Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress
Abstract
1. Introduction
- To explore the influence of mycorrhization on the growth and distribution of Na+ and Cl−.
- To explore the influence of mycorrhization on the gene expression of the CgNHX and CgCLC families.
- To explore whether these genes are related to Na+ and Cl− distribution.
2. Results
2.1. Effect of Rhizophagus irregularis on Mycorrhizal Colonization and Plant Biomass under NaCl Stress
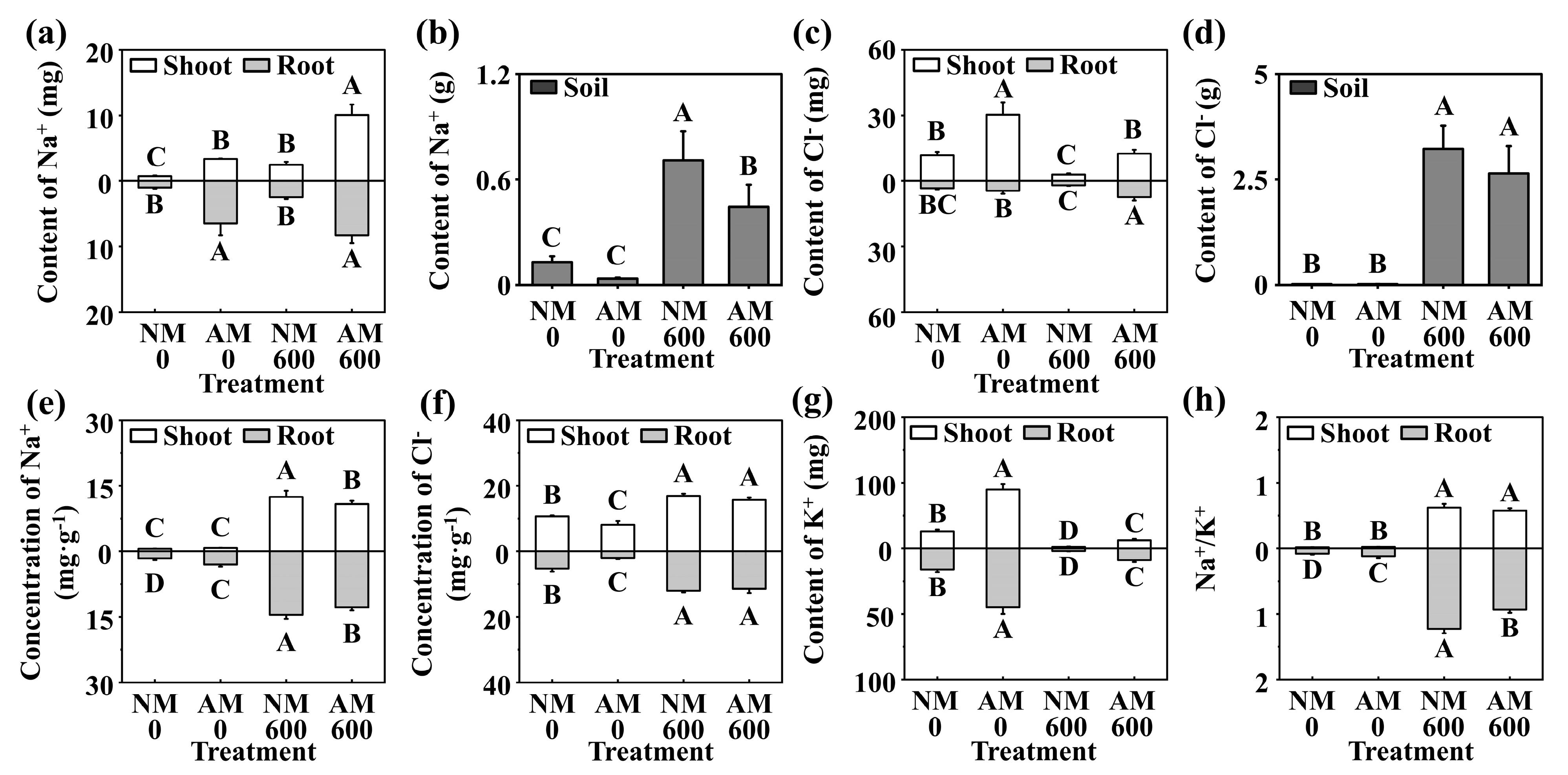
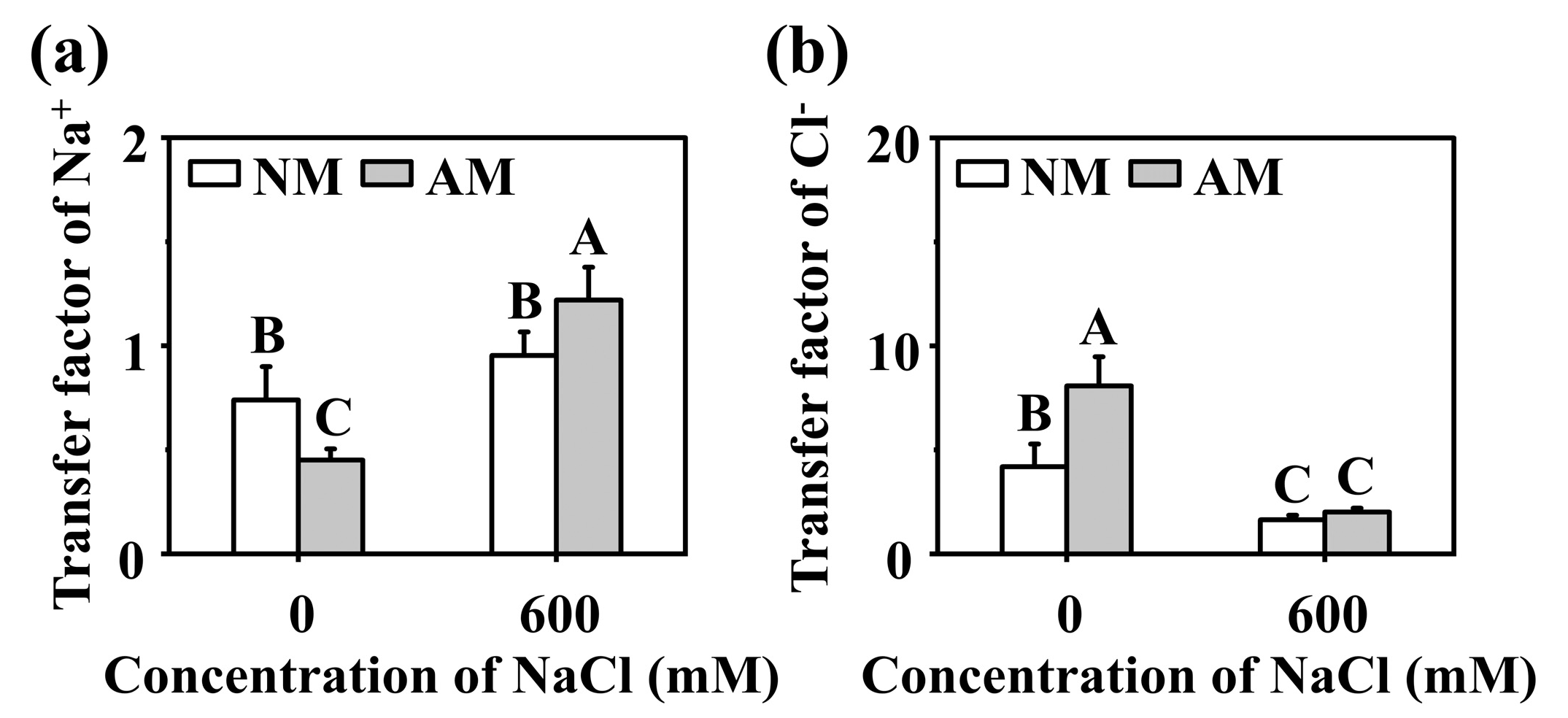
2.2. Effect of R. irregularis on Na+, K+, and Cl− Status under NaCl Stress
2.3. Effect of R. irregularis on Gs and Tr under NaCl Stress
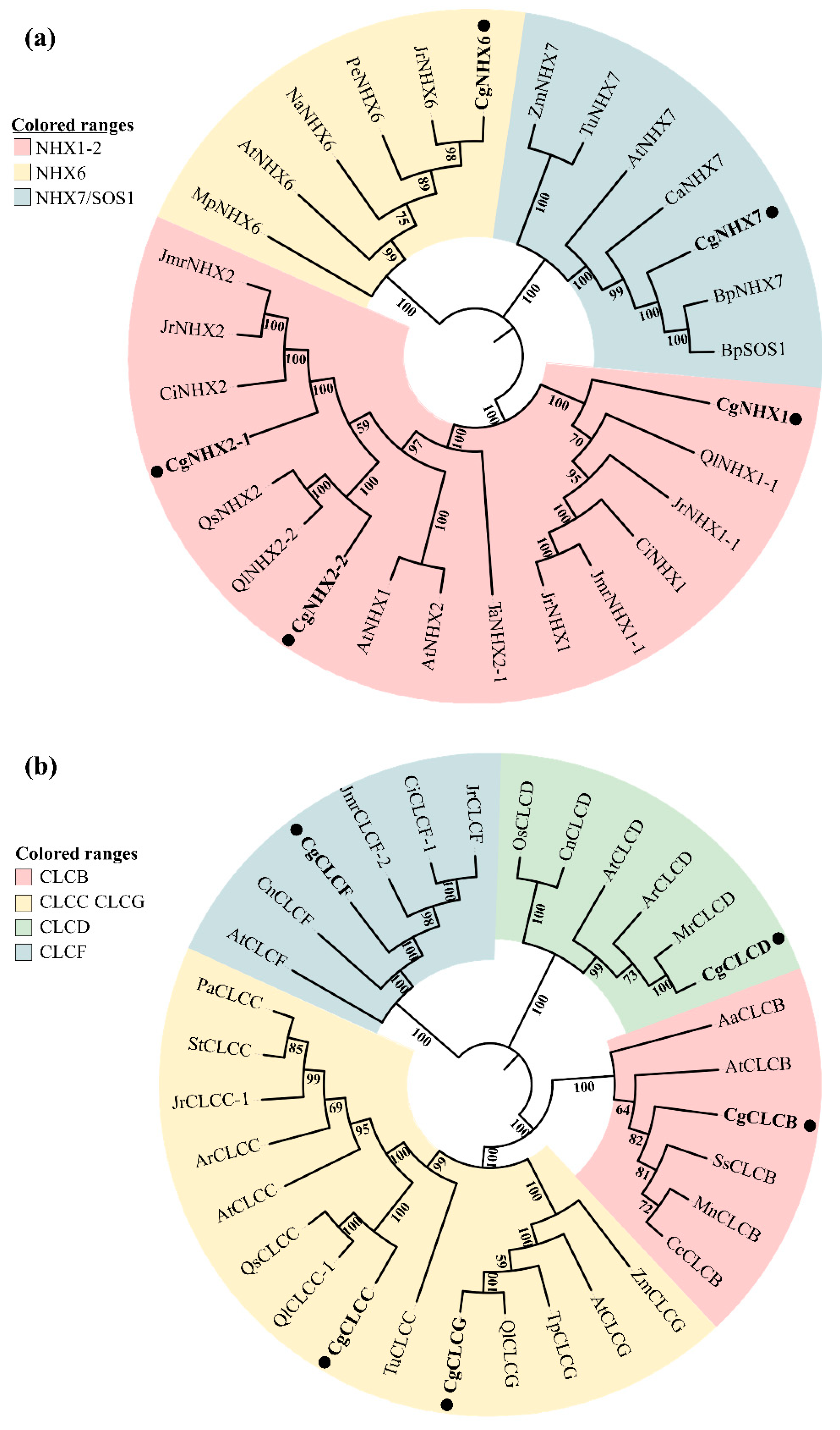
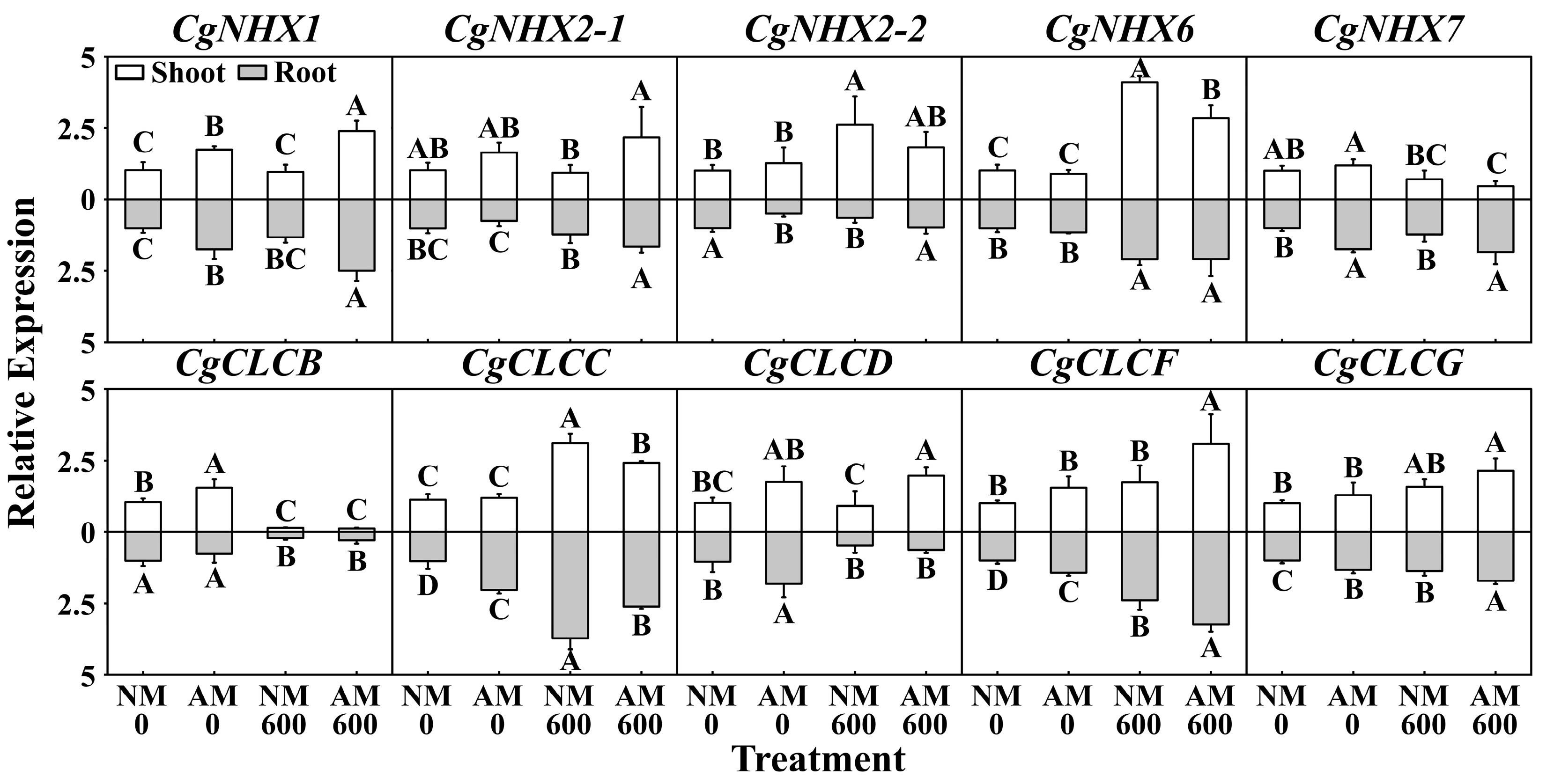
2.4. Effect of R. irregularis on Expression of CgNHXs and CgCLCs under NaCl Stress
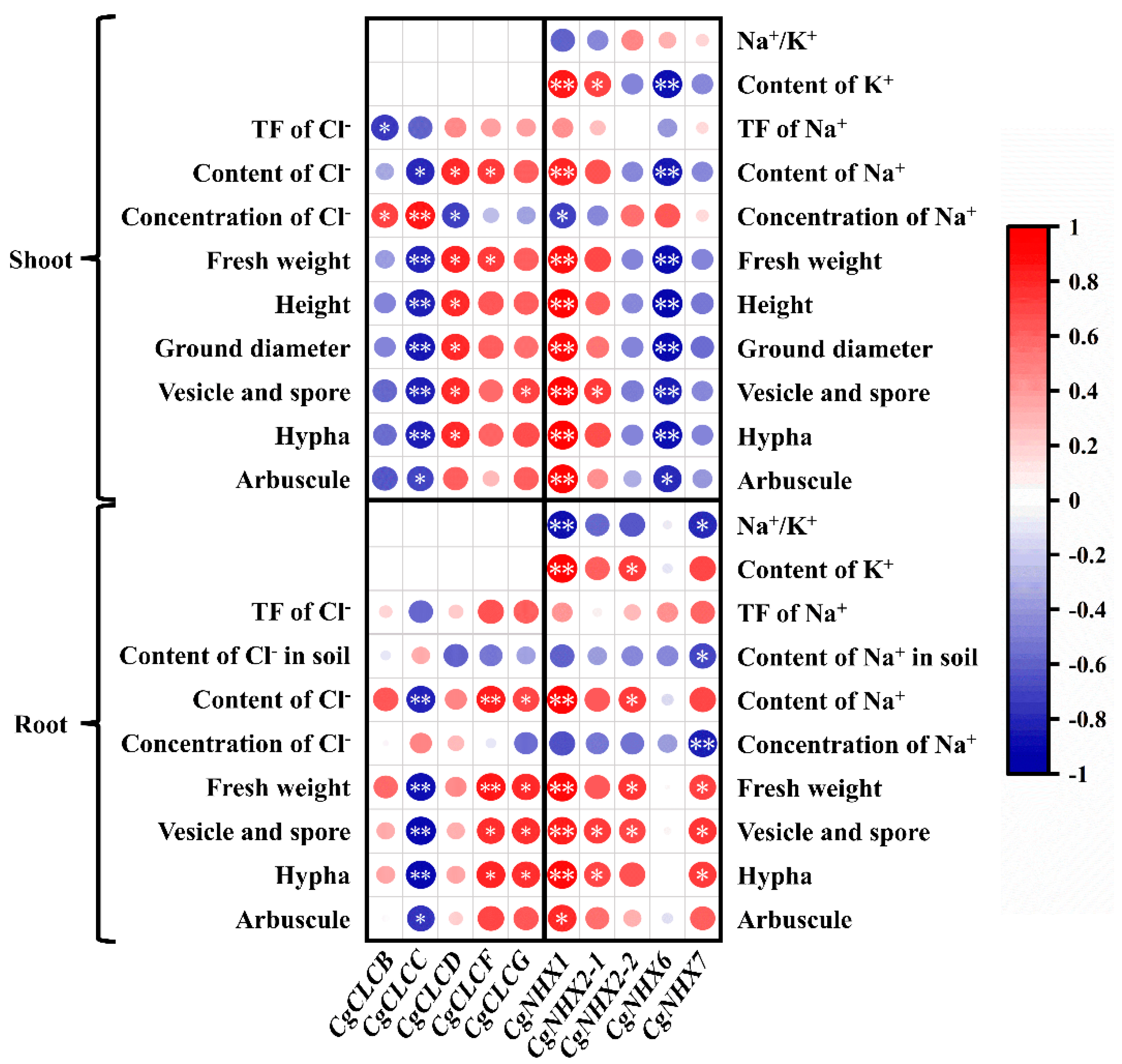
2.5. Correlation Analysis of Gene Expression and Physiological Indicators of C. glauca under NaCl Stress
3. Discussion
3.1. Effects of R. irregularis Inoculation and NaCl Stress on Colonization and Biomass
3.2. Effects of R. irregularis Inoculation and NaCl Stress on Na+, K+, and Cl− Uptake
3.3. Effects of R. irregularis Inoculation and NaCl Stress on Gene Expression
4. Materials and Methods
4.1. Experimental Design and Materials
4.2. Plant Harvest
4.3. Mycorrhizal Colonization
4.4. Plant Biomass and Water Content
4.5. Na+, K+, and Cl− Status
4.6. RNA Extraction, Complementary DNA (cDNA) Synthesis, and Determination of Gene Expression via Quantitative Real-Time PCR (qRT–PCR)
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Cirilloa, V.; Masinb, R.; Maggioa, A.; Zanin, G. Crop-weed interactions in saline environments. Eur. J. Agron. 2018, 99, 51–61. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.; Colla, G.; Rouphael, Y. Grafting tomato as a tool to improve salt tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.H.; Liu, C.; Chen, F.J.; Yang, T.W.; Ma, K.S.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef]
- Wei, X.; Yan, X.; Yang, Z.; Han, G.; Wang, L.; Yuan, F.; Wang, B. Salt glands of recretohalophyte Tamarix under salinity: Their evolution and adaptation. Ecol. Evol. 2020, 10, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhao, H.Y.; Wang, B.X.; Wu, X.Y.; Lan, R.J.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.Q.; Wang, J.L.; et al. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2021, 41, 2108–2121. [Google Scholar] [CrossRef]
- Hassan, M.A.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.P. Polyamines in Halophytes. Front. Plant Sci. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.F.; Song, J.; Feng, G.; Zhao, M.; Liu, J.P. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2010, 342, 495–509. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Akhani, H.; Abedi, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef]
- Ding, F.; Yang, J.C.; Yuan, F.; Wang, B.S. Progress in mechanism of salt excretion in recretohalopytes. Front. Biol. 2010, 5, 164–170. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Myasoedov, N.A.; Shamsutdinov, Z.S.; Shamsutdinov, N.Z. Significance of Na+ and K+ for sustained hydration of organ tissues in ecologically distinct halophytes of the family Chenopodiaceae. Russ. J. Plant Physiol. 2005, 52, 779–787. [Google Scholar] [CrossRef]
- Song, J.; Wang, B.S. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Cui, X.C.; He, S.Y.; Dong, G.; Chen, M.; Wang, J.H.; Lin, X.G. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2021, 41, 1429–1444. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Yang, Y.R.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y.H. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, H.Q.; Fang, F.R.; Wu, N.; Zhang, Y.X.; Tang, M. Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva’. J. Plant Growth Regul. 2017, 36, 824–835. [Google Scholar] [CrossRef]
- Feldmane, D.; Druva-Lūsīte, I.; Pole, V.; Butac, M.M.; Militaru, M.; Missa, I.; Meiere, D.; Rubauskis, E. Rhizophagus irregularis MUCL 41,833 association with green cuttings of Prunus sp. rootstocks. J. Plant Growth Regul. 2020, 40, 533–540. [Google Scholar] [CrossRef]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L. Arbuscular mycorrhizal fungi associated with Phoenix dactylifera L. grown in Tunisian Sahara oases of different salinity levels. Symbiosis 2020, 81, 173–186. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; Ollas, C.d.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Shan, Q.H.; Zhang, J.F.; Sun, S.Y.; Chen, G.C.; Zhang, H.D.; Shen, L.M. Construction of coastline shelterbelts and assessment of their environmental effects in Yuyao, China. Land Degrad. Dev. 2018, 29, 2428–2437. [Google Scholar] [CrossRef]
- Djighaly, P.I.; Diagne, N.; Ngom, M.; Ngom, D.; Hocher, V.; Fall, D.; Diouf, D.; Laplaze, L.; Svistoonoff, S.; Champion, A. Selection of arbuscular mycorrhizal fungal strains to improve Casuarina equisetifolia L. and Casuarina glauca Sieb. tolerance to salinity. Ann. For. Sci. 2018, 75, 72. [Google Scholar] [CrossRef]
- Ye, G.F.; Zhang, H.X.; Chen, B.H.; Nie, S.; Liu, H.; Gao, W.; Wang, H.Y.; Gao, Y.B.; Gu, L.F. De novo genome assembly of the stress tolerant forest species Casuarina equisetifolia provides insight into secondary growth. Plant J. 2019, 97, 779–794. [Google Scholar] [CrossRef]
- Fan, C.; Qiu, Z.; Zeng, B.; Li, X.; Xu, S.H. Physiological adaptation and gene expression analysis of Casuarina equisetifolia under salt stress. Biol. Plant. 2018, 62, 489–500. [Google Scholar] [CrossRef]
- Vikashini, B.; Shanthi, A.; Dasgupta, M.G. Identification and expression profiling of genes governing lignin biosynthesis in Casuarina equisetifolia L. Gene 2018, 676, 37–46. [Google Scholar] [CrossRef]
- Djighaly, P.I.; Ngom, D.; Diagne, N.; Fall, D.; Ngom, M.; Diouf, D.; Hocher, V.; Laplaze, L.; Champion, A.; Farrant, J.M.; et al. Effect of Casuarina plantations inoculated with arbuscular mycorrhizal fungi and Frankia on the diversity of herbaceous vegetation in saline environments in senegal. Diversity 2020, 12, 293. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, F.X.; Tang, M. Transcriptome analysis of arbuscular mycorrhizal Casuarina glauca in damage mitigation of roots on NaCl stress. Microorganisms 2022, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Martins, V.; Ayed, R.B.; Jbir, R.; Feki, M.; Mzid, R.; Géros, H.; Aifa, S.; Hanana, M. Genome wide identification, molecular characterization, and gene expression analyses of grapevine NHX antiporters suggest their involvement in growth, ripening, seed dormancy, and stress response. Biochem. Genet. 2020, 58, 102–128. [Google Scholar] [CrossRef] [PubMed]
- Subba, A.; Tomar, S.; Pareek, A.; Singla-Pareek, S.L. The chloride channels: Silently serving the plants. Physiol. Plant 2020, 171, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Gradogna, A.; Scholz-Starke, J.; Pardo, J.M.; Carpaneto, A. Beyond the patch-clamp resolution: Functional activity of non-electrogenic vacuolar NHX proton/potassium antiporters and inhibition by phosphoinositides. New Phytol. 2020, 229, 3026–3036. [Google Scholar] [CrossRef]
- Bassil, E.; Zhang, S.Q.; Gong, H.J.; Tajima, H.; Blumwald, E. Cation specificity of vacuolar NHX-type cation/H+ antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef]
- Cui, J.Q.; Hua, Y.P.; Zhou, T.; Liu, Y.; Huang, J.Y.; Yue, C.P. Global landscapes of the Na+/H+ antiporter (NHX) family members uncover their potential roles in regulating the rapeseed resistance to salt stress. Int. J. Mol. Sci. 2020, 21, 3429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Tajima, H.; Nambara, E.; Blumwald, E.; Bassil, E. Auxin homeostasis and distribution of the auxin efflux carrier PIN2 require vacuolar NHX-type cation/H+ antiporter activity. Plants 2020, 9, 1311. [Google Scholar] [CrossRef]
- Zhu, X.J.; Pan, T.; Zhang, X.; Fan, L.G.; Quintero, F.J.; Zhao, H.; Su, X.M.; Li, X.; Villalta, I.; Mendoza, I.; et al. K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol. 2018, 178, 1657–1678. [Google Scholar] [CrossRef]
- Lv, S.S.; Wang, L.; Zhang, X.; Li, X.J.; Fan, L.G.; Xu, Y.L.; Zhao, Y.J.; Xie, H.C.; Sawchuk, M.G.; Scarpella, E.; et al. Arabidopsis NHX5 and NHX6 regulate PIN6-mediated auxin homeostasis and growth. J. Plant Physiol. 2020, 255, 153305. [Google Scholar] [CrossRef]
- Sze, H.; Chanroj, S. Plant endomembrane dynamics: Studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhao, Y.J.; Zhao, X.Q.; Dong, J.M.; Yuan, Z.H. Genome-wide identification and expression analysis of the CLC gene family in pomegranate (Punica granatum) reveals its roles in salt resistance. BMC Plant Biol. 2020, 20, 560. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.J.; Gu, Q.Q.; Wang, N.N.; Yang, C.Q.; Peng, S.A. Molecular cloning and characterization of the chloride channel gene family in trifoliate orange. Biol. Plant. 2015, 59, 645–653. [Google Scholar] [CrossRef]
- Jossier, M.; Kroniewicz, L.; Dalmas, F.; Thiec, D.L.; Ephritikhine, G.; Thomine, S.; BarbierBrygoo, H.; Vavasseur, A.; Filleur, S.; Leonhardt, N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010, 64, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Fecht-Bartenbach, J.v.d.; Bogner, M.; Krebs, M.; Stierhof, Y.-D.; Schumacher, K.; Ludewig, U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007, 50, 466–474. [Google Scholar] [CrossRef]
- Nedelyaeva, O.I.; Shuvalov, A.V.; Balnokin, Y.V. Chloride channels and transporters of the CLC family in plants. Russ. J. Plant Physiol. 2020, 67, 767–784. [Google Scholar] [CrossRef]
- Angeli, A.D.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. CLC-mediated anion transport in plant cells. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depre, S.; Thomine, S.; Filleur, S. Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [CrossRef]
- Liu, C.G.; Dai, Z.; Cui, M.Y.; Lu, W.K.; Sun, H.W. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565. [Google Scholar] [CrossRef]
- Bharti, A.; Garg, N. SA and AM symbiosis modulate antioxidant defense mechanisms and asada pathway in chickpea genotypes under salt stress. Ecotoxicol. Environ. Saf. 2019, 178, 66–78. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Baraza, E.; Gulías, J.; Cabot, C. Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Front. Plant Sci. 2019, 10, 843. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; Hessini, K.; Woodrow, P.; Ciarmiello, L.F.; Ferchichi, S.; Fusco, G.M.; Abdelly, C.; Carillo, P. Salinity duration differently modulates physiological parameters and metabolites profile in roots of two contrasting barley genotypes. Plants 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Wang, C.L.; Chiu, J.Y. Proteomic studies in the symbiotic associations between arbuscular mycorrhizal fungi Funneliformis mosseae with melon (Cucumis melo L.) under salt conditions. Acta Sci. Pol. Hortorum Cultus 2021, 20, 17–28. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Cartes, P.; Vidal, G.; Cornejo, P. Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol. Biochem. 2021, 158, 396–409. [Google Scholar] [CrossRef]
- Parvin, S.; Geel, M.V.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Rozentsvet, O.; Nesterov, V.; Bogdanova, E.; Kosobryukhov, A.; Subova, S.; Semenova, G. Structural and molecular strategy of photosynthetic apparatus organisation of wild flora halophytes. Plant Physiol. Biochem. 2018, 129, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.L.; Barrett-Lennard, E.G.; Tian, C.Y. Changes in cell size and tissue hydration (‘succulence’) cause curvilinear growth responses to salinity and watering treatments in euhalophytes. Environ. Exp. Bot. 2019, 159, 87–94. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, H.Y.; Han, B.; Wang, B.; Guo, A.P.; Zheng, D.; Liu, C.J.; Chang, L.L.; Peng, M.; Wang, X.C. Sodium instead of potassium and chloride is an important macronutrient to improve leaf succulence and shoot development for halophyte Sesuvium portulacastrum. Plant Physiol. Biochem. 2012, 51, 53–62. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Jyothi-Prakash, P.A.; Qin, L.; He, J.; Lin, Q.s.; Loh, C.S.; Kumar, P.P. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ. 2014, 37, 1656–1671. [Google Scholar] [CrossRef]
- Munns, R.; Teste, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bryla, D.R.; Lee, J. Salt exclusion and mycorrhizal symbiosis increase tolerance to NaCl and CaCl2 salinity in ‘Siam queen’ basil. HortScience 2017, 52, 278–287. [Google Scholar] [CrossRef]
- Nada, R.M.; Khedr, A.H.A.; Serag, M.S.; El-Qashlan, N.R.; Abogadallah, G.M. Molecular and physiological responses of naturally grown Atriplex halimus L. to drought-stress recovery in the absence or presence of Na+ ions under natural conditions. J. Plant Growth Regul. 2021, 41, 1578–1593. [Google Scholar] [CrossRef]
- Jia, T.T.; Wang, J.; Chang, W.; Fan, X.X.; Sui, X.; Song, F.Q. Proteomics analysis of E. angustifolia seedlings inoculated with arbuscular mycorrhizal fungi under salt stress. Int. J. Mol. Sci. 2019, 20, 788. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.J.; Huang, C.H. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Shawky, B.T. Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Plant. Nutr. Soil Sci. 2011, 174, 283–291. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.Y.; Cheng, K.; Zhang, H.Q.; Tang, M. Arbuscular mycorrhizal fungus and exogenous potassium application improved Lycium barbarum salt tolerance. J. Plant Growth Regul. 2021, 41, 2980–2991. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.Q.; Zhang, X.L.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.-a.; Ushijim, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwal, E. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef]
- Hu, R.B.; Zhu, Y.F.; Wei, J.; Chen, J.; Shi, H.Z.; Shen, G.X.; Zhang, H. Overexpression of PP2A-C5 that encodes the catalytic subunit 5 of protein phosphatase 2A in Arabidopsis confers better root and shoot development under salt conditions. Plant Cell Environ. 2017, 40, 150–164. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.W.; Zhang, F.F.; Huang, Y.H. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Matsushita, N.; Matoh, T. Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 1991, 83, 170–176. [Google Scholar] [CrossRef]
- Zhan, X.C.; Li, C.R.; Li, Z.Y.; Yang, X.C.; Zhong, S.G.; Yi, T. Highly Accurate Nephelometric Titrimetry. J. Pharm. Sci. 2004, 93, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, F.; Chen, H.; Xu, T.; Tang, M. Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress. Int. J. Mol. Sci. 2023, 24, 3680. https://doi.org/10.3390/ijms24043680
Wang Y, Dong F, Chen H, Xu T, Tang M. Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress. International Journal of Molecular Sciences. 2023; 24(4):3680. https://doi.org/10.3390/ijms24043680
Chicago/Turabian StyleWang, Yihan, Fengxin Dong, Hui Chen, Tingying Xu, and Ming Tang. 2023. "Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress" International Journal of Molecular Sciences 24, no. 4: 3680. https://doi.org/10.3390/ijms24043680
APA StyleWang, Y., Dong, F., Chen, H., Xu, T., & Tang, M. (2023). Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress. International Journal of Molecular Sciences, 24(4), 3680. https://doi.org/10.3390/ijms24043680




