Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols
Abstract
1. Introduction
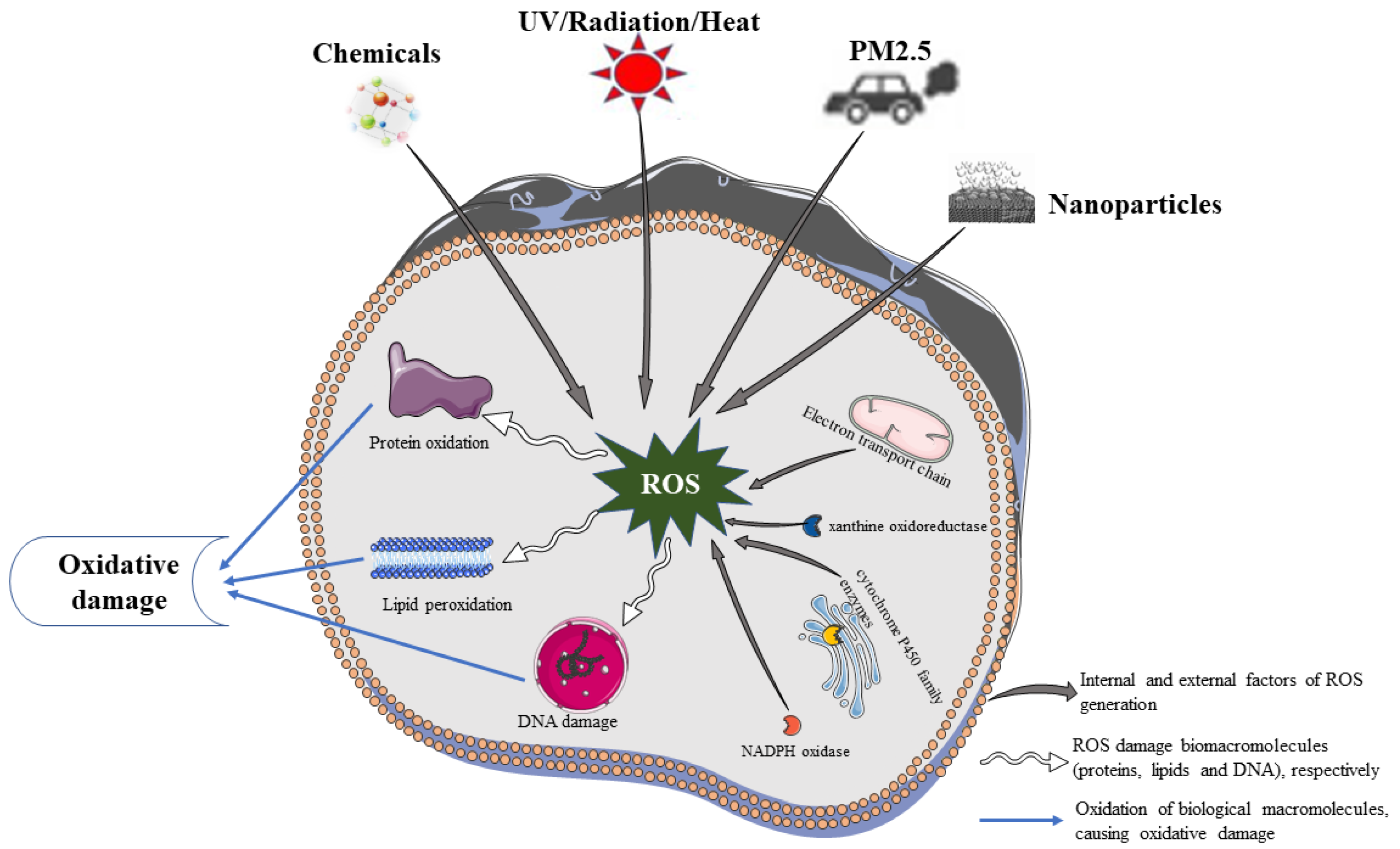
2. Sources of ROS in the Skin
2.1. Endogenous Factors of ROS
2.2. Exogenous Factors of ROS
3. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer
3.1. Oxidative Damage of Biological Macromolecules
3.1.1. Biomacromolecule Damage and Skin Cellular Senescence
3.1.2. Biomacromolecule Damage and Skin Inflammation
3.1.3. Biomacromolecule Damage and Skin Cancer
3.2. Oxidative Stress-Related Signaling Pathway
3.2.1. Skin Cellular Senescence-Related Signaling Pathways
3.2.2. Skin Inflammation-Related Signaling Pathways
3.2.3. Skin Cancer-Related Signaling Pathways
3.2.4. Antioxidant Defense-Related Signaling Pathway
4. Therapeutic Potential of Plant Polyphenols
4.1. Health-Promoting Benefits of Natural Products
4.2. Structure and Classification of Plant Polyphenols
4.3. Antioxidative, Anti-Inflammatory, and Anticancer Activities of Plant Polyphenols
4.4. Regulatory Mechanism of Plant Polyphenols
4.4.1. Curcumin
4.4.2. Catechins
4.4.3. Resveratrol
4.4.4. Quercetin
4.4.5. Ellagic Acid
4.4.6. Honokiol
4.4.7. Proanthocyanidins
4.5. Delivery Systems for Topical Use of Plant Polyphenols
4.6. Clinical Evidence That Plant Polyphenols Prevent Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| B16 | mouse melanoma cells |
| A375 and C8161 | melanoma cells |
| A431 | human epidermoid carcinoma cells |
| HST | human skin tissues |
| JB6 P+ | mouse epithelial cells |
| HFF-1 | skin epithelial cells |
| HaCaT | human immortalized epidermal cells |
| HDF | human dermal fibroblasts |
| BCC | basal cell carcinoma |
| SCC | squamous cell carcinoma |
| AD | atopic dermatitis |
| CD | contact dermatitis |
| SD | solar dermatitis |
| AP-1 | activator protein 1 |
| Bcl-2 | B-cell lymphoma 2 |
| p53 | tumor suppressor proteins and transcription factors |
| BOX | tumor suppressor proteins and transcription factors |
| caspase-3 | terminal shearing enzyme during apoptosis |
| Bax | proapoptotic gene |
| Mcl-1 | proapoptotic gene |
| ARE | antioxidant reaction element |
| ERK | extracellular receptor kinase |
| PTEN | phosphatase and tensin homolog |
| PCNA | proliferating cell nuclear antigen |
| PDCD4 | programmed cell death 4 |
| cdk2 | cyclin-dependent kinases |
| MDA | malondialdehyde |
| MMPs | matrix metalloproteinases |
| SOD | superoxide dismutase |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| GST | glutamate sulfur transferase |
| GR | glutathione reductase |
| TIMP | tissue inhibitor of metalloproteinase |
| TNF-a | tumor necrosis factor alpha |
| EGCG | epigallocatechin gallate |
| CAT | catalase |
| COX-2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| HO-1 | heme oxygenase |
| IκB | NF-κB inhibitory protein |
| IKK | nuclear factor kappa-B inhibitory protein kinase |
| IL- | interleukin- |
| IFN-γ | gamma interferon |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun amino-terminal kinases |
| Keap1 | kelch-like ECH-associated protein 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
References
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible Mechanisms of the Prevention of Doxorubicin Toxicity by Cichoric Acid—Antioxidant Nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.A.; Karimi, J.; Talebi, S.S.; Piri, H. The Association of COVID-19 and Reactive Oxygen Species Modulator 1 (ROMO1) with Oxidative Stress. Chonnam Med. J. 2022, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zarbafian, M.; Dayan, S.; Fabi, S.G. Teachings from COVID-19 and aging-An oxidative process. J. Cosmet. Dermatol. 2020, 19, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Krutmann, J. Air pollution (particulatematter and nitrogen dioxide) and skin aging. Hautarzt 2019, 70, 158–162. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Yu, X.M.; Shoaib, M.; Cheng, X.R.; Cui, Y.L.; Hussain, S.; Yan, J.; Zhou, J.; Chen, Q.; Gu, Y.F.; Zou, L.K.; et al. Role of rhizobia in promoting non-enzymatic antioxidants to mitigate nitrogen-deficiency and nickel stresses in Pongamia pinnata. Ecotoxicol. Environ. Saf. 2022, 241, 113789. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Nevinsky, G.A. Essential Protective Role of Catalytically Active Antibodies (Abzymes) with Redox Antioxidant Functions in Animals and Humans. Int. J. Mol. Sci. 2022, 23, 3898. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.-K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local Melatoninergic System as the Protector of Skin Integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Boehm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Izykowska, I.; Piotrowska, A.; Podhorska-Okolow, M.; Cegielski, M.; Zabel, M.; Dziegiel, P. The protective role of melatonin in the course of UV exposure. Postep. Hig. I Med. Dosw. 2008, 62, 23–27. [Google Scholar]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Perez, L.S.; Yee, N.G.; Peralta-Hernandez, J.M.; Bandala, E.R. Comparison between Fenton and Fenton-like reactions for l-proline degradation. Int. J. Environ. Sci. Technol. 2019, 16, 1515–1526. [Google Scholar] [CrossRef]
- Canas, P.E. The role of xanthine oxidase and the effects of antioxidants in ischemia reperfusion cell injury. Acta Physiol. Pharmacol. Et Ther. Latinoam. Organo Asoc. Latinoam. Cienc. Fisiol. Asoc. Latinoam. Farmacol. 1999, 49, 13–20. [Google Scholar]
- Lewen, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. J. Neurotrauma 2000, 17, 871–890. [Google Scholar] [CrossRef]
- Shaul, P.W. Regulation of endothelial nitric oxide synthase: Location, location, location. Annu. Rev. Physiol. 2002, 64, 749–774. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.F.; Masih, A.P.; Alqasmi, I.; Alsulimani, A.; Khan, F.H.; Haque, S. Oxidative-Stress-Induced Cellular Toxicity and Glycoxidation of Biomolecules by Cosmetic Products under Sunlight Exposure. Antioxidants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Yu, S.-H.; Lee, S.-E.; Park, J.H.; Cho, G.; Choi, C.; Han, K.; Kim, C.-H.; Kang, Y.C. Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts. Platelets 2023, 34, 2151996. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Lee, C.-L.; Korivi, M.; Liao, J.-W.; Rajendran, P.; Wu, J.-J.; Hseu, Y.-C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef]
- Huang, K.-F.; Ma, K.-H.; Chang, Y.-J.; Lo, L.-C.; Jhap, T.-Y.; Su, Y.-H.; Liu, P.-S.; Chueh, S.-H. Baicalein inhibits matrix metalloproteinase 1 expression via activation of TRPV1-Ca-ERK pathway in ultraviolet B-irradiated human dermal fibroblasts. Exp. Dermatol. 2019, 28, 568–575. [Google Scholar] [CrossRef]
- Lee, E.H.; Faulhaber, D.; Hanson, K.M.; Ding, W.H.; Peters, S.; Kodali, S.; Granstein, R.D. Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J. Investig. Dermatol. 2004, 122, 510–517. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Q.; Zhang, Y.; Shi, W.; Wang, H.; Zheng, Z.; Meng, L.; Xin, Y.; Jiang, X. Sulforaphane-Mediated Nrf2 Activation Prevents Radiation-Induced Skin Injury through Inhibiting the Oxidative-Stress-Activated DNA Damage and NLRP3 Inflammasome. Antioxidants 2021, 10, 1850. [Google Scholar] [CrossRef]
- Lin, K.-T.; Chang, T.-C.; Lai, F.-Y.; Lin, C.-S.; Chao, H.-L.; Lee, S.-Y. Rhodiola crenulata Attenuates gamma-Ray Induced Cellular Injury via Modulation of Oxidative Stress in Human Skin Cells. Am. J. Chin. Med. 2018, 46, 175–190. [Google Scholar] [CrossRef]
- Dong, L.; Hu, R.; Yang, D.; Zhao, J.; Kan, H.; Tan, J.; Guan, M.; Kang, Z.; Xu, F. Fine Particulate Matter (PM2.5) upregulates expression of Inflammasome NLRP1 via ROS/NF-kappa B signaling in HaCaT Cells. Int. J. Med. Sci. 2020, 17, 2200–2206. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.-M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, N.; Mao, M.; Zhou, Y.; Wu, Y.; Li, J.; Zhang, W.; Peng, C.; Chen, X.; Li, J. Fine particulate matter (PM2.5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. J. Dermatol. Sci. 2021, 102, 47–57. [Google Scholar] [CrossRef]
- Liang, P.; Xing, X.; Wu, J.; Song, J.; Liu, Q. PM2.5 promotes apoptosis of human epidermal melanocytes through promoting oxidative damage and autophagy. Gen. Physiol. Biophys. 2020, 39, 569–577. [Google Scholar] [CrossRef]
- Balke, J.; Volz, P.; Neumann, F.; Brodwolf, R.; Wolf, A.; Pischon, H.; Radbruch, M.; Mundhenk, L.; Gruber, A.D.; Ma, N.; et al. Visualizing Oxidative Cellular Stress Induced by Nanoparticles in the Subcytotoxic Range Using Fluorescence Lifetime Imaging. Small 2018, 14, e1800310. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Alkahtani, S.; AlKahtane, A.A.; Almalik, A. Cerium Oxide Nanoparticles Induce Oxidative Stress and Genotoxicity in Human Skin Melanoma Cells. Cell Biochem. Biophys. 2015, 71, 1643–1651. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alakhtani, S.; Al Suhaibani, E.S.; Al-Qahtani, A.A. Reactive Oxygen Species-Mediated DNA Damage and Apoptosis in Human Skin Epidermal Cells After Exposure to Nickel Nanoparticles. Biol. Trace Elem. Res. 2014, 157, 84–93. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Chen, W.; Li, N.; Zhai, C.; Liu, Z.; Xiao, Z.; Li, P.; Zhao, J. The expression and roles of chemokine CCL20 in the skin lesions of psoriasis. Immunol. J. 2017, 33, 505–511. [Google Scholar]
- Yoon, Y.S.; Sajo, M.; Ignacio, R.M.C.; Kim, S.K.; Kim, C.S.; Lee, K.J. Positive Effects of Hydrogen Water on 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in NC/Nga Mice. Biol. Pharm. Bull. 2014, 37, 1480–1485. [Google Scholar] [CrossRef]
- Huang, J.K.; Feng, X.Y.; Zeng, J.; Zhang, S.C.; Zhang, J.; Guo, P.; Yu, H.Y.; Sun, M.K.; Wu, J.M.; Li, M.Y.; et al. Aberrant HO-1/NQO1-Reactive Oxygen Species-ERK Signaling Pathway Contributes to Aggravation of TPA-Induced Irritant Contact Dermatitis in Nrf2-Deficient Mice. J. Immunol. 2022, 208, 1424–1433. [Google Scholar] [CrossRef]
- Lou, Q.; Zhang, M.C.; Zhang, K.Y.; Liu, X.A.; Zhang, Z.H.; Zhang, X.; Yang, Y.M.; Gao, Y.H. Arsenic exposure elevated ROS promotes energy metabolic reprogramming with enhanced AKT-dependent HK2 expression. Sci. Total Environ. 2022, 836. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-W.; Liu, F.-C.; Wang, Y.-R.; Tsai, H.-I.; Yu, H.-P. Aloin Protects Skin Fibroblasts from Heat Stress-Induced Oxidative Stress Damage by Regulating the Oxidative Defense System. PLoS ONE 2015, 10, e0143528. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Dahmane, R.G.; Godic, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Et Adriat. 2012, 21, 33–36. [Google Scholar]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.S.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postep. Dermatol. I Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the Redox Balance in Inflammatory Skin Conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Maeda, R.; Munehiro, A.; Fujita, N.; Yokoi, I.; Moriue, J.; Moriue, T.; Kosaka, H.; Kubota, Y. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1405–1408. [Google Scholar] [CrossRef]
- Omata, N.; Tsukahara, H.; Ito, S.; Ohshima, Y.; Yasutomi, M.; Yamada, A.; Jiang, M.; Hiraoka, M.; Nambu, M.; Deguchi, Y.; et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001, 69, 223–228. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Csekes, E.; Rackova, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Thornfeldt, C.R. Chronic inflammation is etiology of extrinsic aging. J. Cosmet. Dermatol. 2008, 7, 78–82. [Google Scholar] [CrossRef]
- He, Y.-L.; Lin, L.; Zheng, H.; Mo, Y.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z.-J. Potential anti-skin aging effect of a peptide AYAPE isolated from Isochrysis zhanjiangensis on UVB-induced HaCaT cells and H2O2-induced BJ cells. J. Photochem. Photobiol. B-Biol. 2022, 233, 112481. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Noh, E.-M.; Jeong, E.-Y.; Yun, S.-K.; Jeong, Y.-J.; Kim, J.-H.; Kwon, K.-B.; Kim, B.-S.; Lee, S.-H.; Park, C.-S.; et al. Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-kappa B pathway in human dermal fibroblasts. Exp. Mol. Med. 2009, 41, 548–554. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.J. TGF-β Signal as Common Targets for Cancer Prevention and Therapy. TGF-β Prevention and Treatment of Cancer through signal Modulators. J. Cancer Prev. 2009, 14, 188–200. [Google Scholar]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Sternlicht, M.; Gerritsen, K.; Goldschmeding, R. Could Aging Human Skin Use a Connective Tissue Growth Factor Boost to Increase Collagen Content? J. Investig. Dermatol. 2010, 130, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Gojniczek, K.; Jurzak, M.; Garncarczyk, A. The role of connective tissue growth factor (CTGF) in fibroproliferative processes and tissues fibrosis. Postep. Biol. Komorki 2008, 35, 113–131. [Google Scholar] [CrossRef]
- Duncan, M.R.; Frazier, K.S.; Abramson, S.; Williams, S.; Klapper, H.; Huang, X.F.; Grotendorst, G.R. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: Downregulation by cAMP. FASEB J. 1999, 13, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, K.; Liu, X.; Chen, H. Identification of key pathways and genes in psoriasis via gene microarray analysis. Mol. Med. Rep. 2016, 13, 2327–2337. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, X.; Wang, X.; Lv, H.; Zhao, J.; Guo, X.; Xian, F.; Ji, Y.; Zhang, G. Plasma MicroRNA Expression Profiles in Psoriasis. J. Immunol. Res. 2020, 2020, 1561278. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, D.H.; Park, H.J. Molecular Mechanisms and Management of a Cutaneous Inflammatory Disorder: Psoriasis. Int. J. Mol. Sci. 2017, 18, 2684. [Google Scholar] [CrossRef]
- Garcia-Melendo, C.; Cubiro, X.; Puig, L. Janus Kinase Inhibitors in Dermatology: Part 2: Applications in Psoriasis, Atopic Dermatitis, and Other Dermatoses. Actas Dermo-Sifiliogr. 2021, 112, 586–600. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, Y.S.; Jeong, M.H. The Role of Nuclear Factor Kappa B Activation in Atherosclerosis and Ischemic Cardiac Injury. Korean Circ. J. 2006, 36, 245–251. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, Z.; Zhang, L.; Wang, Y.; Li, P. 4′-O-beta-D-glucosyl-5-O-methylvisamminol ameliorates imiquimod-induced psoriasis-like dermatitis and inhibits inflammatory cytokines production by suppressing the NF-kappa B and MAPK signaling pathways. Braz. J. Med. Biol. Res. 2020, 53. [Google Scholar] [CrossRef]
- Li, Z.J.; Choi, D.-K.; Sohn, K.-C.; Lim, S.K.; Im, M.; Lee, Y.; Seo, Y.-J.; Kim, C.D.; Lee, J.-H. Induction of Interleukin-22 (IL-22) production in CD4(+) T Cells by IL-17A Secreted from CpG-Stimulated Keratinocytes. Ann. Dermatol. 2016, 28, 579–585. [Google Scholar] [CrossRef]
- Martinez-Torres, I.; Tepale-Segura, A.; Castro-Escamilla, O.; Cancino-Diaz, J.C.; Rodriguez-Martinez, S.; Perez-Tapia, S.M.; Bonifaz, L.C.; Cancino-Diaz, M.E. The Protective Role of pVHL in Imiquimod-Induced Psoriasis-like Skin Inflammation. Int. J. Mol. Sci. 2022, 23, 5226. [Google Scholar] [CrossRef]
- Jendrysik, M.A.; Vasilevsky, S.; Yi, L.; Wood, A.; Zhu, N.; Zhao, Y.; Koontz, S.M.; Jackson, S.H. NADPH Oxidase-2 Derived ROS Dictates Murine DC Cytokine-Mediated Cell Fate Decisions during CD4 T Helper-Cell Commitment. PLoS ONE 2011, 6, e28198. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.-X.; Li, W.; Wu, Y.; Chen, S.-J.; Yue, Q.; Xiao, M.; Li, J.-W. IL-36 Cytokine Expression and Its Relationship with p38 MAPK and NF-kappa B Pathways in Psoriasis Vulgaris Skin Lesions. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2013, 33, 594–599. [Google Scholar] [CrossRef]
- Xiao-hong, M.A.N.; Xiao-yan, Z.; Hang-yu, Y.; Juan, T.; Li-xin, Z.; Li-ping, Y.O.U. High expression of phosphorylated MEK/ERK/NF-kappaB in lesions of psoriasis vulgaris. Chin. J. Dermatol. 2010, 43, 160–163. [Google Scholar]
- Man, X.-Y.; Zheng, M. Advances in pathogenesis of psoriasis. Zhejiang Da Xue Xue Bao. Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2006, 35, 673–677. [Google Scholar]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, M.; Chen, X.; Yan, X. Rehmannia radix Extract Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in a Mouse Model via the Janus-Kinase Signal Transducer and Activator of Transcription Pathway. Pharmacogn. Mag. 2020, 16, 613–619. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; Veerhuis, R.; Rozemuller, A.J.M.; Arendt, T.; Eikelenboom, P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol. Dis. 2004, 15, 492–499. [Google Scholar] [CrossRef]
- Sun, H.; Xu, B.; Inoue, H.; Chen, Q.M. p38 MAPK mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell. Signal. 2008, 20, 1952–1959. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuan, I.H.; Wang, C.H.; Lee, H.M.; Lee, W.S.; Sheu, J.R.; Hsiao, G.; Wu, C.H.; Kuo, H.P. Lipoteichoic acid-induced cyclooxygenase-2 expression requires activations of p44/42 and p38 mitogen-activated protein kinase signal pathways. Eur. J. Pharmacol. 2002, 450, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Choi, M.S.; Lee, H.G.; Lee, S.-H.; Noh, K.H.; Kwon, S.; Jeong, A.J.; Lee, H.; Yi, E.H.; Park, J.Y.; et al. Photoprotective Potential of Penta-O-Galloyl-beta-D-Glucose by Targeting NF-kappa B and MAPK Signaling in UVB Radiation-Induced Human Dermal Fibroblasts and Mouse Skin. Mol. Cells 2015, 38, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, N.; Adamski, H.; Bouvet, R.; Corre, S.; Courbebaisse, Y.; Watier, E.; Mosser, J.; Chesne, C.; Galibert, M.-D. In Vivo Identification of Solar Radiation-Responsive Gene Network: Role of the p38 Stress-Dependent Kinase. PLoS ONE 2010, 5, e10776. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shao, H.; Deng, J.; Liu, Y. Network pharmacology-based analysis to explore the therapeutic mechanism of Cortex Dictamni on atopic dermatitis. J. Ethnopharmacol. 2023, 304, 116023. [Google Scholar] [CrossRef]
- Tanaka, A.; Muto, S.; Jung, K.; Itai, A.; Matsuda, H. Topical application with a new NF-kappa B inhibitor improves atopic dermatitis in NC/NgaTnd mice. J. Investig. Dermatol. 2007, 127, 855–863. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, M.; Ren, J.; Wu, X.; Long, M.; Chen, L.; Chen, Z. Electroacupuncture inhibits mast cell degranulation via cannabinoid CB2 receptors in a rat model of allergic contact dermatitis. Acupunct. Med. 2019, 37, 348–355. [Google Scholar] [CrossRef]
- Sasaki, Y.; Aiba, S. Dendritic cells and contact dermatitis. Clin. Rev. Allergy Immunol. 2007, 33, 27–34. [Google Scholar] [CrossRef]
- Bechara, R.; Nabhan, M.; Antonios, D.; Azouri, H.; Pallardy, M. IL-27 Production and Regulation in Human Dendritic Cells Treated with the Chemical Sensitizer NiSO4. Chem. Res. Toxicol. 2018, 31, 1323–1331. [Google Scholar] [CrossRef]
- Ciuciulete, A.-R.; Stepan, A.E.; Andreiana, B.C.; Simionescu, C.E. Non-Melanoma Skin Cancer: Statistical Associations between Clinical Parameters. Curr. Health Sci. J. 2022, 48, 110–115. [Google Scholar] [CrossRef]
- Durante, G.; Comito, F.; Lambertini, M.; Broseghini, E.; Dika, E.; Ferracin, M. Non-coding RNA dysregulation in skin cancers. Essays Biochem. 2021, 65, 641–655. [Google Scholar]
- Dhanwada, K.R.; Dickens, M.; Neades, R.; Davis, R.; Pelling, J.C. Differential-effects of UV-B and UV-C components of solar-radiation on map kinase signal-transduction pathways in epidermal-keratinocytes. Oncogene 1995, 11, 1947–1953. [Google Scholar]
- Liu, Z.; Zhu, P.; Tao, Y.; Shen, C.; Wang, S.; Zhao, L.; Wu, H.; Fan, F.; Lin, C.; Chen, C.; et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem. Toxicol. 2015, 81, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Malakhova, M.; Mottamal, M.; Reddy, K.; Kurinov, I.; Carper, A.; Langfald, A.; Oi, N.; Kim, M.O.; Zhu, F.; et al. Norathyriol Suppresses Skin Cancers Induced by Solar Ultraviolet Radiation by Targeting ERK Kinases. Cancer Res. 2012, 72, 260–270. [Google Scholar] [CrossRef]
- Zhao, K.; Dai, Q.; Wu, J.; Wei, Z.; Duan, Y.; Chen, B. Morusin enhances the antitumor activity of MAPK pathway inhibitors in BRAF-mutant melanoma by inhibiting the feedback activation of STAT3. Eur. J. Cancer 2022, 165, 58–70. [Google Scholar] [CrossRef]
- Lopez-Bergami, P.; Fitchman, B.; Ronai, Z.e. Understanding signaling cascades in melanoma. Photochem. Photobiol. 2008, 84, 289–306. [Google Scholar] [CrossRef]
- Cohen, C.; Zavala-Pompa, A.; Sequeira, J.H.; Shoji, M.; Sexton, D.G.; Cotsonis, G.; Cerimele, F.; Govindarajan, B.; Macaron, N.; Arbiser, J.L. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002, 8, 3728–3733. [Google Scholar]
- Zipser, M.C.; Eichhoff, O.M.; Widmer, D.S.; Schlegel, N.C.; Schoenewolf, N.L.; Stuart, D.; Liu, W.; Gardner, H.; Smith, P.D.; Nuciforo, P.; et al. A proliferative melanoma cell phenotype is responsive to RAF/MEK inhibition independent of BRAF mutation status. Pigment. Cell Melanoma Res. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Pathria, G.; Verma, S.; Yin, J.; Scott, D.A.; Ronai, Z.E.A. MAPK signaling regulates c-MYC for melanoma cell adaptation to asparagine restriction. Embo Rep. 2021, 22, e51436. [Google Scholar] [CrossRef]
- Xie, J.W.; Aszterbaum, M.; Zhang, X.L.; Bonifas, J.M.; Zachary, C.; Epstein, E.; McCormick, F. A role of PDGFR alpha in basal cell carcinoma proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 9255–9259. [Google Scholar] [CrossRef]
- Geering, B.; Cutillas, P.R.; Vanhaesebroeck, B. Regulation of class IA PI3Ks: Is there a role for monomeric PI3K subunits? Biochem. Soc. Trans. 2007, 35, 199–203. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Long, X.; Jiao, L.; Zhou, M.; Wu, K. MRPS16 facilitates tumor progression via the PI3K/AKT/Snail signaling axis. J. Cancer 2020, 11, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Lai, A.G. An immunoevasive strategy through clinically-relevant pan-cancer genomic and transcriptomic alterations of JAK-STAT signaling components. Mol. Med. 2019, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, F.; Zhang, G.-L. Natural products and their derivatives regulating the janus kinase/signal transducer and activator of transcription pathway. J. Asian Nat. Prod. Res. 2014, 16, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 1–33. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Yan, S.; Zhang, J.; Wang, D.; Shen, J. JAK/STAT signaling regulates the Harmonia axyridis leg regeneration by coordinating cell proliferation. Dev. Biol. 2022, 483, 98–106. [Google Scholar] [CrossRef]
- Bach, E.A.; Ekas, L.A.; Ayala-Camargo, A.; Flaherty, M.S.; Lee, H.; Perrimon, N.; Baeg, G.-H. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 2007, 7, 323–331. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Sun, Y.-X.; Yu, M.; Shen, B.-F. IL-6 inhibits apoptosis of human myeloma cell line XG-7 through activation of JAK/STAT pathway and up-regulation of Mcl-1. Ai Zheng. Chin. J. Cancer 2002, 21, 113–116. [Google Scholar]
- Zhang, M.; Griner, L.A.M.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.N.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 12480–12485. [Google Scholar] [CrossRef]
- Migone, T.S.; Humbert, M.; Rascle, A.; Sanden, D.; D’Andrea, A.; Johnston, J.A. The deubiquitinating enzyme DUB-2 prolongs cytokine-induced signal transducers and activators of transcription activation and suppresses apoptosis following cytokine withdrawal. Blood 2001, 98, 1935–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, S.B.; Ogilvie, J.M.; McCool, D.J.; Sarthy, V. Ciliary neurotrophic factor induces glial fibrillary acidic protein in retinal Muller cells through the JAK/STAT signal transduction pathway. Curr. Eye Res. 2002, 24, 305–312. [Google Scholar] [CrossRef]
- Burgo, M.A.; Roudiani, N.; Chen, J.; Santana, A.L.; Doudican, N.; Proby, C.; Felsen, D.; Carucci, J.A. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight 2018, 3, 120750. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q.; Li, S.; Huang, R.; Wang, X.; Liao, X.; Mo, H.; Zhang, L.; Zhou, X. Prognostic value of key genes of the JAK-STAT signaling pathway in patients with cutaneous melanoma. Oncol. Lett. 2020, 19, 1928–1946. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Ma, T. Mechanisms of JAK-STAT signaling pathway mediated by CXCL8 gene silencing on epithelial-mesenchymal transition of human cutaneous melanoma cells. Oncol. Lett. 2020, 20, 1973–1981. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Chaudhuri, A.; Iqubal, A.; Saleem, S.; Gupta, M.M.; Ahuja, A.; Ali, J.; Baboota, S. Targeted Delivery of Natural Bioactives and Lipid-nanocargos against Signaling Pathways Involved in Skin Cancer. Curr. Med. Chem. 2021, 28, 8003–8035. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Jing, G.; Dong, D. LncRNA HOTAIR Promotes Proliferation of Malignant Melanoma Cells through NF-kappa B Pathway. Iran. J. Public Health 2020, 49, 1931–1939. [Google Scholar]
- Usman, H.A.; Hernowo, B.S.; Tobing, M.D.L.; Hindritiani, R. The Major Role of NF-kappa B in the Depth of Invasion on Acral Melanoma by Decreasing CD8(+) T Cells. J. Pathol. Transl. Med. 2018, 52, 164–170. [Google Scholar] [CrossRef]
- Feehan, R.P.; Shantz, L.M. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem. J. 2016, 473, 2973–2994. [Google Scholar] [CrossRef]
- Schaefer, M.; Werner, S. Nrf2-A regulator of keratinocyte redox signaling. Free. Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef]
- De la Vega, M.R.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Targeting NRF2 for Improved Skin Barrier Function and Photoprotection: Focus on the Achiote-Derived Apocarotenoid Bixin. Nutrients 2017, 9, 1371. [Google Scholar] [CrossRef]
- Motohashi, H.; O’Connor, T.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 2002, 294, 1–12. [Google Scholar] [CrossRef]
- Wong, D.P.W.; Wells, G.; Hagen, T. Heteroaromatic 4-arylquinols are novel inducers of Nuclear factor-erythroid 2-related factor 2 (Nrf2). Eur. J. Pharmacol. 2010, 643, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmstroem, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Et Biophys. Acta-Gen. Subj. 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Hu, Y.; Sheng, R. Autophagy Regulators as Potential Cancer Therapeutic agents: A Review. Curr. Top. Med. Chem. 2015, 15, 720–744. [Google Scholar] [CrossRef]
- Lim, G.-E.; Park, J.E.; Cho, Y.H.; Lim, D.S.; Kim, A.J.; Moh, S.H.; Lee, J.H.; Lee, J.S. Alpha-neoendorphin can reduce UVB-induced skin photoaging by activating cellular autophagy. Arch. Biochem. Biophys. 2020, 689, 108437. [Google Scholar] [CrossRef]
- Hailfinger, S.; Schulze-Osthoff, K. Impaired Autophagy in Psoriasis and Atopic Dermatitis: A New Therapeutic Target. J. Investig. Dermatol. 2021, 141, 2775–2777. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Unterman, T.G.; Shankar, S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol. Cell. Biochem. 2010, 337, 201–212. [Google Scholar] [CrossRef]
- Dansen, T.B. Forkhead Box O Transcription Factors: Key Players in Redox Signaling. Antioxid. Redox Signal. 2011, 14, 559–561. [Google Scholar] [CrossRef]
- Kim, S.; Koh, H. Role of FOXO transcription factors in crosstalk between mitochondria and the nucleus. J. Bioenerg. Biomembr. 2017, 49, 335–341. [Google Scholar] [CrossRef]
- Amin, A.; Adeghate, E.; Lotfy, M. Pancreas-Protective Effects of Chlorella in STZ-Induced Diabetic Animal Model: Insights into the Mechanism. Diabetes 2011, 60, A703. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Murali, C.; Madhoon, A.A.; Amin, A. Salvadora persica (Miswak): Antioxidant and Promising Antiangiogenic Insights. Am. J. Plant Sci. 2018, 9, 1228–1244. [Google Scholar] [CrossRef]
- Hamza, A.A.; Mohamed, M.G.; Lashin, F.M.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 81, 1–13. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Mohamad Anuar, N.N. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical Basis of Anti-Cancer-Effects of PhloretinA Natural Dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Liu, M.; Wu, S.; Fang, L.; Guo, D.; He, J. Research and Application of Proanthocyanidins in Livestock Production. Chin. J. Anim. Nutr. 2018, 30, 2902–2910. [Google Scholar]
- Wan, F.; Hou, F.; Yi, B.; Zhang, H. Physiological Functions of Chlorogenic Acid and Its Application in Livestock and Poultry Production. Chin. J. Anim. Nutr. 2021, 33, 2416–2427. [Google Scholar]
- Djawad, K.; Patellongi, I.J.; Miskad, U.A.; Massi, M.N.; Yusuf, I.; Faruk, M. Single or Daily Application of Topical Curcumin Prevents Ultraviolet B-Induced Apoptosis in Mice. Molecules 2023, 28, 371. [Google Scholar] [CrossRef] [PubMed]
- Minear, S.; O’Donnell, A.F.; Ballew, A.; Giaever, G.; Nislow, C.; Stearns, T.; Cyert, M.S. Curcumin Inhibits Growth of Saccharomyces cerevisiae through Iron Chelation. Eukaryot. Cell 2011, 10, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Meng, Z.; Tao, H.; Bai, Z.; Yan, C.; Li, L. Curcumin inhibits LPS-induced inflammation in VSMCs via Toll-like receptor 4/NADPH oxidase/reactive oxygen species signaling pathway. J. Xi’an Jiaotong Univ. Med. Sci. 2015, 36, 543–548. [Google Scholar]
- Hur, G.-H.; Ryu, A.R.; Kim, Y.-W.; Lee, M.-Y. The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice. Pharmaceutics 2022, 14, 968. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Zheng, X.; Zhang, K.; Du, Z. Chemoprevention effects of a sulindac-based compound on TPA-induced skin inflammation in mice. Medchemcomm 2015, 6, 1605–1611. [Google Scholar] [CrossRef]
- Li, H.; Gao, A.; Jiang, N.; Liu, Q.; Liang, B.; Li, R.; Zhang, E.; Li, Z.; Zhu, H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem. Photobiol. 2016, 92, 808–815. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.Y.; Dong, B.H.; Jiang, Y.X.; Yu, L.Y.; Chen, Z.M.; Hu, C.J.; Xu, R.C. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Zhang, J.C.; Ma, Y.; Li, W. Curcumin reduces inflammation in mice with the psoriasis model by inhibiting NLRP3 inflammatory bodies. Cell. Mol. Biol. 2021, 67, 48–54. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef]
- Limtrakul, P.N.; Anuchapreeda, S.; Lipigorngoson, S.; Dunn, F.W. Inhibition of carcinogen induced c-Ha-ras and c-fos proto-oncogenes expression by dietary curcumin. BMC Cancer 2001, 1. [Google Scholar] [CrossRef]
- Kakar, S.S.; Roy, D. Curcumin inhibits TPA induced expression of c-fos, c-jun and c-myc protooncogenes messenger-RNAs in mouse skin. Cancer Lett. 1994, 87, 85–89. [Google Scholar] [CrossRef]
- Kumar, G.; Tajpara, P.; Maru, G.B. Dietary Turmeric Post-Treatment Decreases DMBA-Induced Hamster Buccal Pouch Tumor Growth by Altering Cell Proliferation and Apoptosis-Related Markers. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 295–312. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-kappa B and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.-A.O. Curcumin Inhibits Skin Squamous Cell Carcinoma Tumor Growth In Vivo. Otolaryngol. Head Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Adhami, V.M.; Fujioka, K.; Mukhtar, H.; Mousa, S.A. Photochemoprevention of ultraviolet Beam Radiation-induced DNA damage in keratinocytes by topical delivery of nanoformulated Epigallocatechin-3-gallate. Nanomed.-Nanotechnol. Biol. Med. 2022, 44, 102580. [Google Scholar] [CrossRef]
- Ding, J.X.; Gao, B.B.; Chen, Z.H.; Mei, X.F. An NIR-Triggered Au Nanocage Used for Photo-Thermo Therapy of Chronic Wound in Diabetic Rats Through Bacterial Membrane Destruction and Skin Cell Mitochondrial Protection. Front. Pharmacol. 2021, 12, 3235. [Google Scholar] [CrossRef]
- Li, D.H.; Martini, N.; Wu, Z.M.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.W.; Wen, J.Y. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics 2022, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Wang, L.X.; Zheng, X.Q.; Xiang, L.P.; Liang, Y.R. Protective Effect of (-)-Epigallocatechin Gallate against Photo-Damage Induced by Ultraviolet A in Human Skin Fibroblasts. Trop. J. Pharm. Res. 2014, 13, 1079–1084. [Google Scholar] [CrossRef]
- Megow, I.; Darvin, M.E.; Meinke, M.C.; Lademann, J. A Randomized Controlled Trial of Green Tea Beverages on the in vivo Radical Scavenging Activity in Human Skin. Ski. Pharmacol. Physiol. 2017, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.-J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-kappa B, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Won, H.-R.; Lee, P.; Oh, S.-r.; Kim, Y.-M. Epigallocatechin-3-Gallate Suppresses the Expression of TNF-alpha-Induced MMP-1 via MAPK/ERK Signaling Pathways in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2021, 44, 18–24. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-alpha-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Yang, W.K.; Park, Y.; Kim, B.K.; Choi, J.J.; Ryu, G.S.; Kim, S.H. Effects of Catechin-rich Green Tea Extract on the MMP-1 Activity of HaCaT Keratinocyte Cells and on UVB-induced Skin Damage in Hairless Mice. Korean J. Med. Crop Sci. 2019, 27, 143–150. [Google Scholar] [CrossRef]
- Nakano, E.; Kamei, D.; Murase, R.; Taki, I.; Karasawa, K.; Fukuhara, K.; Iwai, S. Anti-inflammatory effects of new catechin derivatives in a hapten-induced mouse contact dermatitis model. Eur. J. Pharmacol. 2019, 845, 40–47. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef]
- Bito, T.; Roy, S.; Sen, C.K.; Shirakawa, T.; Gotoh, A.; Ueda, M.; Ichihashi, M.; Packer, L. Flavonoids differentially regulate IFN gamma-induced ICAM-1 expression in human keratinocytes: Molecular mechanisms of action. Febs Lett. 2002, 520, 145–152. [Google Scholar] [CrossRef]
- Monga, J.; Aggarwal, V.; Suthar, S.K.; Monika; Nongalleima, K.; Sharma, M. Topical (+)-catechin emulsified gel prevents DMBA/TPA-induced squamous cell carcinoma of the skin by modulating antioxidants and inflammatory biomarkers in BALB/c mice. Food Funct. 2014, 5, 3197–3207. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-beta-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]
- Leis, K.; Pisanko, K.; Jundzill, A.; Mazur, E.; Mecinska-Jundzill, K.; Witmanowski, H. Resveratrol as a factor preventing skin aging and affecting its regeneration. Postep. Dermatol. I Alergol. 2022, 39, 439–445. [Google Scholar] [CrossRef]
- Lephart, E.D.; Sommerfeldt, J.M.; Andrus, M.B. Resveratrol: Influences on gene expression in human skin. J. Funct. Foods 2014, 10, 377–384. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.-P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.-J.; Song, X.; Chu, W.-M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kalra, N.; Prasad, S.; George, J.; Shukla, Y. Chemopreventive Potential of Resveratrol in Mouse Skin Tumors Through Regulation of Mitochondrial and PI3K/AKT Signaling Pathways. Pharm. Res. 2009, 26, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Madan, E.; Prasad, S.; Roy, P.; George, J.; Shukla, Y. Regulation of apoptosis by resveratrol through JAK/STAT and mitochondria mediated pathway in human epidermoid carcinoma A431 cells. Biochem. Biophys. Res. Commun. 2008, 377, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKC delta and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Sohn, E.-J.; Kim, J.M.; Kang, S.-H.; Kwon, J.; An, H.J.; Sung, J.-S.; Cho, K.A.; Jang, I.-S.; Choi, J.-S. Restoring Effects of Natural Anti-Oxidant Quercetin on Cellular Senescent Human Dermal Fibroblasts. Am. J. Chin. Med. 2018, 46, 853–873. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Lee, E.; Shin, A.; Park, Y.G.; Kim, Y. Effect of Quercetin in the UV-Irradiated Human Keratinocyte HaCaT Cells and A Model of Its Binding To p38 MAPK. Bull. Korean Chem. Soc. 2014, 35, 2787–2790. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J.V. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B-Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Pauff, J.M.; Hille, R. Inhibition Studies of Bovine Xanthine Oxidase by Luteolin, Silibinin, Quercetin, and Curcumin. J. Nat. Prod. 2009, 72, 725–731. [Google Scholar] [CrossRef]
- Lee, K.-M.; Kang, J.H.; Yun, M.; Lee, S.-B. Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-gamma-primed human keratinocytes. Biochem. Biophys. Res. Commun. 2018, 503, 116–122. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Modulation of HMGB1 translocation and RAGE/NFB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp. Dermatol. 2015, 24, 418–423. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Chen, H.M.; Lu, C.J.; Liu, H.Z.; Wang, M.J.; Zhao, H.; Yan, Y.H.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-kappa B pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef]
- Hundsberger, H.; Stierschneider, A.; Sarne, V.; Ripper, D.; Schimon, J.; Weitzenbock, H.P.; Schild, D.; Jacobi, N.; Eger, A.; Atzler, J.; et al. Concentration-Dependent Pro- and Antitumor Activities of Quercetin in Human Melanoma Spheroids: Comparative Analysis of 2D and 3D Cell Culture Models. Molecules 2021, 26, 717. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Choi, J.-S.; Kang, S.-W.; Lee, Y.-J.; Park, J.; Kang, Y.-H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, E182–E190. [Google Scholar] [CrossRef]
- Jimenez, F.; Mitts, T.F.; Liu, K.; Wang, Y.; Hinek, A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J. Investig. Dermatol. 2006, 126, 1272–1280. [Google Scholar] [CrossRef]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef]
- Moon, N.R.; Kang, S.; Park, S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta 1 and wnt signaling pathways. J. Photochem. Photobiol. B-Biol. 2018, 178, 92–100. [Google Scholar] [CrossRef]
- Raudone, L.; Bobinaite, R.; Janulis, V.; Viskelis, P.; Trumbeckaite, S. Effects of raspberry fruit extracts and ellagic acid on respiratory burst in murine macrophages. Food Funct. 2014, 5, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef] [PubMed]
- Lembo, S.; Balato, A.; Di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. The Modulatory Effect of Ellagic Acid and Rosmarinic Acid on Ultraviolet-B-Induced Cytokine/Chemokine Gene Expression in Skin Keratinocyte (HaCaT) Cells. Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical Anti-inflammatory Potential of Standardized Pomegranate Rind Extract and Ellagic Acid in Contact Dermatitis. Phytother. Res. 2014, 28, 629–632. [Google Scholar] [CrossRef]
- Jensen, J.D.; Dunn, J.H.; Luo, Y.; Liu, W.; Fujita, M.; Dellavalle, R.P. Ellagic acid inhibits melanoma growth in vitro. Dermatol. Rep. 2011, 3, e36. [Google Scholar] [CrossRef]
- Huang, Q.; Chai, W.-M.; Ma, Z.-Y.; Deng, W.-L.; Wei, Q.-M.; Song, S.; Zou, Z.-R.; Peng, Y.-Y. Antityrosinase mechanism of ellagic acid in vitro and its effect on mouse melanoma cells. J. Food Biochem. 2019, 43, e12996. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; McClements, D.J.; Xie, B.; Sun, Z.; Deng, Q. Oligomeric Procyanidin Nanoliposomes Prevent Melanogenesis and UV Radiation-Induced Skin Epithelial Cell (HFF-1) Damage. Molecules 2020, 25, 1458. [Google Scholar] [CrossRef]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef]
- Chen, F.; Ye, X.; Yang, Y.; Teng, T.; Li, X.; Xu, S.; Ye, Y. Proanthocyanidins from the bark of Metasequoia glyptostroboides ameliorate allergic contact dermatitis through directly inhibiting T cells activation and Thl/Th17 responses. Phytomedicine 2015, 22, 510–515. [Google Scholar] [CrossRef]
- Katiyar, S.K. Grape seed proanthocyanidines and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol. Nutr. Food Res. 2008, 52, S71–S76. [Google Scholar] [CrossRef]
- Vaid, M.; Singh, T.; Katiyar, S.K. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS ONE 2011, 6, e21539. [Google Scholar] [CrossRef]
- Hah, Y.-S.; Kim, J.G.; Cho, H.Y.; Park, J.S.; Heo, E.P.; Yoon, T.-J. Procyanidins from Vitis vinifera seeds induce apoptotic and autophagic cell death via generation of reactive oxygen species in squamous cell carcinoma cells. Oncol. Lett. 2017, 14, 1925–1932. [Google Scholar] [CrossRef]
- Choi, Y.H. Honokiol attenuates oxidative stress-induced cytotoxicity in human keratinocytes via activating AMPK signaling. Asian Pac. J. Trop. Biomed. 2021, 11, 222–230. [Google Scholar] [CrossRef]
- Costa, A.; Facchini, G.; Pinheiro, A.L.T.A.; da Silva, M.S.; Bonner, M.Y.; Arbiser, J.; Eberlin, S. Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int. J. Dermatol. 2017, 56, 754–761. [Google Scholar] [CrossRef]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Magnolia ovovata extract and its active component magnolol prevent skin photoaging via inhibition of nuclear factor kappaB. Eur. J. Pharmacol. 2007, 565, 212–219. [Google Scholar] [CrossRef]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Chae, S. Magnolol reduces UVB-induced photodamage by regulating matrix metalloproteinase activity. Environ. Toxicol. Pharmacol. 2015, 39, 417–423. [Google Scholar] [CrossRef]
- Lee, J.-H.; Im, D.-S. Honokiol suppresses 2,6-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2022, 289, 115023. [Google Scholar] [CrossRef]
- Chilampalli, C.; Guillermo, R.; Kaushik, R.S.; Young, A.; Chandrasekher, G.; Fahmy, H.; Dwivedi, C. Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells. Exp. Biol. Med. 2011, 236, 1351–1359. [Google Scholar] [CrossRef]
- Stanic, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Zielinska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Med. Lith. 2020, 56, 336. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Wan, M.J.; Li, H.P.; Chen, Q.; Li, R.X.; Liang, B.H.; Zhu, H.L. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef] [PubMed]
- Semita, I.N.; Utomo, D.N.; Suroto, H.; Sudiana, I.K.; Gandi, P. The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: Through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities. Korean J. Pain 2023, 36, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G.; Fang, F.; Kang, S.N.; Wang, Z.C.; Yang, Y.M. Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis. J. Tradit. Chin. Med. 2020, 40, 782–791. [Google Scholar]
- Liu, Y.H.; Lin, Y.S.; Huang, Y.W.; Fang, S.U.; Lin, S.Y.; Hou, W.C. Protective Effects of Minor Components of Curcuminoids on Hydrogen Peroxide-Treated Human HaCaT Keratinocytes. J. Agric. Food Chem. 2016, 64, 3598–3608. [Google Scholar] [CrossRef]
- Greenwald, M.B.Y.; Frusic-Zlotkin, M.; Soroka, Y.; Ben Sasson, S.; Bitton, R.; Bianco-Peled, H.; Kohen, R. Curcumin Protects Skin against UVB-Induced Cytotoxicity via the Keap1-Nrf2 Pathway: The Use of a Microemulsion Delivery System. Oxidative Med. Cell. Longev. 2017, 2017, 5205471. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nacher, A.; Valenti, D.; Fernandez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Alyoussef, A.; El-Gogary, R.I.; Ahmed, R.F.; Farid, O.A.A.; Bakeer, R.M.; Nasr, M. The beneficial activity of curcumin and resveratrol loaded in nanoemulgel for healing of burn-induced wounds. J. Drug Deliv. Sci. Technol. 2021, 62, 102360. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.Z.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.Y.; Zhang, X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Wagn, F.; Li, W. Experimental study of effect of 2 940 nm Er: YAG laser combined with catechin on photo damage in mice skin. J. Clin. Dermatol. 2015, 44, 351–355. [Google Scholar]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Timm, D.; Ozeki, M.; Okubo, T. Green Tea Catechin Association with Ultraviolet Radiation-Induced Erythema: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 3702. [Google Scholar] [CrossRef]
- Tian, B.R.; Liu, J.Y. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Arora, D.; Jaglan, S. Therapeutic applications of resveratrol nanoformulations. Environ. Chem. Lett. 2018, 16, 35–41. [Google Scholar] [CrossRef]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Irnidayanti, Y.; Sutiono, D.R. Tempeh & Soybean Seed Coat: The Alternative Sources of Trans-Resveratrol as Neuroprotective Agents. Int. J. Morphol. 2019, 37, 1164–1171. [Google Scholar] [CrossRef]
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free. Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol Inhibits Particulate Matter-Induced Inflammatory Responses in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Vale, D.L.; Martinez, R.M.; Medeiros, D.C.; da Rocha, C.; Sfeir, N.; Lopez, R.F.V.; Vicentini, F.; Verri, W.A.; Georgetti, S.R.; Baracat, M.M.; et al. A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: Enhancement of activity by microencapsulation. J. Drug Target. 2021, 29, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zi, C.; Chen, D.; Li, J.; He, R.; Hu, J.-M. Target acquisition of anti-aging manno-oligosaccharide that triggers ECM process via TGF-beta/Smads-SIRT1 signalling pathway. Carbohydr. Polym. 2023, 302, 120380. [Google Scholar] [CrossRef] [PubMed]
- Maramaldi, G.; Togni, S.; Pagin, I.; Giacomelli, L.; Cattaneo, R.; Eggenhoffner, R.; Burastero, S.E. Soothing and anti-itch effect of quercetin phytosome in human subjects: A single-blind study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Girme, A.; Saste, G.; Pawar, S.; Ghule, C.; Mirgal, A.; Patel, N.; Singh, R.; Hingorani, L. Development and Validation of a Sensitive Method for Analysis of Ellagic Acid in Dietary Supplements from Punica granatum. Curr. Top. Nutraceutical Res. 2021, 19, 90–105. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Velez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxidative Med. Cell. Longev. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Kumar, K.J.S.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Tang, Q.; Li, D.; Wan, Y.; Li, J.-H.; Gao, X.-H.; Seeram, N.P.; Ma, H.; Chen, H.-D. Pomegranate (Punica granatum) extract and its polyphenols reduce the formation of methylglyoxal-DNA adducts and protect human keratinocytes against methylglyoxal-induced oxidative stress. J. Funct. Foods 2021, 83, 104564. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: A comprehensive review. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 109, 154581. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Wang, L.; Zu, Y.; Wang, S.; Liu, P.; Zhao, X. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int. J. Nanomed. 2018, 13, 5469–5483. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Huang, T.-H.; Hung, C.-F.; Huang, Y.-L.; Aljuffali, I.A.; Liao, W.-C.; Lin, C.-F. Derivatization of honokiol by integrated acetylation and methylation for improved cutaneous delivery and anti-inflammatory potency. Eur. J. Pharm. Sci. 2018, 114, 189–198. [Google Scholar] [CrossRef]
- Lin, C.-F.; Hwang, T.-L.; Al-Suwayeh, S.A.; Huang, Y.-L.; Hung, Y.-Y.; Fang, J.-Y. Maximizing dermal targeting and minimizing transdermal penetration by magnolol/honokiol methoxylation. Int. J. Pharm. 2013, 445, 153–162. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, T.; Katiyar, S.K. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Guillermo, R.F.; Chilampalli, C.; Zhang, X.; Zeman, D.; Fahmy, H.; Dwivedi, C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov. Ther. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Chen, J.; Tao, C.; Huang, X.; Chen, Z.; Wang, L.; Li, X.; Ma, M.; Wu, Z. CT2-3, a novel magnolol analogue suppresses NSCLC cells through triggering cell cycle arrest and apoptosis. Bioorganic Med. Chem. 2020, 28, 115352. [Google Scholar] [CrossRef]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: Development of topical formulation. Carcinogenesis 2010, 31, 2004–2011. [Google Scholar] [CrossRef]
- Chilampalli, S.; Zhang, X.; Fahmy, H.; Kaushik, R.S.; Zeman, D.; Hildreth, M.B.; Dwivedi, C. Chemopreventive Effects of Honokiol on UVB-induced Skin Cancer Development. Anticancer. Res. 2010, 30, 777–783. [Google Scholar]
- Guillermo-Lagae, R.; Santha, S.; Thomas, M.; Zoelle, E.; Stevens, J.; Kaushik, R.S.; Dwivedi, C. Antineoplastic Effects of Honokiol on Melanoma. Biomed. Res. Int. 2017, 2017, 5496398. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, E.P.A.; Van Wijk, R.; Bosman, S. Using ultra-weak photon emission to determine the effect of oligomeric proanthocyanidins on oxidative stress of human skin. J. Photochem. Photobiol. B Biol. 2010, 98, 199–206. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, Y.; Wang, Q.; Lei, X.; Zhao, J.; Zeng, K.; Ming, J. Construction of food hydrogel-polyphenol delivery system and their enhancement of polyphenol bioavailability: A review. Food Ferment. Ind. 2022, 48, 304–311. [Google Scholar]
- Gupta, N.K.; Dixit, V.K. Development and evaluation of vesicular system for curcumin delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef]
- Agrawal, R.; Sandhu, S.K.; Sharma, I.; Kaur, I.P. Development and Evaluation of Curcumin-loaded Elastic Vesicles as an Effective Topical Anti-inflammatory Formulation. AAPS PharmSciTech 2015, 16, 364–374. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanic, Z.; Skalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Song, Z.; Wen, Y.; Teng, F.; Wang, M.; Liu, N.; Feng, R. Carbopol 940 hydrogel containing curcumin-loaded micelles for skin delivery and application in inflammation treatment and wound healing. New J. Chem. 2022, 46, 3674–3686. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced transdermal efficiency of curcumin-loaded peptide-modified liposomes for highly effective antipsoriatic therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef]
- Thuy, L.T.; Kang, N.; Choi, M.; Lee, M.; Choi, J.S. Dendrimeric micelles composed of polyamidoamine dendrimer-peptide-cholesterol conjugates as drug carriers for the treatment of melanoma and bacterial infection. J. Ind. Eng. Chem. 2022, 114, 361–376. [Google Scholar] [CrossRef]
- Wolf, J.R.; Gewandter, J.S.; Bautista, J.; Heckler, C.E.; Strasser, J.; Dyk, P.; Anderson, T.; Gross, H.; Speer, T.; Dolohanty, L.; et al. Utility of topical agents for radiation dermatitis and pain: A randomized clinical trial. Support. Care Cancer 2020, 28, 3303–3311. [Google Scholar] [CrossRef]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric tonic as a treatment in scalp psoriasis: A randomized placebo-control clinical trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef]
- Chatterjee, S.; Datta, R.N.; Bhattacharyya, B.; Bandopadhyay, S.K. Emollient and antipruritic effect of Itch cream in dermatological disorders: A randomized controlled trial. Indian J. Pharmacol. 2005, 37, 253–254. [Google Scholar] [CrossRef]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controllecl trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–859. [Google Scholar] [CrossRef]


| ROS Stimulators | Experimental Models | Experimental Results | References |
|---|---|---|---|
| UV | UVA (15 K/cm2)-induced HaCaT cells, UVA (15 J/cm2/every 2 days/14 days)-induced nude mice | UVA toxicity, DNA single-strand breaks, apoptotic DNA fragmentation, dysregulated Bax/Bcl-2 ratio | [25] |
| UVB (15 mJ/cm2, 24 h)-irradiated human dermal fibroblasts | Photoaging: increased production of MMP-1, decreased Nrf2 protein levels, ERK and JNK phosphorylation | [26] | |
| Mice exposed to 1700 J/m2 UVB radiation four times per day. | Inflammation and immune response | [27] | |
| Radiation | The left thigh skin of mice irradiated with X-ray (40 Gy 180 kV) 5 days a week for 1 month | Increased fibrosis, inflammation, and oxidative stress injury indices, and decreased expression of Nrf2 and its regulatory antioxidant enzymes | [28] |
| Human skin cells induced by gamma-ray | Production of p53, p21, oxidative stress markers, and apoptosis and expression of MMP and cytokine genes | [29] | |
| PM2.5 | HaCaT cells induced with 100 μg/mL PM2.5 for 24 h | Inflammation: upregulation of the inflammasome NLRP1 and IL-1β expression via ROS/NF-κB | [30] |
| HaCaT cells cultured with 50 μg/mL PM2.5 for 7 days | Skin cellular senescence: decreased DNA methyltransferase expression, increased DNA demethylase, decreased histone H3 lysine 27 trimethylation (H3K27Me3) | [31] | |
| BALB/c mice were treated with PM2.5 solution (0.187, 0.375, and 0.75 mg/kg body weight (b.w.)) or saline through a pipette tip into the nose for fifteen days; The RBL-2H3 cells treated with PM2.5 (0, 25, 50, or 100 mg/mL) for 24 h | Increased cytokine expression in mast cells; MEKK4 and JNK1/2 were activated | [32] | |
| Human epidermal melanocytes treated with PM2.5 at various concentrations (0, 1, 10, 100, and 250 µg/mL) for 48 h | Inhibited the proliferation of melanocytes and induced their apoptosis | [33] | |
| Nanoparticles | Cationic AuNPs with a diameter of 25 nm induced cellular stress in macrophages | Increased cytokine expression | [34] |
| Human skin melanoma (A375) cells induced by cerium oxide nanoparticles | Cytotoxicity, malondialdehyde, and SOD production; decreased glutathione levels; DNA double-strand breaks | [35] | |
| Human skin cells (A431) induced by nickel carriers (NiNPs) | Lipid peroxidation; genotoxicity; apoptosis; and increased catalase, SOD, and caspase-3 activities | [36] | |
| Chemicals | Hydrogen peroxide-induced injury of human skin fibroblasts | Human skin fibroblasts have decreased viability, cell apoptosis | [37] |
| Imiquimod-induced mice | Psoriasis | [38] | |
| NC/Nga mice induced by 2, 4-dinitrochlorobenzene | Atopic dermatitis | [39] | |
| Mice induced by 12-O-tetracyl-cutoene-13-acetate | Irritant contact dermatitis | [40] | |
| Rat model exposed to trivalent arsenic (iAs (3+)) | Skin cancer | [41] | |
| Heat | Skin fibroblasts (Hs68) incubated for 30 min at 43 °C | Elevated thiobarbituric acid reactive substances and 8-OH-dG | [42] |
| Polyphenols | Cell or Animal Types | Skin Cellular Senescence, Inflammation, and Cancer | Molecular Targets/Mechanisms | References |
|---|---|---|---|---|
| Curcumin | HaCaT; Mice | Skin cellular senescence, inflammation, cancer | ↓ROS, lipid peroxidation, DNA damage, NADPH oxidase Chelate iron ions | [142,143,144] |
| Fibroblasts; Mice; HDFs | Skin cellular senescence | ↓ MMPs ↓ MAPK/p38, NF-κB pathways ↑ Collagen I, HO-1 ↑ TGF-β, Nrf2 pathways | [145,146,147] | |
| Mice | Inflammation (SD) | ↓ PI3K/AKT/NF-κB pathway ↑ Nrf2 pathway | [148,149] | |
| HaCaT | Inflammation (Psoriasis) | ↓ MAPK, NF-κB pathways ↓ IL-17, TNF-α, AP-1, IL-22, IL-18 ↓ IFN-γ, IL-6, iNOS | [150,151] | |
| Mice | Inflammation (AD) | ↓ IL-33, IL-4, IL-5, IL-13,IL-31 | [152] | |
| Mice; Hamster | Skin cancer | ↓ C-fos, c-jun, c-myc, PCNA, cyclin D1 ↓ Bcl-2 ↑ p53, Box, caspase-3 ↓ MAPK/ERK, JAK/STAT pathways | [114,153,154,155] | |
| B16 | Skin cancer (Melanoma) | ↓ NF-κB pathway ↑ PTEN, PDCD4 ↓ Cyclin D1, Ki67 | [156] | |
| A375 and C8161 | Skin cancer (Melanoma) | ↓ AKT/mTOR pathway ↓ Mcl-1, Bcl-2 ↑ Bax | [149,157] | |
| Mice | Skin cancer (SCC) | ↓ PI3K/AKT/mTOR pathway | [158] | |
| Catechins | Keratinocytes; Rats | Skin cellular senescence, inflammation, cancer | ↓ DNA damage, mitochondrial ↓ damage, lipid peroxidation Chelate iron ions | [159,160,161,162,163] |
| HaCaT; Mice; HDF | Skin cellular senescence | ↓ ROS, MMP-1, MMP-2, MMP-9, AP-1 ↓ IL-1β, IL-6 ↓ MAPK, NF-κB pathways ↓ Lipid peroxidation | [164,165,166,167] | |
| Mice | Inflammation (CD) | ↓ TNF-α, IL-1β, IL-4 ↓ ROS | [168] | |
| Mice; HaCaT | Inflammation (Psoriasis) | ↓ IL-17A, IL-17F, IL-22, IL-23 ↑ SOD, CAT ↓ JAK/STAT pathway ↓ MDA, DNA damage | [169,170,171] | |
| Catechins | Mice | Skin cancer (SCC) | ↑ GSH, SOD, CAT, GST, GR, GPx ↑ Nrf2 pathway ↓ COX-2, iNOS, TNF-α, IL-1β, IL-6 ↓ NF-κB pathway ↓ MDA | [172] |
| A375 cells | Skin cancer (Melanoma) | ↑ PI3K/AKT/mTOR pathway ↓ Bcl-2 | [173] | |
| Resveratrol | HaCaT; Mice; Fibroblasts; Keratinocytes | Skin cellular senescence | ↑ SOD, GSH-Px, collagen I ↑ SIRT1/FOXO pathway ↓ COX-2, MMPs ↓ JNK MAPK pathway | [174,175,176,177,178] |
| Human keratinocytes; Mice | Inflammation (AD) | ↓ MAPK pathway ↓ IL-31, IL-8 ↓ ROS, DNA damage ↓ lipid peroxidation | [179,180] | |
| Mice | Inflammation (Psoriasis) | ↓ NF-κB pathway ↓ IL-17A, IL-19 | [181] | |
| Mice | Skin cancer | ↑ Bax ↓ Bcl-2 | [182] | |
| Mice | Skin cancer | ↑ p53 ↓ MAPK pathway | [183] | |
| A431 | Skin cancer | ↓ JAK/STAT pathway | [184] | |
| Quercetin | HST; JB6 P+; HDF; HaCaT; Mice | Skin cellular senescence | ↓ ROS, MMP-1, COX-2, AP-1, XOR ↓ NF-κB, MAPK pathways ↑ SIRT1/FOXO pathway | [185,186,187,188,189,190] |
| Human keratinoytes | Inflammation | ↓ JAK/STAT pathway ↓ IL-1β, IL-18 | [191] | |
| Mice; HaCaT | Inflammation (AD) | ↓ NF-κB, MAPK pathways ↓ COX2, TNF-α, IL-1, IL-2R, IL-1β ↓ IL-6, IL-8 ↑ Nrf2 pathway ↑ SOD, CAT, GPx | [192,193] | |
| Mice | Inflammation (Psoriasis) | ↓ TNF-α, IL-6, IL-17 ↑ GSH, CAT, SOD | [194] | |
| Human melanoma cells | Skin cancer (Melanoma) | ↑ Bax ↓ Bcl-2 ↑ Nrf2 pathway ↓ DNA damage | [195,196] | |
| Ellagic acid | Mice; Fibroblasts; Human skin; HaCaT; Macrophages | Skin cellular senescence | ↓ IL-1β, IL-6, MMPs, ROS ↑ TGF-β pathway ↓ NADPH oxidase, DNA damage ↑ HO-1, SOD | [197,198,199,200,201] |
| Ellagic acid | HaCaT; Mice | Inflammation (AD) | ↓ MAPK, JAK/STAT pathways | [202,203] |
| Mice | Inflammation (CD) | ↓ IL-1β, IL-4 ↓ DNA damage, ROS | [204] | |
| A375; B16 | Skin cancer (Melanoma) | ↑ Apoptosis ↓ PI3K/AKT/mTOR, NF-κB pathways | [205,206,207] | |
| Proanthocyanidins | HFF-1 | Skin cellular senescence | ↑ SOD | [208] |
| Th17 and Treg cells | Inflammation (Psoriasis) | ↓ IL-17, IL-22, TNF-γ, IFN-α, VEGF | [209] | |
| Mice | Inflammation (CD) | ↓ IL-2, IFN-γ, IL-17 | [210] | |
| Mice | Skin cancer (Photocarcinogenesis) | ↓ MAPK, NF-κB pathways | [211] | |
| A375 and Hs294t | Skin cancer (Melanoma) | ↓ ERK, MAPK, NF-κB pathways ↓ COX-2 | [212] | |
| Keratinocytic | Skin cancer (SCC) | ↑ Autophagy | [213] | |
| Honokiol | HaCaT; HFF-1; Keratinocyte; Mice | Skin cellular senescence | ↓ ROS, MMP-1 ↓ NF-κB, MAPK pathways | [214,215,216,217] |
| Mice | Inflammation (AD) | ↓ IL-4, IL-13, IL-17, TNF-γ | [218] | |
| HaCat | Inflammation | ↓ IL-1, IL-8 | [215] | |
| A431 | Skin cancer (SCC) | ↓ Cyclin D1, cyclin D2, cdk2, cd | [219] |
| Polyphenol | Delivery Systems | Skin Model | Main Results | Potential Therapeutic Application | Reference |
|---|---|---|---|---|---|
| Curcumin | Microemulsion | HaCaT cells; Human skin | Significant curcumin concentrations were found in the dermis and curcumin microemulsion-reduced, UV-induced epidermal cytotoxicity. | To protect the skin from oxidative stress-related diseases | [227] |
| Phytovesicles | Mice | The phytovesicles were found to be most effective compared to all other formulations and plain curcumin in providing enhanced antioxidant and antiaging effects. | To enhance the antiaging, antioxidant, and antiwrinkle effects of curcumin | [266] | |
| Elastic vesicle | Mice | Compared with the marketed formula (VICCOA® turmeric skin cream), curcumin ointment (1.64%) had a higher skin retention rate (51.66%). The developed ointment displayed similar effects as the marketed diclofenac sodium ointment (Omni-g (R)) in suppressing acute inflammation in mice. | To treat skin inflammation | [267] | |
| Liposome (DLs) nanocarriers | Isolated human skin | Continuously penetrated the skin and enhanced its biological properties. | To provide more effective treatment | [268] | |
| Carbopol 940 hydrogel | Mice | The anti-inflammatory activity of the gel was better than that of the dexamethasone sodium phosphate cream. The hydrogel promoted collagen enrichment and improved the re-epithelialization of wound epidermis. | To treat skin inflammation and full-thickness wound healing | [269] | |
| Transparent plastid nanovesicles | Human keratinocytes | It protected human keratinocytes from oxidative stress damage in vitro, counteracted inflammation and injury caused by 12-0-tetracyl-chlorowave, reduced edema formation, and improved the biocompatibility and safety of the components. | To increase curcumin’s biocompatibility and safety, as well as its anti-inflammatory activity | [229] | |
| Nanoemulsion loaded polymeric hydrogel | Mice | Compared with curcumin and betamethasone-17-valerate gels, nanolatex preparations showed faster and earlier healing in psoriatic mice. | To treat psoriasis | [270] | |
| Peptide-modified curcumin-loaded liposome (CRC-TD-Lip) | Mice | It exhibited high stability and high curcumin encapsulation efficiency, accelerated the transdermal delivery of curcumin, and enhanced the inhibition of psoriasis. | To treat psoriasis | [271] | |
| Dendritic micellar polymer | B16 and melanoma cells | It has good bioavailability and bioactivity. | To treat melanoma | [272] |
| Participants/ N. (Years). | Products Containing Plant Polyphenols | Topical or Ingested Products | Dosage/ Duration | Outcomes | Potential Therapeutic Application | Reference |
|---|---|---|---|---|---|---|
| Skin of breast cancer patients with radiation/191 (36–81) | Curcumin gel | Topical | (Unknown)/three times a day for a week | Topical curcumin prophylactic therapy may treat radiation dermatitis and pain. | To treat skin inflammation | [273] |
| Patients with moderate scalp psoriasis/30 (18–75) | Turmeric supplements | Ingested | (Unknown)/twice a day for nine weeks | Dermatology Life Quality Index (DLQI) questionnaire and PASI (Psoriasis Area and Severity Index) scores were assessed and turmeric tonic was found to significantly reduce erythema, dandruff, and skin lesions. | To treat skin inflammation | [274] |
| Children with AD/64 (2–12) | Multi-herb formula anti-itch cream with 16% turmeric extract and 0.1% turmeric oil | Topical | 16% turmeric/twice a day | The treatment group (anti-itching cream) and the control group (Moisturex) exhibited statistically significant improvements in all parameters (subjective itching severity clinical evaluation and health). | To treat skin inflammation | [275] |
| People with aging skin caused by UV radiation/39 (20–40) | Pomegranate extract rich in ellagic acid made into round tablets | Ingested | High dose (200 mg/d ellagic acid), low dose (100 mg/d ellagic acid)/once a day for four weeks | The results of the questionnaire showed that the decline rates of skin luminance values in the low-dose group and high-dose group were suppressed by 1.35% and 1.73%, respectively, relative to the baseline. In addition, an improvement trend in some items, such as “facial brightness” and “spots and freckles”, was observed. | To treat skin photoaging | [199] |
| Women with moderate photoaging/10 (unknown) | Green tea oral supplement and green tea cream | Topical and ingested | 10% green tea cream and 300 mg twice-daily green tea oral supplementation for eight weeks. | Participants receiving a combined topical and oral green tea regimen showed histological improvements in elastin content, but no clinically significant changes could be detected. | To treat skin photoaging | [276] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. https://doi.org/10.3390/ijms24043755
Liu H-M, Cheng M-Y, Xun M-H, Zhao Z-W, Zhang Y, Tang W, Cheng J, Ni J, Wang W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. International Journal of Molecular Sciences. 2023; 24(4):3755. https://doi.org/10.3390/ijms24043755
Chicago/Turabian StyleLiu, Hui-Min, Ming-Yan Cheng, Meng-Han Xun, Zhi-Wei Zhao, Yun Zhang, Wei Tang, Jun Cheng, Jia Ni, and Wei Wang. 2023. "Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols" International Journal of Molecular Sciences 24, no. 4: 3755. https://doi.org/10.3390/ijms24043755
APA StyleLiu, H.-M., Cheng, M.-Y., Xun, M.-H., Zhao, Z.-W., Zhang, Y., Tang, W., Cheng, J., Ni, J., & Wang, W. (2023). Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. International Journal of Molecular Sciences, 24(4), 3755. https://doi.org/10.3390/ijms24043755






