Targeting Breast Cancer: An Overlook on Current Strategies
Abstract
:1. Introduction
2. Classification of BCs
3. Genetic Mutations in BCs
4. Breast Cancer Therapies
4.1. Endocrine Therapy
4.1.1. Selective Estrogen-Receptor Modulators (SERMs)
4.1.2. Selective Estrogen Receptor Downregulators or Degraders (SERDs)
4.1.3. Aromatase Inhibitors (AIs)
4.1.4. Gonadotropin-Releasing Hormone (GnRH) Agonists
4.1.5. Complete Estrogen Receptor Antagonists (CERANs)
4.1.6. Selective Estrogen Receptor Covalent Antagonists (SERCAs)
4.1.7. Selective Human Estrogen Receptor Partial Agonist (ShERPA)
4.2. Chemotherapy
4.2.1. Targeted Therapy
Topoisomerase (TOP) Inhibitors
Drugs Targeting Tubulin
Drugs Targeting Actin
Trophoblast Cell Surface Antigen 2 (TROP2) Inhibitors
4.2.2. HR+ Targeted Therapies
mTOR Inhibitors
Phosphatidylinositol 3-Kinase (PI3K) Inhibitors
Protein Kinase B (Akt) Inhibitors
PI3K/Akt/mTOR (PAM) Inhibitors
4.2.3. HER2-Positive (HER2+) Targeted Therapies
Tyrosine Kinase Inhibitors (TKIs)
Monoclonal Antibodies
Antibody-Drug Conjugates (ADCs)
4.2.4. HER2-Negative (HER2−) Targeted Therapies
Poly(ADP-Ribose) Polymerase Inhibitors (PARPi)
Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors
Antibody-Drug Conjugates (ADCs)
4.2.5. Targeted Protein Degradation (TPD) Technologies
Proteolysis-Targeting Chimeras (PROTACs)
Lysosome Targeting Chimeras (LYTACs)
Metronomic Chemotherapy (MCT)
5. Androgen Receptor Targeted Therapies
6. Immune Checkpoint Inhibitors (ICIs)
7. Treatment of Breast Cancer Stem Cells
8. Natural Products
9. Nanomedicines and Nanopharmaceuticals
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer–epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 7 December 2022).
- Giaquinto, A.N.; Miller, K.D.; Tossas, K.Y.; Winn, R.A.; Jemal, A.; Siegel, R.L. Cancer statistics for African American/Black people 2022. CA Cancer J. Clin. 2022, 72, 202–229. [Google Scholar] [CrossRef]
- Miller, K.D.; Ortiz, A.P.; Pinheiro, P.S.; Bandi, P.; Minihan, A.; Fuchs, H.E.; Tyson, D.M.; Tortolero-Luna, G.; Fedewa, S.A.; Jemal, A.M.; et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J. Clin. 2021, 71, 466–487. [Google Scholar] [CrossRef]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef]
- Harvey-Jones, E.J.; Lord, C.J.; Tutt, A.N. Systemic therapy for hereditary breast cancers. Hematol. Oncol. Clin. 2021, 37, 203–224. [Google Scholar] [CrossRef]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer Targets Ther. 2021, 13, 241. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Xu, C.; Ganesan, K.; Liu, X.; Ye, Q.; Cheung, Y.; Liu, D.; Zhong, S.; Chen, J. Prognostic value of negative emotions on the incidence of breast cancer: A systematic review and meta-analysis of 129,621 patients with breast cancer. Cancers 2022, 14, 475. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef]
- Moo, T.A.; Sanford, R.; Dang, C.; Morrow, M. Overview of breast cancer therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef]
- Arya, N.; Saha, S. Multi-modal advanced deep learning architectures for breast cancer survival prediction. Knowl. Based Syst. 2021, 221, 106965. [Google Scholar] [CrossRef]
- Ihle, C.L.; Wright-Hobart, S.J.; Owens, P. Therapeutics targeting the metastatic breast cancer bone microenvironment. Pharmacol. Ther. 2022, 239, 108280. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, E180. [Google Scholar] [CrossRef]
- Tagliamento, M.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Saini, K.S.; de Azambuja, E.; Punie, K.; Westphalen, C.B.; Morgan, G.; Pronzato, P.; et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103365. [Google Scholar] [CrossRef]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef]
- Pal, S.K.; Childs, B.H.; Pegram, M. Triple negative breast cancer: Unmet medical needs. Breast Cancer Res. Treat. 2011, 125, 627–636. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Fougner, C.; Bergholtz, H.; Norum, J.H.; Sørlie, T. Re-definition of claudin-low as a breast cancer phenotype. Nat. Commun. 2020, 11, 1787. [Google Scholar] [CrossRef]
- Mohanapure, N.S.; Khandeparkar, S.G.S.; Saragade, P.B.; Gogate, B.P.; Joshi, A.R.; Mehta, S.R. Immunohistochemical study of epidermal growth factor receptor, human epidermal growth factor receptor 2/neu, p53, and Ki67 in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2022, 26, 127. [Google Scholar] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Meisel, J.L.; Venur, V.A.; Gnant, M.; Carey, L. Evolution of targeted therapy in breast cancer: Where precision medicine began. Am. Soc. Clin. Oncol. Educat. Book 2018, 38, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Narayan, P.; Osgood, C.L.; Wedam, S.; Prowell, T.M.; Gao, J.J.; Shah, M.; Krol, D.; Wahby, S.; Royce, M.; et al. U.S. FDA drug approvals for breast cancer: A decade in review. Clin. Cancer Res. 2022, 28, 1072–1086. [Google Scholar] [CrossRef]
- Nabieva, N.; Fasching, P.A. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers 2021, 13, 5643. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef]
- Muggia, F.; Safra, T.; Dubeau, L. BRCA genes: Lessons learned from experimental and clinical cancer. Ann. Oncol. 2011, 22, i7–i10. [Google Scholar] [CrossRef] [PubMed]
- De Sabando, A.R.; Lafuente, E.U.; García-Amigot, F.; Alonso Sánchez, A.; Morales Garofalo, L.; Moreno, S.; Ardanaz, E.; Ramos-Arroyo, M.A. Genetic and clinical characterization of BRCA-associated hereditary breast and ovarian cancer in Navarra (Spain). BMC Cancer 2019, 19, 1145. [Google Scholar] [CrossRef] [PubMed]
- National Breast Cancer Foundation. BRCA: The Breast Cancer Gene. Available online: https://www.nationalbreastcancer.org/what-is-brca (accessed on 22 December 2022).
- Menghi, F.; Banda, K.; Kumar, P.; Straub, R.; Dobrolecki, L.; Rodriguez, I.V.; Yost, S.E.; Chandok, H.; Radke, M.R.; Somlo, G.; et al. Genomic and Epigenomic BRCA alterations predict adaptive resistance and response to platinum-based therapy in aptients with triple-negative breast and ovarian carcinomas. Sci. Trans. Med. 2022, 14, eabn1926. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Yoshida, H.; Kitagishi, Y.; Nishimura, Y.; Matsuda, S. Alternative splicings on p53, BRCA1 and PTEN genes involved in breast cancer. Biochem. Biophys. Res. Comm. 2011, 413, 395–399. [Google Scholar] [CrossRef]
- Chai, C.; Wu, H.H.; Abuetabh, Y.; Sergi, C.; Leng, R. Regulation of the tumor suppressor PTEN in triple-negative breast cancer. Cancer Lett. 2021, 527, 41–48. [Google Scholar] [CrossRef]
- Lau, K.H.; Tan, A.M.; Shi, Y. New and emerging targeted therapies for advanced breast cancer. Int. J. Mol. Sci. 2022, 23, 2288. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast cancer treatments: Updates and new challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The mechanistic roles of sirtuins in breast and prostate cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- Weiss, A.; King, T.A. What is the role of neoadjuvant endocrine therapy for breast cancer? Adv. Surg. 2022, 56, 275–286. [Google Scholar] [CrossRef]
- Cucciniello, L.; Gerratana, L.; del Mastro, L.; Puglisi, F. Tailoring adjuvant endocrine therapy in early breast cancer: When, how, and how long? Cancer Treat. Rev. 2022, 110, 102445. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Walsh, E.M.; Tao, J.J.; Chandarlapaty, S.; Jhaveri, K. Accelerating drug development in breast cancer: New frontiers for ER inhibition. Cancer Treat. Rev. 2022, 109, 102432. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer (Primer). Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Grinshpun, A.; Chen, V.; Sandusky, Z.M.; Fanning, S.W.; Jeselsohn, R. ESR1 activating mutations: From structure to clinical application. Biochim. Biophys. Acta BBA Rev. Cancer 2023, 1878, 188830. [Google Scholar] [CrossRef]
- Liu, M.; Xie, F.; Liu, M.; Zhang, Y.; Wang, S. Association between BRCA mutational status and survival in patients with breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2021, 186, 591–605. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; Andre, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) dagger. Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- NICE Pathway. Managing Advanced Breast Cancer. 2020. Available online: https://breastcancernow.org/sites/default/files/advanced-breast-cancer-managing-advanced-breast-cancer.pdf (accessed on 13 December 2022).
- Dustin, D.; Gu, G.; Fuqua, S.A. ESR1 mutations in breast cancer. Cancer 2019, 125, 3714–3728. [Google Scholar] [CrossRef] [PubMed]
- López González, A.; del Barco Berrón, S.; Grau, I.; Galan, M.; Castelo Fernández, B.; Cortés, A.; Sánchez Rovira, P.; Martinez-Bueno, A.; Gonzalez, X.; García, A.; et al. Challenging endocrine sensitivity of hormone receptor-positive/HER2-negative advanced breast cancer with the combination of eribulin and endocrine therapy: The REVERT study. Cancers 2022, 14, 5880. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kulkarni, S.; Singh, Y.; Kumar, P.; Thareja, S. Selective Estrogen Receptor Modulators (SERMs) for the treatment of ER+ breast cancer: An overview. J. Mol. Struct. 2022, 1270, 133853. [Google Scholar] [CrossRef]
- Abel, M.K.; Morgan, T.; Angeles, A.; Goldman, M. Gynecological management of the breast cancer survivor. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 82, 69–80. [Google Scholar] [CrossRef]
- Murthy, A.; Rao Ravi, P.; Kathuria, H.; Malekar, S. Oral Bioavailability Enhancement of Raloxifene with Nanostructured Lipid Carriers. Nanomaterials 2020, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Ahmed, O.A.; Alhakamy, N.A.; Neamatallah, T.; Okbazghi, S.Z.; Fahmy, U.A. Optimized semisolid self-nanoemulsifying system based on glyceryl behenate: A potential nanoplatform for enhancing antitumor activity of raloxifene hydrochloride in MCF-7 human breast cancer cells. Int. J. Pharm. 2021, 600, 120493. [Google Scholar] [CrossRef] [PubMed]
- Lainé, M.; Fanning, S.W.; Chang, Y.-F.; Green, B.; Greene, M.E.; Komm, B.; Kurleto, J.D.; Phung, L.; Greene, G.L. Lasofoxifene as a Potential Treatment for Therapy-Resistant ER-Positive Metastatic Breast Cancer. Breast Cancer Res. 2021, 23, 54. [Google Scholar] [CrossRef]
- Goetz, M.P.; Plourde, P.; Stover, D.G.; Bagegni, N.; Vidal, G.A.; Brufsky, A.; Rugo, H.S.; Portman, D.J.; Gal-Yam, E. LBA20 Open-label, randomized study of lasofoxifene (LAS) vs. fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2-breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. Ann. Oncol. 2022, 33, S1387–S1388. [Google Scholar]
- Zafar, E.; Maqbool, M.F.; Iqbal, A.; Maryam, A.; Shakir, H.A.; Irfan, M.; Khan, M.; Ma, T. A comprehensive review on anticancer mechanism of bazedoxifene. Biotechnol. Appl. Biochem. 2022, 69, 767–782. [Google Scholar] [CrossRef]
- Tsuji, J.; Li, T.; Grinshpun, A.; Coorens, T.; Russo, D.; Anderson, L.; Rees, R.; Nardone, A.; Patterson, C.; Lennon, N.J.; et al. Clinical efficacy and whole-exome sequencing of liquid biopsies in a phase IB/II study of bazedoxifene and palbociclib in advanced hormone receptor—positive breast cancer. Clin. Cancer Res. 2022, 28, 5066–5078. [Google Scholar] [CrossRef]
- Das, A.; Lavanya, K.J.; Kaur, K.; Jaitak, V. Effectiveness of selective estrogen receptor modulators in breast cancer therapy: An update. Curr. Med. Chem. 2022, in press. [CrossRef]
- Lu, Y.; Liu, W. Selective estrogen receptor degraders (SERDs): A promising strategy for estrogen receptor positive endocrine-resistant breast cancer. J. Med. Chem. 2020, 63, 15094–15114. [Google Scholar] [CrossRef]
- Robertson, J.F.; Lindemann, J.; Garnett, S.; Anderson, E.; Nicholson, R.I.; Kuter, I.; Gee, J.M. A Good drug made better: The fulvestrant dose-response story. Clin. Breast Cancer 2014, 14, 381–389. [Google Scholar] [CrossRef]
- Boer, K. Fulvestrant in advanced breast cancer: Evidence to date and place in therapy. Adv Med. Oncol. 2017, 9, 465–479. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Tsuboi, K.; Kaneko, Y.; Nagatomo, T.; Fujii, R.; Hanamura, T.; Gohno, T.; Yamaguchi, Y.; Niwa, T.; Hayashi, S.I. Different epigenetic mechanisms of ERalpha implicated in the fate of fulvestrant-resistant breast cancer. J. Steroid. Biochem. Mol. Biol. 2017, 167, 115–125. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Wardell, S.E.; Chang, C.Y.; Norris, J.D. Next-generation endocrine therapies for breast cancer. J. Clin Oncol. 2021, 39, 1383. [Google Scholar] [CrossRef] [PubMed]
- Downton, T.; Zhou, F.; Segara, D.; Jeselsohn, R.; Lim, E. Oral Selective Estrogen Receptor Degraders (SERDs) in Breast Cancer: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2022, 16, 2933–2948. [Google Scholar] [CrossRef]
- Garcia-Fructuoso, I.; Gomez-Bravo, R.; Schettini, F. Integrating new oral selective oestrogen receptor degraders in the breast cancer treatment. Curr. Opin. Oncol. 2022, 34, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.R.; Wander, S.A.; Hamilton, E.; Razavi, P.; Bardia, A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: Current and emerging role. Ther. Adv. Med. Oncol. 2022, in press. [CrossRef]
- Hernando, C.; Ortega-Morillo, B.; Tapia, M.; Moragón, S.; Martínez, M.T.; Eroles, P.; Garrido-Cano, I.; Adam-Artigues, A.; Lluch, A.; Bermejo, B.; et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: Present and future from a clinical perspective. Int. J. Mol. Sci. 2021, 22, 7812. [Google Scholar] [CrossRef]
- Liang, J.; Zbieg, J.R.; Blake, R.A.; Chang, J.H.; Daly, S.; DiPasquale, A.G.; Friedman, L.S.; Gelzleichter, T.; Gill, M.; Giltnane, J.M.; et al. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J. Med. Chem. 2021, 64, 11841–11856. [Google Scholar] [CrossRef]
- Jimenez, M.M.; Lim, E.; Mac Gregor, M.C.; Bardia, A.; Wu, J.; Zhang, Q.; Nowecki, Z.; Cruz, F.; Safin, R.; Kim, S.B.; et al. 211MO Giredestrant (GDC-9545) vs. physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2–locally advanced/metastatic breast cancer (LA/mBC): Primary analysis of the phase II, randomised, open-label acelERA BC study. Ann. Oncol. 2022, 33, S633–S634. [Google Scholar] [CrossRef]
- Bardia, A.; Schmid, P.; Harbeck, N.; Rimawi, M.F.; Hurvitz, S.A.; Loi, S.; Saji, S.; Jung, K.H.; Werutsky, G.; Stroyakovskii, D.; et al. Abstract OT2-11-09: Lidera breast cancer: A phase III adjuvant study of giredestrant (GDC-9545) vs. physician’s choice of endocrine therapy (ET) in patients (pts) with estrogen receptor-positive, HER2-negative early breast cancer (ER+/HER2-EBC). Cancer Res. 2022, 82, OT2-11-09. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Quiroga, V.; Park, Y.H.; Bardia, A.; López-Valverde, V.; Steinseifer, J.; Fernando, T.M.; Spera, G.; Xue, C.; Fasching, P.A.; et al. Abstract PD13-06: Neoadjuvant giredestrant (GDC-9545)+ palbociclib versus anastrozole+ palbociclib in postmenopausal women with estrogen receptor-positive, HER2-negative, untreated early breast cancer: Primary analysis of the randomized, open-label, phase II coopERA breast cancer study. Cancer Res. 2022, 82, PD13-06. [Google Scholar]
- Bardia, A.; Fernando, T.M.; Fasching, P.A.; Garcia, V.Q.; Park, Y.H.; Giltnane, J.M.; Xue, C.; Lopez Valverde, V.; Steinzeifer-Szabo, J.; Pérez-Moreno, P.D.; et al. 144P–Neoadjuvant giredestrant (GDC-9545) + palbociclib (P) vs. anastrozole (A)+ P in postmenopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2–eBC): Biomarker subgroup analysis of the randomised, phase II coopERA BC study. Ann. Oncol. 2022, 33, S605–S606. [Google Scholar]
- Fasching, P.A.; Bardia, A.; Quiroga, V.; Park, Y.H.; Blancas, I.; Alonso, J.L.; Vasilyev, A.; Adamchuk, H.; Salgado, M.R.T.; Yardley, D.A.; et al. Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2− eBC): Final analysis of the randomized, open-label, international phase 2 coopERA BC study. J. Clin. Oncol. 2022, 40, 589. [Google Scholar]
- Neilan, T.G.; Villanueva-Vázquez, R.; Bellet, M.; López-Miranda, E.; García-Estévez, L.; Kabos, P.; Bond, J.; Gates, M.R.; Chang, C.W.; Boni, V.; et al. Abstract P5-18-07: Heart rate changes, cardiac safety, and exercise tolerance from a phase Ia/b study of giredestrant (GDC-9545) ± palbociclib in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Cancer Res. 2022, 82, P5–P18. [Google Scholar] [CrossRef]
- Liang, J.; Ingalla, E.R.; Yao, X.; Wang, B.E.; Tai, L.; Giltnane, J.; Liang, Y.; Daemen, A.; Moore, H.M.; Aimi, J.; et al. Giredestrant reverses progesterone hypersensitivity driven by estrogen receptor mutations in breast cancer. Sci. Transl. Med. 2022, 14, eabo5959. [Google Scholar] [CrossRef]
- Andreano, K.J.; Wardell, S.E.; Baker, J.G.; Desautels, T.K.; Baldi, R.; Chao, C.A. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res. Treat. 2020, 180, 635–646. [Google Scholar] [CrossRef]
- Dees, E.C.; Aftimos, P.G.; van Oordt, H.; de Vries, E.G.E.; Neven, P.; Pegram, M.D.; Iqbal, R.; Boers, J.; Xiao, J.; Sipes, C.; et al. Dose-escalation study of G1T48, an oral selective estrogen receptor degrader (SERD), in postmenopausal women with ER+/HER2-locally advanced or metastatic breast cancer (ABC). Ann. Oncol. 2019, 30, v121–v122. [Google Scholar] [CrossRef]
- Aftimos, P.; Neven, P.; Pegram, M.; van Menke-van der Houven Oordt, C.W.; Jager, A.; Bulat, I.; Chap, L.; Maglakelidze, M.; Dees, E.C.; Schorder, C.; et al. Abstract PS12-04: Rintodestrant (G1T48), an oral selective estrogen receptor degrader in ER+/HER2-locally advanced or metastatic breast cancer: Updated phase 1 results and dose selection. Cancer Res. 2021, 81, PS12-04. [Google Scholar] [CrossRef]
- Beelen, A.P.; Li, C.; Jarugula, P.; Gopalakrishnan, M.; Sorrentino, J.A.; Wolfgang, C.D.; Jain, S.; Tao, W. Abstract PS17-08: Population pharmacokinetic and exposure-response modeling of the oral selective estrogen receptor degrader, rintodestrant (G1T48), in patients with ER+/HER2-advanced breast cancer. Cancer Res. 2021, 81, PS17-08. [Google Scholar] [CrossRef]
- Bardia, A.; Chandarlapaty, S.; Linden, H.M.; Ulaner, G.A.; Gosselin, A.; Cartot-Cotton, S.; Cohen, P.; Doroumian, S.; Paux, G.; Celanovic, M.; et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat. Commun. 2022, 13, 4116. [Google Scholar] [CrossRef]
- Bardia, A.; Cortes, J.; Hurvitz, S.A.; Delaloge, S.; Iwata, H.; Shao, Z.M.; Kanavagel, D.; Cohen, P.; Liu, Q.; Cartot-Cotton, S.; et al. AMEERA-5: A randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2–advanced breast cancer. Ther. Adv. Med. Oncol. 2022, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.S.; Moss, T.A.; Balazs, A.; Barlaam, B.; Breed, J.; Carbajo, R.J.; Chiarparin, E.; Davey, P.R.J.; Delpuech, O.; Fawell, S.; et al. Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J. Med. Chem. 2020, 63, 14530–14559. [Google Scholar] [CrossRef]
- André, F.; Im, S.A.; Neven, P.; Baird, R.D.; Ettl, J.; Goetz, M.P.; Hamilton, E.; Iwata, H.; Jiang, Z.; Joy, A.A.; et al. Abstract OT2-11-06: SERENA-4: A Phase III comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive/HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. Cancer Res. 2022, 82, OT2-11. [Google Scholar]
- Bidard, F.C.; Kalinsky, K.; Cristofanilli, M.; Bianchini, G.; Chia, S.K.; Janni, W.; Ma, C.X.; Mayer, E.L.; Park, Y.H.; Fox, S.; et al. Abstract OT2-11-05: SERENA-6: A Phase III study to assess the efficacy and safety of AZD9833 (camizestrant) compared with aromatase inhibitors when given in combination with palbociclib or abemaciclib in patients with HR+/HER2-metastatic breast cancer with detectable ESR1 m who have not experienced disease progression on first-line therapy. Cancer Res. 2022, 82, OT2-11. [Google Scholar]
- Bardia, A.; Aftimos, P.; Bihani, T.; Anderson-Villaluz, A.T.; Jung, J.; Conlan, M.G.; Kaklamani, V.G. EMERALD: Phase III trial of elacestrant (RAD1901) vs. endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019, 15, 3209–3218. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Jeselsohn, R.; Lim, E.; Hamilton, E.P.; Yonemori, K.; Beck, J.T.; Kaufman, P.A.; Sammons, S.; Bhave, M.A.; Saura, C.; et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Monotherapy results from EMBER. J. Clin. Oncol. 2022, 40, 1021. [Google Scholar] [CrossRef]
- Weir, H.M.; Bradbury, R.H.; Lawson, M.; Rabow, A.A.; Buttar, D.; Callis, R.J.; Curwen, J.O.; de Almeida, C.; Ballard, P.; Hulse, M.; et al. AZD9496: An oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models pharmacology of SERD inhibitor in breast cancer models. Cancer Res. 2016, 76, 3307–3318. [Google Scholar] [CrossRef]
- Paoletti, C.; Schiavon, G.; Dolce, E.M.; Darga, E.P.; Carr, T.H.; Geradts, J.; Hoch, M.; Klinowska, T.; Lindemann, J.; Marshall, G.; et al. Circulating biomarkers and resistance to endocrine therapy in metastatic breast cancers: Correlative results from AZD9496 oral SERD phase I trial liquid biopsies, metastatic breast cancer, oral SERD AZD9496. Clin. Cancer Res. 2018, 24, 5860–5872. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Patel, M.R.; Armstrong, A.C.; Baird, R.D.; Jhaveri, K.; Hoch, M.; Klinowska, T.; Lindemann, J.P.O.; Morgan, S.R.; Schiavon, G.; et al. A first-in-human study of the new oral selective estrogen receptor degrader AZD9496 for ER+/HER2− advanced breast cancer. Clin. Cancer Res. 2018, 24, 3510–3518. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, J.; Metcalfe, C.; de Bruyn, T.; Gelzleichter, T.; Malhi, V.; Perez-Moreno, P.D.; Wang, X. Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Exp. Opin. Investig. Drugs 2022, 31, 515–529. [Google Scholar] [CrossRef]
- Ratre, P.; Mishra, K.; Dubey, A.; Vyas, A.; Jain, A.; Thareja, S. Aromatase inhibitors for the treatment of breast cancer: A journey from the scratch. Anticancer Agents Med. Chem. 2020, 20, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S. Clinical utilities of aromatase inhibitors in breast cancer. Int. J. Women’s Health 2015, 7, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Raheja, K.; Luxami, V.; Paul, K. A review on diverse heterocyclic compounds as the privileged scaffolds in non-steroidal aromatase inhibitors. Bioorg. Chem. 2021, 113, 105017. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Catalano, A.; Agostino, R.M.; Bellone, F.; Morabito, N.; Lasco, C.G.; Vicario, C.M.; Schwarz, P.; Feldt-Rasmussen, U. Quality of life and psychological functioning in postmenopausal women undergoing aromatase inhibitor treatment for early breast cancer. PLoS ONE 2020, 15, e0230681. [Google Scholar] [CrossRef]
- Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. [Google Scholar] [CrossRef]
- Caprioli, M.; Carrara, G.; Sakellariou, G.; Silvagni, E.; Scirè, C.A. Influence of aromatase inhibitors therapy on the occurrence of rheumatoid arthritis in women with breast cancer: Results from a large population-based study of the Italian Society for Rheumatology. RMD Open 2017, 3, e000523. [Google Scholar] [CrossRef]
- Mascella, F.; Gianni, L.; Affatato, A.; Fantini, M. Aromatase inhibitors and antisynthetase syndrome. Int. J. Immunopathol. Pharmacol. 2016, 29, 494–497. [Google Scholar] [CrossRef]
- Tenti, S.; Giordano, N.; Cutolo, M.; Giannini, F.; Fioravanti, A. Primary antiphospholipid syndrome during aromatase inhibitors therapy: A case report and review of the literature. Medicine 2019, 98, e15052. [Google Scholar] [CrossRef]
- Boszkiewicz, K.; Piwowar, A.; Petryszyn, P. Aromatase inhibitors and risk of metabolic and cardiovascular adverse effects in breast cancer patients—A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 3133. [Google Scholar] [CrossRef]
- Ghaly, H.S.A.; Varamini, P. New drug delivery strategies targeting GnRH receptor in breast and other cancers. Endocr. Relat. Cancer 2021, 28, R251–R269. [Google Scholar] [CrossRef]
- Robertson, J.; Blamey, R. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur. J. Cancer 2003, 39, 861–869. [Google Scholar] [CrossRef]
- Ulrich, N.D.; Raja, N.S.; Moravek, M.B. A review of fertility preservation in patients with breast cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 82, 60–68. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Maya-Núñez, G.; Pérez-Solis, M.A.; López-Muñoz, E.; Guillén, N.; Olivo-Marin, J.C.; Aguilar-Rojas, A. Treatment of breast cancer with gonadotropin-releasing hormone analogs. Front. Oncol. 2019, 9, 943. [Google Scholar] [CrossRef]
- Di Lauro, L.; Pizzuti, L.; Barba, M.; Sergi, D.; Sperduti, I.; Mottolese, M.; Amoreo, C.A.; Belli, F.; Vici, P.; Speirs, V.; et al. Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: Results from a pooled analysis. J. Hematol. Oncol. 2015, 8, 53. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Forat-Yazdi, M.; Vafaeenasab, M.R.; Nakhaie-Moghadam, M.; Miratashi-Yazdi, A.; Teimoori, S.; Dehghani-Tafti, A. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur. J. Gynaecol. Oncol. 2014, 35, 59–61. [Google Scholar] [PubMed]
- Jonat, W.; Kaufmann, M.; Sauerbrei, W.; Blamey, R.; Cuzick, J.; Namer, M.; de Haes, F.J.C.; de Matteis, A.; Stewart, A.; Eiermann, W.; et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The zoladex early breast cancer research association study. J. Clin. Oncol. 2002, 20, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pei, L.; Hu, T.; Jia, M.; Wang, S. Protective effect of goserelin on ovarian reserve during (neo) adjuvant chemotherapy in young breast cancer patients: A prospective cohort study in China. Hum. Reproduct. 2021, 36, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, M.; Gambo, M.A.; Salama, M.Y.; Ismail, N.H.; Tavalla, P.; Uppal, P.; Mohammed, S.A.; Rajashekar, S.; Ravindran, S.G.; Hamid, P.; et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer patients: A systematic review. Cureus 2022, 14, e26209. [Google Scholar] [CrossRef]
- Boland, J.; Choi, W.; Lee, M.; Lin, J. Cardiovascular toxicity of androgen deprivation therapy. Curr. Cardiol. Rep. 2021, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.S.; Wang, C.C.; Hsu, L.F.; Chuang, P.H.; Cheng, C.F.; Li, N.H.; Chen, C.C.; Chen, C.L.; Lai, Y.J.; Yen, Y.F.; et al. Gonadotropin—Releasing hormone agonist treatment and ischemic heart disease among female patients with breast cancer: A cohort study. Cancer Med. 2022, in press. [CrossRef] [PubMed]
- Alemany, C.; Patel, M.; Mitri, Z.; Sparano, J.; Borges, V.; Makower, D.; Klein, P.; Lawrence, J.; Le, T.; Zujewski, J.A.; et al. Abstract P037: A phase 1/2 dose escalation and expansion study of OP-1250 in adults with advanced and/or metastatic hormone receptor-positive (HR+), HER2-negative (HER2−) breast cancer. Mol. Cancer Ther. 2021, 20, P037. [Google Scholar] [CrossRef]
- Parisian, A.D.; Miller, C.; Ortega, F.; Hodges-Gallagher, L.; Barratt, S.; Azofeifa, J.; Kushner, P.J.; Kulp, D.; Harmon, C.L. Precision run-on sequencing (PRO-seq) analysis of a treatment time course in ER+ breast cancer cell lines reveals the transcriptional changes underlying response to complete estrogen receptor antagonist (CERAN) OP-1250. Cancer Res. 2022, 82, 5375. [Google Scholar] [CrossRef]
- Patel, M.; Alemany, C.; Mitri, Z.; Makower, D.; Borges, V.; Sparano, J.; Le, T.; Klein, P.; Lawrence, J.; Khushner, P.J.; et al. Preliminary data from a phase 1/2, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in patients with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer. Cancer Res. 2022, 82, P1-17-12. [Google Scholar]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04505826 (accessed on 22 December 2022).
- Harlow, K.W.; Smith, D.N.; Katzenellenbogen, J.A.; Greene, G.L.; Katzenellenbogen, B.S. Identification of cysteine 530 as the covalent attachment site of an affinity-labeling estrogen (ketononestrol aziridine) and antiestrogen (tamoxifen aziridine) in the human estrogen receptor. J. Biol. Chem. 1989, 264, 17476–17485. [Google Scholar] [CrossRef]
- Furman, C.; Hao, M.H.; Prajapati, S.; Reynolds, D.; Rimkunas, V.; Zheng, G.Z.; Zhu, P.; Korpal, M. Estrogen receptor covalent antagonists: The best is yet to come. Cancer Res. 2019, 79, 1740–1745. [Google Scholar] [CrossRef]
- Scott, J.S.; Barlaam, B. Selective estrogen receptor degraders (SERDs) and covalent antagonists (SERCAs): A patent review (2015-present). Exp. Opin. Ther. Pat. 2022, 32, 131–151. [Google Scholar] [CrossRef]
- Puyang, X.; Furman, C.; Zheng, G.Z.; Wu, Z.J.; Banka, D.; Aithal, K.; Agoulnik, S.; Bolduc, D.M.; Buonamici, S.; Caleb, B.; et al. Discovery of selective estrogen receptor covalent antagonists for the treatment of ERalpha (WT) and ERalpha (MUT) breast cancer. Cancer Discov. 2018, 8, 1176–1193. [Google Scholar] [CrossRef]
- Furman, C.; Puyang, X.; Zhang, Z.; Wu, Z.J.; Banka, D.; Aithal, K.B.; Albacker, L.A.; Hao, M.H.; Irwin, S.; Kim, A.; et al. Covalent ERα antagonist H3B-6545 demonstrates encouraging preclinical activity in therapy-resistant breast cancer. Mol. Cancer Ther. 2022, 21, 890–902. [Google Scholar] [CrossRef]
- Colomer-Saucedo, J.B.; Loulousis, M.M.; Copello, V.A.; Krager, S.L.; Tischkau, S.L.; Copello, J.A. Pharmacological targeting of SERCA in breast cancer. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- So, C.L.; Saunus, J.M.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2019, 94, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, P.; Yiallouris, A.; Michail, A.; Christodoulou, M.I.; Politis, P.K.; Patrikios, I. Altered SERCA expression in breast cancer. Medicina 2021, 57, 1074. [Google Scholar] [CrossRef]
- Xiong, R.; Patel, H.K.; Gutgesell, L.M.; Zhao, J.; Delgado-Rivera, L.; Pham, T.N.; Zhao, H.; Carlson, K.; Martin, T.; Katzenellenbogen, J.A.; et al. Selective human estrogen receptor partial agonists (ShERPAs) for tamoxifen-resistant breast cancer. J. Med. Chem. 2016, 59, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, M.; Donato, M.; D’Amato, A.L.; Rosanova, M.; Russo, A.O.M.; Scafetta, R.; de Angelis, C.; Trivedi, M.V.; André, F.; Arpino, G.; et al. New steps on an old path: Novel estrogen receptor inhibitors in breast cancer. Crit. Rev. Oncol. Hematol. 2022, 180, 103861. [Google Scholar] [CrossRef] [PubMed]
- Abderrahman, B.; Maximov, P.Y.; Curpan, R.F.; Hanspal, J.S.; Fan, P.; Xiong, R.; Tonetti, D.A.; Thatcher, G.R.; Jordan, V.C. Pharmacology and molecular mechanisms of clinically relevant estrogen estetrol and estrogen mimic BMI-135 for the treatment of endocrine-resistant breast cancer. Mol. Pharmacol. 2020, 98, 364–381. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Liu, L.C.; Fischer, J.H.; Wiley, E.L.; Sachdev, J.C.; Bleeker, J.; Hurley, R.W.; Tonetti, D.A.; Thatcher, G.R.J.; Venuti, R.P.; et al. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res. Treat. 2020, 183, 617–627. [Google Scholar] [CrossRef]
- Moy, B.; Rumble, R.B.; Come, S.E.; Davidson, N.E.; di Leo, A.; Gralow, J.R.; Hortobagyi, G.N.; Yee, D.; Smith, I.E.; Chavez-MacGregor, M.; et al. Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline update. J. Clin. Oncol. 2021, 39, 3938–3958. [Google Scholar] [CrossRef]
- Jacquet, E.; Lardy-Cléaud, A.; Pistilli, B.; Franck, S.; Cottu, P.; Delaloge, S.; Debled, M.; Vanlemmens, L.; Leheurteur, M.; Guizard, A.; et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor–positive HER2-negative metastatic breast cancer patients. Eur. J. Cancer 2018, 95, 93–101. [Google Scholar] [CrossRef]
- Trapani, D.; Gandini, S.; Corti, C.; Crimini, E.; Bellerba, F.; Minchella, I.; Criscitiello, C.; Tarantino, P.; Curigliano, G. Benefit of adjuvant chemotherapy in patients with lobular breast cancer: A systematic review of the literature and metanalysis. Cancer Treat. Rev. 2021, 97, 102205. [Google Scholar] [CrossRef]
- Rossi, P.G.; Lebeau, A.; Canelo-Aybar, C.; Saz-Parkinson, Z.; Quinn, C.; Langendam, M.; McGarrigle, H.; Warman, S.; Rigau, D.; Alonso-Coello, P.; et al. Recommendations from the European Commission Initiative on Breast Cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, HER-2 negative. Br. J. Cancer 2021, 124, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Tsimberidou, A.M. Targeted therapy in cancer. Cancer Chemother. Pharmacol. 2015, 76, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Simmons, C.; Rayson, D.; Joy, A.A.; Henning, J.W.; Lemieux, J.; McArthur, H.; Card, P.B.; Dent, R.; Bresden-Masley, C. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther. Adv. Med. Oncol. 2022, in press. [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Buzun, K.; Bielawska, A.; Bielawski, K.; Gornowicz, A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2020, 35, 1781–1799. [Google Scholar] [CrossRef]
- Thomas, A.; Pommier, Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef]
- Tesauro, C.; Simonsen, A.K.; Andersen, M.B.; Petersen, K.W.; Kristoffersen, E.L.; Algreen, L.; Hansen, N.Y.; Andersen, A.B.; Jakobsen, A.K.; Stougaard, M.; et al. Topoisomerase I activity and sensitivity to camptothecin in breast cancer-derived cells: A comparative study. BMC Cancer 2019, 19, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kümler, I.; Brünner, N.; Stenvang, J.; Balslev, E.; Nielsen, D.L. A systematic review on topoisomerase 1 inhibition in the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 2013, 138, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Tolaney, S.M.; Seidman, A.D.; Anders, C.K.; Ibrahim, N.; Rugo, H.S.; Twelves, C.; Diéras, V.; Müller, V.; Du, Y.; et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases. JAMA Oncol. 2022, 8, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S. Etirinotecan Pegol treatment for patients with metastatic breast cancer and brain metastases. JAMA Oncol. 2022, 8, 1700–1701. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, S.M.; Roshdy, J.; Ibrahim, A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J. Egypt. Natl. Cancer Inst. 2020, 32, 20. [Google Scholar] [CrossRef]
- Hu, G.; Wang, D. Analysis of cardiac adverse reactions caused by different doses of adriamycin chemotherapy in patients with breast cancer. J. Healthc. Eng. 2022, 2022, 1642244. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.-M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard anthracycline-and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Elshimali, Y.I.; Wu, Y.; Khaddour, H.; Wu, Y.; Gradinaru, D.; Sukhija, H.; Chung, S.S.; Vadgama, J.V. Optimization of cancer treatment through overcoming drug resistance. J. Cancer Res. Oncobiol. 2018, 1, 107. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Li, Y.; Ruan, Y.; Quan, C. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol. Med. Rep. 2018, 17, 6211–6226. [Google Scholar] [CrossRef]
- Yang, F.; Hu, Y.; Shao, L.; Zhuang, J.; Huo, Q.; He, S.; Chen, S.; Wang, J.; Xie, N. SIRT7 interacts with TEK (TIE2) to promote adriamycin induced metastasis in breast cancer. Cell Oncol. 2021, 44, 1405–1424. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, J.; Gu, R.; Shi, Y.; Hu, H.; Liu, J.; Huang, J.; Zhong, C.; Zhou, W.; Yang, Y.; et al. Comprehensive analysis of transcriptomics and genetic alterations identifies potential mechanisms underlying anthracycline therapy resistance in breast cancer. Biomolecules 2022, 12, 1834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, P.; Zhang, J.; Li, Y.; Ma, J.; Zhang, Y.; Liu, H. CircRNA_0044556 diminishes the sensitivity of triple-negative breast cancer cells to adriamycin by sponging miR-145 and regulating NRAS. Mol. Med. Rep. 2022, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Ni, K.; Xue, H.; Li, J. RBM38 is negatively regulated by miR-320b and enhances Adriamycin resistance in breast cancer cells. Oncol. Lett. 2022, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Y.; Mao, M.; Gu, Y.; Xu, C.; Yang, J.; Hu, W.; Shen, J.; Hu, D.; Chen, C.; et al. PLAC8 promotes adriamycin resistance via blocking autophagy in breast cancer. J. Cell. Mol. Med. 2021, 25, 6948–6962. [Google Scholar] [CrossRef]
- Chen, W.-X.; Wang, D.-D.; Zhu, B.; Zhu, Y.-Z.; Zheng, L.; Feng, Z.-Q.; Qin, X.-H. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging 2021, 13, 10415–10430. [Google Scholar] [CrossRef] [PubMed]
- Masood, S. Neoadjuvant chemotherapy in breast cancers. Women’s Health 2016, 12, 480–491. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar]
- Pourkarim, F.; Rahimpour, E.; Alvani, S.; Jouyban, A. Solubilization methods for paclitaxel and docetaxel: An overview. Pharm. Sci. 2021, 28, 208–223. [Google Scholar] [CrossRef]
- Risinger, A.L.; Riffle, S.M.; Lopus, M.; Jordan, M.A.; Wilson, L.; Mooberry, S.L. The taccalonolides and paclitaxel cause distinct effects on microtubule dynamics and aster formation. Mol. Cancer 2014, 13, 41. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef]
- Klein, I.; Lehmann, H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Tang, W.; Yang, J.; Yuan, Y.; Zhao, Z.; Lian, Z.; Liang, G. Paclitaxel nanoparticle awakens immune system to fight against cancer. Nanoscale 2017, 9, 6529–6536. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Li, C.; Song, Y.; Liu, G.; Huang, Q.; Yu, L.; Zhu, D.; Lu, C.; Lu, A.; et al. Nab-paclitaxel promotes the cancer-immunity cycle as a potential immunomodulator. Am. J. Cancer Res. 2021, 11, 3445. [Google Scholar] [PubMed]
- Denis, J.N.; Greene, A.E.; Guenard, D.; Gueritte-Voegelein, F.; Mangatal, L.; Potier, P. Highly efficient, practical approach to natural taxol. J. Am. Chem. Soc. 1988, 110, 5917–5919. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; Vermunt, M.A.; van Werkhoven, E.; Wilthagen, E.A.; Huitema, A.D.; Beijnen, J.H. The influence of docetaxel schedule on treatment tolerability and efficacy in patients with metastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2022, 22, 104. [Google Scholar] [CrossRef]
- Vrignaud, P.; Semiond, D.; Benning, V.; Beys, E.; Bouchard, H.; Gupta, S. Preclinical profile of cabazitaxel. Drug Des. Dev. Ther. 2014, 8, 1851–1867. [Google Scholar] [CrossRef]
- Koutras, A.; Zagouri, F.; Koliou, G.; Psoma, E.; Chryssogonidis, I.; Lazaridis, G.; Tryfonopoulos, D.; Kotsakis, A.; Res, E.; Kentepozidis, N.; et al. Phase 2 study of cabazitaxel as second-line treatment in patients with HER2-negative metastatic breast cancer previously treated with taxanes-a Hellenic Cooperative Oncology Group (HeCOG) trial. Br. J. Cancer 2020, 123, 355–361. [Google Scholar] [CrossRef]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009, 26, 273–287. [Google Scholar] [CrossRef]
- Suresh, R.; Diaz, R.J. The remodelling of actin composition as a hallmark of cancer. Translat. Oncol. 2021, 14, 101051. [Google Scholar] [CrossRef]
- Arumugam, A.; Subramani, R.; Lakshmanaswamy, R. Involvement of actin cytoskeletal modifications in the inhibition of triple-negative breast cancer growth and metastasis by nimbolide. Mol. Ther. Oncolytics 2021, 20, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Parks, D.R.; Rouse, R.V.; Herzenberg, L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1981, 78, 5147–5150. [Google Scholar] [CrossRef]
- McDougall, A.R.; Tolcos, M.; Hooper, S.B.; Cole, T.J.; Wallace, M.J. Trop2: From development to disease. Dev. Dyn. 2015, 244, 99–109. [Google Scholar] [CrossRef]
- Cortesi, M.; Zanoni, M.; Maltoni, R.; Ravaioli, S.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. TROP2 (trophoblast cell-surface antigen 2): A drug target for breast cancer. Exp. Opin. Ther. Targets 2022, 26, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Trerotola, M.; Guerra, E. The Hu2G10 tumor-selective anti-Trop-2 monoclonal antibody targets the cleaved-activated Trop-2 and shows therapeutic efficacy against multiple human cancers. Cancer Res. 2022, 82, 340. [Google Scholar] [CrossRef]
- Sayama, Y.; Kaneko, M.K.; Kato, Y. Development and characterization of TrMab-6, a novel anti-TROP2 monoclonal antibody for antigen detection in breast cancer. Mol. Med. Rep. 2021, 23, 92. [Google Scholar] [CrossRef]
- Sayama, Y.; Kaneko, M.K.; Takei, J.; Hosono, H.; Sano, M.; Asano, T.; Kato, Y. Establishment of a novel anti-TROP2 monoclonal antibody TrMab-29 for immunohistochemical analysis. Biochem. Biophys. Rep. 2021, 25, 100902. [Google Scholar] [CrossRef]
- Kauffman, H.M.; Cherikh, W.S.; Cheng, Y.; Hanto, D.W.; Kahan, B.D. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 2005, 80, 883–889. [Google Scholar] [CrossRef]
- Hare, S.H.; Harvey, A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017, 7, 383. [Google Scholar]
- Chaturvedi, D.; Gao, X.; Cohen, M.S.; Taunton, J.; Patel, T.B. Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene 2009, 28, 1187–1196. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Biomarkers of everolimus efficacy in breast cancer therapy. J. Oncol. Pharm. Pract. 2022, 28, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Honda, A.; Itoi, N.; Lee, T. Everolimus for treating hormone receptor-positive metastatic breast cancer previously treated with cyclin-dependent kinase 4/6 inhibitors. Anticancer Res. 2022, 42, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Bahleda, R.; Tolaney, S.M.; Kwak, E.L.; Cleary, J.M.; Pandya, S.S.; Hollebecque, A.; Abbas, R.; Ananthakrishnan, R.; Berkenblit, A.; et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 2014, 32, 68–75. [Google Scholar] [CrossRef]
- Diamond, J.; David, P.; Salkeni, M.; Silverman, P.; Haddad, T.; Forget, F.; Awada, A.; Canon, J.L.; Danso, M.; Lortholary, A.; et al. Phase 2 safety and efficacy results of TAK-228 in combination with exemestane or fulvestrant in postmenopausal women with ER-positive/HER2-negative metastatic breast cancer previously treated with everolimus. Cancer Res. 2019, 79, PD1-09. [Google Scholar] [CrossRef]
- Starks, D.; Rojas-Espaillat, L.; Meissner, T.; Williams, C. Patient reported outcomes and final results of phase 1 evaluation of the safety and clinical activity of sapanisertib in combination with serabelisib and paclitaxel in patients with advanced ovarian, endometrial, or breast cancer (044). Gynecol. Oncol. 2022, 166, S30–S31. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef]
- Akinleye, A.; Avvaru, P.; Furqan, M.; Song, Y.; Liu, D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013, 6, 88. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H.A. Phosphatidylinositol 3-kinase (PI3K) inhibitors: A recent update on inhibitor design and clinical trials (2016–2020). Expert Opin. Ther. Pat. 2021, 31, 877–892. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K inhibitors in cancer: Clinical implications and adverse effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- Narayan, P.; Prowell, T.M.; Gao, J.J.; Fernandes, L.L.; Li, E.; Jiang, X.; Qiu, J.; Fan, J.; Song, P.; Yu, J.; et al. FDA approval summary: Alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin. Cancer Res. 2021, 27, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.J.; Braun, M.G.; Heald, R.A.; MacLeod, C.; Chan, C.; Clausen, S.; Hedgar, K.A.; Eigenbrot, C.; Elliott, R.; Endres, N.; et al. Discovery of GDC-0077 (inavolisib), a highly selective inhibitor and degrader of mutant PI3Kα. J. Med. Chem. 2022, in press. [CrossRef] [PubMed]
- Bedard, P.L.; Accordino, M.K.; Cervantes, A.; Gambardella, V.; Hamilton, E.P.; Italiano, A.; Juric, D.; Kalinsky, K.; Krop, I.E.; Oliveira, M.; et al. Long-term safety of inavolisib (GDC-0077) in an ongoing phase 1/1b study evaluating monotherapy and in combination (combo) with palbociclib and/or endocrine therapy in patients (pts) with PIK3CA-mutated, hormone receptor-positive/HER2-negative (HR+/HER2−) metastatic breast cancer (BC). J. Clin. Oncol. 2022, 40, 1052. [Google Scholar]
- Moore, H.M.; Savage, H.M.; O’Brien, C.; Zhou, W.; Sokol, E.S.; Goldberg, M.E.; Metcalfe, C.; Friedman, L.S.; Leckner, M.R.; Wilson, T.R.; et al. Predictive and pharmacodynamic biomarkers of response to the phosphatidylinositol 3-kinase inhibitor Taselisib in breast cancer preclinical models. Mol. Cancer Ther. 2019, 19, 292–303. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Ang, J.E.; Baird, R.; Kristeleit, R.; Shah, K.; Moreno, V.; Clarke, P.A.; Raynaud, F.I.; Levy, G.; Ware, J.A.; et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2015, 21, 77–86. [Google Scholar] [CrossRef]
- Schoffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar] [CrossRef]
- Sirohi, B.; Rastogi, S.; Dawood, S. Buparlisib in Breast Cancer. Future Oncol. 2015, 11, 1463–1470. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, H.; Zhou, X.; Wang, Y. The efficacy and safety of neoadjuvant buparlisib for breast cancer: A meta-analysis of randomized controlled studies. Medicine 2019, 98, e17614. [Google Scholar] [CrossRef]
- Lu, Y.S.; Lee, K.S.; Chao, T.Y.; Tseng, L.M.; Chitapanarux, I.; Chen, S.C.; Liu, C.T.; Sohn, J.; Kim, J.H.; Chang, Y.C.; et al. A Phase Ib study of alpelisib or buparlisib combined with tamoxifen plus goserelin in premenopausal women with HR-positive HER2-negative advanced breast cancer. Clin. Cancer Res. 2021, 27, 408–417. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Im, Y.H.; Calvo, E.; Lu, Y.S.; Hamilton, E.; Forero-Torres, A.; Bachelot, T.; Maur, M.; Fasolo, A.; Tiedt, R.; et al. Phase Ib study of ribociclib plus fulvestrant and ribociclib plus fulvestrant plus PI3K inhibitor (alpelisib or buparlisib) for HR(+) advanced breast cancer. Clin. Cancer Res. 2021, 27, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a therapeutic target for cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT inhibitors: New weapons in the fight against breast cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Hinz, N.; Jücker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell. Commun. Signal 2019, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prever, L.; Hirsch, E.; Gulluni, F. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers 2021, 13, 3517. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mtor pathway and its role in cancer therapeutics: Are we making headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Jelovac, D.; Wolff, A.C. The adjuvant treatment of HER2-positive breast cancer. Curr. Treat. Options Oncol. 2012, 13, 230–239. [Google Scholar] [CrossRef]
- Schlam, I.; Swain, S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer 2021, 7, 56. [Google Scholar] [CrossRef]
- Ryan, Q.; Ibrahim, A.; Cohen, M.H.; Johnson, J.; Ko, C.W.; Sridhara, R.; Justice, R.; Pazdur, R. FDA drug approval summary: Lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist 2008, 13, 1114–1119. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. Recent Results Cancer Res. 2018, 211, 19–44. [Google Scholar] [PubMed]
- Guarneri, V.; Dieci, M.V.; Griguolo, G.; Miglietta, F.; Girardi, F.; Bisagni, G.; Generali, D.G.; Cagossi, K.; Sarti, S.; Frassoldati, A.; et al. Trastuzumab-lapatinib as neoadjuvant therapy for HER2-positive early breast cancer: Survival analyses of the CHER-Lob trial. Eur. J. Cancer 2021, 153, 133–141. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Orally effective FDA-approved protein kinase targeted covalent inhibitors (TCIs). Pharmacol. Res. 2021, 165, 105422. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Chen, S.; Zhu, W.; Fan, Y.; Wang, J.; Luo, Y.; Xing, P.; Lan, B.; Li, M.; et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J. Clin. Oncol. 2017, 35, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, S.; Chen, J.; Xu, X.; Hu, X.; Kong, D.; Liang, G.; Yuan, X.; Li, Y.; Wang, X.; et al. The synergistic effects of pyrotinib combined with adriamycin on HER2-positive breast cancer. Front. Oncol. 2021, 11, 1603. [Google Scholar] [CrossRef]
- Lee, A. Tucatinib: First approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef]
- Ulrich, L.; Okines, A.F.C. Treating advanced unresectable or metastatic HER2-positive breast cancer: A spotlight on tucatinib. Breast Cancer 2021, 13, 361–381. [Google Scholar] [CrossRef]
- Capelan, M.; Pugliano, L.; de Azambuja, E.; Bozovic, I.; Saini, K.S.; Sotiriou, C.; Loi, S.; Piccart-Gebhart, M.J. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann. Oncol. 2013, 24, 273–282. [Google Scholar] [CrossRef]
- Dang, C.; Ewer, M.S.; Delaloge, S.; Ferrero, J.-M.; Colomer, R.; de la Cruz-Merino, L.; Werner, T.L.; Dadswell, K.; Verrill, M.; Eiger, D.; et al. BERENICE final analysis: Cardiac safety study of neoadjuvant pertuzumab, trastuzumab, and chemotherapy followed by adjuvant pertuzumab and trastuzumab in HER2-positive early breast cancer. Cancers 2022, 14, 2596. [Google Scholar] [CrossRef]
- Husna, S.M.N.; Wong, K.K. Margetuximab and trastuzumab deruxtecan: New generation of anti-HER2 immunotherapeutic agents for breast cancer. Mol. Immunol. 2022, 152, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA approval summary: Margetuximab plus chemotherapy for advanced or metastatic HER2-positive breast cancer. Clin. Cancer Res. 2022, 28, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-Chacon, R.; Hughes, P.; et al. FDA approval: Ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 2014, 20, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Osgood, C.L.; Singh, H.; Chiu, H.J.; Ricks, T.K.; Chiu Yuen Chow, E.; Qui, J.; Song, P.; Yu, J.; Namuswe, F.; et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast CancerFDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki. Clin. Cancer Res. 2021, 27, 4478–4485. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Hurvitz, S.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. GS3-01. Trastuzumab Deruxtecan (T-DXd; DS-8201a) vs. Trastuzumab Emtansine (T-DM1) in Patients (pts) with HER2+ Metastatic Breast Cancer (mBC): Subgroup Analyses from the Randomized Phase 3 Study DESTINY-Breast03. 2021. Available online: https://www.abstractsonline.com/pp8/#!/10462/presentation/649 (accessed on 16 December 2022).
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef]
- Chen, N.; Michaels, E.; Howard, F.; Nanda, R. The evolving therapeutic landscape of antibody-drug conjugates in breast cancer. Exp. Rev. Anticancer Ther. 2022, 12, 1235–1331. [Google Scholar] [CrossRef]
- Sharma, P.; Klemp, J.R.; Kimler, B.F.; Mahnken, J.D.; Geier, L.J.; Khan, Q.J.; Elia, M.; Connor, C.S.; McGinness, M.K.; Mammen, J.M.; et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res. Treat. 2014, 145, 707–714. [Google Scholar] [CrossRef]
- Deeks, E.D. Olaparib: First global approval. Drugs 2015, 75, 231–240. [Google Scholar] [CrossRef]
- U.S Food and Drug Administration. FDA Approves Olaparib for Germline BRCA-Mutated Metastatic Breast Cancer. 2018. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm592357.htm (accessed on 16 January 2023).
- McCann, K.E. Advances in the use of PARP inhibitors for BRCA1/2-associated breast cancer: Talazoparib. Future Oncol. 2019, 15, 1707–1715. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Ponce Lorenzo, J.; et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Arun, B.K.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.P.; Puhalla, S.L.; Bach, B.A.; Kundu, M.G.; Khandelwal, N.; et al. Veliparib monotherapy following carboplatin/paclitaxel plus veliparib combination therapy in patients with germline BRCA-associated advanced breast cancer: Results of exploratory analyses from the phase III BROCADE3 trial. Ann. Oncol. 2022, 33, 299–309. [Google Scholar] [CrossRef]
- Stodtmann, S.; Eckert, D.; Joshi, R.; Nuthalapati, S.; Ratajczak, C.K.; Menon, R.; Mensing, S.; Xiong, H. Exposure-response model with time-varying predictors to estimate the effects of veliparib in combination with carboplatin/paclitaxel and as monotherapy: Veliparib phase 3 study in BRCA-mutated advanced breast cancer (BROCADE3) trial. J. Clin. Pharmacol. 2022, in press. [CrossRef] [PubMed]
- Turner, N.C.; Balmaña, J.; Poncet, C.; Goulioti, T.; Tryfonidis, K.; Honkoop, A.H.; Zoppoli, G.; Razis, E.; Johannsson, O.T.; Colleoni, M.; et al. Niraparib for Advanced Breast Cancer with Germline BRCA1 and BRCA2 Mutations: The EORTC 1307-BCG/BIG5-13/TESARO PR-30-50-10-C BRAVO Study. Clin. Cancer Res. 2021, 27, 5482–5491. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Cescon, D.W.; Loibl, S.; Janni, W.; Rugo, H.; Balmaña, J.; Crowley, C.; Chung, J.; Fucli, G.; Hofstatter, E.; et al. Abstract OT2-24-02: ZEST: Randomized phase III study evaluating efficacy and safety of niraparib in patients with HER2-negative BRCA-mutated or triple-negative breast cancer with detectable circulating tumor DNA after definitive therapy. Cancer Res. 2022, 82, OT2-24. [Google Scholar] [CrossRef]
- Markham, A. Pamiparib: First approval. Drugs 2021, 81, 1343–1348. [Google Scholar] [CrossRef]
- Xu, B.; Sun, T.; Shi, Y.; Cui, J.; Yin, Y.; Ouyang, Q.; Liu, Q.; Zhang, Q.; Chen, Y.; Wang, S.; et al. Pamiparib in patients with locally advanced or metastatic HER2-negative breast cancer with germline BRCA mutations: A phase II study. Breast Cancer Res. Treat. 2022, in press. [CrossRef]
- Poratti, M.; Marzaro, G. Third-generation CDK inhibitors: A review on the synthesis and binding modes of palbociclib, ribociclib and abemaciclib. Eur. J. Med. Chem. 2019, 172, 143–153. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.; Koolen, S.L.; Jager, A. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: Similarities and differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab govitecan: First approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; Huang, H.; Liang, B.; Ming, T.; Wen, J.; et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022, 239, 108296. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.M.; Tanaka, T.; Zanon, P.R.; Baisden, J.T.; Abegg, D.; Yang, X.; Akahori, Y.; Alshakarchi, Z.; Cameron, M.D.; Adibekian, A.; et al. DNA-encoded library screening to inform design of a Ribonuclease Targeting Chimera (RiboTAC). J. Am. Chem. Soc. 2022, 144, 21096–21102. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, S.; Dong, G.; Sheng, C. Nucleic-acid-based targeted degradation in drug discovery. J. Med. Chem. 2022, 65, 10217–10232. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Seghetti, F.; Caciolla, J.; Uliassi, E.; Testi, E.; Guardigni, M.; Roberti, M.; Milelli, A.; Bolognesi, M.L. Enriching proteolysis targeting chimeras with a second modality: When two are better than one. J. Med. Chem. 2022, 65, 9507–9530. [Google Scholar] [CrossRef]
- Kessler, D.; Gmachl, M.; Mantoulidis, A.; Martin, L.J.; Zoephel, A.; Mayer, M.; Gollner, A.; Covini, D.; Fischer, S.; Gerstberger, T.; et al. Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. USA 2019, 116, 15823–15829. [Google Scholar] [CrossRef]
- Rudolph, J.; Settleman, J.; Malek, S. Emerging trends in cancer drug discovery-from drugging the “undruggable” to overcoming resistance. Cancer Discov. 2021, 11, 815–821. [Google Scholar] [CrossRef]
- Grimster, N.P. Covalent PROTACs: The best of both worlds? RSC Med. Chem. 2021, 12, 1452–1458. [Google Scholar] [CrossRef]
- Gao, H.; Sun, X.; Rao, Y. PROTAC technology: Opportunities and challenges. ACS Med. Chem. Lett. 2020, 11, 237–240. [Google Scholar] [CrossRef]
- LaPlante, G.; Zhang, W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule inhibitors. Cancers 2021, 13, 3079. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. PROTACs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Kim, K.B.; Verma, R.; Ransick, A.; Stein, B.; Crews, C.M.; Deshaies, R. Development of Protacs to Target Cancer-promoting Proteins for Ubiquitination and Degradation. Mol. Cell. Proteom. 2003, 2, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Vahdat, L.; Han, H.S.; Ranciato, J.; Gedrich, R.; Keung, C.F.; Chirnomas, D.; Hurvitz, S. Abstract PD13-08: First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2-locally advanced or metastatic breast cancer. Cancer Res. 2022, 82, PD13-08. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Schott, A.F.; Nanda, R.; Lu, H.; Keung, C.F.; Gedrich, R.; Parameswaran, J.; Han, H.S.; Hurvitz, S.A. ARV-471, an estrogen receptor (ER) PROTAC degrader, combined with palbociclib in advanced ER+/human epidermal growth factor receptor 2–negative (HER2-) breast cancer: Phase 1b cohort (part C) of a phase 1/2 study. J. Clin. Oncol. 2022, 40, TPS1120. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Chen, C.; Nie, J.; Jiao, B. Can EGFR be a therapeutic target in breast cancer? Biochim. Biophys. Acta BBA Rev. Cancer 2022, 1877, 188789. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Capici, S.; Cordani, N.; Cogliati, V.; Pepe, F.F.; Riva, F.; Cerrito, M.G. Metronomic chemotherapy for metastatic breast cancer treatment: Clinical and preclinical data between lights and shadows. J. Clin. Med. 2022, 11, 4710. [Google Scholar] [CrossRef]
- Krajnak, S.; Battista, M.J.; Hasenburg, A.; Schmidt, M. Metronomic Chemotherapy for Metastatic Breast Cancer. Oncol. Res. Treat. 2022, 45, 12–17. [Google Scholar] [CrossRef]
- Liu, J.; He, M.; Wang, Z.; Li, Q.; Xu, B. Current research status of metronomic chemotherapy in combination treatment of breast cancer. Oncol. Res. Treat. 2022, 45, 681–692. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- You, C.P.; Leung, M.H.; Tsang, W.C.; Khoo, U.S.; Tsoi, H. Androgen receptor as an emerging feasible biomarker for breast cancer. Biomolecules 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Forooshani, M.K.; Scarpitta, R.; Fanelli, G.N.; Miccoli, M.; Naccarato, A.G.; Scatena, C. Is it time to consider the androgen receptor as a therapeutic target in breast cancer? Anti-Cancer Agents Med. Chem. 2021, 22, 775–786. [Google Scholar] [CrossRef]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310. [Google Scholar] [CrossRef]
- Kolyvas, E.A.; Caldas, C.; Kelly, K.; Ahmad, S.S. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. 2022, 24, 79. [Google Scholar] [CrossRef]
- Wardley, A.; Cortes, J.; Provencher, L.; Miller, K.; Chien, A.J.; Rugo, H.S.; Steinberg, J.; Sugg, J.; Tudor, I.C.; Huizing, M.; et al. The efficacy and safety of enzalutamide with trastuzumab in patients with HER2+ and androgen receptor-positive metastatic or locally advanced breast cancer. Breast Cancer Res. Treat. 2021, 187, 155–165. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Chen, F.; Chen, N.; Gao, Y.; Jia, L.; Lyu, Z.; Cui, J. Clinical progress of PD-1/L1 inhibitors in breast cancer immunotherapy. Front. Oncol. 2022, 11, 724424. [Google Scholar] [CrossRef]
- Mezni, E.; Behi, K.; Gonçalves, A. Immunotherapy and breast cancer: An overview. Curr. Opin. Oncol. 2022, 34, 587–594. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh Kaboli, P.; Salimian, F.; Aghapour, S.; Xiang, S.; Zhao, Q.; Li, M.; Wu, X.; Du, F.; Zhao, Y.; Shen, J.; et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020, 156, 104806. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, H.I.; Kim, S.J.; Park, S.H.; Kim, H.S. Paclitaxel suppresses the viability of breast tumor MCF7 cells through the regulation of EF1á and FOXO3a by AMPK signaling. Int. J. Oncol. 2015, 47, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, L.; Dattilo, V.; Catalogna, G.; Scumaci, D.; Fiumara, C.V.; Musumeci, F.; Perrotti, G.; Schenone, S.; Tallerico, R.; Spoleti, C.B.; et al. In Preclinical Model of Ovarian Cancer, the SGK1 Inhibitor SI113 Counteracts the Development of Paclitaxel Resistance and Restores Drug Sensitivity. Transl. Oncol. 2019, 12, 1045–1055. [Google Scholar] [CrossRef]
- Uprety, B.; Abrahamse, H. Targeting breast cancer and their stem cell population through AMPK activation: Novel insights. Cells 2022, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The role of natural products and their multitargeted approach to treat solid cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef]
- González-Burgos, E.; Gómez-Serranillos, M.P. Vinca alkaloids as chemotherapeutic agents against breast cancer. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 69–101. [Google Scholar]
- Roskoski, R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacolog. Res. 2019, 139, 395–411. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A.; et al. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; de la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Lozza, I.; Torres-Suárez, A.I. Actively targeted nanomedicines in breast cancer: From pre-clinical investigation to clinic. Cancers 2022, 14, 1198. [Google Scholar] [CrossRef]

| Characteristics | Adjuvant | Neoadjuvant |
|---|---|---|
| Definition | Adjuvant therapy is the treatment after tumor removal | Neoadjuvant therapy is the treatment before tumor removal |
| Advantages | Can help prevent cancer recurrence and kills the remaining cancerous cells | Helps shrink the size of tumors making them easier to cut out |
| Disadvantages | The treatments can further weaken the organism due to unpleasant side effects | Delay in surgical removal of the tumor could mean the cancer spread |
| Cancer caused by genetic mutations | Most often recommended because the risk of cancer recurrence is high where mutations are inherited | Not often recommended if cancer is not due to inherited mutations but is likely due to environmental factors |
| Early insight into treatment plan | Does not provide early insight into the effects of chemotherapy and radiation until after surgery | Useful in indicating how a patient does with certain chemotherapy or radiation treatments before surgery, allowing for changes to be made after surgery |
| Structure | Name | Class | Phase Study |
|---|---|---|---|
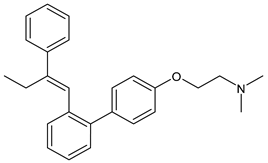 | Tamoxifen (Nolvadex®, Kessar®, Nomafen®, Tamoxene®) | SERM | FDA approved |
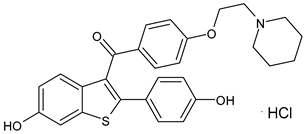 | Raloxifene Hydrochloride (Evista®) | SERM | FDA approved |
 | Lasofoxifene (Fablyn®) | SERM | FDA approved |
 | Bazedoxifene (Duavive®) | SERM | FDA approved |
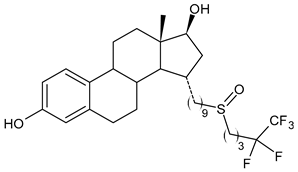 | Fulvestrant (Faslodex®) | SERD | FDA approved |
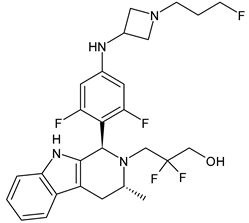 | Giredestrant (GDC-9545) | SERD | Phase III |
 | Rintodestrant (GIT48) | SERD | Phase I |
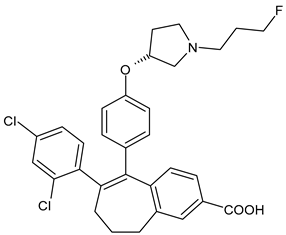 | Amcenestrant (SAR439859) | SERD | Phase III |
 | Camizestrant (AZD9833) | SERD | Phase III |
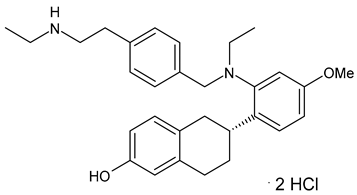 | Elacestrant (RAD1901) | SERD | Phase III |
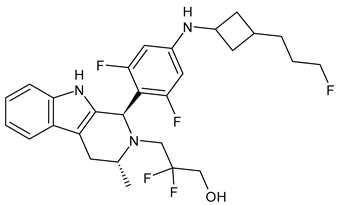 | Imlunestrant (LY3484356) | SERD | Phase I |
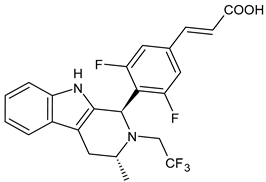 | AZD9496 | SERD | Phase I |
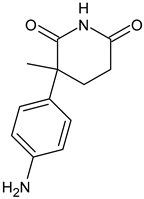 | Aminoglutethimide (Orimeten) | AI First generation type II (non-steroidal) | FDA approved |
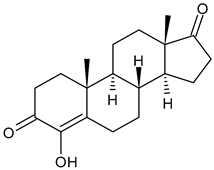 | Formestane (Lentaron) | AI Second generation type I (steroidal) | FDA approved |
 | Fadrazole (Afema®) | AI Second generation type II (non-steroidal) | FDA approved |
 | Exemestane (Aromasin®) | AI Third generation type I (steroidal) | FDA approved |
 | Anastrozole (Arimidex®) | AI Third generation type II (non-steroidal) | FDA approved |
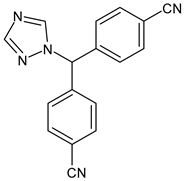 | Letrozole (Femara®) | AI Third generation type II (non-steroidal) | FDA approved |
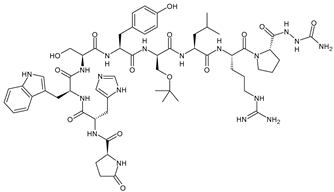 | Goserelin | GnRH agonist | FDA approved |
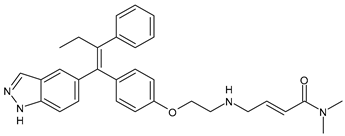 | H3B-5942 | SERCA | Precursor of SERCA H3B-6545 |
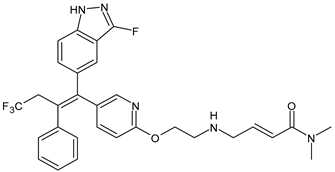 | H3B-6545 | SERCA | Phase II |
 | BMI-135 | ShERPA | Phase I |
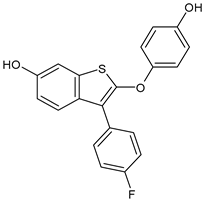 | TTC-352 | ShERPA | Phase I |
| Structure | Name | Activity |
|---|---|---|
 | Camptothecin | TOPOI inhibitor |
 | Topotecan | TOPOI inhibitor |
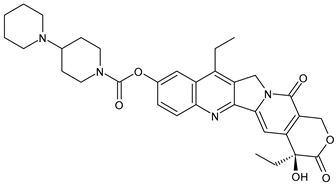 | Irinotecan | TOPOI inhibitor |
 | Adriamycin or doxorubicin | TOPOII inhibitor |
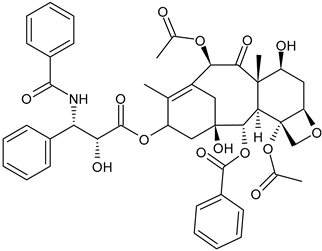 | Paclitaxel | Tubulin stabilizer |
 | Nimbolide | Actin regulator |
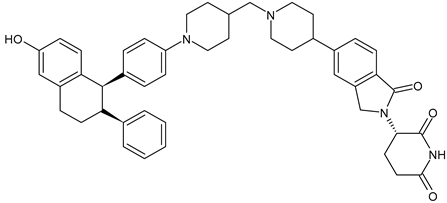 | ARV-471 | PROTACs |
| Structure | Name | Class | Reference Number of Clinical Trials |
|---|---|---|---|
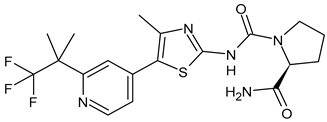 | Alpelisib | PI3K inhibitor (selective PI3Kα) | NCT02437318 |
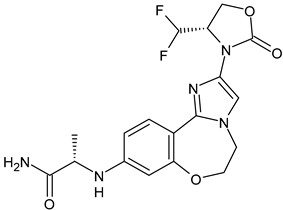 | Inavolisib | PI3K inhibitor (selective PI3Kα) | NCT03006172 |
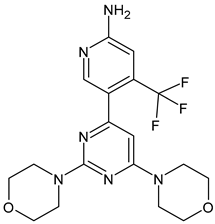 | Buparlisib | PI3K inhibitor (pan-PI3K) | NCT01790932, NCT01629615 |
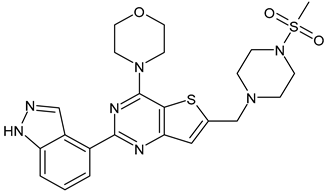 | Pictilisib (GDC-0941) | PI3K inhibitor (pan-PI3K) | NCT01740336 |
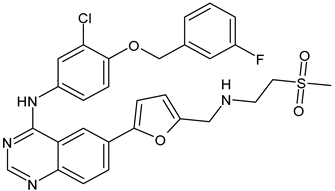 | Lapatinib (GW-572016, Tyverb) | TKI | NCT03894410 |
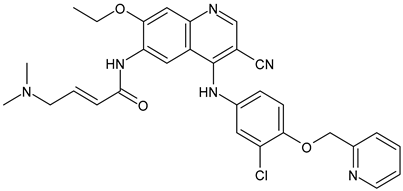 | Neratinib (HKI-272) (Nerlynx™) | TKI | NCT01670877 |
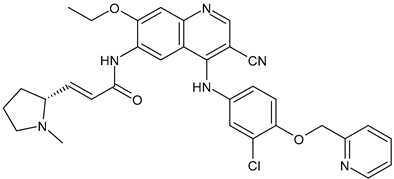 | Pyrotinib | TKI | NCT03863223 |
 | Tucatinib (Tukysa®) | TKI | NCT02614794 |
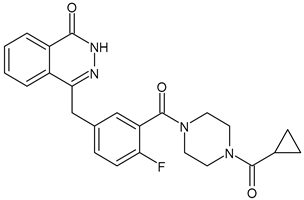 | Olaparib (AZD-2281, Lynparza) | PARPi | NCT01034033 |
 | Talazoparib (BMN-673, Talzenna) | PARPi | NCT04039230 |
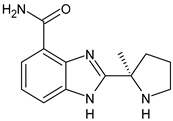 | Veliparib (ABT-888) | PARPi | NCT01149083 |
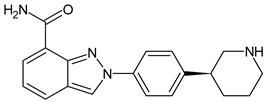 | Niraparib (MK-4827) | PARPi | NCT04837209 |
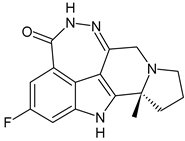 | Pamiparib (BGB-290) | PARPi | NCT03575065 |
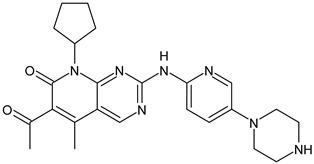 | Palbociclib (Ibrance) | CDK4/6 inhibitor | NCT04711252 |
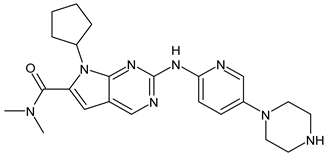 | Ribociclib (Kisqali) | CDK4/6 inhibitor | NCT03577197 |
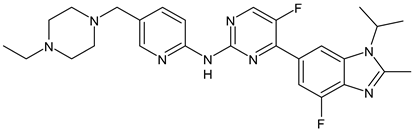 | Abemaciclib (Verzenios) | CDK4/6 inhibitor | NCT02246621 |
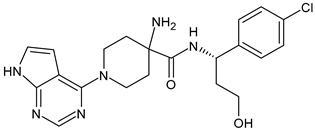 | Capivasertib | Akt inhibitor | NCT03742102 |
 | Ipatasertib | Akt inhibitor | NCT03337724, NCT04177108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting Breast Cancer: An Overlook on Current Strategies. Int. J. Mol. Sci. 2023, 24, 3643. https://doi.org/10.3390/ijms24043643
Iacopetta D, Ceramella J, Baldino N, Sinicropi MS, Catalano A. Targeting Breast Cancer: An Overlook on Current Strategies. International Journal of Molecular Sciences. 2023; 24(4):3643. https://doi.org/10.3390/ijms24043643
Chicago/Turabian StyleIacopetta, Domenico, Jessica Ceramella, Noemi Baldino, Maria Stefania Sinicropi, and Alessia Catalano. 2023. "Targeting Breast Cancer: An Overlook on Current Strategies" International Journal of Molecular Sciences 24, no. 4: 3643. https://doi.org/10.3390/ijms24043643
APA StyleIacopetta, D., Ceramella, J., Baldino, N., Sinicropi, M. S., & Catalano, A. (2023). Targeting Breast Cancer: An Overlook on Current Strategies. International Journal of Molecular Sciences, 24(4), 3643. https://doi.org/10.3390/ijms24043643









