Atypical Preeclampsia before 20 Weeks of Gestation—A Systematic Review
Abstract
1. Introduction
PLGF and sFlt-1
2. Case Presentation
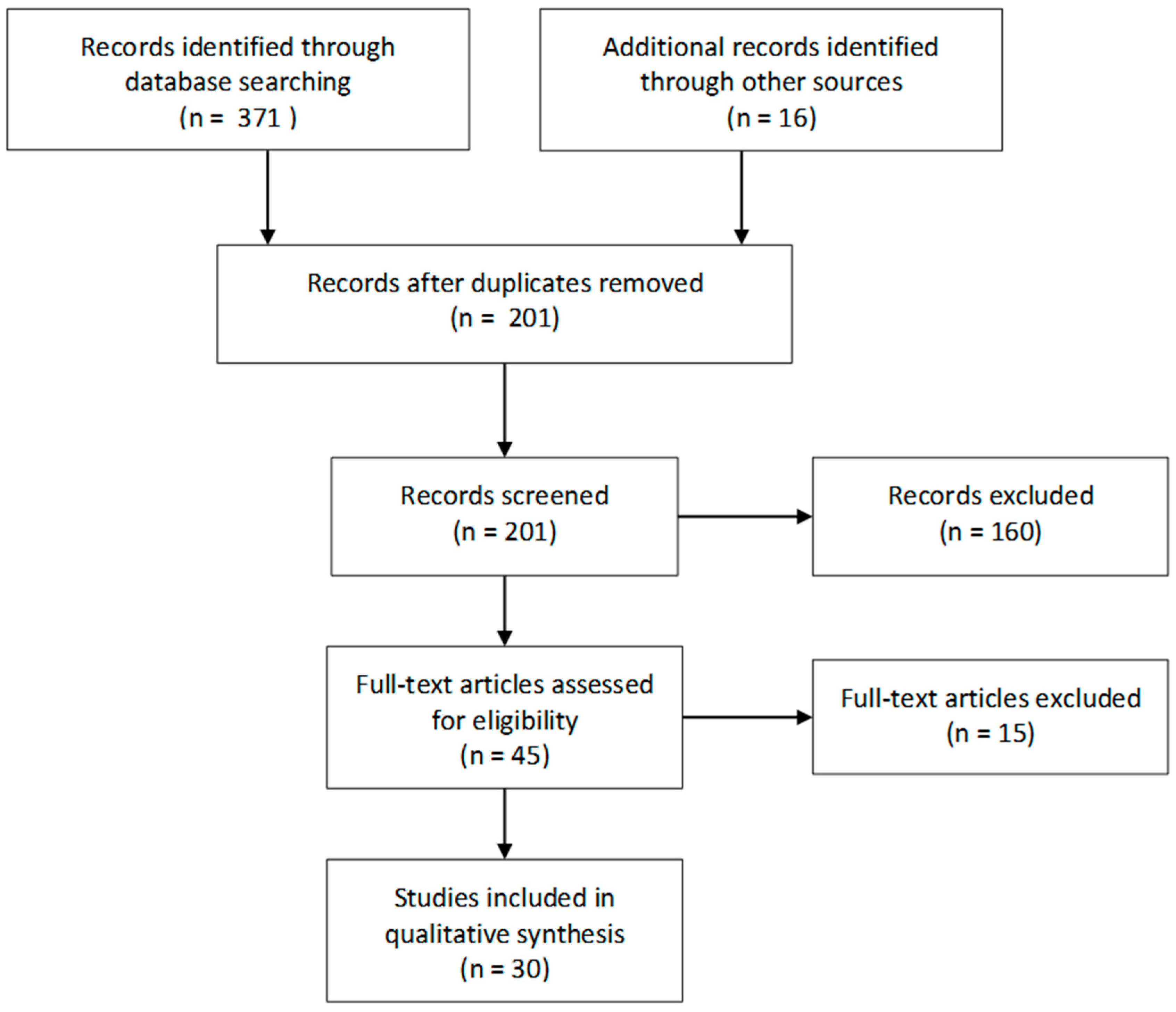
3. Materials and Methods
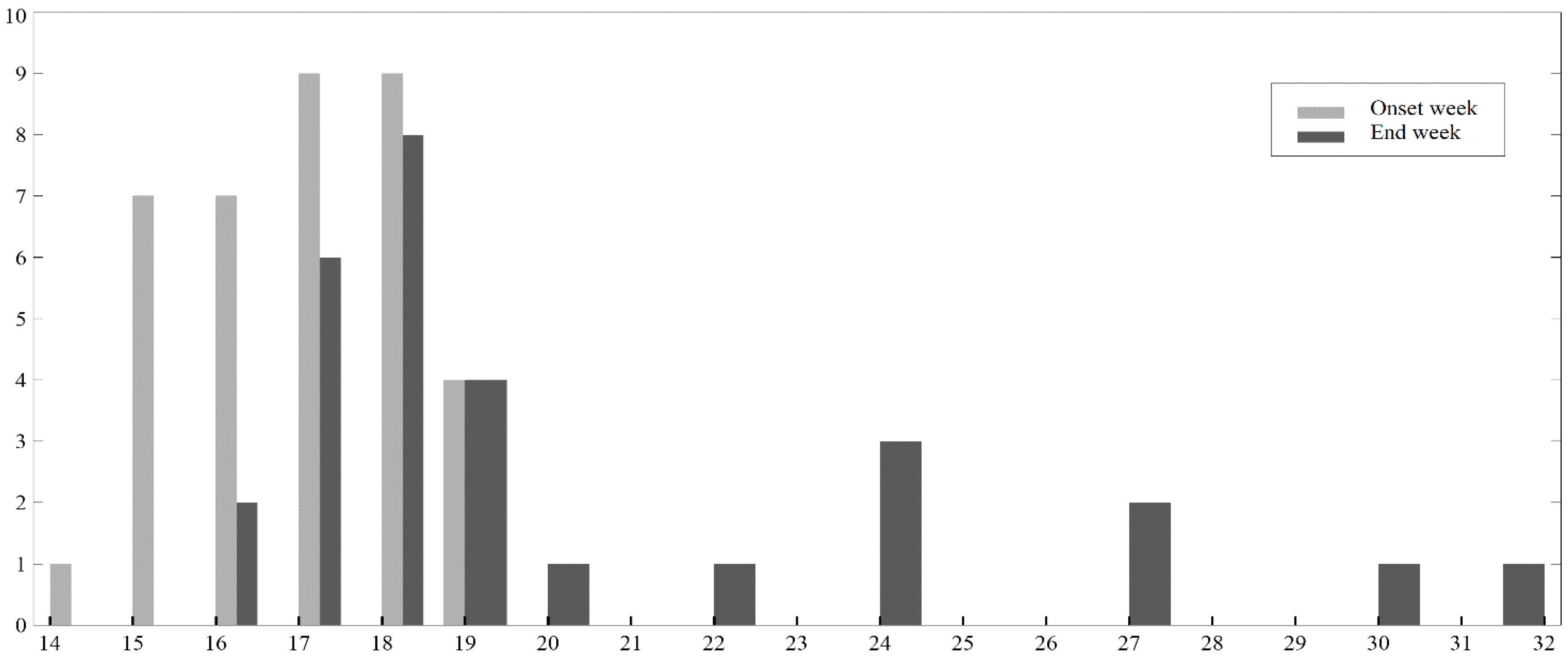
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gómez, T.; Castillo-Marco, N.; Cordero, T.; Simón, C. Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S886–S894. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Kim, S.H.; Cha, D.H.; Park, H.J. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 11202. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Woods, A.; Jauniaux, E.; Kingdom, J. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Meta-Analysis and Systematic Review to Assess the Role of Soluble FMS-Like Tyrosine Kinase-1 and Placenta Growth Factor Ratio in Prediction of Preeclampsia. Hypertension 2018, 71, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Kaczyńska, K. Placental Syndromes—A New Paradigm in Perinatology. Int. J. Environ. Res. Public Health 2022, 19, 7392. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Gurung, S.; Goulwara, S.S.; Whitley, G.S.; Johnstone, A.P.; Cartwright, J.E. Increased angiogenic factor secretion by decidual natural killer cells from pregnancies with high uterine artery resistance alters trophoblast function. Hum. Reprod. 2014, 29, 652–660. [Google Scholar] [CrossRef]
- Nejabati, H.R.; Latifi, Z.; Ghasemnejad, T.; Fattahi, A.; Nouri, M. Placental growth factor (PlGF) as an angiogenic/inflammatory switcher: Lesson from early pregnancy losses. Gynecol. Endocrinol. 2017, 33, 668–674. [Google Scholar] [CrossRef]
- Villalain, C.; Herraiz, I.; Valle, L.; Mendoza, M.; Delgado, J.L.; Vazquez-Fernandez, M.; Martinez-Uriarte, J.; Melchor, I.; Caamiña, S.; Fernandez-Oliva, A.; et al. Maternal and Perinatal Outcomes Associated With Extremely High Values for the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio. J. Am. Heart Assoc. 2020, 9, e015548. [Google Scholar] [CrossRef]
- Vuorela, P.; Hatva, E.; Lymboussaki, A.; Kaipainen, A.; Joukov, V.; Persico, M.G.; Alitalo, K.; Halmesmäki, E. Expression of Vascular Endothelial Growth Factor and Placenta Growth Factor in Human Placenta1. Biol. Reprod. 1997, 56, 489–494. [Google Scholar] [CrossRef] [PubMed]
- MIM Number: 601121: Updated 20/02/2015, access 01/01/2023. In Online Mendelian Inheritance in Man, OMIM®; Johns Hopkins University: Baltimore, MD, USA, 2015.
- Frang, H.; Hurskainen, P.; Nicolaides, K.; Sairanen, M. PlGF isoform 3 in maternal serum and placental tissue. Pregnancy Hypertens. 2019, 18, 9–13. [Google Scholar] [CrossRef]
- Albonici, L.; Benvenuto, M.; Focaccetti, C.; Cifaldi, L.; Miele, M.T.; Limana, F.; Manzari, V.; Bei, R. PlGF Immunological Impact during Pregnancy. Int. J. Mol. Sci. 2020, 21, 8714. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar] [CrossRef]
- MIM Number: 165070: Updated 24/09/2018, access 01/01/2023. In Online Mendelian Inheritance in Man, OMIM®; Johns Hopkins University: Baltimore, MD, USA, 2018.
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac Angiogenic Imbalance Leads to Peri-partum Cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Muehlenbachs, A.; Fried, M.; Lachowitzer, J.; Mutabingwa, T.K.; Duffy, P.E. Natural selection of FLT1 alleles and their association with malaria resistance in utero. Proc. Natl. Acad. Sci. USA 2008, 105, 14488–14491. [Google Scholar] [CrossRef]
- Jebbink, J.; Keijser, R.; Veenboer, G.; van der Post, J.; Ris-Stalpers, C.; Afink, G. Expression of Placental FLT1 Transcript Variants Relates to Both Gestational Hypertensive Disease and Fetal Growth. Hypertension 2011, 58, 70–76. [Google Scholar] [CrossRef]
- Souders, C.A.; Maynard, S.E.; Yan, J.; Wang, Y.; Boatright, N.K.; Sedan, J.; Balyozian, D.; Cheslock, P.S.; Molrine, D.C.; Simas, T.A.M. Circulating Levels of sFlt1 Splice Variants as Predictive Markers for the Development of Preeclampsia. Int. J. Mol. Sci. 2015, 16, 12436–12453. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, L.; Neuman, R.; Broekhuizen, M.; Schoenmakers, S.; Lu, X.; Danser, A. Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas. Int. J. Mol. Sci. 2021, 22, 7407. [Google Scholar] [CrossRef]
- Neuman, R.I.; Baars, M.D.; Saleh, L.; Broekhuizen, M.; Nieboer, D.; Cornette, J.; Schoenmakers, S.; Verhoeven, M.; Koch, B.C.; Russcher, H.; et al. Omeprazole Administration in Preterm Preeclampsia: A Randomized Controlled Trial to Study Its Effect on sFlt-1 (Soluble Fms-Like Tyrosine Kinase-1), PlGF (Placental Growth Factor), and ET-1 (Endothelin-1). Hypertension 2022, 79, 1297–1307. [Google Scholar] [CrossRef]
- Cluver, C.A.; Hannan, N.J.; van Papendorp, E.; Hiscock, R.; Beard, S.; Mol, B.W.; Theron, G.B.; Hall, D.R.; Decloedt, E.H.; Stander, M.; et al. Esomeprazole to treat women with preterm preeclampsia: A randomized placebo controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 388.e1–388.e17. [Google Scholar] [CrossRef]
- Hastie, R.; Bergman, L.; Cluver, C.A.; Wikman, A.; Hannan, N.J.; Walker, S.P.; Wikström, A.-K.; Tong, S.; Hesselman, S. Proton Pump Inhibitors and Preeclampsia Risk Among 157 720 Women. Hypertension 2019, 73, 1097–1103. [Google Scholar] [CrossRef]
- de Alwis, N.; Fato, B.R.; Beard, S.; Binder, N.K.; Kaitu’U-Lino, T.J.; Onda, K.; Hannan, N.J. Assessment of the Proton Pump Inhibitor, Esomeprazole Magnesium Hydrate and Trihydrate, on Pathophysiological Markers of Preeclampsia in Preclinical Human Models of Disease. Int. J. Mol. Sci. 2022, 23, 9533. [Google Scholar] [CrossRef]
- Khalil, A.; Muttukrishna, S.; Harrington, K.; Jauniaux, E. Effect of Antihypertensive Therapy with Alpha Methyldopa on Levels of Angiogenic Factors in Pregnancies with Hypertensive Disorders. PLoS ONE 2008, 3, e2766. [Google Scholar] [CrossRef]
- Gangooly, S.; Muttukrishna, S.; Jauniaux, E. In-Vitro Study of the Effect of Anti-Hypertensive Drugs on Placental Hormones and Angiogenic Proteins Synthesis in Pre-Eclampsia. PLoS ONE 2014, 9, e107644. [Google Scholar] [CrossRef]
- Alzoubi, O.; Maaita, W.; Madain, Z.; Alzoubi, M.; Sweis, J.J.G.; Arar, A.R.; Sweis, N.W.G. Association between placenta accreta spectrum and third-trimester serum levels of vascular endothelial growth factor, placental growth factor, and soluble Fms-like tyrosine kinase-1: A meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 2363–2376. [Google Scholar] [CrossRef]
- Meakin, A.; Cuffe, J.; Darby, J.; Morrison, J.; Clifton, V. Let’s Talk about Placental Sex, Baby: Understanding Mechanisms That Drive Female- and Male-Specific Fetal Growth and Developmental Outcomes. Int. J. Mol. Sci. 2021, 22, 6386. [Google Scholar] [CrossRef] [PubMed]
- Imasawa, T.; Nishiwaki, T.; Nishimura, M.; Shikama, N.; Matsumura, R.; Nagai, M.; Soyama, A.; Koike, K.; Kitamura, H.; Joh, K. A Case of “Pure” Preeclampsia With Nephrotic Syndrome Before 15 Weeks of Gestation in a Patient Whose Renal Biopsy Showed Glomerular Capillary Endotheliosis. Am. J. Kidney Dis. 2006, 48, 495–501. [Google Scholar] [CrossRef]
- Suzuki, T.; Ichikawa, D.; Nakata, M.; Watanabe, S.; Han, W.; Kohatsu, K.; Shirai, S.; Imai, N.; Koike, J.; Shibagaki, Y. Nephrotic syndrome due to preeclampsia before 20 weeks of gestation: A case report. BMC Nephrol. 2020, 21, 240. [Google Scholar] [CrossRef]
- Stefos, T.; Plachouras, N.; Mari, G.; Cosmi, E.; Lolis, D. A case of partial mole and atypical type I triploidy associated with severe HELLP syndrome at 18 weeks’ gestation. Ultrasound Obstet. Gynecol. 2002, 20, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, N.; Shiozaki, A.; Miura, K.; Yonezawa, R.; Takemura, K.; Yoneda, S.; Masuzaki, H.; Saito, S. A triploid partial mole placenta from paternal isodisomy with a diploid fetus derived from one sperm and one oocyte may have caused angiogenic imbalance leading to preeclampsia-like symptoms at 19 weeks of gestation. Placenta 2013, 34, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Myer, E.; Hill, J. First trimester hemolysis, elevated liver enzymes, low platelets syndrome in a surrogate pregnancy. AJP Rep. 2015, 5, e212–e214. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Waugh, J.; Bosio, P. “Pure” pre-eclampsia before 20 weeks of gestation: A unique entity. Int. J. Obstet. Gynaecol. 2003, 110, 1034–1035. [Google Scholar] [CrossRef]
- Bornstein, E.; Barnhard, Y.; Atkin, R.; Divon, M.Y. HELLP syndrome: A rare, early presentation at 17 weeks of gestation. Obstet. Gynecol. 2007, 110, 525–527. [Google Scholar] [CrossRef]
- Alsulyman, O.M.; Amescastro, M.; Zuckerman, E.; McGehee, W.; Murphygoodwin, T. Preeclampsia and liver infarction in early pregnancy associated with the antiphospholipid syndrome. Obstet. Gynecol. 1996, 88, 644–646. [Google Scholar] [CrossRef]
- Stillman, I.E.; Karumanchi, S.A. The Glomerular Injury of Preeclampsia. J. Am. Soc. Nephrol. 2007, 18, 2281–2284. [Google Scholar] [CrossRef]
- Parrott, J.; Fields, T.A.; Parrish, M. Previable Preeclampsia Diagnosed by Renal Biopsy in Setting of Novel Diagnosis of C4 Glomerulopathy. Case Rep. Obstet. Gynecol. 2017, 2017, 8698670. [Google Scholar] [CrossRef]
- Tanaka, M.; Tsujimoto, Y.; Goto, K.; Kumahara, K.; Onishi, S.; Iwanari, S.; Fumihara, D.; Miki, S.; Ikeda, M.; Sato, K.; et al. Preeclampsia before 20 weeks of gestation: A case report and review of the literature. CEN Case Rep. 2015, 4, 55–60. [Google Scholar] [CrossRef]
- Maya, I.D. Hypertension and proteinuria in a 17-year-old at 19 weeks’ gestation. Am. J. Kidney Dis. 2008, 51, 155–159. [Google Scholar] [CrossRef]
- Mayer-Pickel, K.; Stern, C.; Cervar-Zivkovic, M.; Schöll, W.; Moertl, M. Preeclampsia before fetal viability in women with primary antiphospholipid syndrome- materno-fetal outcomes in a series of 7 cases. J. Reprod. Immunol. 2020, 138, 103101. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.N.; Nelson, D.B.; Spong, C.Y.; McIntire, D.D.; Reddy, M.; Cunningham, F.G. 1012 Acute kidney injury in pregnancies complicated by preeclampsia with severe features. Am. J. Obstet. Gynecol. 2021, 224, S627. [Google Scholar] [CrossRef]
- Romero-Arauz, J.F.; Carranco-Salinas, C.; Leaños-Miranda, A.; Martínez-Rodríguez, O.A. Atypical preeclampsia: Case report. Ginecol. Obstet. Mex. 2014, 82, 354–360. [Google Scholar] [PubMed]
- Konstantopoulos, A.; Sfakianoudis, K.; Simopoulou, M.; Kontogeorgi, A.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Bathrellos, N.; Grammatis, A.; Pantos, K. Early Onset Preeclampsia Diagnosis Prior to the 20th Week of Gestation in a Twin Pregnancy Managed via Selective Reduction of an Intrauterine Growth Restriction Fetus: A Case Report and Literature Review. Diagnostics 2020, 10, 531. [Google Scholar] [CrossRef]
- Khan, B.S.A.; Sharma, Y.; Soman, S.S.; Jandali, H.A.; Reddy, S. In vitro fertilization (IVF): Early-onset preeclampsia before 16 weeks’ gestation. J. Am. Soc. Nephrol. 2019, 30, 861. [Google Scholar]
- Thomas, W.; Griffiths, M.; Nelson-Piercy, C.; Sinnamon, K. Pre-eclampsia before 20-week gestation: Diagnosis, investigation and management. Clin. Kidney J. 2012, 5, 597–599. [Google Scholar] [CrossRef]
- Craig, K.; Pinette, M.G.; Blackstone, J.; Chard, R.; Cartin, A. Highly abnormal maternal inhibin and β-human chorionic gonadotropin levels along with severe HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome at 17 weeks’ gestation with triploidy. Am. J. Obstet. Gynecol. 2000, 182, 737–739. [Google Scholar] [CrossRef]
- Nwosu, E.C.; Ferriman, E.; McCormack, M.J.; Williams, J.H.; Gosden, C.M. Partial hydatidiform mole and hypertension associated with a live fetus—Variable presentation in two cases. Hum. Reprod. 1995, 10, 2459–2462. [Google Scholar] [CrossRef]
- Billieux, M.-H.; Petignat, P.; Fior, A.; Mhawech, P.; Blouin, J.-L.; Dahoun, S.; Vassilakos, P. Pre-eclampsia and peripartum cardiomyopathy in molar pregnancy: Clinical implication for maternally imprinted genes. Ultrasound Obstet. Gynecol. 2004, 23, 398–401. [Google Scholar] [CrossRef]
- Brittain, P.C.; Bayliss, P. Partial Hydatidiform Molar Pregnancy Presenting with Severe Preeclampsia Prior to Twenty Weeks Gestation: A Case Report and Review of the Literature. Mil. Med. 1995, 160, 42–44. [Google Scholar] [CrossRef]
- Saad, O.E.; Tanouti, S.; Bkiyar, H.; Mimouni, A.; Housni, B. Near death 18 weeks preeclampsia in molar pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 2020, 9, 877. [Google Scholar] [CrossRef]
- Rahimpanah, F.; Smoleniec, J. Partial mole, triploidy and proteinuric hypertension: Two case reports. Aust. N. Z. J. Obstet. Gynaecol. 2000, 40, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Nugent, C.E.; Punch, M.R.; Barr, M.; LeBlanc, L.; Johnson, M.P.; Evans, M.I. Persistence of partial molar placenta and severe preeclampsia after selective termination in a twin pregnancy. Obstet. Gynecol. 1996, 87, 829–831. [Google Scholar]
- Prasannan-Nair, C.; Reynolds, S.F.; Budden, G. Partial molar pregnancy with severe pre-eclampsia at 19 weeks’ gestation. J. Obstet. Gynaecol. 2006, 26, 817. [Google Scholar] [CrossRef]
- Sherer, D.M.; Dalloul, M.; Stimphil, R.; Hellmann, M.; Khoury-Collado, F.; Osho, J.; Fomitcheva, L.; Brennan, K.J.; Abulafia, O. Acute Onset of Severe Hemolysis, Elevated Liver Enzymes, and Low Platelet Count Syndrome in a Patient with a Partial Hydatidiform Mole at 17 Weeks Gestation. Am. J. Perinatol. 2006, 23, 163–166. [Google Scholar] [CrossRef]
- Haram, K.; Trovik, J.; Sandset, P.M.; Hordnes, K. Severe syndrome of hemolysis, elevated liver enzymes and low platelets (HELLP) in the 18th week of pregnancy associated with the antiphospholipid–antibody syndrome. Acta Obstet. Gynecol. Scand. 2003, 82, 679–680. [Google Scholar] [CrossRef]
- McMahon, L.P.; Smith, J. The HELLP Syndrome at 16 weeks’Gestation: Possible Association with the Antiphospholipid Syndrome. Aust. N. Z. J. Obstet. Gynaecol. 1997, 37, 313–314. [Google Scholar] [CrossRef]
- De Weg, J.B.; De Groot, C.; Pajkrt, E.; De Vries, H.; De Boer, M. 143. Recovery of second trimester preeclampsia in triplet after foetal reduction; a case history and review of the literature. Pregnancy Hypertens. 2018, 13, S84. [Google Scholar] [CrossRef]
- Stevens, A.B.; Brasuell, D.M.; Higdon, R.N. Atypical preeclampsia—Gestational proteinuria. J. Fam. Med. Prim. Care 2017, 6, 669–671. [Google Scholar] [CrossRef]
- Albayrak, M.; Özdemir, I.; Demiraran, Y.; Dikici, S. Atypical preeclampsia and eclampsia: Report of four cases and review of the literature. J. Turk. Ger. Gynecol. Assoc. Artemis 2010, 11, 115–117. [Google Scholar] [CrossRef]
- Castelazo-Morales, E.; Monzalbo-Núñez, D.E.; López-Rioja, M.J.; Castelazo-Alatorre, S. Atypical preeclampsia and perinatal success: A case report. Ginecol. Obstet. Mex. 2014, 82, 70–74. [Google Scholar]
- Ditisheim, A.; Boulvain, M.; Irion, O.; Pechère-Bertschi, A. Atypical presentation of preeclampsia. Rev. Med. Suisse. 2015, 11, 1655–1658. [Google Scholar] [PubMed]
- Quintero-Loaiza, C.A.; Parra-Saavedra, M.; Molina-Giraldo, S.; Figueras, F.; Rojas-Arias, J.L.; Ortiz-López, L.D.; Orduña-Aparicio, W.J.; Acuña-Osorio, E.; Franco-Hernández, A. Characterization of atypical preeclampsia. Fetal Diagn. Ther. 2015, 38, 119–125. [Google Scholar] [CrossRef]
- Valle Tejero, A.; Monfort, I.R.; Calvo, P.; Aguilar, A.; Romero, A.; Diago, V.; Perales, A. Classic vs Atipic HELLP sydrome. Obstetric and perinatal results. J. Perinat. Med. 2015, 43, 432–1299. [Google Scholar] [CrossRef]
- Sibai, B.M.; Stella, C.L. Diagnosis and management of atypical preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 2009, 200, 481.e1–481.e7. [Google Scholar] [CrossRef]
- van Scheltinga, J.A.T.; Krabbendam, I.; Spaanderman, M.E. Differentiating between gestational and chronic hypertension; An explorative study. Acta Obstet. Gynecol. Scand. 2013, 92, 312–317. [Google Scholar] [CrossRef]
- Van Scheltinga, J.A.T.; Krabbendam, I.; Lotgering, F.K.; Spaanderman, M.E.A. Hypertension before 20 weeks gestation and chronic hypertension. Reprod. Sci. 2010, 17, 238A. [Google Scholar] [CrossRef]
- Seguro, F.; Duly Bouhanick, B.; Chamontin, B.; Amar, J. Management of arterial hypertension before 20 weeks gestation in pregnant women. Presse. Med. 2016, 45, 627–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Long, Y.; Ma, Q.; Chen, R. Plasma Level of Placenta-Derived Macrophage-Stimulating Protein -Chain in Preeclampsia before 20 Weeks of Pregnancy. PLoS ONE 2016, 11, e0161626. [Google Scholar] [CrossRef]
- Sáez Cantero, V.L.C. Preeclampsia and eclampsia with atypical presentation. Prog. Obstet. Ginecol. 2012, 55, 326–328. [Google Scholar] [CrossRef]
- Stella, C.L.; Sibai, B.M. Preeclampsia: Diagnosis and management of the atypical presentation. J. Matern. Fetal. Neonatal. Med. 2006, 19, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M.; Abdella, T.H.; Taylor, H.A. Eclampsia in the first half of pregnancy. A report of three cases and review of the literature. J. Reprod. Med. 1982, 27, 706–708. [Google Scholar] [PubMed]
- Garland, J.S.; Smith, G.N.; Moran, S.M. TMA in pregnancy before 20 weeks gestation: Is this preeclampsia or primary TMA? Can. J. Kidney Health Dis. 2020, 7, 8–9. [Google Scholar] [CrossRef]

| Case 1 (G1P0) | Case 2 (G4P1) | Case 3 (G1P0) | |
|---|---|---|---|
| Onset week | 16 | 15 | 17 |
| Comorbidities | APS, history of thrombosis | DM, obesity | Idiopathic thrombocytopenia |
| First-trimester screening—risks, according to FMF protocol | Trisomy—low; no PE screening, no fetal malformations | Trisomy—low; PE—1:4, PLGF—13.3 pg/ml (0.33 MoM) | Trisomy 21 1:340, NIPT—low risk; no PE screening, PAPP-A 0.7 MoM |
| Angiogenesis markers at the onset | Sflt-1—8956 pg/mL; PLGF—9.96 pg/mL; sFlt-1/PLGF ratio—899 | sFlt-1—3540 pg/mL; PlGF—9.21 pg/mL; sFlt-1/PlGF ratio—393 | PLGF—20.5 pg/mL; sFlt—10610.0 pg/mL; sFlt-1/PLGF ratio—517.6 |
| Uterine artery flow at the onset | Left UtA PI—3.04; right UtA PI—3.55; mean PI—3.295 > 95th percentile | Left UtA PI—3.7; right UtA PI—2.89; mean PI—3.295 > 95th percentile | Left UtA PI—2.25; right UtA PI—2.1; mean PI—2.175 > 95th percentile |
| Outcome | IUFD at 19 weeks, placental aCGH normal | IUFD at 15 weeks, placental aCGH normal | IUFD at 24 weeks, anhydramniosis, birth of a stillborn female fetus 370 g |
| Case | Yoneda et al. [31] | Romero-Arauz et al. [32] | Suzuki et al. [33] |
|---|---|---|---|
| Reported angiogenesis markers | At onset: sFlt-1—4196 pg/mL; PLGF—127 ng/mL, sFlt-1/PLGF ratio—476 | At onset: sFLT-1/PLGF ratio—895.5 | At 21 weeks (onset at 16): sFlt-1—13,400 pg/mL; PlGF, —21.9 pg/mL; sFlt-1/PlGF ratio—611.9 |
| Case | Authors: | Onset Week | Pregnancy | Comorbidities | Outcome |
|---|---|---|---|---|---|
| 1 | Imasawa et al. [31] | 15 | G1P0, dichorionic twin | 12 cm leiomyoma | TOP 18 HBD |
| 2 | Suzuki et al. [32] | 16 | G5P1, singleton | Protein S deficiency, infertility | Cesarean section at 24 + 3 HBD, LB 303 g, retinopathy, RDS, rickets |
| 3 | Stefos et al. [33] | 18 | G1P0, partial molar, growth-restricted fetus | none | TOP 18 HBD |
| 4 | Yoneda et al. [34] | 19 | G1P0, partial molar, growth-restricted fetus | none | TOP 20 HBD |
| 5 | Myer et al. [35] | 15 | G3P2, twin surrogate IVF pregnancy | Chronic hypertension, asthma, positive ANA-antibodies | IUFD 9 and 16 HBD |
| 6 | Hazra et al. [36] | 18 | G4P3, singleton | Thyroidectomy at 9-year-old | TOP 18 HBD |
| 7 | Bornstein et al. [37] | 15 | G4P0, singleton | Obesity | TOP 17 HBD |
| 8 | Alsulyman et al. [38] | 17 | G2P1, singleton, admitted because of upper abdominal pain and biochemical markers of HELLP | Antiphospholipid syndrome | IUFD 17 HBD |
| 9 | Alsulyman et al. [38] | 19 | G3P2, singleton | Antiphospholipid syndrome | IUFD 19 HBD |
| 10 | Alsulyman et al. [38] | 17 | G3P2, singleton | Antiphospholipid syndrome | IUFD 17 HBD |
| 11 | Stillman et al. [39] | 15 | G1P0, fetal reduction to twins, IVF | PCOS | TOP, HBD not given |
| 12 | Parrott et al. [40] | 18 | G3P0, singleton | None | TOP 18 HBD |
| 13 | Tanaka et al. [41] | 17 | G1P0, singleton, admission because of isolated leg edema | None | IUFD 22 HBD |
| 14 | Maya et al. [42] | 19 | G1P0, singleton | None | TOP, HBD not given |
| 15 | Mayer-Picke et al. [43] | 17 | G2P1, singleton, admitted because of abdominal pain; plasma exchange after admission | Antiphospholipid syndrome | LB 27 HBD, normal development |
| 16 | Mayer-Picke et al. [43] | 17 | G1P0, singleton, admitted because of abdominal pain; plasma exchange after admission | Antiphospholipid syndrome | TOP 24 HBD |
| 17 | Mayer-Picke et al. [43] | 19 | G1P0, singleton, admitted because of thrombocytopenia; plasma exchange after admission | Antiphospholipid syndrome | LB 27 HBD, normal development |
| 18 | Rodríguez et al. [44] | 16 | G1P0, singleton, partial molar | None | TOP 16 HBD |
| 19 | Romero-Arauz et al. [45] | 18 | G1P0, singleton, IVF | Chronic hypertension, infertility | TOP 18 HBD |
| 20 | Konstantopoulos et al. [46] | 18 | G1P0, twin after double donated embryo transfer, sFGR of one fetus | Infertility | Selective reduction of sFGR twin, CS 30 HBD, retinopathy of prematurity |
| 21 | Khan et al. [47]—no full text available, data from abstract | 15 | G2P0, singleton, IVF | Infertility | TOP 17 HBD |
| 22 | Thomas et al. [48] | 14 | G6P4, singleton | Chronic hypertension, obesity, cholecystectomy | TOP 22 HBD |
| 23 | Craig et al. [49] | 17 | G1P0, singleton, kariotype 69 XXY | None | TOP 17 HBD |
| 24 | Nwosu et al. [50] | 18 | G2P1, dichornionic twin | None | TOP, HBD not given |
| 25 | Billieux et al. [51] | 18 | G2P1, partial molar | None | TOP 18 HBD |
| 26 | Brittain and Bayliss [52] | 18 | G7P2, partial molar | No information given | TOP, HBD not given |
| 27 | Es Saad et al. [53] | 16 | G2P1, complete molar | None | TOP, HBD not given |
| 28 | Rahimpanah and Smoleniec [54] | 16 | G2P1, partial molar | No information given | TOP, HBD not given |
| 29 | Nugent et al. [55]—no full text available, data from abstract | 15 | Twin pregnancy, one fetus normal, another partial molar | No information given | TOP of the partial molar fetus 15 HBD, normal twin 19 HBD |
| 30 | Prasannan-Nair et al. [56] | 17 | G1P0, partial molar | No information given | TOP at 19 HBD |
| 31 | Sherer et al. [57] | 17 | G2P1, partial molar, fetal karyotype 69, XXY | No information given | TOP at 17 HBD |
| 32 | Haram et al. [58] | 18 | G4P0, singleton, admitted due to epigastric pain | Antiphospholipid syndrome, protein S deficiency | TOP at 18 HBD |
| 33 | McMahon et al. [59] | 16 | G1P0, singleton, admitted due to epigastric pain | None | IUFD at 18 HBD |
| 34 | de Weg et al. [60]—no full text available, data from abstract | 16 | Triplet pregnancy | No information given | Selective reduction of one monochorionic twin, delivery in 32 HBD |
| Author | Title | Reason for Rejection | |
|---|---|---|---|
| 1 | Stevens et al. [61] | Atypical preeclampsia—gestational proteinuria | PE > 20 weeks of gestation |
| 2 | Albayrak et al. [62] | Atypical preeclampsia and eclampsia: report of four cases and review of the literature | PE > 20 weeks of gestation |
| 3 | Castelazo-Morales et al. [63] | Atypical preeclampsia and perinatal success: a case report | PE > 20 weeks of gestation |
| 4 | Ditisheim et al. [64] | Atypical presentation of preeclampsia | No text available |
| 5 | Rojas-Arias et al. [65] | Characterization of atypical preeclampsia | PE > 20 weeks of gestation |
| 6 | Valle Tejero et al. [66] | Classic vs atypical HELLP syndrome. Obstetric and perinatal results | No text available |
| 7 | Sibai and Stella [67] | Diagnosis and management of atypical preeclampsia-eclampsia | No data on PE < 20 weeks presented |
| 8 | Van Scheltinga et al. [68] | Differentiating between gestational and chronic hypertension; an explorative study | No data on the timing of PE onset |
| 9 | Van Scheltinga et al. [69] | Hypertension before 20 weeks’ gestation and chronic hypertension | No text available |
| 10 | Seguro et al. [70] | Management of arterial hypertension before 20 weeks’ gestation in pregnant women | No data on PE < 20 weeks presented |
| 11 | Zhang et al. [71] | Plasma level of placenta-derived macrophage-stimulating protein-chain in preeclampsia before 20 weeks of pregnancy | No data on PE < 20 weeks presented |
| 12 | Sáez Cantero et al. [72] | Preeclampsia and eclampsia with atypical presentation | No data on PE < 20 weeks presented |
| 13 | Stella and Sibai [73] | Preeclampsia: Diagnosis and management of the atypical presentation | No data on PE < 20 weeks presented |
| 14 | Sibai et al. [74] | Eclampsia in the first half of pregnancy. A report of three cases and a review of the literature | No text available |
| 15 | Garland et al. [75] | TMA in pregnancy before 20 weeks’ gestation: Is this preeclampsia or primary TMA? | No data on PE < 20 weeks presented |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modzelewski, J.; Siarkowska, I.; Pajurek-Dudek, J.; Feduniw, S.; Muzyka-Placzyńska, K.; Baran, A.; Kajdy, A.; Bednarek-Jędrzejek, M.; Cymbaluk-Płoska, A.; Kwiatkowska, E.; et al. Atypical Preeclampsia before 20 Weeks of Gestation—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3752. https://doi.org/10.3390/ijms24043752
Modzelewski J, Siarkowska I, Pajurek-Dudek J, Feduniw S, Muzyka-Placzyńska K, Baran A, Kajdy A, Bednarek-Jędrzejek M, Cymbaluk-Płoska A, Kwiatkowska E, et al. Atypical Preeclampsia before 20 Weeks of Gestation—A Systematic Review. International Journal of Molecular Sciences. 2023; 24(4):3752. https://doi.org/10.3390/ijms24043752
Chicago/Turabian StyleModzelewski, Jan, Iga Siarkowska, Justyna Pajurek-Dudek, Stepan Feduniw, Katarzyna Muzyka-Placzyńska, Arkadiusz Baran, Anna Kajdy, Magdalena Bednarek-Jędrzejek, Aneta Cymbaluk-Płoska, Ewa Kwiatkowska, and et al. 2023. "Atypical Preeclampsia before 20 Weeks of Gestation—A Systematic Review" International Journal of Molecular Sciences 24, no. 4: 3752. https://doi.org/10.3390/ijms24043752
APA StyleModzelewski, J., Siarkowska, I., Pajurek-Dudek, J., Feduniw, S., Muzyka-Placzyńska, K., Baran, A., Kajdy, A., Bednarek-Jędrzejek, M., Cymbaluk-Płoska, A., Kwiatkowska, E., & Kwiatkowski, S. (2023). Atypical Preeclampsia before 20 Weeks of Gestation—A Systematic Review. International Journal of Molecular Sciences, 24(4), 3752. https://doi.org/10.3390/ijms24043752





