The Ketogenic Diet in Colorectal Cancer: A Means to an End
Abstract
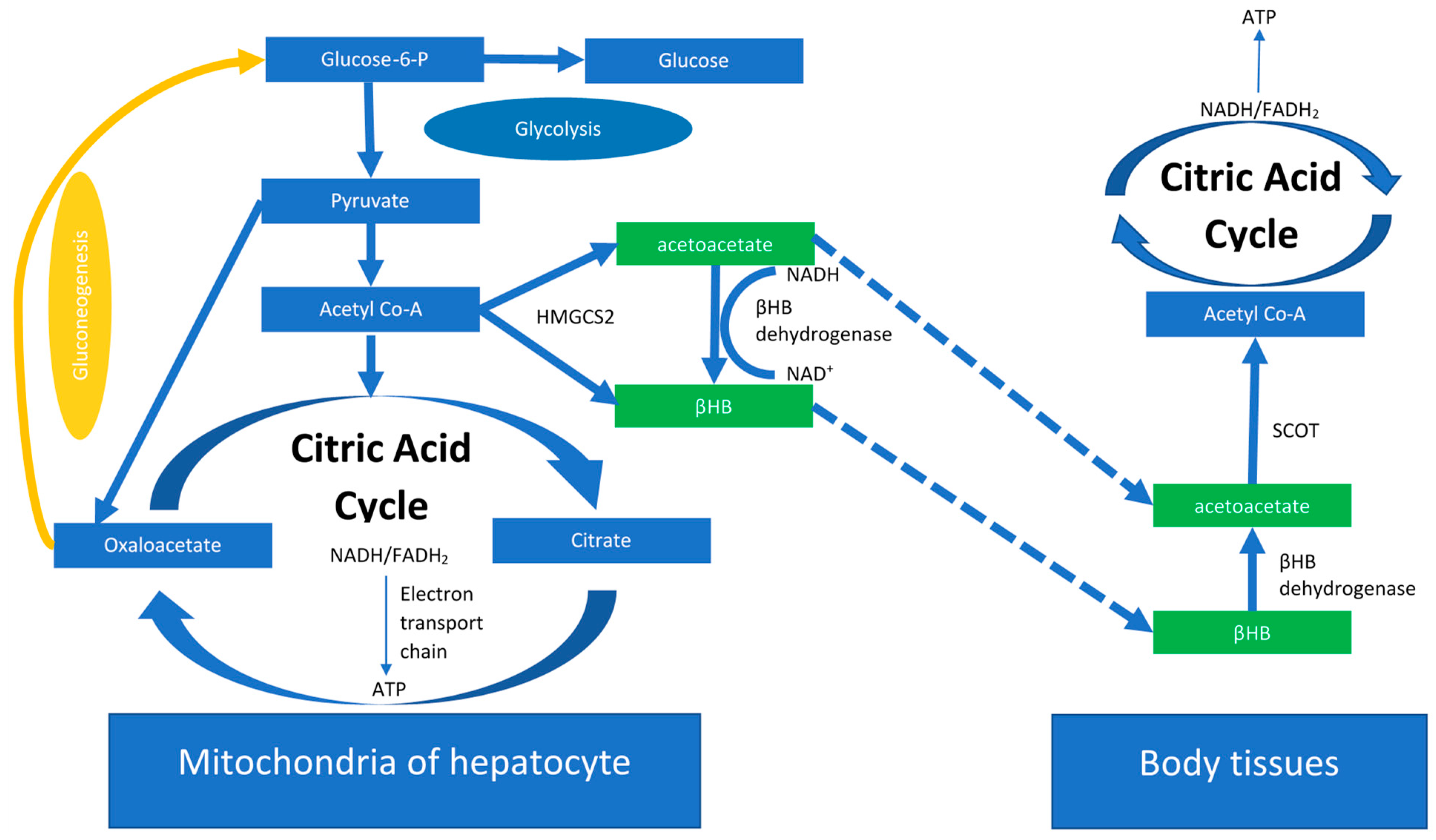
:1. Introduction
2. The Ketogenic Diet
The KD Anti-Tumor Potential
3. Mechanism by Which KD Influences Growth and Proliferation of Tumor Cells
4. KD as Adjunct Treatment in CRC Patients
5. The Downfalls to the Clinical Application of KD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Lin, Y.; Bien, S.A.; Figueiredo, J.C.; Harrison, T.A.; Guinter, M.A.; Berndt, S.I.; Brenner, H.; Chan, A.T.; Chang-Claude, J.; et al. Association of Body Mass Index with Colorectal Cancer Risk by Genome-Wide Variants. JNCI J. Natl. Cancer Inst. 2020, 113, 38–47. [Google Scholar] [CrossRef]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, Y.; Wu, H.; Duerksen-Hughes, P.; Zhang, H.; Li, P.; Huang, J.; Yang, J.; Wu, Y.; Xia, D. Association between markers of glucose metabolism and risk of colorectal cancer. BMJ Open 2016, 6, e011430. [Google Scholar] [CrossRef]
- Cassim, S.; Vučetić, M.; Ždralević, M.; Pouyssegur, J. Warburg and Beyond: The Power of Mitochondrial Metabolism to Collaborate or Replace Fermentative Glycolysis in Cancer. Cancers 2020, 12, 1119. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Hong, J.T.; Kim, T.J.; Pyo, J.H.; Kim, E.R.; Hong, S.N.; Kim, Y.-H.; Ahn, H.S.; Sohn, I.; Chang, D.K. Impact of Sarcopenia on the Risk of Advanced Colorectal Neoplasia. J. Gastroenterol. Hepatol. 2019, 34, 162–168. [Google Scholar] [CrossRef]
- van Vugt, J.L.; Braak, R.R.C.V.D.; Lalmahomed, Z.S.; Vrijland, W.W.; Dekker, J.W.; Zimmerman, D.D.; Vles, W.J.; Coene, P.-P.L.; Ijzermans, J.N. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur. J. Surg. Oncol. 2018, 44, 1354–1360. [Google Scholar] [CrossRef]
- Abbass, T.; Ho, Y.T.T.; Horgan, P.G.; Dolan, R.D.; McMillan, D.C. The relationship between computed tomography derived skeletal muscle index, psoas muscle index and clinical outcomes in patients with operable colorectal cancer. Clin. Nutr. ESPEN 2020, 39, 104–113. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer: Results from the C Scans Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Weng, M.-L.; Chen, W.-K.; Chen, X.-Y.; Lu, H.; Sun, Z.-R.; Yu, Q.; Sun, P.-F.; Xu, Y.-J.; Zhu, M.-M.; Jiang, N.; et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1α pathway suppression. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, F.; Fucà, G.; Provenzano, L.; Lobefaro, R.; Zanenga, L.; Vingiani, A.; Belfiore, A.; Lorenzoni, A.; Alessi, A.; Pruneri, G.; et al. Exceptional tumour responses to fasting-mimicking diet combined with standard anticancer therapies: A sub-analysis of the NCT03340935 trial. Eur. J. Cancer 2022, 172, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic Diets for Drug-Resistant Epilepsy. Cochrane Database Syst. Rev. 2020, 6, cd001903. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2013, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.G.; Bhatia, S.K.; Anderson, C.M.; Eichenberger-Gilmore, J.M.; Sibenaller, Z.A.; Mapuskar, K.A.; Schoenfeld, J.D.; Buatti, J.M.; Spitz, D.R.; Fath, M.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014, 2, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-D.; Wu, H.; Huang, S.; Zhang, H.-L.; Qin, C.-J.; Zhao, L.-H.; Fu, G.-B.; Zhou, X.; Wang, X.-M.; Tang, L.; et al. HBx regulates fatty acid oxidation to promote hepatocellular carcinoma survival during metabolic stress. Oncotarget 2016, 7, 6711–6726. [Google Scholar] [CrossRef] [PubMed]
- Cassim, S.; Raymond, V.-A.; Dehbidi-Assadzadeh, L.; Lapierre, P.; Bilodeau, M. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle 2018, 17, 903–916. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef]
- Sihui, M.; Suzuki, K. Potential Application of Ketogenic Diet to Metabolic Status and Exercise Performance: A Review. EC Nutr. 2018, 13, 496–499. [Google Scholar]
- Saris, C.G.J.; Timmers, S. Ketogenic diets and Ketone suplementation: A strategy for therapeutic intervention. Front. Nutr. 2022, 9, 947567. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone Bodies as Signaling Metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117, 114–125. [Google Scholar] [CrossRef]
- Poff, A.; Ari, C.; Arnold, P.; Seyfried, T.; D’Agostino, D. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int. J. Cancer 2014, 135, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Magee, B.A.; Potezny, N.; Rofe, A.M.; Conyers, R.A. The inhibition of malignant cell growth by ketone bodies. Aust. J. Exp. Biol. Med. Sci. 1979, 57, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Maurer, G.D.; Brucker, D.P.; Bähr, O.; Harter, P.N.; Hattingen, E.; Walenta, S.; Mueller-Klieser, W.; Steinbach, J.P.; Rieger, J. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, J.C.; Buschemeyer, W.C.; Tewari, A.K.; Rokhfeld, D.; Pollak, M.; Zhao, Y.; Febbo, P.G.; Cohen, P.; Hwang, D.; Devi, G.; et al. The Effects of Varying Dietary Carbohydrate and Fat Content on Survival in a Murine Lncap Prostate Cancer Xenograft Model. Cancer Prev. Res. 2009, 2, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, J.C.; Isaacs, W.B.; Pizzo, S.V.; Freedland, S.J. Is There a Role for a Low-Carbohydrate Ketogenic Diet in the Management of Prostate Cancer? Urology 2006, 68, 15–18. [Google Scholar] [CrossRef]
- Masko, E.M.; Thomas, J.A.; Antonelli, J.A.; Lloyd, J.C.; Phillips, T.E.; Poulton, S.H.; Dewhirst, M.W.; Pizzo, S.V.; Freedland, S.J. Freedland. Low-Carbohydrate Diets and Prostate Cancer: How Low Is “Low Enough”? Cancer Prev. Res. 2010, 3, 1124–1131. [Google Scholar] [CrossRef]
- Kim, H.S.; Masko, E.M.; Poulton, S.L.; Kennedy, K.M.; Pizzo, S.V.; Dewhirst, M.W.; Freedland, S.J. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int. 2012, 110, 1062–1069. [Google Scholar] [CrossRef]
- Caso, J.; Masko, E.M.; Ii, J.A.T.; Poulton, S.H.; Dewhirst, M.; Pizzo, S.V.; Freedland, S.J.; Thomas, J.A. The effect of carbohydrate restriction on prostate cancer tumor growth in a castrate mouse xenograft model. Prostate 2012, 73, 449–454. [Google Scholar] [CrossRef]
- Ho, V.W.; Leung, K.; Hsu, A.; Luk, B.; Lai, J.; Shen, S.Y.; Minchinton, A.I.; Waterhouse, D.; Bally, M.B.; Lin, W.; et al. A Low Carbohydrate, High Protein Diet Slows Tumor Growth and Prevents Cancer Initiation. Cancer Res. 2011, 71, 4484–4493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, B.G.; Bhatia, S.K.; Buatti, J.M.; Brandt, K.E.; Lindholm, K.E.; Button, A.M.; Szweda, L.I.; Smith, B.J.; Spitz, D.R.; Fath, M.A. Ketogenic Diets Enhance Oxidative Stress and Radio-Chemo-Therapy Responses in Lung Cancer Xenografts. Clin. Cancer Res. 2013, 19, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Kaemmerer, U.; Illert, B.; Muehling, B.; Pfetzer, N.; Wittig, R.; Voelker, H.U.; Thiede, A.; Coy, J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, K.E.; Williams, E.A.; Smith, N.C.; Dillard, A.; Park, E.Y.; Nunez, N.P.; Hursting, S.D.; Lane, M.A. Low-Carbohydrate Diet Versus Caloric Restriction: Effects on Weight Loss, Hormones, and Colon Tumor Growth in Obese Mice. Nutr. Cancer 2008, 60, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE 2013, 8, e65522. [Google Scholar] [CrossRef]
- Nebeling, L.C.; Miraldi, F.; Shurin, S.B.; Lerner, E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J. Am. Coll. Nutr. 1995, 14, 202–208. [Google Scholar] [CrossRef]
- Zuccoli, G.; Marcello, N.; Pisanello, A.; Servadei, F.; Vaccaro, S.; Mukherjee, P.; Seyfried, T.N. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr. Metab. 2010, 7, 33. [Google Scholar] [CrossRef]
- Schmidt, M.; Pfetzer, N.; Schwab, M.; Strauss, I.; Kämmerer, U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011, 8, 54. [Google Scholar] [CrossRef]
- Fine, E.J.; Segal-Isaacson, C.J.; Feinman, R.D.; Herszkopf, S.; Romano, M.C.; Tomuta, N.; Bontempo, A.F.; Negassa, A.; Sparano, J.A. Targeting insulin inhibition as a metabolic therapy in advanced cancer: A pilot safety and feasibility dietary trial in 10 patients. Nutrition 2012, 28, 1028–1035. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. Ergo: A Pilot Study of Ketogenic Diet in Recurrent Glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef]
- Cully, M.; You, H.; Levine, A.J.; Mak, T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 2006, 6, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Marie, S.K.; Shinjo, S.M. Metabolism and Brain Cancer. Clinics 2011, 66, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.B.; Hay, N. Is Akt the “Warburg Kinase”?-Akt-Energy Metabolism Interactions and Oncogenesis. Semin. Cancer Biol. 2009, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.J.; Pan, J.; Lee, M.H. Roles of P53, Myc and Hif-1 in Regulating Glycolysis—The Seventh Hallmark of Cancer. Cell. Mol. Life Sci. 2008, 65, 3981–3999. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 2009, 19, 12–16. [Google Scholar] [CrossRef]
- Agarwal, A.; Das, K.; Lerner, N.; Sathe, S.; Cicek, M.; Casey, G.; Sizemore, N. The Akt/Iκb Kinase Pathway Promotes Angiogenic/Metastatic Gene Expression in Colorectal Cancer by Activating Nuclear Factor-Κb and Β-Catenin. Oncogene 2005, 24, 1021–1031. [Google Scholar] [CrossRef]
- Wong, D.; Teixeira, A.; Oikonomopoulos, S.; Humburg, P.; Lone, I.N.; Saliba, D.; Siggers, T.; Bulyk, M.; Angelov, D.; Dimitrov, S.; et al. Extensive Characterization of Nf-Κb Binding Uncovers Non-Canonical Motifs and Advances the Interpretation of Genetic Functional Traits. Genome Biol. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The Two Nf-Κb Activation Pathways and Their Role in Innate and Adaptive Immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Lin, Y.; He, Z.; Ye, J.; Liu, Z.; She, X.; Gao, X.; Liang, R. Progress in Understanding the Il-6/Stat3 Pathway in Colorectal Cancer. Onco Targets Ther. 2020, 13, 13023–13032. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Xu, S.; Chheda, C.; Morvaridi, S.; Hu, R.W.; Grippo, P.J.; Mascariñas, E.; Principe, D.R.; Knudsen, B.; Xue, J.; et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget 2016, 7, 7747–7760. [Google Scholar] [CrossRef]
- Van Dalen, F.J.; Van Stevendaal, M.H.M.E.; Fennemann, F.L.; Verdoes, M.; Ilina, O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules 2018, 24, 9. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Quatromoni, J.G.; Eruslanov, E. Tumor-Associated Macrophages: Function, Phenotype, and Link to Prognosis in Human Lung Cancer. Am. J. Transl. Res. 2012, 4, 376. [Google Scholar]
- Dong, H.; Diao, H.; Zhao, Y.; Xu, H.; Pei, S.; Gao, J.; Wang, J.; Hussain, T.; Zhao, D.; Zhou, X.; et al. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Prolif. 2019, 52, e12633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, C.; Jin, L.; Zhang, R.; Wang, T.; Wang, Q.; Chen, J.; Yang, F.; Siebert, H.C.; Zheng, X. Ketogenic Diet Elicits Antitumor Properties through Inducing Oxidative Stress, Inhibiting Mmp-9 Expression, and Rebalancing M1/M2 Tumor-Associated Macrophage Phenotype in a Mouse Model of Colon Cancer. J. Agric. Food Chem. 2020, 68, 11182–11196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Rychahou, P.; Fan, T.W.-M.; Lane, A.N.; Weiss, H.L.; Evers, B.M. Ketogenesis contributes to intestinal cell differentiation. Cell Death Differ. 2017, 24, 458–468. [Google Scholar] [CrossRef]
- Kim, J.T.; Napier, D.L.; Kim, J.; Li, C.; Lee, E.Y.; Weiss, H.L.; Wang, Q.; Evers, B.M. Ketogenesis alleviates TNFα-induced apoptosis and inflammatory responses in intestinal cells. Free Radic. Biol. Med. 2021, 172, 90–100. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Wong, A.C.; Lundgren, P.; Golos, A.M.; Descamps, H.C.; Dohnalová, L.; Cramer, Z.; Tian, Y.; Yueh, B.; Eskiocak, O.; et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 2022, 605, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Yamashita, K.; Waraya, M.; Margalit, O.; Ooki, A.; Tamaki, H.; Sakagami, H.; Kokubo, K.; Sidransky, D.; Watanabe, M. Epigenetic Silencing of HOPX Promotes Cancer Progression in Colorectal Cancer. Neoplasia 2012, 14, 559–571. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, M.; Miao, H. Ketogenic diet: New avenues to overcome colorectal cancer. Signal Transduct. Target. Ther. 2022, 7, 1–2. [Google Scholar] [CrossRef]
- Hao, G.-W.; Chen, Y.-S.; He, D.-M.; Wang, H.-Y.; Wu, G.-H.; Zhang, B. Growth of Human Colon Cancer Cells in Nude Mice is Delayed by Ketogenic Diet With or Without Omega-3 Fatty Acids and Medium-chain Triglycerides. Asian Pac. J. Cancer Prev. 2015, 16, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tonouchi, H.; Sasayama, A.; Ashida, K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients 2018, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, G.; Wang, H.; He, D.; Chen, Y.; Wu, G.; Zhang, B. Effect of Ketogenic Diet on Growth of Human Colon Cancer Cells in Nude Mice. Chin. J. Clin. Oncol. 2014, 24, 1154–1157. [Google Scholar]
- Kadochi, Y.; Mori, S.; Fujiwara-Tani, R.; Luo, Y.; Nishiguchi, Y.; Kishi, S.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Remodeling of energy metabolism by a ketone body and medium-chain fatty acid suppressed the proliferation of CT26 mouse colon cancer cells. Oncol. Lett. 2017, 14, 673–680. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Sato, K.; Niedzwiecki, D.; Ye, C.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Dietary Glycemic Load and Cancer Recurrence and Survival in Patients with Stage III Colon Cancer: Findings From CALGB 89803. Gynecol. Oncol. 2012, 104, 1702–1711. [Google Scholar] [CrossRef]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Yilmaz, O.; Wang, M.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI Cancer Spectr. 2018, 2, pky077. [Google Scholar] [CrossRef]
- Kato, I.; Dyson, G.; Snyder, M.; Kim, H.-R.; Severson, R.K. Differential effects of patient-related factors on the outcome of radiation therapy for rectal cancer. J. Radiat. Oncol. 2016, 5, 279–286. [Google Scholar] [CrossRef]
- Giovannucci, E. Metabolic Syndrome, Hyperinsulinemia, and Colon Cancer: A Review. Am. J. Clin. Nutr. 2007, 86, S836–S842. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Chan, J.A.; Wu, K.; Pollak, M.N.; Giovannucci, E.L.; Fuchs, C.S. Insulin, the Insulin-Like Growth Factor Axis, and Mortality in Patients With Nonmetastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 176–185. [Google Scholar] [CrossRef]
- Volkova, E.; Willis, J.; Wells, J.E.; Robinson, B.; Dachs, G.U.; Currie, M.J. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br. J. Cancer 2010, 104, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Naveed, S.; Ahmed, A.; Abbas, Z.; Gull, I.; Athar, M.A. Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J. Cancer Ther. 2014, 05, 817–822. [Google Scholar] [CrossRef]
- Tulipan, J.; Kofler, B. Implementation of a Low-Carbohydrate Diet Improves the Quality of Life of Cancer Patients—An Online Survey. Front. Nutr. 2021, 8, 661253. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Sweeney, R.A. Impact of a Ketogenic Diet Intervention During Radiotherapy on Body Composition: I. Initial Clinical Experience with Six Prospectively Studied Patients. BMC Res. Notes 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Klement, R.J.; Sweeney, R.A. Impact of a Ketogenic Diet Intervention During Radiotherapy on Body Composition: II. Protocol of a Randomised Phase I Study (Ketocomp). Clin. Nutr. ESPEN 2016, 12, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Shigematus, K.; Iwase, Y.; Mikami, W.; Hoshi, H.; Nishiyama, T.; Ohtuka, A.; Abe, H. Clinical effects of one year of chemotherapy with a modified medium-chain triglyceride ketogenic diet on the recurrence of stage IV colon cancer. Am. Soc. Clin. Oncol. 2018, 36, e15709. [Google Scholar] [CrossRef]
- Klement, R.J.; Koebrunner, P.S.; Meyer, D.; Kanzler, S.; Sweeney, R.A. Impact of a Ketogenic Diet Intervention During Radiotherapy on Body Composition: IV. Final Results of the Ketocomp Study for Rectal Cancer Patients. Clin. Nutr. 2021, 40, 4674–4684. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Wei, R.; Zhou, Y.; Li, C.; Rychahou, P.; Zhang, S.; Titlow, W.B.; Bauman, G.; Wu, Y.; Liu, J.; Wang, C.; et al. Ketogenesis Attenuates KLF5-Dependent Production of CXCL12 to Overcome the Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Cancer Res. 2022, 82, 1575–1588. [Google Scholar] [CrossRef]
- Klement, R.J.; Sweeney, R.A.; Gross, E.C.; Champ, C.E. Problems Associated with a Highly Artificial Ketogenic Diet: Letter to the Editor Re: Van der Louw EJTM, Olieman JF, van den Bemt PMLA; et al. ‘Ketogenic Diet Treatment as Adjuvant to Standard Treatment of Glioblastoma Multiforme: A Feasibility and Safety Study’. Ther. Adv. Med. Oncol. 2019, 11, 1758835919879268. [Google Scholar] [CrossRef]
- Cai, H.; Sobue, T.; Kitamura, T.; Ishihara, J.; Nanri, A.; Mizoue, T.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Tsugane, S.; et al. Low-carbohydrate diet and risk of cancer incidence: The Japan Public Health Center-based prospective study. Cancer Sci. 2021, 113, 744–755. [Google Scholar] [CrossRef]
- Römer, M.; Dörfler, J.; Huebner, J. The use of ketogenic diets in cancer patients: A systematic review. Clin. Exp. Med. 2021, 21, 501–536. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Allen, J.; Jarman, A.; Oyekunle, T.; Armstrong, A.J.; Moul, J.W.; Sandler, H.M.; Posadas, E.; Levin, D.; Wiggins, E.; et al. A Randomized Controlled Trial of a 6-Month Low-Carbohydrate Intervention on Disease Progression in Men with Recurrent Prostate Cancer: Carbohydrate and Prostate Study 2 (CAPS2). Clin. Cancer Res. 2020, 26, 3035–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodabakhshi, A.; Akbari, M.E.; Mirzaei, H.R.; Mehrad-Majd, H.; Kalamian, M.; Davoodi, S.H. Feasibility, Safety, and Beneficial Effects of MCT-Based Ketogenic Diet for Breast Cancer Treatment: A Randomized Controlled Trial Study. Nutr. Cancer 2019, 72, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Alvarez, R.D.; Leath, C.A., III; Huh, W.K.; Bevis, K.S.; Kim, K.H.; Straughn, J.M., Jr.; Gower, B.A. A Ketogenic Diet Reduces Central Obesity and Serum Insulin in Women with Ovarian or Endometrial Cancer. J. Nutr. 2018, 148, 1253–1260. [Google Scholar] [CrossRef]
- Mullins, G.; Hallam, C.; Broom, I. Ketosis, ketoacidosis and very-low-calorie diets: Putting the record straight. Nutr. Bull. 2011, 36, 397–402. [Google Scholar] [CrossRef]
- Ok, J.H.; Lee, H.; Chung, H.-Y.; Lee, S.H.; Choi, E.J.; Kang, C.M.; Lee, S.M. The Potential Use of a Ketogenic Diet in Pancreatobiliary Cancer Patients After Pancreatectomy. Anticancer Res. 2018, 38, 6519–6527. [Google Scholar] [CrossRef]
- Klement, R.J.; Schäfer, G.; Sweeney, R.A. A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: An interim analysis of the KETOCOMP study. J. Tradit. Complement. Med. 2019, 10, 180–187. [Google Scholar] [CrossRef]
- Brinkworth, G.; Noakes, M.; Clifton, P.; Bird, A.R. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Nutrition and colonic health: The critical role of the microbiota. Curr. Opin. Gastroenterol. 2008, 24, 51–58. [Google Scholar] [CrossRef]
- Johnston, C.S.; Tjonn, S.L.; Swan, P.D.; White, A.; Sears, B. Low-carbohydrate, high-protein diets that restrict potassium-rich fruits and vegetables promote calciuria. Osteoporos. Int. 2006, 17, 1820–1821. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; King, M.T.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Newport, M.T.; VanItallie, T.B.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A New Way to Produce Hyperketonemia: Use of Ketone Ester in a Case of Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Ward, N.; Seyfried, T.N.; Arnold, P.; D’Agostino, D.P. Non-Toxic Metabolic Management of Metastatic Cancer in Vm Mice: Novel Combination of Ketogenic Diet, Ketone Supplementation, and Hyperbaric Oxygen Therapy. PLoS ONE 2015, 10, e0127407. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Vansant, H.; Evans, R.D.; Clarke, K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul. Toxicol. Pharmacol. 2019, 109, 104506. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Castillo-García, A.; Lucia, A. Acute Ketone Supplementation and Exercise Performance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Sports Physiol. Perform. 2020, 15, 298–308. [Google Scholar] [CrossRef]
- Thomsen, H.H.; Rittig, N.; Johannsen, M.; Møller, A.B.; Jørgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: Anticatabolic impact of ketone bodies. Am. J. Clin. Nutr. 2018, 108, 857–867. [Google Scholar] [CrossRef]
- Khoziainova, S.; Rozenberg, G.; Levy, M. Ketogenic Diet and Beta-Hydroxybutyrate in Colorectal Cancer. DNA Cell Biol. 2022, 41, 1007–1011. [Google Scholar] [CrossRef]
- Ungaro, P.; Nettore, I.C.; Franchini, F.; Palatucci, G.; Muscogiuri, G.; Colao, A.; Macchia, P.E. Epigenome Modulation Induced by Ketogenic Diets. Nutrients 2022, 14, 3245. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamraz, M.; Al Ghossaini, N.; Temraz, S. The Ketogenic Diet in Colorectal Cancer: A Means to an End. Int. J. Mol. Sci. 2023, 24, 3683. https://doi.org/10.3390/ijms24043683
Tamraz M, Al Ghossaini N, Temraz S. The Ketogenic Diet in Colorectal Cancer: A Means to an End. International Journal of Molecular Sciences. 2023; 24(4):3683. https://doi.org/10.3390/ijms24043683
Chicago/Turabian StyleTamraz, Magie, Najib Al Ghossaini, and Sally Temraz. 2023. "The Ketogenic Diet in Colorectal Cancer: A Means to an End" International Journal of Molecular Sciences 24, no. 4: 3683. https://doi.org/10.3390/ijms24043683
APA StyleTamraz, M., Al Ghossaini, N., & Temraz, S. (2023). The Ketogenic Diet in Colorectal Cancer: A Means to an End. International Journal of Molecular Sciences, 24(4), 3683. https://doi.org/10.3390/ijms24043683






