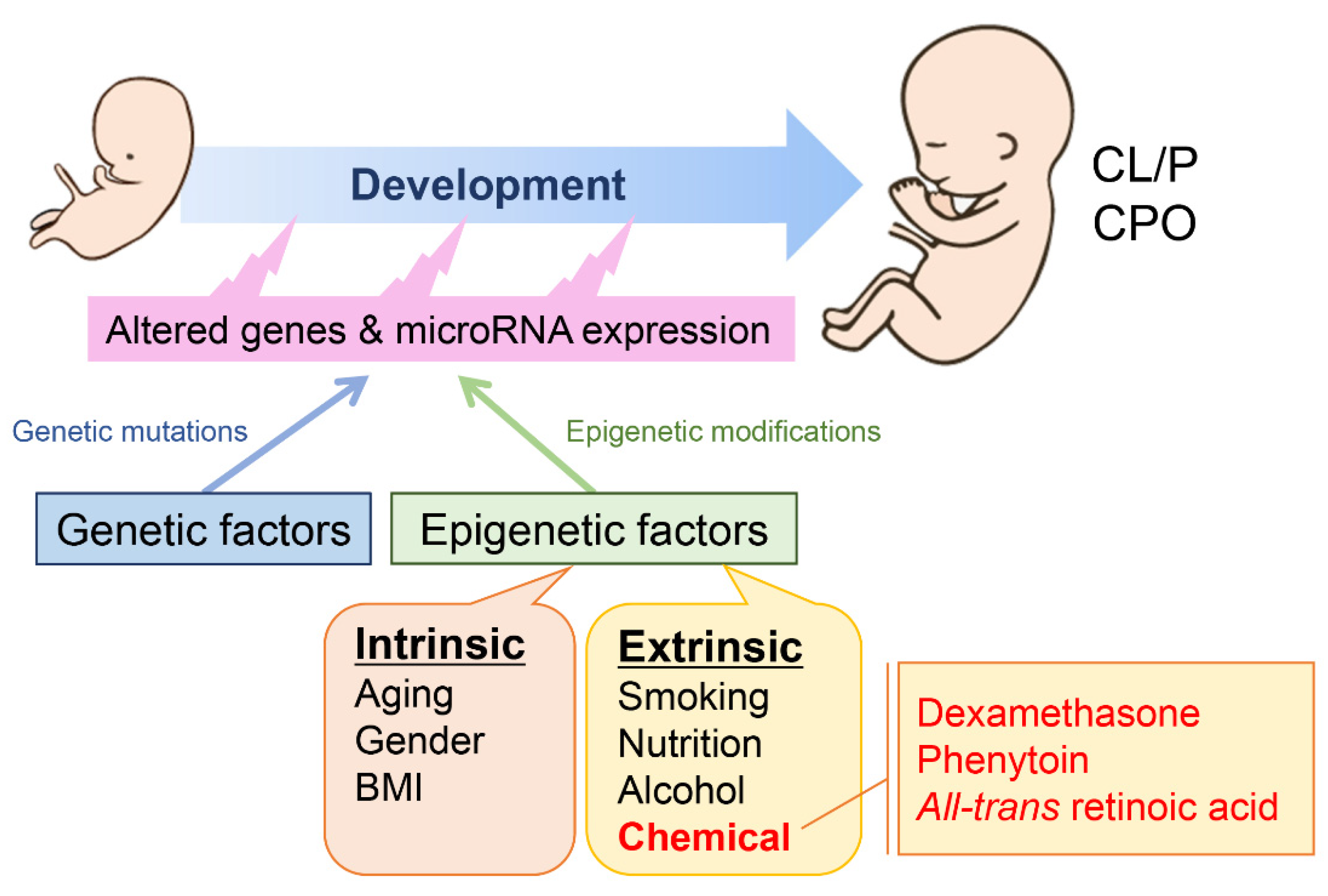
:1. Introduction
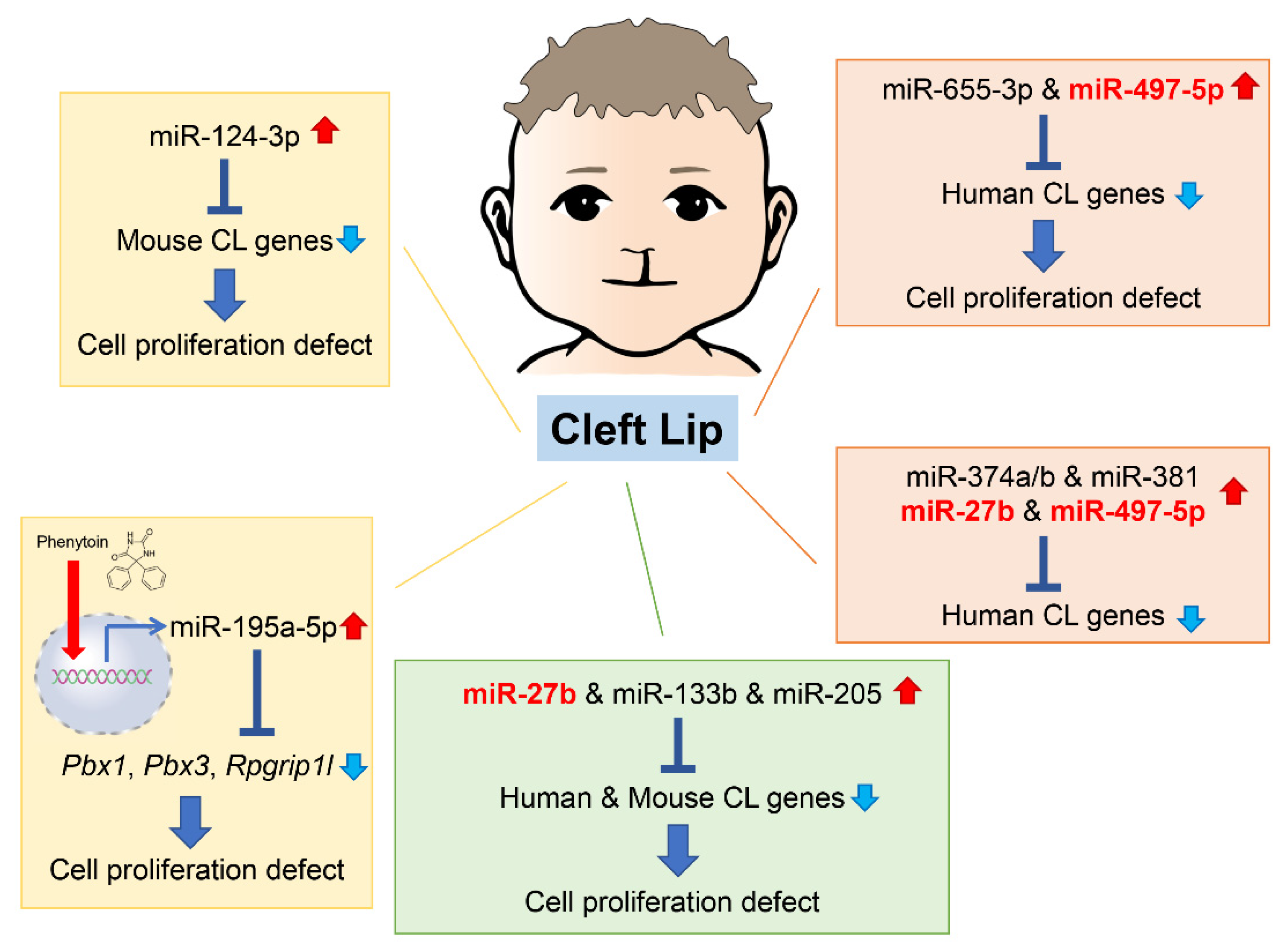
2. microRNAs Related to Cleft Lip
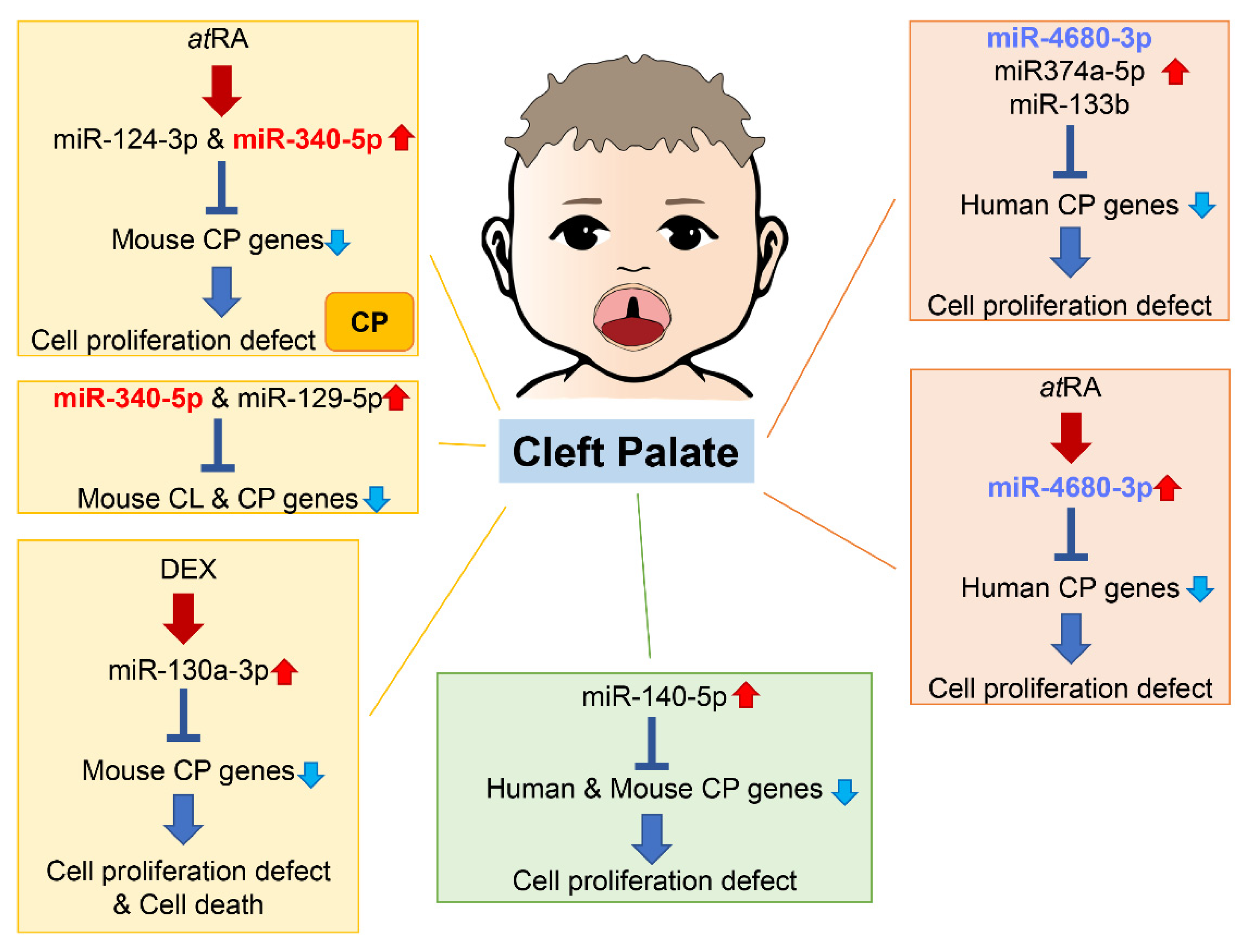
3. microRNAs Related to Cleft Palate
4. microRNAs Involved in Chemical-Induced Cleft Lip and Cleft Palate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPDTOC Working Group. Prevalence at birth of cleft lip with or without cleft palate: Data from the International Perinatal Database of Typical Oral Clefts (IPDTOC). Cleft Palate-Craniofacial J. 2011, 48, 66–81. [Google Scholar] [CrossRef]
- Gonseth, S.; Shaw, G.; Roy, R.; Segal, M.; Asrani, K.; Rine, J.; Wiemels, J.; Marini, N. Epigenomic profiling of newborns with isolated orofacial clefts reveals widespread DNA methylation changes and implicates metastable epiallele regions in disease risk. Epigenetics 2019, 14, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Sun, B.; Zhang, S.; Reynolds, K.; Ji, Y.; Zhou, C. Role of epigenetics and miRNAs in orofacial clefts. Birth. Defects. Res. 2020, 112, 1635–1659. [Google Scholar] [CrossRef] [PubMed]
- Alvizi, L.; Ke, X.; Brito, L.; Seselgyte, R.; Moore, G.; Stanier, P.; Passos-Bueno, M. Differential methylation is associated with non-syndromic cleft lip and palate and contributes to penetrance effects. Sci. Rep. 2017, 7, 2441. [Google Scholar] [CrossRef] [PubMed]
- Beaty, T.; Marazita, M.; Leslie, E. Genetic factors influencing risk to orofacial clefts: Today’s challenges and tomorrow’s opportunities. F1000Research 2016, 5, 2800. [Google Scholar] [CrossRef]
- Jiang, R.; Bush, J.O.; Lidral, A.C. Development of the upper lip: Morphogenetic and molecular mechanisms. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1152–1166. [Google Scholar] [CrossRef]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Raterman, S.T.; Metz, J.R.; Wagener, F.; Von den Hoff, J.W. Zebrafish Models of Craniofacial Malformations: Interactions of Environmental Factors. Front. Cell. Dev. Biol. 2020, 8, 600926. [Google Scholar] [CrossRef]
- Li, K.; Fan, L.; Tian, Y.; Lou, S.; Li, D.; Ma, L.; Wang, L.; Pan, Y. Application of zebrafish in the study of craniomaxillofacial developmental anomalies. Birth. Defects. Res. 2022, 114, 583–595. [Google Scholar] [CrossRef]
- Fell, M.; Dack, K.; Chummun, S.; Sandy, J.; Wren, Y.; Lewis, S. Maternal Cigarette Smoking and Cleft Lip and Palate: A Systematic Review and Meta-Analysis. Cleft Palate-Craniofacial J. 2022, 59, 1185–1200. [Google Scholar] [CrossRef]
- Lie, R.T.; Wilcox, A.J.; Taylor, J.; Gjessing, H.K.; Saugstad, O.D.; Aabyholm, F.; Vindenes, H. Maternal smoking and oral clefts: The role of detoxification pathway genes. Epidemiology 2008, 19, 606–615. [Google Scholar] [CrossRef]
- DeRoo, L.A.; Wilcox, A.J.; Lie, R.T.; Romitti, P.A.; Pedersen, D.A.; Munger, R.G.; Moreno Uribe, L.M.; Wehby, G.L. Maternal alcohol binge-drinking in the first trimester and the risk of orofacial clefts in offspring: A large population-based pooling study. Eur. J. Epidemiol. 2016, 31, 1021–1034. [Google Scholar] [CrossRef]
- Lorente, C.; Cordier, S.; Goujard, J.; Ayme, S.; Bianchi, F.; Calzolari, E.; De Walle, H.E.; Knill-Jones, R. Tobacco and alcohol use during pregnancy and risk of oral clefts. Occupational Exposure and Congenital Malformation Working Group. Am. J. Public Health 2000, 90, 415–419. [Google Scholar] [CrossRef]
- Izedonmwen, O.M.; Cunningham, C.; Macfarlane, T.V. What is the Risk of Having Offspring with Cleft Lip/Palate in Pre-Maternal Obese/Overweight Women When Compared to Pre-Maternal Normal Weight Women? A Systematic Review and Meta-Analysis. J. Oral. Maxillofac. Res. 2015, 6, e1. [Google Scholar] [CrossRef]
- Kutbi, H.; Wehby, G.L.; Moreno Uribe, L.M.; Romitti, P.A.; Carmichael, S.; Shaw, G.M.; Olshan, A.F.; DeRoo, L.; Rasmussen, S.A.; Murray, J.C.; et al. Maternal underweight and obesity and risk of orofacial clefts in a large international consortium of population-based studies. Int. J. Epidemiol. 2017, 46, 190–199. [Google Scholar] [CrossRef]
- Parker, S.E.; Werler, M.M.; Shaw, G.M.; Anderka, M.; Yazdy, M.M.; National Birth Defects Prevention Study. Dietary glycemic index and the risk of birth defects. Am. J. Epidemiol. 2012, 176, 1110–1120. [Google Scholar] [CrossRef]
- Krapels, I.P.; van Rooij, I.A.; Ocke, M.C.; van Cleef, B.A.; Kuijpers-Jagtman, A.M.; Steegers-Theunissen, R.P. Maternal dietary B vitamin intake, other than folate, and the association with orofacial cleft in the offspring. Eur. J. Nutr. 2004, 43, 7–14. [Google Scholar] [CrossRef]
- Alade, A.; Ismail, W.; Nair, R.; Schweizer, M.; Awotoye, W.; Oladayo, A.; Ryckman, K.; Butali, A. Periconceptional use of vitamin A and the risk of giving birth to a child with nonsyndromic orofacial clefts-A meta-analysis. Birth. Defects. Res. 2022, 114, 467–477. [Google Scholar] [CrossRef]
- Dolk, H.M.; Nau, H.; Hummler, H.; Barlow, S.M. Dietary vitamin A and teratogenic risk: European Teratology Society discussion paper. Eur. J. Obs. Gynecol. Reprod. Biol. 1999, 83, 31–36. [Google Scholar] [CrossRef]
- Xu, W.; Yi, L.; Deng, C.; Zhao, Z.; Ran, L.; Ren, Z.; Zhao, S.; Zhou, T.; Zhang, G.; Liu, H.; et al. Maternal periconceptional folic acid supplementation reduced risks of non-syndromic oral clefts in offspring. Sci. Rep. 2021, 11, 12316. [Google Scholar] [CrossRef]
- Zhou, Y.; Sinnathamby, V.; Yu, Y.; Sikora, L.; Johnson, C.Y.; Mossey, P.; Little, J. Folate intake, markers of folate status and oral clefts: An updated set of systematic reviews and meta-analyses. Birth. Defects. Res. 2020, 112, 1699–1719. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl Huber, A.; Van Hoeck, A.; Van Boxtel, R. The Mutagenic Impact of Environmental Exposures in Human Cells and Cancer: Imprints through Time. Front. Genet. 2021, 12, 760039. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell. Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Liu, F.; Tian, Q.; Hu, M.; Li, P.; Zeng, Y. Conservation of Differential Animal MicroRNA Processing by Drosha and Dicer. Front. Mol. Biosci. 2021, 8, 730006. [Google Scholar] [CrossRef]
- Antonaci, M.; Wheeler, G.N. MicroRNAs in neural crest development and neurocristopathies. Biochem. Soc. Trans. 2022, 50, 965–974. [Google Scholar] [CrossRef]
- Schoen, C.; Aschrafi, A.; Thonissen, M.; Poelmans, G.; Von den Hoff, J.W.; Carels, C.E.L. MicroRNAs in Palatogenesis and Cleft Palate. Front. Physiol. 2017, 8, 165. [Google Scholar] [CrossRef] [Green Version]
- Seelan, R.S.; Pisano, M.M.; Greene, R.M. MicroRNAs as epigenetic regulators of orofacial development. Differ. Res. Biol. Divers. 2022, 124, 1–16. [Google Scholar] [CrossRef]
- Fu, C.; Lou, S.; Zhu, G.; Fan, L.; Yu, X.; Zhu, W.; Ma, L.; Wang, L.; Pan, Y. Identification of New miRNA-mRNA Networks in the Development of Non-syndromic Cleft Lip with or without Cleft Palate. Front. Cell. Dev. Biol. 2021, 9, 631057. [Google Scholar] [CrossRef]
- Stussel, L.G.; Hollstein, R.; Laugsch, M.; Hochfeld, L.M.; Welzenbach, J.; Schroder, J.; Thieme, F.; Ishorst, N.; Romero, R.O.; Weinhold, L.; et al. MiRNA-149 as a Candidate for Facial Clefting and Neural Crest Cell Migration. J. Dent. Res. 2022, 101, 323–330. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Q.; Jiao, K. Dicer activity in neural crest cells is essential for craniofacial organogenesis and pharyngeal arch artery morphogenesis. Mech. Dev. 2011, 128, 200–207. [Google Scholar] [CrossRef]
- Zehir, A.; Hua, L.L.; Maska, E.L.; Morikawa, Y.; Cserjesi, P. Dicer is required for survival of differentiating neural crest cells. Dev. Biol. 2010, 340, 459–467. [Google Scholar] [CrossRef]
- Barritt, L.C.; Miller, J.M.; Scheetz, L.R.; Gardner, K.; Pierce, M.L.; Soukup, G.A.; Rocha-Sanchez, S.M. Conditional deletion of the human ortholog gene Dicer1 in Pax2-Cre expression domain impairs orofacial development. Indian J. Hum. Genet. 2012, 18, 310–319. [Google Scholar] [CrossRef]
- Weiner, A.M.J.; Scampoli, N.L.; Steeman, T.J.; Dooley, C.M.; Busch-Nentwich, E.M.; Kelsh, R.N.; Calcaterra, N.B. Dicer1 is required for pigment cell and craniofacial development in zebrafish. Biochim. Biophys. Acta. Gene. Regul. Mech. 2019, 1862, 472–485. [Google Scholar] [CrossRef]
- Grassia, V.; Lombardi, A.; Kawasaki, H.; Ferri, C.; Perillo, L.; Mosca, L.; Delle Cave, D.; Nucci, L.; Porcelli, M.; Caraglia, M. Salivary microRNAs as new molecular markers in cleft lip and palate: A new frontier in molecular medicine. Oncotarget 2018, 9, 18929–18938. [Google Scholar] [CrossRef]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Warner, D.R.; Mukhopadhyay, P.; Brock, G.; Webb, C.L.; Michele Pisano, M.; Greene, R.M. MicroRNA expression profiling of the developing murine upper lip. Dev. Growth Differ. 2014, 56, 434–447. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Meng, Y.; Zhang, B.; Wang, X.; Su, Y.; Shi, L.; Zhao, E. A pilot study: Screening target miRNAs in tissue of nonsyndromic cleft lip with or without cleft palate. Exp. Med. 2017, 13, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yoshioka, H.; Summakia, D.; Desai, N.G.; Jun, G.; Jia, P.; Loose, D.S.; Ogata, K.; Gajera, M.V.; Zhao, Z.; et al. MicroRNA-124-3p suppresses mouse lip mesenchymal cell proliferation through the regulation of genes associated with cleft lip in the mouse. BMC Genom. 2019, 20, 852. [Google Scholar] [CrossRef] [PubMed]
- Gajera, M.; Desai, N.; Suzuki, A.; Li, A.; Zhang, M.; Jun, G.; Jia, P.; Zhao, Z.; Iwata, J. MicroRNA-655-3p and microRNA-497-5p inhibit cell proliferation in cultured human lip cells through the regulation of genes related to human cleft lip. BMC Med. Genom. 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, C.; Zeng, B.; Tang, X.; Zhang, Y.; Xiang, L.; Mi, L.; Pan, Y.; Wang, H.; Yang, Z. miR124-3p/FGFR2 axis inhibits human keratinocyte proliferation and migration and improve the inflammatory microenvironment in psoriasis. Mol. Immunol. 2020, 122, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, R.; Su, W.; Yang, X.; Geng, Q.; Guo, C.; Wang, Z.; Wang, J.; Kresty, L.A.; Beer, D.G.; et al. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKalpha signaling in STK11 mutant lung cancer. Autophagy 2020, 16, 659–671. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, N.; Hui, L.; Song, M.; Miao, Z.W.; Jiang, X.J. MicroRNA-124-3p inhibits the growth and metastasis of nasopharyngeal carcinoma cells by targeting STAT3. Oncol. Rep. 2016, 35, 1385–1394. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, W.; Wang, Q.; Chen, Z.; Huang, S.; Zhao, F.; Yao, M.; Zhao, Y.; He, X. MicroRNA-124 reduces the pentose phosphate pathway and proliferation by targeting PRPS1 and RPIA mRNAs in human colorectal cancer cells. Gastroenterology 2015, 149, 1587–1598 e1511. [Google Scholar] [CrossRef]
- Wang, X.; Yin, J. The biological function of the long non-coding RNA endogenous born avirus-like nucleoprotein in lung adenocarcinoma is mediated through the microRNA-655-3p/B-cell lymphoma-2 axis. Bioengineered 2022, 13, 10679–10690. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Pan, A.; He, L.; Wang, J.; Zhou, F.; Lei, Y.; Wang, Y. Long non-coding RNA NHEG1/hsa-miR-665/HMGB1 axis is involved in the regulation of neuroblastoma progression. Bioengineered 2021, 12, 11584–11596. [Google Scholar] [CrossRef]
- Bai, M.; He, C.; Shi, S.; Wang, M.; Ma, J.; Yang, P.; Dong, Y.; Mou, X.; Han, S. Linc00963 Promote Cell Proliferation and Tumor Growth in Castration-Resistant Prostate Cancer by Modulating miR-655/TRIM24 Axis. Front. Oncol. 2021, 11, 636965. [Google Scholar] [CrossRef]
- Yu, L.; Huo, L.; Shao, X.; Zhao, J. lncRNA SNHG5 promotes cell proliferation, migration and invasion in oral squamous cell carcinoma by sponging miR-655-3p/FZD4 axis. Oncol. Lett. 2020, 20, 310. [Google Scholar] [CrossRef]
- Lu, M.; Gao, Q.; Wang, Y.; Ren, J.; Zhang, T. LINC00511 promotes cervical cancer progression by regulating the miR-497-5p/MAPK1 axis. Apoptosis. Int. J. Program. Cell. Death 2022, 27, 800–811. [Google Scholar] [CrossRef]
- Song, M.; Liu, J. Circ_0067717 promotes colorectal cancer cell growth, invasion and glutamine metabolism by serving as a miR-497-5p sponge to upregulate SLC7A5. Histol. Histopathol. 2022, 38, 53–64. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, W.; Gong, Q.; Luo, L.; Jing, W. Long Non-Coding RNA Cancer Susceptibility Candidate 9 Regulates the Malignant Biological Behavior of Nasopharyngeal Carcinoma Cells by Targeting miR-497-5p/Wnt3a/beta-catenin Signaling Pathway. Front. Oncol. 2022, 12, 807052. [Google Scholar] [CrossRef]
- Yoshioka, H.; Li, A.; Suzuki, A.; Ramakrishnan, S.S.; Zhao, Z.; Iwata, J. Identification of microRNAs and gene regulatory networks in cleft lip common in humans and mice. Hum. Mol. Genet. 2021, 30, 1881–1893. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Z.; Wei, Y.; Xue, L.; Wang, Y.; Liao, J.; Liang, J.; Chen, X.; Chu, L.; Zhang, B. Hsa-microRNA-27b-3p inhibits hepatocellular carcinoma progression by inactivating transforming growth factor-activated kinase-binding protein 3/nuclear factor kappa B signalling. Cell. Mol. Biol. Lett. 2022, 27, 79. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, L.; Yu, H.; Wang, W.; Wang, H.; Hu, Y.; Qin, L.; Deng, G.; Xie, B.; Li, G.; et al. M2-like tumor-associated macrophages transmit exosomal miR-27b-3p and maintain glioblastoma stem-like cell properties. Cell. Death Discov. 2022, 8, 350. [Google Scholar] [CrossRef]
- Bao, C.; Guo, L. TP73-AS1 promotes gastric cancer proliferation and invasion by regulation miR-27b-3p/TMED5 axis. J. Cancer 2022, 13, 1324–1335. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P.; Zeng, C.; Zhu, J.; Zhou, Y.; Lu, Y.; Xue, Q. Long Intergenic Non-Protein Coding RNA 02381 Promotes the Proliferation and Invasion of Ovarian Endometrial Stromal Cells through the miR-27b-3p/CTNNB1 Axis. Genes 2022, 13, 433. [Google Scholar] [CrossRef]
- Wu, T.; Han, N.; Zhao, C.; Huang, X.; Su, P.; Li, X. The long non-sacoding RNA TMEM147-AS1/miR-133b/ZNF587 axis regulates the Warburg effect and promotes prostatic carcinoma invasion and proliferation. J. Gene. Med. 2022, 24, e3453. [Google Scholar] [CrossRef]
- Liu, M.; Shen, A.; Zheng, Y.; Chen, X.; Wang, L.; Li, T.; Ouyang, X.; Yu, X.; Sun, H.; Wu, X. Long non-coding RNA lncHUPC1 induced by FOXA1 promotes tumor progression by inhibiting apoptosis via miR-133b/SDCCAG3 in prostate cancer. Am. J. Cancer Res. 2022, 12, 2465–2491. [Google Scholar] [PubMed]
- Jiang, L.; Wang, X. The miR-133b/brefeldin A-inhibited guanine nucleotide-exchange protein 1 (ARFGEF1) axis represses proliferation, invasion, and migration in cervical cancer cells. Bioengineered 2022, 13, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, J.; Wang, L.; Liu, X.; Da, B.; Liu, Y.; Huang, L.; Chen, Q.; Tong, Y.; Jiang, Z. miR-133b inhibits cell proliferation, migration, and invasion of lung adenocarcinoma by targeting CDCA8. Pathol. Res. Pract. 2021, 223, 153459. [Google Scholar] [CrossRef] [PubMed]
- Mytidou, C.; Koutsoulidou, A.; Zachariou, M.; Prokopi, M.; Kapnisis, K.; Spyrou, G.M.; Anayiotos, A.; Phylactou, L.A. Age-Related Exosomal and Endogenous Expression Patterns of miR-1, miR-133a, miR-133b, and miR-206 in Skeletal Muscles. Front. Physiol. 2021, 12, 708278. [Google Scholar] [CrossRef]
- Zhong, R.; Miao, R.; Meng, J.; Wu, R.; Zhang, Y.; Zhu, D. Acetoacetate promotes muscle cell proliferation via the miR-133b/SRF axis through the Mek-Erk-MEF2 pathway. Acta. Biochim. Biophys. Sin. 2021, 53, 1009–1016. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Liu, C.; Fu, Y.; Zhu, K.; Niu, Z.; Liu, J. Investigation of the Mechanism of hsa_circ_000 1429 Adsorbed miR-205 to Regulate KDM4A and Promote Breast Cancer Metastasis. Contrast. Media. Mol. Imaging 2022, 2022, 4657952. [Google Scholar] [CrossRef]
- Cheng, R.; Ji, L.; Su, H.; Wang, L.; Jia, D.; Yao, X.; Ji, H. Silencing of Long Noncoding RNA HLA Complex P5 (HCP5) Suppresses Glioma Progression through the HCP5-miR-205-Vascular Endothelial Growth Factor A Feedback Loop. Biomed. Res. Int. 2022, 2022, 3092063. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Geng, J.; Chen, Z.; Cui, X. MiR-205-5p Functions as a Tumor Suppressor in Gastric Cancer Cells through Downregulating FAM84B. J. Oncol. 2022, 2022, 8267891. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, X.; Zhao, Y.; Xue, H.; Wang, Z.; Wu, B.; Li, X.; Shen, Y. lncRNA LINC00284 promotes nucleus pulposus cell proliferation and ECM synthesis via regulation of the miR-205-3p/Wnt/beta-catenin axis. Mol. Med. Rep. 2022, 25, 179. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Pang, X.; Chen, Z.; Zhang, Z.; Lei, L.; Xu, H.; Wen, L.; Zhu, J.; Jiang, Y.; et al. MiR-205-5p suppresses angiogenesis in gastric cancer by downregulating the expression of VEGFA and FGF1. Exp. Cell. Res. 2021, 404, 112579. [Google Scholar] [CrossRef]
- Toro, A.U.; Shukla, S.K.; Bansal, P. Micronome Revealed miR-205-5p as Key Regulator of VEGFA during Cancer Related Angiogenesis in Hepatocellular Carcinoma. Mol. Biotechnol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Fu, W.; Wang, X.; Chen, H.; Wu, X.; Lao, G.; Wu, Y.; Hu, M.; Yang, C.; et al. MiR-195-5p and miR-205-5p in extracellular vesicles isolated from diabetic foot ulcer wound fluid decrease angiogenesis by inhibiting VEGFA expression. Aging 2021, 13, 19805–19821. [Google Scholar] [CrossRef]
- Liang, G.; Qin, Z.; Luo, Y.; Yin, J.; Shi, Z.; Wei, R.; Ma, W. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Gene. Ther. 2022, 29, 710–719. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.; Wang, Z.; Liu, J. MiRNA-27b Regulates Angiogenesis by Targeting AMPK in Mouse Ischemic Stroke Model. Neuroscience 2019, 398, 12–22. [Google Scholar] [CrossRef]
- Liu, W.; Lv, C.; Zhang, B.; Zhou, Q.; Cao, Z. MicroRNA-27b functions as a new inhibitor of ovarian cancer-mediated vasculogenic mimicry through suppression of VE-cadherin expression. RNA 2017, 23, 1019–1027. [Google Scholar] [CrossRef]
- Liu, H.T.; Xing, A.Y.; Chen, X.; Ma, R.R.; Wang, Y.W.; Shi, D.B.; Zhang, H.; Li, P.; Chen, H.F.; Li, Y.H.; et al. MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget 2015, 6, 37458–37470. [Google Scholar] [CrossRef]
- Suzuki, A.; Li, A.; Gajera, M.; Abdallah, N.; Zhang, M.; Zhao, Z.; Iwata, J. MicroRNA-374a, -4680, and -133b suppress cell proliferation through the regulation of genes associated with human cleft palate in cultured human palate cells. BMC Med. Genom. 2019, 12, 93. [Google Scholar] [CrossRef]
- Suzuki, A.; Jun, G.; Abdallah, N.; Gajera, M.; Iwata, J. Gene datasets associated with mouse cleft palate. Data Brief. 2018, 18, 655–673. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, H.; Xu, Y.; Wang, M.; Tian, Z. miR-374a-5p inhibits non-small cell lung cancer cell proliferation and migration via targeting NCK1. Exp. Med. 2021, 22, 943. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Z.; He, Z.; Li, X.; Wu, Z.; Xu, J.; Li, Q. High expression of miR-374a-5p inhibits the proliferation and promotes differentiation of Rencell VM cells by targeting Hes1. Neurosci. Res. 2021, 170, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yang, M.; Wu, C.; Wang, J. Potential Roles of miR-374a-5p in Mediating Neuroprotective Effects and Related Molecular Mechanism. J. Mol. Neurosci. 2019, 69, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, X. miR-374a-5p alleviates sepsis-induced acute lung injury by targeting ZEB1 via the p38 MAPK pathway. Exp. Med. 2022, 24, 564. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacIntyre, D.A.; Binkhamis, R.; Cook, J.; Sykes, L.; Bennett, P.R.; Terzidou, V. Maternal plasma miRNAs as potential biomarkers for detecting risk of small-for-gestational-age births. EBioMedicine 2020, 62, 103145. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Bennett, P.R.; Kim, S.H.; Teoh, T.G.; Sykes, L.; Kindinger, L.M.; Garrett, A.; Binkhamis, R.; MacIntyre, D.A.; Terzidou, V. First Trimester Circulating MicroRNA Biomarkers Predictive of Subsequent Preterm Delivery and Cervical Shortening. Sci. Rep. 2019, 9, 5861. [Google Scholar] [CrossRef]
- Li, A.; Jia, P.; Mallik, S.; Fei, R.; Yoshioka, H.; Suzuki, A.; Iwata, J.; Zhao, Z. Critical microRNAs and regulatory motifs in cleft palate identified by a conserved miRNA-TF-gene network approach in humans and mice. Brief Bioinform. 2020, 21, 1465–1478. [Google Scholar] [CrossRef]
- Yan, Y.; Yuan, J.; Luo, X.; Yu, X.; Lu, J.; Hou, W.; He, X.; Zhang, L.; Cao, J.; Wang, H. microRNA-140 Regulates PDGFRalpha and Is Involved in Adipocyte Differentiation. Front. Mol. Biosci. 2022, 9, 907148. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Shi, J.; Liu, S.; Zou, H. MicroRNA-140-5p represses chondrocyte pyroptosis and relieves cartilage injury in osteoarthritis by inhibiting cathepsin B/Nod-like receptor protein 3. Bioengineered 2021, 12, 9949–9964. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, J.Y.; Guo, B.; Lin, X.; Wu, F.; Li, F.X.; Shan, S.K.; Zheng, M.H.; Wang, Y.; Xu, Q.S.; et al. H19 Promotes Osteoblastic Transition by Acting as ceRNA of miR-140-5p in Vascular Smooth Muscle Cells. Front. Cell. Dev. Biol. 2022, 10, 774363. [Google Scholar] [CrossRef]
- Mahajan, M.; Sitasawad, S. miR-140-5p Attenuates Hypoxia-Induced Breast Cancer Progression by Targeting Nrf2/HO-1 Axis in a Keap1-Independent Mechanism. Cells 2021, 11, 12. [Google Scholar] [CrossRef]
- Eberhart, J.K.; He, X.; Swartz, M.E.; Yan, Y.L.; Song, H.; Boling, T.C.; Kunerth, A.K.; Walker, M.B.; Kimmel, C.B.; Postlethwait, J.H. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat. Genet. 2008, 40, 290–298. [Google Scholar] [CrossRef]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes. Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef]
- Mulder, P.; Dompeling, E.C.; van Slochteren-van der Boor, J.C.; Kuipers, W.D.; Smit, A.J. Transcutaneous electrical nerve stimulation (TENS) in Raynaud’s phenomenon. Angiology 1991, 42, 414–417. [Google Scholar] [CrossRef]
- Rattanasopha, S.; Tongkobpetch, S.; Srichomthong, C.; Siriwan, P.; Suphapeetiporn, K.; Shotelersuk, V. PDGFRa mutations in humans with isolated cleft palate. Eur. J. Hum. Genet. 2012, 20, 1058–1062. [Google Scholar] [CrossRef]
- Liao, J.; Kochilas, L.; Nowotschin, S.; Arnold, J.S.; Aggarwal, V.S.; Epstein, J.A.; Brown, M.C.; Adams, J.; Morrow, B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004, 13, 1577–1585. [Google Scholar] [CrossRef]
- Herman, S.B.; Guo, T.; McGinn, D.M.; Blonska, A.; Shanske, A.L.; Bassett, A.S.; Chow, E.W.; Bowser, M.; Sheridan, M.; Beemer, F.; et al. Overt cleft palate phenotype and TBX1 genotype correlations in velo-cardio-facial/DiGeorge/22q11.2 deletion syndrome patients. Am. J. Med. Genet. Part A 2012, 158A, 2781–2787. [Google Scholar] [CrossRef]
- Yagi, H.; Furutani, Y.; Hamada, H.; Sasaki, T.; Asakawa, S.; Minoshima, S.; Ichida, F.; Joo, K.; Kimura, M.; Imamura, S.; et al. Role of TBX1 in human del22q11.2 syndrome. Lancet 2003, 362, 1366–1373. [Google Scholar] [CrossRef]
- Funato, N.; Yanagisawa, H. TBX1 targets the miR-200-ZEB2 axis to induce epithelial differentiation and inhibit stem cell properties. Sci. Rep. 2022, 12, 20188. [Google Scholar] [CrossRef]
- Miyoshi, T.; Maruhashi, M.; Van De Putte, T.; Kondoh, H.; Huylebroeck, D.; Higashi, Y. Complementary expression pattern of Zfhx1 genes Sip1 and deltaEF1 in the mouse embryo and their genetic interaction revealed by compound mutants. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1941–1952. [Google Scholar] [CrossRef]
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 2012, 21, 2524–2537. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bai, Y.; Li, H.; Greene, S.B.; Klysik, E.; Yu, W.; Schwartz, R.J.; Williams, T.J.; Martin, J.F. MicroRNA-17-92, a direct Ap-2alpha transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 2013, 9, e1003785. [Google Scholar] [CrossRef]
- Li, L.; Shi, B.; Chen, J.; Li, C.; Wang, S.; Wang, Z.; Zhu, G. An E2F1/MiR-17-92 Negative Feedback Loop mediates proliferation of Mouse Palatal Mesenchymal Cells. Sci. Rep. 2017, 7, 5148. [Google Scholar] [CrossRef] [PubMed]
- Ries, R.J.; Yu, W.; Holton, N.; Cao, H.; Amendt, B.A. Inhibition of the miR-17-92 Cluster Separates Stages of Palatogenesis. J. Dent. Res. 2017, 96, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Alvizi, L.; Brito, L.A.; Kobayashi, G.S.; Bischain, B.; da Silva, C.B.F.; Ramos, S.L.G.; Wang, J.; Passos-Bueno, M.R. mir152 hypomethylation as a mechanism for non-syndromic cleft lip and palate. Epigenetics 2022, 17, 2278–2295. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, S.K.; Raman, R. A novel non-coding RNA within an intron of CDH2 and association of its SNP with non-syndromic cleft lip and palate. Gene 2018, 658, 123–128. [Google Scholar] [CrossRef]
- Reis, L.M.; Houssin, N.S.; Zamora, C.; Abdul-Rahman, O.; Kalish, J.M.; Zackai, E.H.; Plageman, T.F., Jr.; Semina, E.V. Novel variants in CDH2 are associated with a new syndrome including Peters anomaly. Clin. Genet. 2020, 97, 502–508. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Ma, L.; Han, Y.; Xu, M.; Wang, Z.; Jiang, H.; Zhang, W.; Wang, L.; Pan, Y. Associations between microRNA binding site SNPs in FGFs and FGFRs and the risk of non-syndromic orofacial cleft. Sci. Rep. 2016, 6, 31054. [Google Scholar] [CrossRef]
- Ma, L.; Xu, M.; Li, D.; Han, Y.; Wang, Z.; Yuan, H.; Ma, J.; Zhang, W.; Jiang, H.; Pan, Y.; et al. A miRNA-binding-site SNP of MSX1 is Associated with NSOC Susceptibility. J. Dent. Res. 2014, 93, 559–564. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, Q.; Wang, Y.; Wei, X.; Gu, H.; Liu, D.; Ma, W.; He, Y.; Luo, W.; Yuan, Z. Identification by RNA-Seq of let-7 clusters as prenatal biomarkers for nonsyndromic cleft lip with palate. Ann. N. Y. Acad. Sci. 2022, 1516, 234–246. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, B.; Shi, J.; Li, J.; Zhou, C.; Lu, W.; Xu, F.; He, F. Distinct Expression of miR-378 in Nonsyndromic Cleft Lip and/or Cleft Palate: A Cogitation of Skewed Sex Ratio in Prevalence. Cleft Palate-Craniofacial J. 2021, 58, 61–71. [Google Scholar] [CrossRef]
- Buser, M.C.; Pohl, H.R. Windows of Sensitivity to Toxic Chemicals in the Development of Cleft Palates. J. Toxicol. Env. Health B Crit. Rev. 2015, 18, 242–257. [Google Scholar] [CrossRef]
- Garland, M.A.; Reynolds, K.; Zhou, C.J. Environmental mechanisms of orofacial clefts. Birth. Defects. Res. 2020, 112, 1660–1698. [Google Scholar] [CrossRef]
- Leskow, A.; Nawrocka, M.; Latkowska, M.; Tarnowska, M.; Galas, N.; Matejuk, A.; Calkosinski, I. Can contamination of the environment by dioxins cause craniofacial defects? Hum. Exp. Toxicol. 2019, 38, 1014–1023. [Google Scholar] [CrossRef]
- Van Lang, Q.C.; Tassinari, M.S.; Keith, D.A.; Holmes, L.B. Effect of in utero exposure to anticonvulsants on craniofacial development and growth. J. Craniofacial Genet. Dev. Biol. 1984, 4, 115–133. [Google Scholar]
- Puho, E.H.; Szunyogh, M.; Metneki, J.; Czeizel, A.E. Drug treatment during pregnancy and isolated orofacial clefts in hungary. Cleft Palate-Craniofacial J. 2007, 44, 194–202. [Google Scholar] [CrossRef]
- Pradat, P.; Robert-Gnansia, E.; Di Tanna, G.L.; Rosano, A.; Lisi, A.; Mastroiacovo, P.; All Contributors to the MADRE Database. First trimester exposure to corticosteroids and oral clefts. Birth Defects Res. Part A Clin. Mol. Teratol. 2003, 67, 968–970. [Google Scholar] [CrossRef]
- Honein, M.A.; Rasmussen, S.A.; Reefhuis, J.; Romitti, P.A.; Lammer, E.J.; Sun, L.; Correa, A. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology 2007, 18, 226–233. [Google Scholar] [CrossRef]
- Carlson, J.C.; Shaffer, J.R.; Deleyiannis, F.; Hecht, J.T.; Wehby, G.L.; Christensen, K.; Feingold, E.; Weinberg, S.M.; Marazita, M.L.; Leslie, E.J. Genome-wide Interaction Study Implicates VGLL2 and Alcohol Exposure and PRL and Smoking in Orofacial Cleft Risk. Front. Cell Dev. Biol. 2022, 10, 621261. [Google Scholar] [CrossRef]
- Suhl, J.; Leonard, S.; Weyer, P.; Rhoads, A.; Siega-Riz, A.M.; Renee Anthony, T.; Burns, T.L.; Conway, K.M.; Langlois, P.H.; Romitti, P.A. Maternal arsenic exposure and nonsyndromic orofacial clefts. Birth Defects Res. 2018, 110, 1455–1467. [Google Scholar] [CrossRef]
- Linnenkamp, B.D.W.; Raskin, S.; Esposito, S.E.; Herai, R.H. A comprehensive analysis of AHRR gene as a candidate for cleft lip with or without cleft palate. Mutat. Res. Rev. Mutat. Res. 2020, 785, 108319. [Google Scholar] [CrossRef]
- Kayano, S.; Suzuki, Y.; Kanno, K.; Aoki, Y.; Kure, S.; Yamada, A.; Matsubara, Y. Significant association between nonsyndromic oral clefts and arylhydrocarbon receptor nuclear translocator (ARNT). Am. J. Med. Genet. Part A 2004, 130A, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Christensen, K.; Weinberg, C.R.; Romitti, P.; Bathum, L.; Lozada, A.; Morris, R.W.; Lovett, M.; Murray, J.C. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am. J. Hum. Genet. 2007, 80, 76–90. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, I.A.; Wegerif, M.J.; Roelofs, H.M.; Peters, W.H.; Kuijpers-Jagtman, A.M.; Zielhuis, G.A.; Merkus, H.M.; Steegers-Theunissen, R.P. Smoking, genetic polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: A gene-environment interaction. Epidemiology 2001, 12, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, C.; Imura, H.; Yamada, T.; Hirata, A.; Ikeda, Y.; Ito, M.; Natsume, N. Histological and Immunohistochemical Studies to Determine the Mechanism of Cleft Palate Induction after Palatal Fusion in Mice Exposed to TCDD. Int. J. Mol. Sci. 2022, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Huang, P.; Wang, X.; Meng, L. Susceptibility to DNA damage caused by abrogation of Rad54 homolog B: A putative mechanism for chemically induced cleft palate. Toxicology 2021, 456, 152772. [Google Scholar] [CrossRef]
- Scheller, K.; Kalmring, F.; Schubert, J. Sex distribution is a factor in teratogenically induced clefts and in the anti-teratogenic effect of thiamine in mice, but not in genetically determined cleft appearance. J. Cranio-Maxillo-Facial Surg. 2016, 44, 104–109. [Google Scholar] [CrossRef]
- Yoshioka, H.; Mikami, Y.; Ramakrishnan, S.S.; Suzuki, A.; Iwata, J. MicroRNA-124-3p Plays a Crucial Role in Cleft Palate Induced by Retinoic Acid. Front. Cell. Dev. Biol. 2021, 9, 621045. [Google Scholar] [CrossRef]
- Yoshioka, H.; Suzuki, A.; Iwaya, C.; Iwata, J. Suppression of microRNA 124-3p and microRNA 340-5p ameliorates retinoic acid-induced cleft palate in mice. Development 2022, 149, dev200476. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Z.; Xing, Y.; Zhao, H.; Liang, Y.; Chen, J.; Zhong, X.; Shi, L.; Wan, X.; Zhou, J.; et al. MiR-106a-5p modulates apoptosis and metabonomics changes by TGF-beta/Smad signaling pathway in cleft palate. Exp. Cell Res. 2020, 386, 111734. [Google Scholar] [CrossRef]
- Yoshioka, H.; Jun, G.; Suzuki, A.; Iwata, J. Dexamethasone Suppresses Palatal Cell Proliferation through miR-130a-3p. Int. J. Mol. Sci. 2021, 22, 12453. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Liu, W. MicroRNA-130a-3p promotes the proliferation and inhibits the apoptosis of cervical cancer cells via negative regulation of RUNX3. Mol. Med. Rep. 2020, 22, 2990–3000. [Google Scholar] [CrossRef]
- Shao, L.; Ye, Q.; Jia, M. miR-130-3p Promotes MTX-Induced Immune Killing of Hepatocellular Carcinoma Cells by Targeting EPHB4. J. Health Eng. 2021, 2021, 4650794. [Google Scholar] [CrossRef]
- Li, L.; Zhan, M.; Li, M. Circular RNA circ_0130438 suppresses TNF-alpha-induced proliferation, migration, invasion and inflammation in human fibroblast-like MH7A synoviocytes by regulating miR-130a-3p/KLF9 axis. Transpl. Immunol. 2022, 72, 101588. [Google Scholar] [CrossRef]
- Nakatomi, M.; Ludwig, K.U.; Knapp, M.; Kist, R.; Lisgo, S.; Ohshima, H.; Mangold, E.; Peters, H. Msx1 deficiency interacts with hypoxia and induces a morphogenetic regulation during mouse lip development. Development 2020, 147, dev189175. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ramakrishnan, S.S.; Suzuki, A.; Iwata, J. Phenytoin Inhibits Cell Proliferation through microRNA-196a-5p in Mouse Lip Mesenchymal Cells. Int. J. Mol. Sci. 2021, 22, 1746. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Yang, H.; Cao, Y.; Yu, D.; Zhao, Y.; Cao, Y. MicroRNA-196a-5p overexpression in Wharton’s jelly umbilical cord stem cells promotes their osteogenic differentiation and new bone formation in bone defects in the rat calvarium. Cell Tissue Res. 2022, 390, 245–260. [Google Scholar] [CrossRef]
- Li, K.; Cao, H.; Fan, M.; Li, Q.; Zhang, Q.; Jia, C.; Wang, D.; Jiang, W. LncRNA KCNQ1OT1 Participates in Ox-LDL-Induced Proliferation/Apoptosis Imbalance in Vascular Smooth Muscle Cells by Regulating the MiR-196a-5p/FOXO1 Axis. J. Stroke Cereb. Dis. 2022, 31, 106622. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.; An, L.; Fang, G.; Hong, D.; Jiao, T.; Yang, H.; Wang, Z. Serum exosomal microRNA-370-3p and microRNA-196a-5p are potential biomarkers for the diagnosis and prognosis of hepatocellular carcinoma. Folia Histochem. Cytobiol. 2022, 60, 215–225. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, F. Circular RNA circCRKL inhibits the proliferation of acute myeloid leukemia cells via the miR-196a-5p/miR-196b-5p/p27 axis. Bioengineered 2021, 12, 7704–7713. [Google Scholar] [CrossRef]
- Editorial, O. Erratum to tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 4047–4048. [Google Scholar] [CrossRef]
- Tuysuz, E.C.; Ozbey, U.; Gulluoglu, S.; Kuskucu, A.; Sahin, F.; Bayrak, O.F. miRNAs as cell fate determinants of lateral and paraxial mesoderm differentiation from embryonic stem cells. Dev. Biol. 2021, 478, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Tatsumi, K.; Kawao, N.; Okada, K.; Muratani, M.; Kaji, H. MicroRNA-196a-5p in Extracellular Vesicles Secreted from Myoblasts Suppresses Osteoclast-like Cell Formation in Mouse Cells. Calcif. Tissue Int. 2021, 108, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, H.; Li, S. LncRNA LOXL1-AS1 controls osteogenic and adipocytic differentiation of bone marrow mesenchymal stem cells in postmenopausal osteoporosis through regulating the miR-196a-5p/Hmga2 axis. J. Bone Min. Metab. 2020, 38, 794–805. [Google Scholar] [CrossRef] [PubMed]

| Cleft Type (# of Genes) | Mouse Type | Genes |
|---|---|---|
| Complete CPO (367 genes) | Single gene mutation | Acan Acvr1 Acvr2a Adamts3 Adamts6 Adamts20 Adgra2 Afdn Amer1 Anp32b Ap2b1 Arhgap29 Asxl1 Barx1 Bcor Bmp2 Bmp4 Bmp7 Bmpr1a Bnc2 Cask Casp3 Ccn2 Ccp110 Cdc42 Cdk20 Cdkn1c Chd7 Chrd Chuk Col2a1 Colgalt1 Crampl Crebbp Crk Ctnnb1 Ctnnbip1 Cycsp Cyp26b1 Cyp51 Dhcr7 Dhrs3 Dicer1 Dlg1 Dlx1 Dlx2 Dlx5Dnmt3b Dph1 Edn1 Edn2 Ednra Efnb1 Efnb2 Egfr Ermp1 Esrp1 Eya1 Fam20b Fbxo11 Fbxw7 Fgf8 Fgf9 Fgf10 Fgf18 Fgfr1 Fgfr2 Fgfr2c Fign Flna Foxc2 Foxe1 Foxf2 Fras1 Fst Fuz Fzd2 Gab1 Gabrb3 Gad1 Gas1 Gbx2 Gdf11 Glce Glg1 Gli2 Golb1 Gpc6 Grb2 Grhl3 Gsk3b Gskip Haao Hand2 Has2 Hdac3 Hoxa2 Hoxb7 Hs2st1 Hsd17b7 Hspb11 Hspg2 Ift88 Ift140 Igf2 Ilk Impad1 Inhba Inpp5e Irf6 Itga5 Itgav Ift140 Ift 172 Igf2 Ilk Impad1 Inha Inpp5e Irf6 Itga5 Itgb1 Itgb8 Jag2 Jmjd6 Kat6a Kcnj2 Kcnj13 Kdf1 Kif7 Kif20b Kifbp Ldb1 Lhx8 Loxl3 Lrp2 Luzp1 Map3k7 Mapk1 Med23 Megf8 Meis2 Men1 Meox2 Mfcs4 Midn Mirc1 Mir17-18 Msk1 Mn1 Mnt Msx1 Msx2 Mybphl Nabp2 Mectin1 Mectin4 Nog Nosip Nprl3 Nrp1 Nsd2 Nxn Oca2 Ofd1 Osr2 Pax3 Pax9 Pcnt Pcsk5 Pdgfc Pdgfra Pds5a Pds5b Pdss2 Phc1 Piga Pigv Pitx1 Pitx2 Pkdcc Plod3 Plxnd1 Pnn Porcn Prdm16 Prickle1 Prrx1 Ptch1 Pygo2 Qrich1 Qsox1 Rad23b Rbfox2 Rdh10 Recql4 Robo1 Ror2 Rpgrip1l Rspo2 Runx2 Ryk Ryr1 Satb2 Sc5d Sclt1 Serpinh1 Sfn Sh3pxd2a Shh Sim2 Skor2 Slc13a4 Slc32a1 Slc35d1 Slmap Smad7 Smo Smoc1 Snai2 Snx3 Sos1 Sox2 Sox5 Sox9 Sox11 Spry2 Sufu Sumo1 Tapt1 Tbc1d32 Tbx1 Tbx2 Tbx22 Tcof1 Tctn2 Tent5c Tfap2a Tgds Tgfb2 Tgfb3 Tgfbr2 Tgfbr3 Tmem107 Trppc10 Trp53 Trp63 Trps1 Ttc21b Twist1 Ugdh Vax1 Vegfa Wdpcp Wdr19 Wls Wnt5a Wen Zeb1 Zmynd11 |
| Spontaneous | Abn Acan Am Cacnal2 Col11a1 Crn Csp2 Far Fgf9 Gli3 Hpmd Lmbr1 M9bei Mut1679 Oca2 Oel Pad Pc Pcp Ptd Rpl38 Sho Sme Srn Srt Ur Zeb1 | |
| Compound mutant | Adamts9;Adamts20 Adamts20;Ptch1 Adamts20;Vcan Akap8;Fign Arid5b;Pdgfra Ard5b;Zfp950 Bmi1;Pcgf2 Bmp2;Bmp4 Bmp4;Bmp7 Bmp2;Bmp4;Bmp7 Boc;Cdon Chrd;Nog Chrd;Tbx1 Dlx1;Clx2 Clx5;Msx1 Dph1;Ovca2 Dph1;Ovca2;Trp53 Ednrb;Spry2 Ephb2;Ephb3 Eya1;Six1 Eya1;Sumo1 Fgfr1;Fgfr2 Fuddle;TCZ-tau Fzd1;Fzd2 Fzd2;Fzd7 Fzd2;Vangl2 Fzd2;Fzd7;Wnt3a Fzd2;Fzd7;Wnt11 Gab1;Met Gad1;Gad2 Gas1;Shh Gdf11;Mstn Gdf11;Wfikkn1 Gdf11;Wfikkn2 Golga5;Golgb1 Gsc;Gsk3a H19;Igf2r Hspa5;TCZ-tau Hoxa1;Hoxa2 Igf2;Rr27 Inhba;Inhbb Insig1;Insig2 Irf6;Sfn Itga5;Itgav Itgb6;Itgb8 Kat6a;Tbx1 Kdf1;Sfn Kif20b;TCZ-tau Lbr;Tm7sf2 Lgr4;Lgr5;Lgr6 Lgr5;Lgr6 Lhx6;Lhx8 Lrp6;Rspo2 Mapk1;Mapk3 Mdm2;Mdm4 Mmp14;Mmp16 Msc;Tcf21 Ncor2;Ncor2 Nectin1;Nectin4 Osr2;Pax9 Pax9;Msx1 Pax9;Sostdc1 Pbx1;Pbx2 Pbx1;Pbx2;Pbx3 Pdgfra;Pdgfrb Pdgfra;Plekha1 Phc1;Phc2 Prrx1;Prrx2 Ptprf;Ptprs Pygo1;Pygo2 Ror1Ror2 Ror2;Wnt5a Ror1;Wnt9a Shh;Six3 Six1;Six4 Snai1;Snai2 Sox5;Sox6 Spry1;Spry2 Tbx2;Tbx3 Tfap2a;Tfap2b Tgfb1;Tgfb3 Vax1;Vax2 Yap;Taz | |
| Partial CPO: anterior (16 genes) | Single gene mutation | Codn Ctnnb1 Fgfr2 Gsc Lims1 Runx1 Shh Shox2 Sox11 Tbx1 Tbx3 Tgfb3 |
| Compound mutant | Boc;Cdon Map3k7;Smad4 | |
| Partial CPO: posterior/soft palate (15 genes) | Single gene mutation | Bnc2 Dlx5 Foxf2 Hic1 Hox3a Mef2c Mfcs4 Pax3 Rspo2 Sim2 Smo Tbx1 Tgfbr1 Tgfbr2 Tshz1 |
| Compound mutant | Dlx5;Mef2c | |
| Submucous cleft palate (37 genes) | Single gene mutation | Acvr1 Amer1 Apaf1 Arid5b Asph Bmp4 Csrnp1 Dlx5 Eda Eya4 Fras1 Inhba Krt5 Lrp4 Meis2 Ndst1 Nog Recql4 Schip1 Six3 Sgpl1 Smad4 Smo Sostdc1 Tbx1 Tbx3 Tbx22 Tgfb3 Tgfbr1 Tgfbr2 Tiparp Zfp640 Zfp950 |
| Compound mutant | Map3k7;Smad4 Shh;Six3 Smad4;Irf6 Smad4;Trim33 | |
| CLO (23 genes) | Single gene mutation | Bmp4 Cplane2 Ermp1 Folr1 Gli3 Kynu Mks1 Pbx1 Pgap2 Ptch1 Rpgrip1l Sp8 Tbx1 Tgfbr1 |
| Spontaneous | Clf2 Knyn Rpl38 Wnt9b | |
| Compound mutant | Aldh1a2;Aldh1a3 Bbs7;Ift88 Gdf1;Nodal | |
| CLP (44 genes) | Single gene mutation | Bmpr1a Cdc42 Cplane1 Ctnnb1 Dzip1l Ednrb Ermp1 Esrp1 Folr1 Ift88 Ihh Kif3a Kynu Lrp6 Mirc1 Mks1 Pbx1 Pgap2 Rpgrip1l Rspo2 Sox11 Tfap2a Tgfbr1 Trp53 Trp63 Ttc21b Wdr19 Wnt9b |
| Spontaneous | Clf2 Knyn Rpl38 Tbx10 Zeb1 | |
| Compound mutant | Bbs7;Ift88 Esrp1;Esrp2 Fgf8;Tfap2 Hhat;Ptch1 Lrp6;Rspo2 Mirc1;Mirc3 Msx1;Pax9 Pbx1;Pbx2 Pbx1;Pbx3 Pbx1;Wnt9b Rspo2;Wnt9b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaya, C.; Suzuki, A.; Iwata, J. MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate. Int. J. Mol. Sci. 2023, 24, 3552. https://doi.org/10.3390/ijms24043552
Iwaya C, Suzuki A, Iwata J. MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate. International Journal of Molecular Sciences. 2023; 24(4):3552. https://doi.org/10.3390/ijms24043552
Chicago/Turabian StyleIwaya, Chihiro, Akiko Suzuki, and Junichi Iwata. 2023. "MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate" International Journal of Molecular Sciences 24, no. 4: 3552. https://doi.org/10.3390/ijms24043552
APA StyleIwaya, C., Suzuki, A., & Iwata, J. (2023). MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate. International Journal of Molecular Sciences, 24(4), 3552. https://doi.org/10.3390/ijms24043552








