The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana
Abstract
1. Introduction
2. Results and Discussion
2.1. Promoter Analysis of AtSAH7
2.2. Differential Expression of AtSAH7 under Se and Cd Exposure
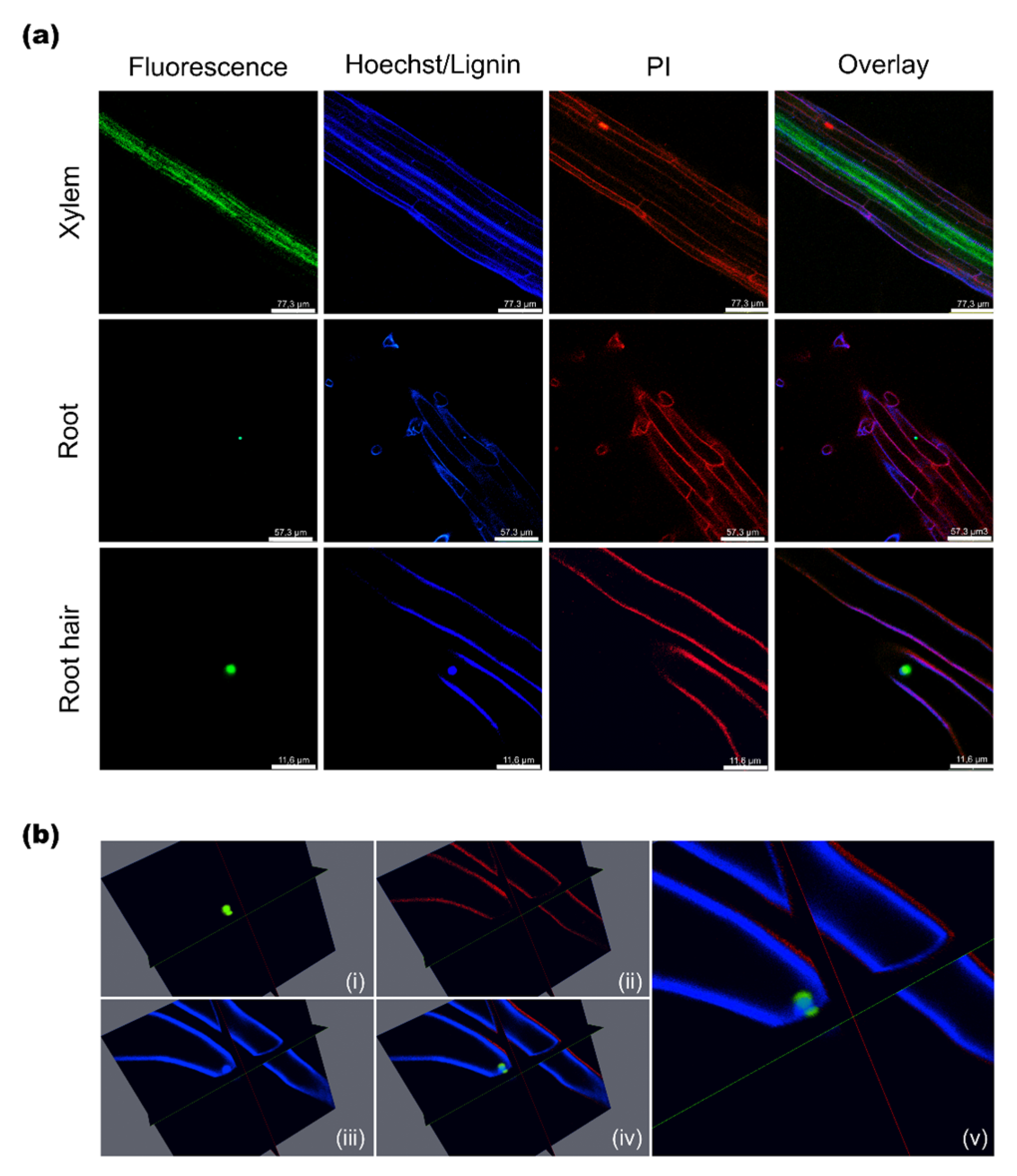
2.3. Subcellular Localization of AtSAH7
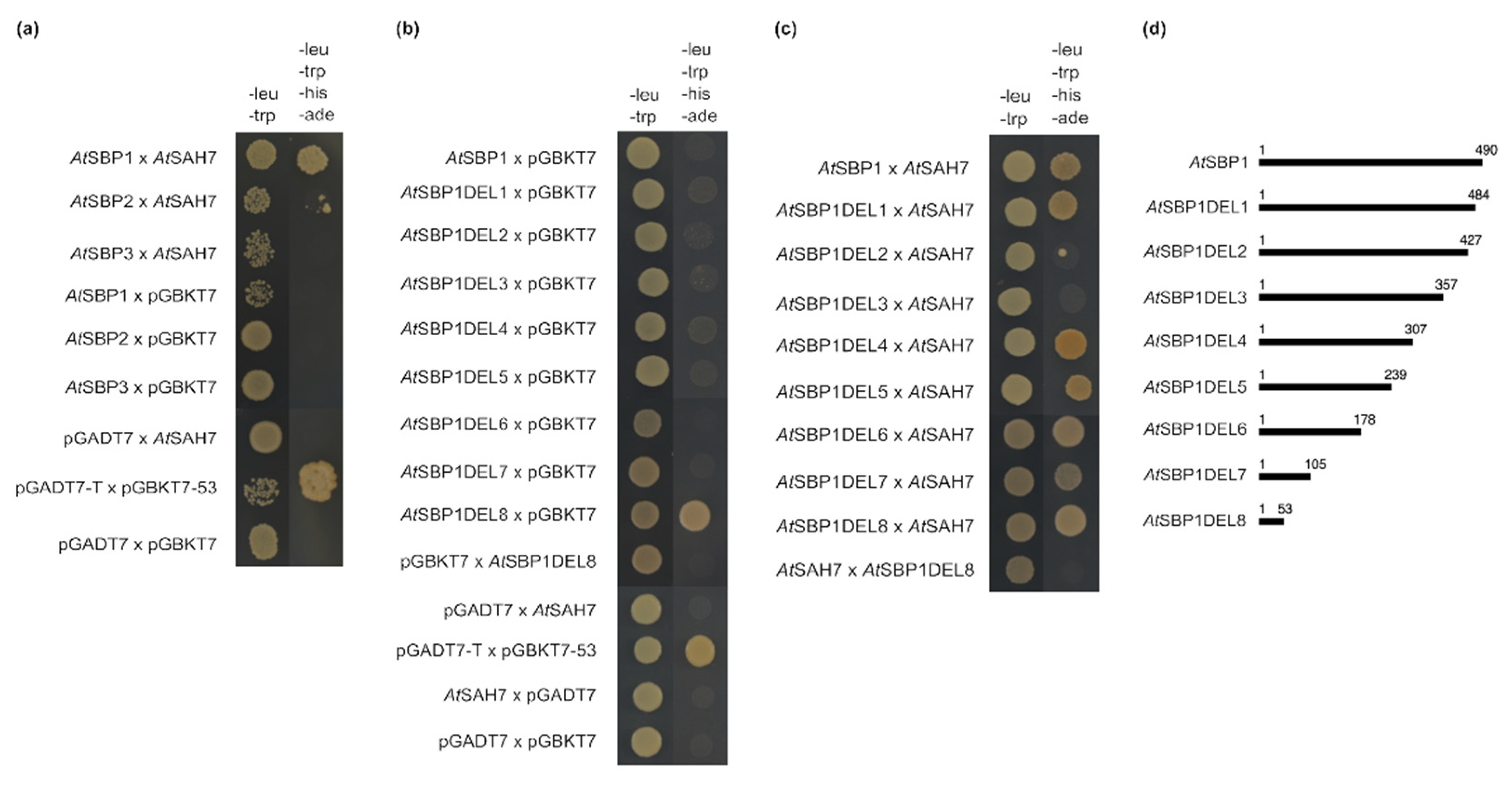
2.4. AtSAH7 Interacts Only with AtSBP1 in a Yeast Two-Hybrid Assay
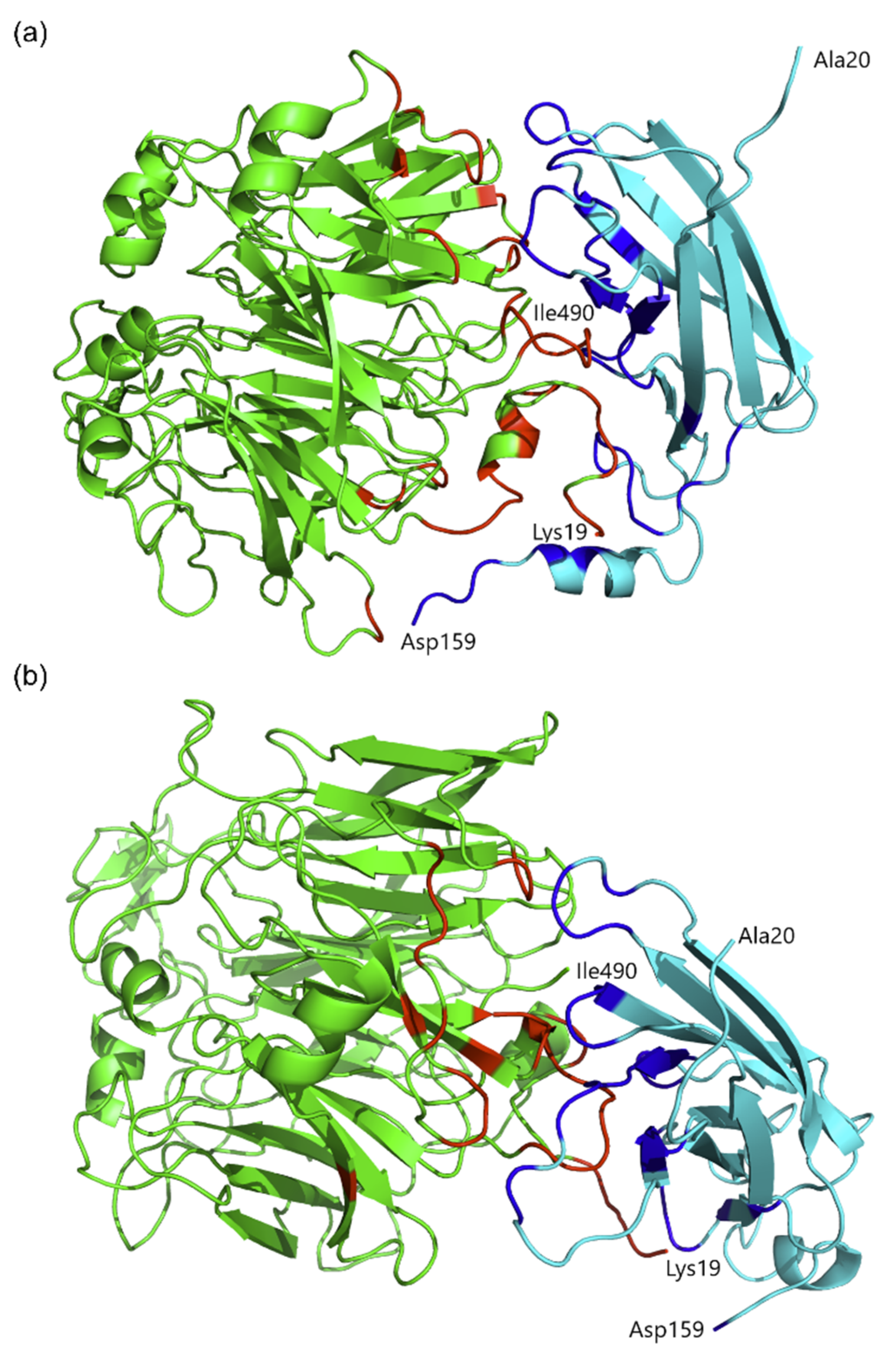
2.5. Protein Molecular Modeling and Structural Prediction of Protein–Protein Interactions
2.6. AtSBP1 Interacts in Planta with AtSAH7
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Construction of Vectors
3.3. Chemical Treatment of Plants
3.4. Stainings
3.5. Yeast Two-Hybrid Assays
3.6. Structural Prediction of Protein–Protein Interactions by Molecular Docking and Molecular Dynamics Simulations
3.7. Protoplast Analysis
3.8. Microscopy
3.9. Confocal Microscopy
3.10. RNA Extraction, cDNA Synthesis and Gene Expression Analysis
3.11. Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2010, 38, D211–D222. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.C.; Rodríguez-García, M.I.; de Dios Alché, J. Systematic and Phylogenetic Analysis of the Ole e 1 Pollen Protein Family Members in Plants. In Systems and Computational Biology; Intech Open: London, UK, 2011. [Google Scholar]
- Lauzurica, P.; Gurbindo, C.; Maruri, N.; Galocha, B.; Diaz, R.; Gonzalez, J.; García, R.; Lahoz, C. Olive (Olea europea) Pollen Allergens-I Immunochemical Characterization by Immunoblotting, Crie and Immunodetection by a Monoclonal Antibody. Mol. Immunol. 1988, 25, 329–335. [Google Scholar] [CrossRef]
- King, T.; Hoffman, D.; Lowenstein, H.; Marsh, D.G.; Platts-Mills, T.A.E.; Thomas, W. Allergen Nomenclature. Int. Arch. Allergy Immunol. 1994, 105, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Villalba, M.; Batanero, E.; González, E.M.; Monsalve, R.I.; Huecas, S.; Tejera, M.L.; Ledesma, A. Allergenic Diversity of the Olive Pollen. Allergy Eur. J. Allergy Clin. Immunol. Suppl. 2002, 57, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Batanero, E.; López-Otín, C.; Sánchez, L.M.; Monsalve, R.I.; González De La Peña, M.A.; Lahoz, C.; Rodríguez, R. The Amino Acid Sequence of Ole e I, the Major Allergen from Olive Tree (Olea europaea) Pollen. Eur. J. Biochem. 1993, 216, 863–869. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Jasmin, P.X.H.; Ting, Y.Y.; Ramachandran, S. Genome-Wide Identification and Molecular Characterization of Ole_e_i, Allerg_1 and Allerg_2 Domain-Containing Pollen-Allergen-like Genes in Oryza Sativa. DNA Res. 2005, 12, 167–179. [Google Scholar] [CrossRef]
- Hu, B.; Liu, B.; Liu, L.; Liu, C.; Xu, L.; Ruan, Y. Epigenetic Control of Pollen Ole e 1 Allergen and Extensin Family Gene Expression in Arabidopsis Thaliana. Acta Physiol. Plant. 2014, 36, 2203–2209. [Google Scholar] [CrossRef]
- Alché, J.D.D.; M’rani-Alaoui, M.; Castro, A.J.; Rodríguez-García, M.I. Ole e 1, the Major Allergen from Olive (Olea europaea L.) Pollen, Increases Its Expression and Is Released to the Culture Medium during in Vitro Germination. Plant Cell Physiol. 2004, 45, 1149–1157. [Google Scholar] [CrossRef]
- Agalou, A.; Roussis, A.; Spaink, H.P. The Arabidopsis Selenium-Binding Protein Confers Tolerance to Toxic Levels of Selenium. Funct. Plant Biol. 2005, 32, 881–890. [Google Scholar] [CrossRef]
- Dutilleul, C.; Jourdain, A.; Bourguignon, J.; Hugouvieux, V. The Arabidopsis Putative Selenium-Binding Protein Family: Expression Study and Characterization of SBP1 as a Potential New Player in Cadmium Detoxification Processes. Plant Physiol. 2008, 147, 239–251. [Google Scholar] [CrossRef]
- Agalou, A.; Spaink, H.P.; Roussis, A. Novel Interaction of Selenium-Binding Protein with Glyceraldehyde-3- Phosphate Dehydrogenase and Fructose-Bisphosphate Aldolase of Arabidopsis Thaliana. Funct. Plant Biol. 2006, 33, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Valassakis, C.; Livanos, P.; Minopetrou, M.; Haralampidis, K.; Roussis, A. Promoter Analysis and Functional Implications of the Selenium Binding Protein (SBP) Gene Family in Arabidopsis Thaliana. J. Plant Physiol. 2018, 224–225, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Schild, F.; Kieffer-Jaquinod, S.; Palencia, A.; Cobessi, D.; Sarret, G.; Zubieta, C.; Jourdain, A.; Dumas, R.; Forge, V.; Testemale, D.; et al. Biochemical and Biophysical Characterization of the Selenium-Binding and Reducing Site in Arabidopsis thaliana Homologue to Mammals Selenium-Binding Protein 1. J. Biol. Chem. 2014, 289, 31765–31776. [Google Scholar] [CrossRef]
- Hugouvieux, V.; Dutilleul, C.; Jourdain, A.; Reynaud, F.; Lopez, V.; Bourguignon, J. Arabidopsis Putative Selenium-Binding Protein1 Expression Is Tightly Linked to Cellular Sulfur Demand and Can Reduce Sensitivity to Stresses Requiring Glutathione for Tolerance. Plant Physiol. 2009, 151, 768–781. [Google Scholar] [CrossRef]
- Valassakis, C.; Dervisi, I.; Agalou, A.; Papandreou, N.; Kapetsis, G.; Podia, V.; Haralampidis, K.; Iconomidou, V.A.; Spaink, H.P.; Roussis, A. Novel Interactions of Selenium Binding Protein Family with the PICOT Containing Proteins AtGRXS14 and AtGRXS16 in Arabidopsis Thaliana. Plant Sci. 2019, 281, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dervisi, I.; Valassakis, C.; Agalou, A.; Papandreou, N.; Podia, V.; Haralampidis, K.; Iconomidou, V.A.; Kouvelis, V.N.; Spaink, H.P.; Roussis, A. Investigation of the Interaction of DAD1-LIKE LIPASE 3 (DALL3) with Selenium Binding Protein 1 (SBP1) in Arabidopsis Thaliana. Plant Sci. 2020, 291, 110357. [Google Scholar] [CrossRef]
- Dervisi, I.; Haralampidis, K.; Roussis, A. Investigation of the Interaction of a Papain-like Cysteine Protease (RD19c) with Selenium-Binding Protein 1 (SBP1) in Arabidopsis Thaliana. Plant Sci. 2022, 315, 111157. [Google Scholar] [CrossRef]
- Koletti, A.; Dervisi, I.; Kalloniati, C.; Zografaki, M.E.; Rennenberg, H.; Roussis, A.; Flemetakis, E. Selenium-Binding Protein 1 (SBD1): A Stress Response Regulator in Chlamydomonas Reinhardtii. Plant Physiol. 2022, 189, 2368–2381. [Google Scholar] [CrossRef]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Minãmbres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis Messenger RNAs Transported to Distant Tissues. Nat. Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Ilegems, M.; Douet, V.; Meylan-Bettex, M.; Uyttewaal, M.; Brand, L.; Bowman, J.L.; Stieger, P.A. Interplay of Auxin, KANADI and Class III HD-ZIP Transcription Factors in Vascular Tissue Formation. Development 2010, 137, 975–984. [Google Scholar] [CrossRef]
- Seo, M.; Kim, H.; Lee, J.Y. Information on the Move: Vascular Tissue Development in Space and Time during Postembryonic Root Growth. Curr. Opin. Plant Biol. 2020, 57, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Carlsbecker, A.; Etchells, J.P.; Turner, S. Class III HD-ZIPs Govern Vascular Cell Fate: An HD View on Patterning and Differentiation. J. Exp. Bot. 2017, 68, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Feild, T.S.; Sage, T.L.; Czerniak, C.; Iles, W.J.D. Hydathodal Leaf Teeth of Chloranthus japonicus (Chloranthaceae) Prevent Guttation-Induced Flooding of the Mesophyll. Plant Cell Environ. 2005, 28, 1179–1190. [Google Scholar] [CrossRef]
- Cerutti, A.; Jauneau, A.; Laufs, P.; Leonhardt, N.; Schattat, M.H.; Berthome, R.; Routaboul, J.M.; Noediel, L.D. Mangroves in the Leaves: Anatomy, Physiology, and Immunity of Epithemal Hydathodes. Annu. Rev. Phytopathol. 2019, 57, 91–116. [Google Scholar] [CrossRef]
- Bellenot, C.; Routaboul, J.M.; Laufs, P.; Noël, L.D. Hydathodes. Curr. Biol. 2022, 32, R763–R764. [Google Scholar] [CrossRef]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An Auxin-Dependent Distal Organizer of Pattern and Polarity in the Arabidopsis Root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R.; Schwalm, K.; Langhans, M.; Ullrich, C.I. Gradual Shifts in Sites of Free-Auxin Production during Leaf-Primordium Development and Their Role in Vascular Differentiation and Leaf Morphogenesis in Arabidopsis. Planta 2003, 216, 841–853. [Google Scholar] [CrossRef]
- Alvarez, J.P.; Goldshmidt, A.; Efroni, I.; Bowman, J.L.; Esheda, Y. The NGATHA Distal Organ Development Genes Are Essential for Style Specification in Arabidopsis. Plant Cell 2009, 21, 1373–1393. [Google Scholar] [CrossRef]
- Eklund, D.M.; Thelander, M.; Landberg, K.; Ståldal, V.; Nilsson, A.; Johansson, M.; Valsecchi, I.; Pederson, E.R.A.; Kowalczyk, M.; Ljung, K.; et al. Homologues of the Arabidopsis thaliana SHI/STY/LRP1 Genes Control Auxin Biosynthesis and Affect Growth and Development in the Moss Physcomitrella Patens. Development 2010, 137, 1275–1284. [Google Scholar] [CrossRef]
- Wang, W.; Xu, B.; Wang, H.; Li, J.; Huang, H.; Xu, L. YUCCA Genes Are Expressed in Response to Leaf Adaxial-Abaxial Juxtaposition and Are Required for Leaf Margin Development. Plant Physiol. 2011, 157, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Müller-Moulé, P.; Nozue, K.; Pytlak, M.L.; Palmer, C.M.; Covington, M.F.; Wallace, A.D.; Harmer, S.L.; Maloof, J.N. YUCCA Auxin Biosynthetic Genes Are Required for Arabidopsis Shade Avoidance. PeerJ 2016, 2016, e2574. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, J.-M.; Bellenot, C.; Clément, G.; Citerne, S.; Remblière, C.; Charvin, M.; Franke, L.; Chiarenza, S.; Vasselon, D.; Jardinaud, M.-F.; et al. Arabidopsis Hydathodes Are Sites of Intense Auxin Metabolism and Nutrient Scavenging. bioRxiv 2022. [Google Scholar] [CrossRef]
- Van Hengel, A.J.; Roberts, K. AtAGP30, an Arabinogalactan-Protein in the Cell Walls of the Primary Root, Plays a Role in Root Regeneration and Seed Germination. Plant J. 2003, 36, 256–270. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, K.; Chen, Y.; Wang, X.; Wang, Y. Gerbera POE1 (GhPOE1) Is Involved in Leaf Senescence in Arabidopsis. S. Afr. J. Bot. 2021, 143, 33–41. [Google Scholar] [CrossRef]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium Channels Activated by Hydrogen Peroxide Mediate Abscisic Acid Signalling in Guard Cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Guan, L.M.; Zhao, J.; Scandalios, J.G. Cis-Elements and Trans-Factors That Regulate Expression of the Maize Cat1 Antioxidant Gene in Response to ABA and Osmotic Stress: H2O2 Is the Likely Intermediary Signaling Molecule for the Response. Plant J. 2000, 22, 87–95. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.-P. Hydrogen Peroxide Is Involved in Abscisic Acid-Induced Stomatal Closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of Plasma-Membrane NADPH Oxidase in Abscisic Acid- and Water Stress-Induced Antioxidant Defense in Leaves of Maize Seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Cross-Talk between Calcium and Reactive Oxygen Species Originated from NADPH Oxidase in Abscisic Acid-Induced Antioxidant Defence in Leaves of Maize Seedlings. Plant Cell Environ. 2003, 26, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The Interaction of ABA and ROS in Plant Growth and Stress Resistances. Front. Plant Sci. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic Acid Prevents Pollen Abortion under High-Temperature Stress by Mediating Sugar Metabolism in Rice Spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Blum, T.; Briesemeister, S.; Kohlbacher, O. MultiLoc2: Integrating Phylogeny and Gene Ontology Terms Improves Subcellular Protein Localization Prediction. BMC Bioinform. 2009, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- de Dios Alché, J.; Castro, A.J.; Olmedilla, A.; Fernandez, M.C.; Rodriguez, R.; Villalba, M.; Rodriguez-Garcia, M.I. The Major Olive Pollen Allergen (Ole e I) Shows Both Gametophytic and Sporophytic Expression during Anther Development, and Its Synthesis and Storage Takes Place in the RER. J. Cell Sci. 1999, 112, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Alche Ramírez, J.D.D.; Calabozo, B.; Rodríguez-García, M.I.; Polo, F. Pla l 1 and Ole e 1 Pollen Allergens Share Common Epitopes and Similar Ultrastructural Localization. J. Investig. Allergol. Clin. Immunol. 2007, 17, 93–99. [Google Scholar]
- Raucci, R.; Colonna, G.; Guerriero, E.; Capone, F.; Accardo, M.; Castello, G.; Costantini, S. Structural and Functional Studies of the Human Selenium Binding Protein-1 and Its Involvement in Hepatocellular Carcinoma. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 513–522. [Google Scholar] [CrossRef]
- Stemeseder, T.; Freier, R.; Wildner, S.; Fuchs, J.E.; Briza, P.; Lang, R.; Batanero, E.; Lidholm, J.; Liedl, K.R.; Campo, P.; et al. Crystal Structure of Pla l 1 Reveals Both Structural Similarity and Allergenic Divergence within the Ole e 1–like Protein Family. J. Allergy Clin. Immunol. 2017, 140, 277–280. [Google Scholar] [CrossRef]
- Kerppola, T.K. Bimolecular Fluorescence Complementation (BiFC) Analysis as a Probe of Protein Interactions in Living Cells. Annu. Rev. Biophys. 2008, 37, 465–487. [Google Scholar] [CrossRef]
- Lee, L.Y.; Fang, M.J.; Kuang, L.Y.; Gelvin, S.B. Vectors for Multi-Color Bimolecular Fluorescence Complementation to Investigate Protein-Protein Interactions in Living Plant Cells. Plant Methods 2008, 4, 1–11. [Google Scholar] [CrossRef]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic Reticulum Stress Triggers ROS Signalling, Changes the Redox State, and Regulates the Antioxidant Defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Hall, A.; Millar, A.J.; Darrah, C.; Davis, S.J. Protocol: Streamlined Sub-Protocols for Floral-Dip Transformation and Selection of Transformants in Arabidopsis thaliana. Plant Methods 2009, 5, 1–7. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for the rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 474–497. [Google Scholar] [CrossRef]
- Citovsky, V.; Lee, L.Y.; Vyas, S.; Glick, E.; Chen, M.H.; Vainstein, A.; Gafni, Y.; Gelvin, S.B.; Tzfira, T. Subcellular Localization of Interacting Proteins by Bimolecular Fluorescence Complementation in Planta. J. Mol. Biol. 2006, 362, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.G.; McPherson, M.J. Developmental Expression and Biochemical Analysis of the Arabidopsis Atao1 Gene Encoding an H2O2-Generating Diamine Oxidase. Plant J. 1998, 13, 781–791. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; Van Dijk, M.; De Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2015, 428, 720–725. [Google Scholar] [CrossRef]
- Wassenaar, T.A.; Van Dijk, M.; Gijs, N.L.; Sjoerd, V.D.S.; Schmitz, C.; Van Der Zwan, J.; Boelens, R.; Giachetti, A.; Ferella, L.; Rosato, A.; et al. WeNMR: Structural Biology on the Grid. IWSG-Life 2011: Sci. Gatew. Life Sci. 2012, 10, 743–767. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Green, T.; et al. NAR Breakthrough Article AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, 439–444. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Armenteros Almagro, J.J.; Rosenberg Johansen, A.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- De Vries, S.J.; Bonvin, A.M.J.J. CPORT: A Consensus Interface Predictor and Its Performance in Prediction-Driven Docking With. PLoS ONE 2011, 6, e17695. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, C.; Páll, S.; Fechner, M.; Esztermann, A.; de Groot, B.L.; Grubmüller, H. More Bang for Your Buck: Improved Use of GPU Nodes for GROMACS 2018. J. Comput. Chem. 2019, 40, 2418–2431. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Berendsen, H.J.C. Transport Properties Computed by Linear Response through Weak Coupling to a Bath. Comput. Simul. Mater. Sci. 1991, 205, 139–155. [Google Scholar]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 10, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich-A Simpler Arabidopsis Protoplast Isolation Method. Plant Methods 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System, Version 2.4; Shrödinger, LLC.: New York, NY, USA, 2010.
- Bargmann, B.O.R.; Birnbaum, K.D. Fluorescence Activated Cell Sorting of Plant Protoplasts. J. Vis. Exp. 2010, 36, e1673. [Google Scholar]
- Oñate-Sánchez, L.; Vicente-Carbajosa, J. DNA-Free RNA Isolation Protocols for Arabidopsis thaliana, Including Seeds and Siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Oualline, S.; Oualline, G. Drawing with Inkscape. In Practical Free Alternatives to Commercial Software; Apress: Berkeley, CA, USA, 2018; pp. 187–219. [Google Scholar]



| A | B | C | D |
|---|---|---|---|
| 19–21, 23–25, 30–31, 33–34, 36–40, 68–69, 99–106, 160–161, 185–186, 191, 194, 196, 211–212, 214, 250,252, 254, 277, 412–413, 415–416 | 19–23, 30–31, 99–106, 137, 159–161, 163, 183, 185–187, 191, 193–196, 250,252 | 34, 40–45, 47, 49, 62–65, 90–91, 93, 95, 111–119, 121, 123–126, 130–135, 151, 154, 156–159 | 42–45, 47, 49, 61–63, 65, 90–93, 112–113, 115–121, 123–124, 131–135,159 |
| A | B | C | D | |
|---|---|---|---|---|
| Residues that participate in hydrogen bonds | Gly20, Lys23, Tyr24, Gly25, Thr31, Ser40, Gly102, Asp103, Pro251, Lys415 | Lys19, Cys22, Gly102, Asp103, Ala104, Ser105, Glu163, Trp194, Gly252 | Tyr34, Gly41, Glu43, Thr44, Ser47, Arg62, Thr119, His113, Asp114, Thr119, Asn124, Phe132, Asn134, Asn135, Tyr154, Glu156, Glu158 | Glu43, Pro45, Arg62, Arg63, Arg117, Thr119, Arg131, Phe132, Asn134 |
| Residues that participate in salt bridges | Lys23, Arg212, Lys415 | Lys19, Glu163 | Glu43, Asp114, Glu158 | Glu43, Arg62, Asp159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervisi, I.; Petropoulos, O.; Agalou, A.; Podia, V.; Papandreou, N.; Iconomidou, V.A.; Haralampidis, K.; Roussis, A. The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 3580. https://doi.org/10.3390/ijms24043580
Dervisi I, Petropoulos O, Agalou A, Podia V, Papandreou N, Iconomidou VA, Haralampidis K, Roussis A. The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(4):3580. https://doi.org/10.3390/ijms24043580
Chicago/Turabian StyleDervisi, Irene, Orfeas Petropoulos, Adamantia Agalou, Varvara Podia, Nikolaos Papandreou, Vassiliki A. Iconomidou, Kosmas Haralampidis, and Andreas Roussis. 2023. "The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 4: 3580. https://doi.org/10.3390/ijms24043580
APA StyleDervisi, I., Petropoulos, O., Agalou, A., Podia, V., Papandreou, N., Iconomidou, V. A., Haralampidis, K., & Roussis, A. (2023). The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana. International Journal of Molecular Sciences, 24(4), 3580. https://doi.org/10.3390/ijms24043580







