The EphA1 and EphA2 Signaling Modulates the Epithelial Permeability in Human Sinonasal Epithelial Cells and the Rhinovirus Infection Induces Epithelial Barrier Dysfunction via EphA2 Receptor Signaling
Abstract
:1. Introduction
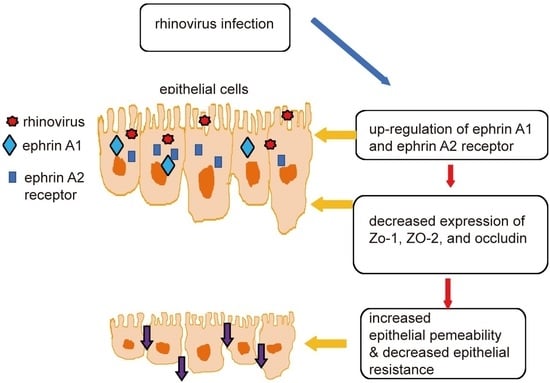
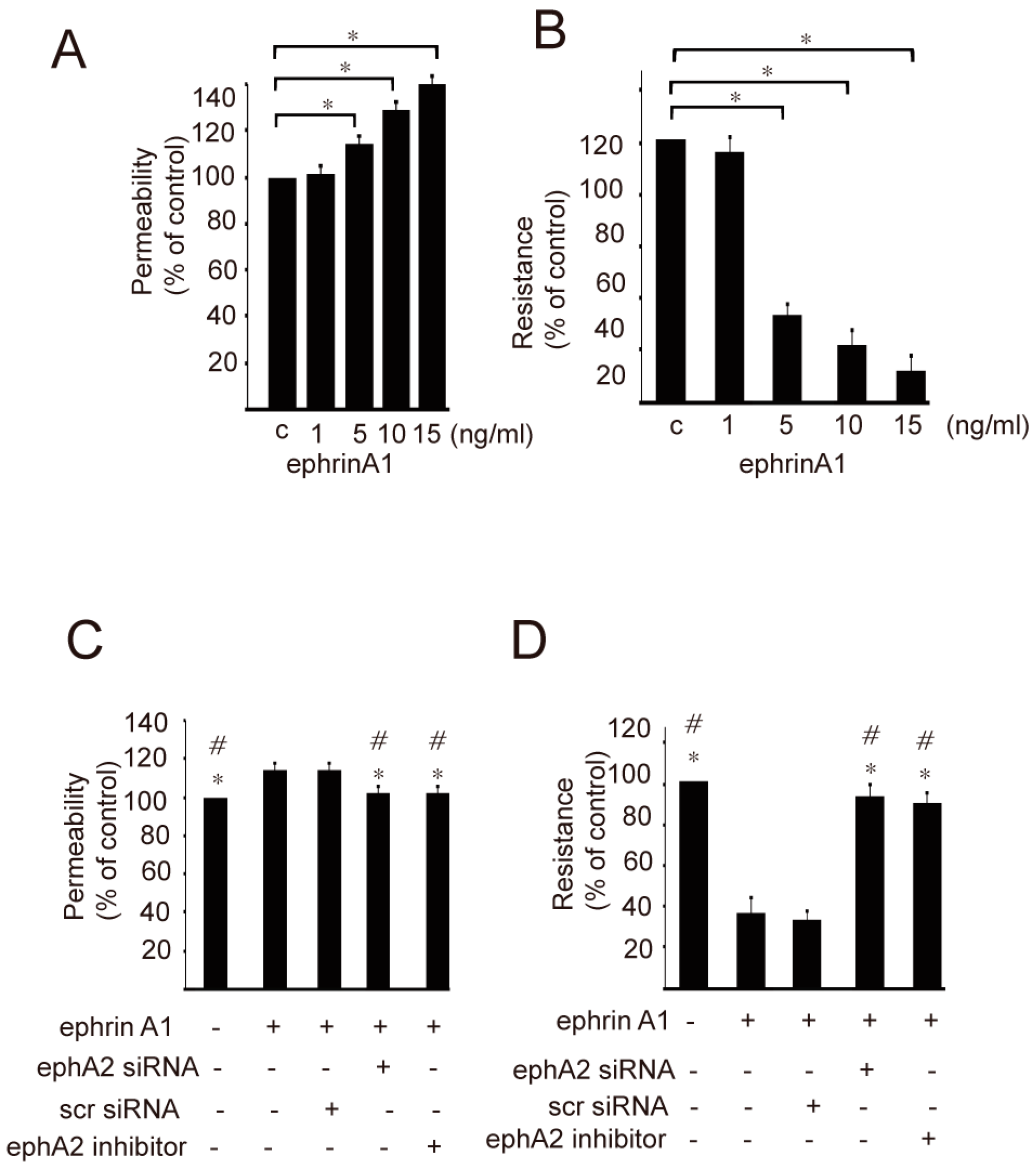
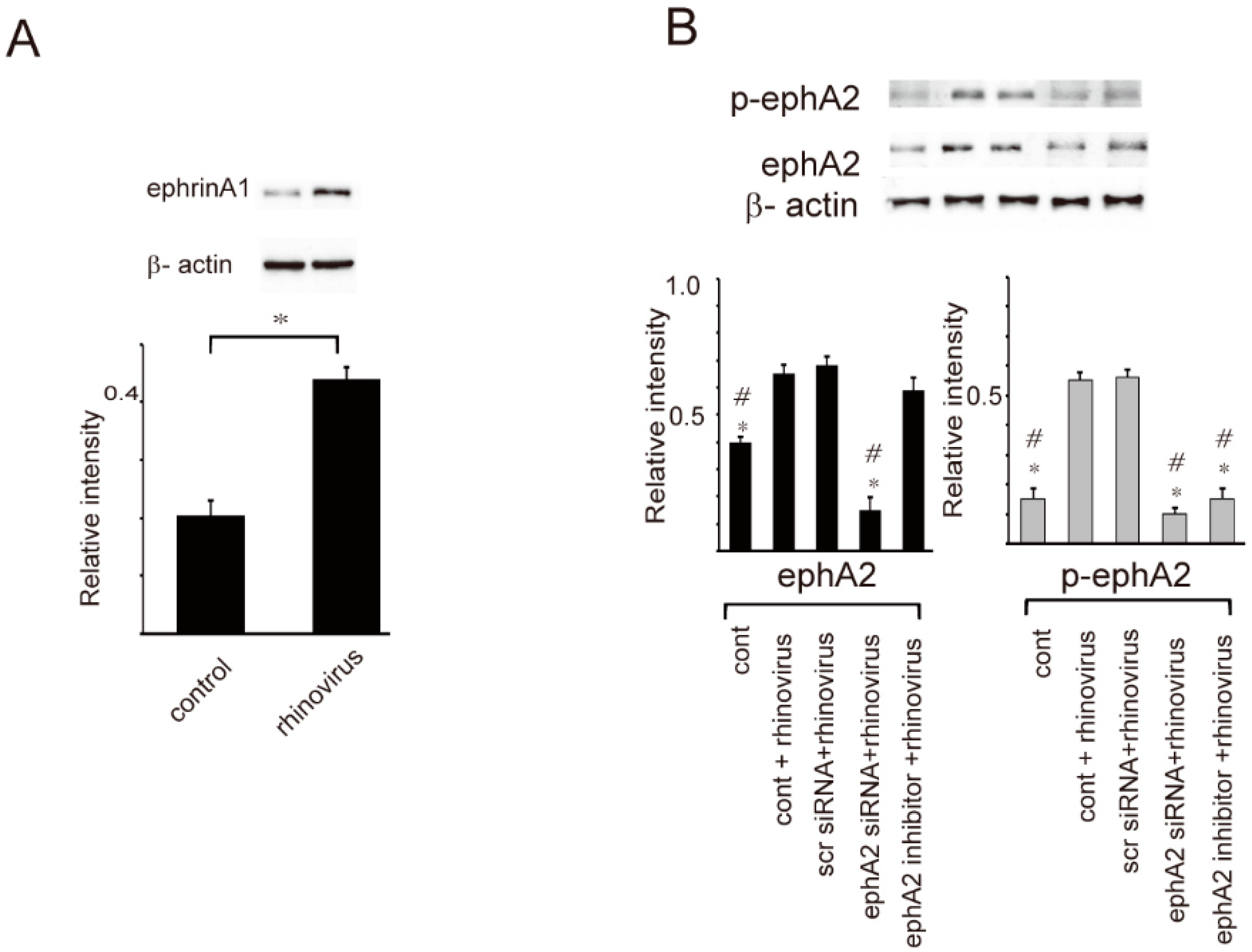
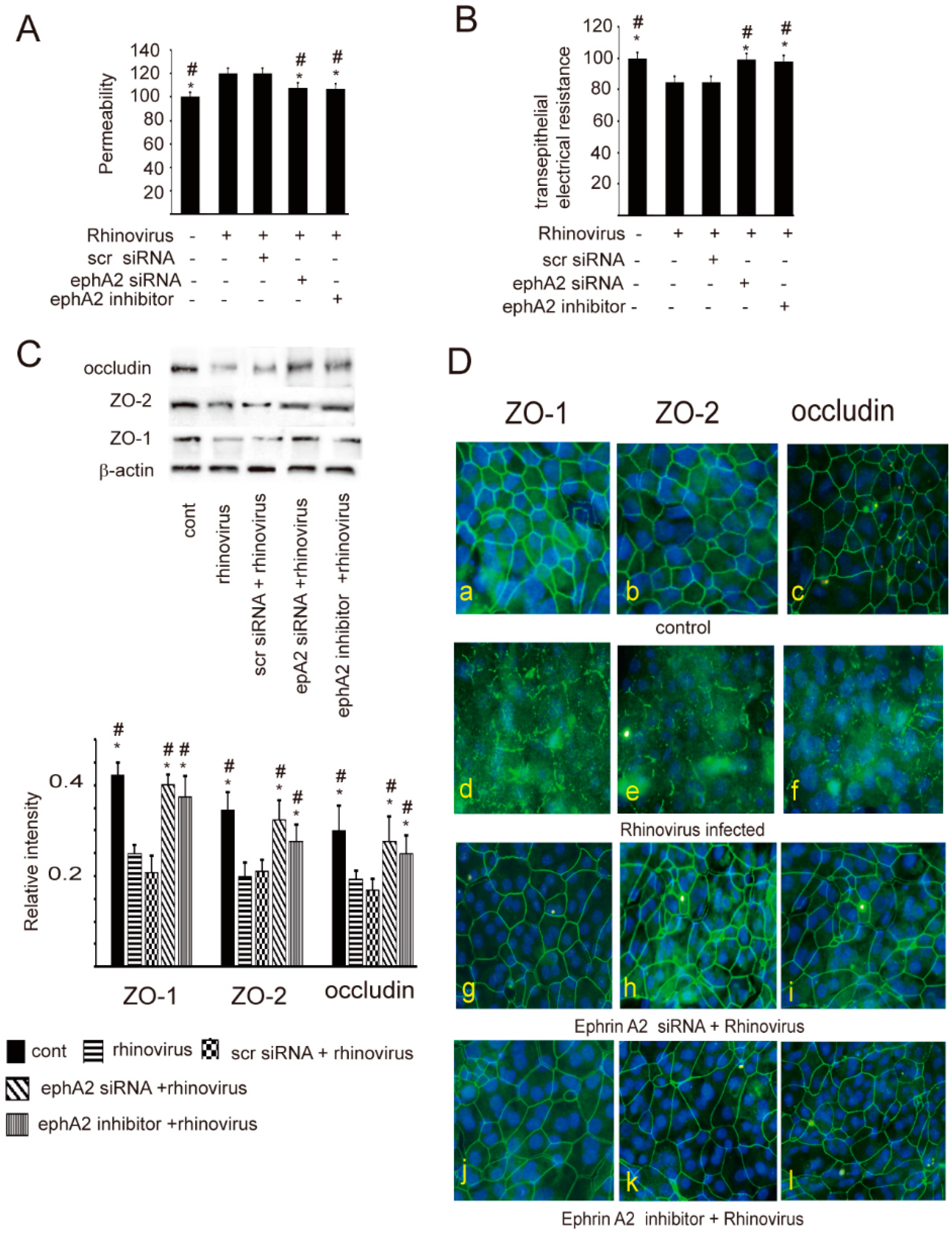
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Sampling
4.2. Epithelial Cell Culture
4.3. RV Propagation and Infection
4.4. Effect of RV Infection on Expression of EphrinA1 and EphA2 Receptor
4.5. Effect of EphrinA1/EphA2 Pathway on the Maintenance of the Epithelial Barrier Function
4.6. Small Interfering RNA (siRNA) Transfection Experiments
4.7. Western Blot
4.8. Confocal Microscopy
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Bachert, C.; Baraniuk, J.; Baroody, F.M.; Benninger, M.S.; et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J. Allergy Clin. Immunol. 2004, 114 (Suppl. S6), 155–212. [Google Scholar] [CrossRef] [PubMed]
- Kern, R.C.; Conley, D.B.; Walsh, W.; Chandra, R.; Kato, A.; Tripathi-Peters, A.; Grammer, L.C.; Schleimer, R.P. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am. J. Rhinol. 2008, 22, 549–559. [Google Scholar] [CrossRef]
- Ramanathan, M., Jr.; Lane, A.P. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2007, 136, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L. Drivers of chronic rhinosinusitis: Inflammation versus infection. J. Allergy Clin. Immunol. 2015, 136, 1454–1459. [Google Scholar] [CrossRef]
- Hulse, K.E. Immune mechanisms of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.D.; Lee, W.Y.; DelGaudio, J.M.; Moore, C.E.; Nava, P.N.; Nusrat, A.; Parkos, C.A. Pathophysiology of nasal polyposis: The role of desmosomal junctions. Am. J. Rhinol. 2008, 22, 589–597. [Google Scholar] [CrossRef]
- Winther, B.; Gwaltney, J.M., Jr.; Mygind, N.; Hendley, J.O. Viral-induced rhinitis. Am. J. Rhinol. 1998, 12, 17–20. [Google Scholar] [CrossRef]
- Greenberg, S.B. Respiratory viral infections in adults. Curr. Opin. Pulm. Med. 2002, 8, 201–208. [Google Scholar] [CrossRef]
- Koyama, S.; Ishii, K.J.; Coban, C.; Akira, S. Innate immune response to viral infection. Cytokine 2008, 43, 336–341. [Google Scholar] [CrossRef]
- Sajjan, U.; Wang, Q.; Zhao, Y.; Gruenert, D.C.; Hershenson, M.B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 2008, 178, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Yeo, N.K.; Jang, Y.J. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope 2010, 120, 346–352. [Google Scholar] [CrossRef]
- Fokkens, W.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef]
- Parri, M.; Buricchi, F.; Taddei, M.L.; Giannoni, E.; Raugei, G.; Ramponi, G.; Chiarugi, P. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J. Biol. Chem. 2005, 280, 34008–34018. [Google Scholar] [CrossRef]
- Leite, M.; Marques, M.S.; Melo, J.; Pinto, M.T.; Cavadas, B.; Aroso, M.; Gomez-Lazaro, M.; Seruca, R.; Figueiredo, C. Helicobacter pylori targets the EphA2 receptor tyrosine kinase in gastric cells modulating key cellular functions. Cells 2020, 9, 513. [Google Scholar] [CrossRef]
- Swidergall, M.; Solis, N.V.; Lionakis, M.S.; Filler, S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat. Microbiol. 2018, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.B.; Zhang, A.; Chen, M.L.; Fang, Z.X.; Dong, X.D.; Li, S.B.; Du, Y.; Xiong, D.; et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nat. Microbiol. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Chakraborty, S.; Veettil, M.V.; Bottero, V.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. Proc. Natl. Acad. Sci. USA 2012, 109, E1163–E1172. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.S.; Kaufmann, J.K.; Wies, E.; Naschberger, E.; Panteleev-lelev, J.; Schmidt, K.; Holzer, A.; Schmidt, M.; Chen, J.; Konig, S.; et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma–associated herpesvirus. Nat. Med. 2012, 18, 961–966. [Google Scholar] [CrossRef]
- Cercone, M.A.; Schroeder, W.; Schomberg, S.; Carpenter, T.C. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L856–L863. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.C.; Schroeder, W.; Stenmark, K.R.; Schmidt, E.P. Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 46, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Nasreen, N.; Khodayari, N.; Sriram, P.S.; Patel, J.; Mohammed, K.A. Tobacco smoke induces epithelial barrier dysfunction via receptor EphA2 signaling. Am. J. Physiol. Cell Physiol. 2014, 306, C1154–C1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Zhang, Y.; Bai, T.; Song, J.; Qian, W.; Hou, X. EphrinA1/ephA2 promotes epithelial hyperpermeability involving in lipopolysaccharide-induced intestinal barrier dysfunction. J. Neurogastroenterol. Motil. 2020, 26, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.H.; Han, M.S.; Kwak, J.W.; Kim, H.G.; Lee, T.H.; Lee, D.B.; Kim, T.H. The expression of ephrin A1/ephA2 receptor increases in chronic rhinosinusitis and ephrinA1/ephA2 signaling affects rhinovirus-induced innate immunity in human sinonasal epithelial cells. Front. Immunol. 2021, 12, 793517. [Google Scholar] [CrossRef] [PubMed]
- Linfield, D.T.; Raduka, A.; Aghapour, M.; Rezaee, F. Airway tight junctions as targets of viral infections. Tissue Barriers 2021, 9, e1883965. [Google Scholar] [CrossRef] [PubMed]
- Tieu, D.D.; Kern, R.C.; Schleimer, R.P. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2009, 124, 37–42. [Google Scholar] [CrossRef]
- Kim, K.A.; Jung, J.H.; Kang, I.G.; Choi, Y.S.; Kim, S.T. ROS is involved in disruption of tight junctions of human nasal epithelial cells induced by HRV16. Laryngoscope 2018, 128, E393–E401. [Google Scholar] [CrossRef]
- Kojima, T.; Go, M.; Takano, K.; Kurose, M.; Ohkuni, T.; Koizumi, J.I.; Kamekura, R.; Ogasawara, N.; Masaki, T.; Fuchimoto, J.; et al. Regulation of tight junctions in upper airway epithelium. BioMed Res. Int. 2013, 2013, 947072. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wang, C.; Zhang, L. Epithelial physical barrier defects in chronic rhinosinusitis. Exp. Rev. Clin. Immunol. 2019, 15, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Shin, M.H.; Douglas, I.S.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, S.K.; Chang, J.; Kim, Y.S.; et al. Inhibition of ephA2/ephrinA1 signal attenuates lipopolysaccharide-induced lung injury. Clin. Sci. 2016, 130, 1993–2003. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Schomberg, S.; Schroeder, W.; Carpenter, T.C. Endothelial EphA receptor stimulation increases lung vascular permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L431–L439. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.G.; Dowling, H.F.; Spiesman, I.G.; Boand, A.V. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch. Intern. Med. 1958, 101, 267–278. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.M.; Han, M.S.; Park, J.H.; Lee, S.H.; Kim, T.H.; Lee, S.H. The EphA1 and EphA2 Signaling Modulates the Epithelial Permeability in Human Sinonasal Epithelial Cells and the Rhinovirus Infection Induces Epithelial Barrier Dysfunction via EphA2 Receptor Signaling. Int. J. Mol. Sci. 2023, 24, 3629. https://doi.org/10.3390/ijms24043629
Shin JM, Han MS, Park JH, Lee SH, Kim TH, Lee SH. The EphA1 and EphA2 Signaling Modulates the Epithelial Permeability in Human Sinonasal Epithelial Cells and the Rhinovirus Infection Induces Epithelial Barrier Dysfunction via EphA2 Receptor Signaling. International Journal of Molecular Sciences. 2023; 24(4):3629. https://doi.org/10.3390/ijms24043629
Chicago/Turabian StyleShin, Jae Min, Moon Soo Han, Jae Hyung Park, Seung Hyeok Lee, Tae Hoon Kim, and Sang Hag Lee. 2023. "The EphA1 and EphA2 Signaling Modulates the Epithelial Permeability in Human Sinonasal Epithelial Cells and the Rhinovirus Infection Induces Epithelial Barrier Dysfunction via EphA2 Receptor Signaling" International Journal of Molecular Sciences 24, no. 4: 3629. https://doi.org/10.3390/ijms24043629