Gata2a Mutation Causes Progressive Microphthalmia and Blindness in Nile Tilapia
Abstract
:1. Introduction
2. Results
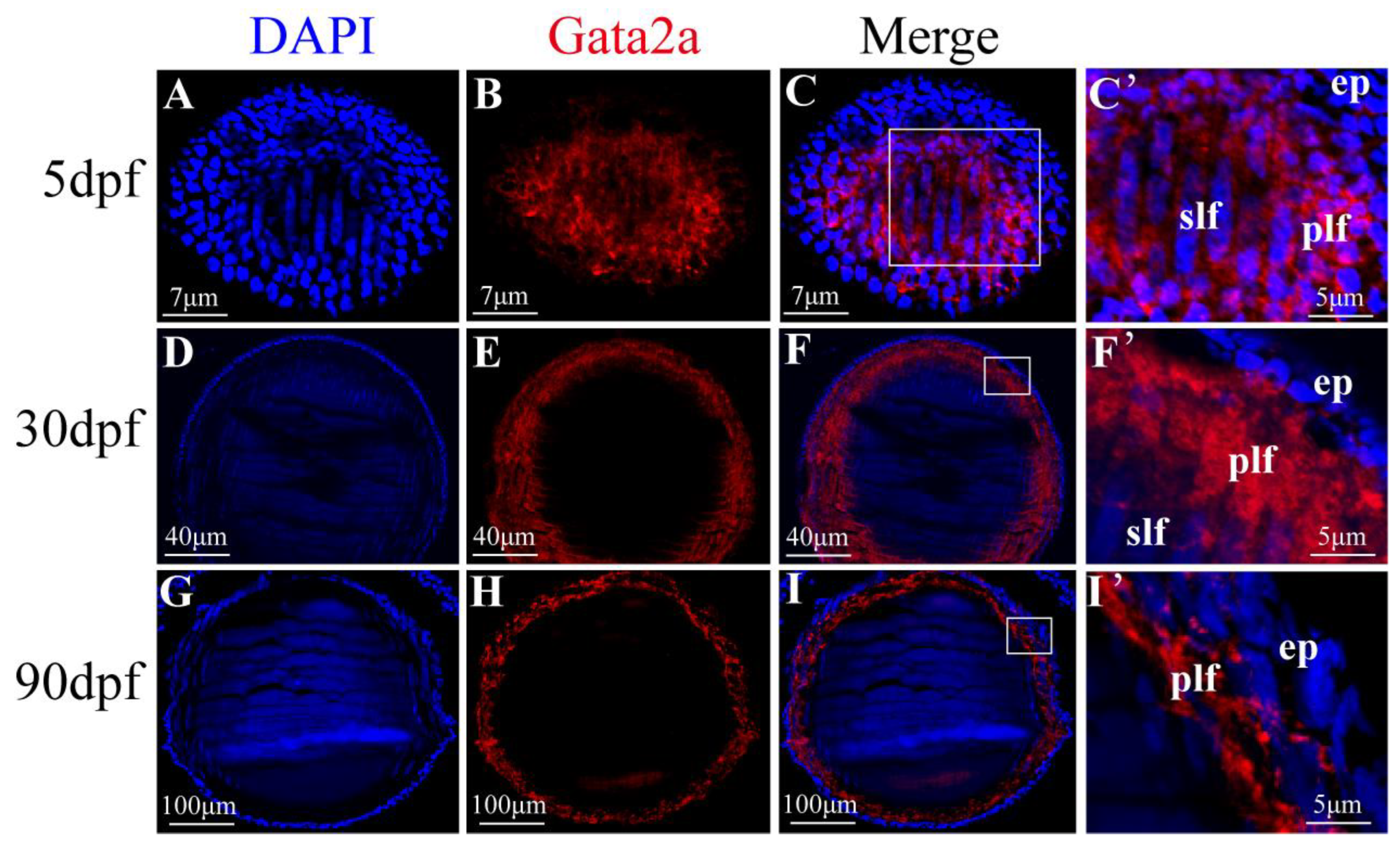
2.1. Expression Pattern of Gata2a in the Lens of Nile Tilapia
2.2. Establishment of Gata2a and Gata2b Homozygous Mutants in Nile Tilapia
2.3. Gata2a Mutation Causes Microphthalmia and Blindness in Nile Tilapia
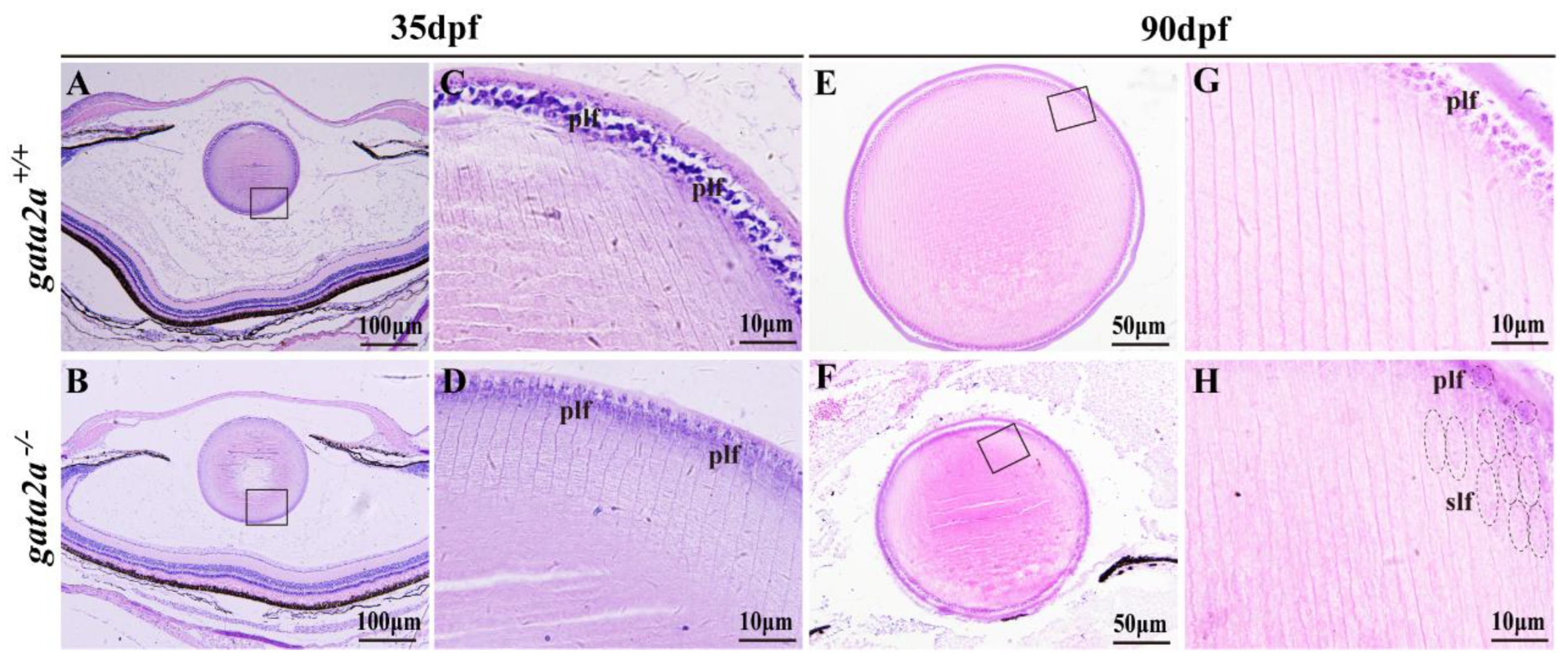
2.4. Gata2a Mutation Disrupts the Development of the Lens Fiber Cells
2.5. Gata2a Mutation Down-Regulates Genes Related to Crystallin Synthesis and Up-Regulates Genes Related to Visual Perception and Iron Ion Binding
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Construction of Gata2a and Gata2b Homozygous Mutants by CRISPR/Cas9
4.3. Immunofluorescence
4.4. Western Blot
4.5. Transcriptome Sequencing and Analysis
4.6. Histological Analysis
4.7. Real-Time PCR
4.8. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Dpf | Day post fertilization |
| Plf | Primary lens fiber cells |
| Slf | Secondary lens fiber cells |
| Ep | epithelial cell |
| PAM | protospacer adjacent motif |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEGs | differentially expressed genes |
| FDR | false discovery rate |
| DAPI | 4′,6′-diamidine-2-phenylindole-dihydrochloride |
| WT | wild type |
References
- Cvekl, A.; Ashery-Padan, R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014, 141, 4432–4447. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Wang, Z.; He, Y.; Zhang, Q. Origin and evolution of GATA2a and GATA2b in teleosts: Insights from tongue sole, Cynoglossus semilaevis. PeerJ 2016, 4, e1790. [Google Scholar] [CrossRef] [PubMed]
- Miesfeld, J.B.; Brown, N.L. Eye organogenesis: A hierarchical view of ocular development. Curr. Top Dev. Biol. 2019, 132, 351–393. [Google Scholar] [PubMed]
- Graw, J. Mouse models for microphthalmia, anophthalmia and cataracts. Hum. Genet. 2019, 138, 1007–1018. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar]
- Berry, V.; Ionides, A.; Pontikos, N.; Georgiou, M.; Yu, J.; Ocaka, L.A.; Moore, A.T.; Quinlan, R.A.; Michaelides, M. The genetic landscape of crystallins in congenital cataract. Orphanet J. Rare Dis. 2020, 15, 333. [Google Scholar] [CrossRef]
- Beyer, E.C.; Ebihara, L.; Berthoud, V.M. Connexin mutants and cataracts. Front. Pharmacol. 2013, 4, 43. [Google Scholar] [CrossRef]
- Pei, R.; Liang, P.-F.; Ye, W.; Li, J.; Ma, J.-Y.; Zhou, J. A novel mutation of LIM2 causes autosomal dominant membranous cataract in a Chinese family. Int. J. Ophthalmol. 2020, 13, 1512–1520. [Google Scholar] [CrossRef]
- Anand, D.; Agrawal, S.A.; Slavotinek, A.; Lachke, S.A. Mutation update of transcription factor genes FOXE3, HSF4, MAF, and PITX3 causing cataracts and other developmental ocular defects. Human Mutat. 2018, 39, 471–494. [Google Scholar] [CrossRef]
- Song, S.; Landsbury, A.; Dahm, R.; Liu, Y.; Zhang, Q.; Quinlan, R.A. Functions of the intermediate filament cytoskeleton in the eye lens. J. Clin. Investig. 2009, 119, 1837–1848. [Google Scholar] [CrossRef]
- Greiling, T.M.S.; Aose, M.; Clark, J.I. Cell fate and differentiation of the developing ocular lens. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1540–1546. [Google Scholar] [CrossRef] [Green Version]
- Bassnett, S. On the mechanism of organelle degradation in the vertebrate lens. Exp. Eye Res. 2009, 88, 133–139. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Wride, M.A. The ocular lens: A classic model for development, physiology and disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 1190–1192. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Riazuddin, S.A.; McGreal, R.; Liu, W.; Cvekl, A.; Shiels, A. Lens Biology and Biochemistry. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar]
- Dahm, R.; Schonthaler, H.B.; Soehn, A.S.; van Marle, J.; Vrensen, G.F. Development and adult morphology of the eye lens in the zebrafish. Exp. Eye Res. 2007, 85, 74–89. [Google Scholar] [CrossRef]
- Audette, D.S.; Scheiblin, D.A.; Duncan, M.K. The molecular mechanisms underlying lens fiber elongation. Exp. Eye Res. 2017, 156, 41–49. [Google Scholar] [CrossRef]
- Vorontsova, I.; Gehring, I.; Hall, J.E.; Schilling, T.F. Aqp0a Regulates Suture Stability in the Zebrafish Lens. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2869–2879. [Google Scholar] [CrossRef]
- Wride, M.A. Lens fibre cell differentiation and organelle loss: Many paths lead to clarity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 1219–1233. [Google Scholar] [CrossRef]
- Costello, M.J.; Brennan, L.A.; Basu, S.; Chauss, D.; Mohamed, A.; Gilliland, K.O.; Johnsen, S.; Menko, A.S.; Kantorow, M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp. Eye Res. 2013, 116, 141–150. [Google Scholar] [CrossRef]
- He, C.; Cheng, H.; Zhou, R. GATA family of transcription factors of vertebrates: Phylogenetics and chromosomal synteny. J. Biosci. 2007, 32, 1273–1280. [Google Scholar] [CrossRef]
- Charles, M.A.; Saunders, T.L.; Wood, W.M.; Owens, K.; Parlow, A.F.; Camper, S.A.; Ridgway, E.C.; Gordon, D.F. Pituitary-specific Gata2 knockout: Effects on gonadotrope and thyrotrope function. Mol. Endocrinol. 2006, 20, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, D.; Suzuki, N.; Kobayashi-Osaki, M.; Pan, X.; Engel, J.D.; Yamamoto, M. GATA2-dependent and region-specific regulation of Gata2 transcription in the mouse midbrain. Genes Cells 2009, 14, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.P.; McReynolds, L.J.; Holland, S.M. GATA2 deficiency. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C.; Patel, S.Y.; Uzel, G.; Anderson, V.L.; Freeman, A.F.; Olivier, K.N.; Spalding, C.; Hughes, S.; Pittaluga, S.; Raffeld, M.; et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 2009, 115, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Bigley, V.; Collin, M. Dendritic cell, monocyte, B and NK lymphoid deficiency defines the lost lineages of a new GATA-2 dependent myelodysplastic syndrome. Haematologica 2011, 96, 1081–1083. [Google Scholar] [CrossRef]
- Hahn, C.N.; Chong, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Keller, G.; Kuo, F.C.; Weiss, M.; Chen, J.; Rosenblatt, M.; Alt, F.W.; Orkin, S.H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 1994, 371, 221–226. [Google Scholar] [CrossRef]
- Peña, O.A.; Lubin, A.; Rowell, J.; Hoade, Y.; Khokhar, N.; Lemmik, H.; Mahony, C.; Dace, P.; Umamahesan, C.; Payne, E.M. Differential Requirement of Gata2a and Gata2b for Primitive and Definitive Myeloid Development in Zebrafish. Front. Cell. Dev. Biol. 2021, 9, 708113. [Google Scholar] [CrossRef]
- Gioacchino, E.; Koyunlar, C.; Zink, J.; de Looper, H.; de Jong, M.; Dobrzycki, T.; Mahony, C.B.; Hoogenboezem, R.; Bosch, D.; van Strien, P.M.H.; et al. Essential role for Gata2 in modulating lineage output from hematopoietic stem cells in zebrafish. Blood Adv. 2021, 5, 2687–2700. [Google Scholar] [CrossRef]
- Leonard, M.; Lim, K.; Engel, J. Expression of the chicken GATA factor family during early erythroid development and differentiation. Development 1993, 119, 519–531. [Google Scholar] [CrossRef]
- Maeda, A.; Moriguchi, T.; Hamada, M.; Kusakabe, M.; Fujioka, Y.; Nakano, T.; Yoh, K.; Lim, K.C.; Engel, J.D.; Takahashi, S. Transcription factor GATA-3 is essential for lens development. Dev. Dyn. 2009, 238, 2280–2291. [Google Scholar] [CrossRef]
- El Wakil, A.; Francius, C.; Wolff, A.; Pleau-Varet, J.; Nardelli, J. The GATA2 transcription factor negatively regulates the proliferation of neuronal progenitors. Development 2006, 133, 2155–2165. [Google Scholar] [CrossRef] [Green Version]
- Crispino, J.D. GATA1 in normal and malignant hematopoiesis. Semin. Cell Dev. Biol. 2005, 16, 137–147. [Google Scholar] [CrossRef]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef]
- Ghosh, K.S.; Chauhan, P. Crystallins and Their Complexes. Subcell Biochem. 2019, 93, 439–460. [Google Scholar]
- Slingsby, C.; Wistow, G.J. Functions of crystallins in and out of lens: Roles in elongated and post-mitotic cells. Prog. Biophys Mol. Biol. 2014, 115, 52–67. [Google Scholar] [CrossRef]
- Domínguez-Calva, J.A.; Pérez-Vázquez, M.L.; Serebryany, E.; King, J.A.; Quintanar, L. Mercury-induced aggregation of human lens γ-crystallins reveals a potential role in cataract disease. J. Biol. Inorg. Chem. 2018, 23, 1105–1118. [Google Scholar] [CrossRef]
- Rao, P.V.; Maddala, R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin. Cell Dev. Biol. 2006, 17, 698–711. [Google Scholar] [CrossRef]
- Rocha, M.A.; Kwok, A.O.; Martin, R.W. α-Crystallins in the Vertebrate Eye Lens: Complex Oligomers and Molecular Chaperones. Annu. Rev. Phys. Chem. 2021, 72, 143–163. [Google Scholar]
- Nishiguchi, S.; Wood, H.; Kondoh, H.; Lovell-Badge, R.; Episkopou, V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998, 12, 776–781. [Google Scholar] [CrossRef]
- Goring, D.; Breitman, M.L.; Tsui, L.-C. Temporal regulation of six crystallin transcripts during mouse lens development. Exp. Eye Res. 1992, 54, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Ring, B.; Cordes, S.; Overbeek, P.; Barsh, G. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development 2000, 127, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Avunduk, A.; Yardimci, S.; Avunduk, M.; Kurnaz, L.; Koçkar, M. Determinations of some trace and heavy metals in rat lenses after tobacco smoke exposure and their relationships to lens injury. Exp. Eye Res. 1997, 65, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, H.; Zhao, J.; Fang, L.; Shi, H.; Li, M.; Sun, Y.; Zhang, X.; Jiang, D.; Zhou, L.; et al. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics 2014, 197, 591–599. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, L.; Li, W.; Wu, J.; Wang, D. Gata2a Mutation Causes Progressive Microphthalmia and Blindness in Nile Tilapia. Int. J. Mol. Sci. 2023, 24, 3567. https://doi.org/10.3390/ijms24043567
Liu X, Zhou L, Li W, Wu J, Wang D. Gata2a Mutation Causes Progressive Microphthalmia and Blindness in Nile Tilapia. International Journal of Molecular Sciences. 2023; 24(4):3567. https://doi.org/10.3390/ijms24043567
Chicago/Turabian StyleLiu, Xingyong, Li Zhou, Wenbo Li, Jiahong Wu, and Deshou Wang. 2023. "Gata2a Mutation Causes Progressive Microphthalmia and Blindness in Nile Tilapia" International Journal of Molecular Sciences 24, no. 4: 3567. https://doi.org/10.3390/ijms24043567




