Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles
Abstract
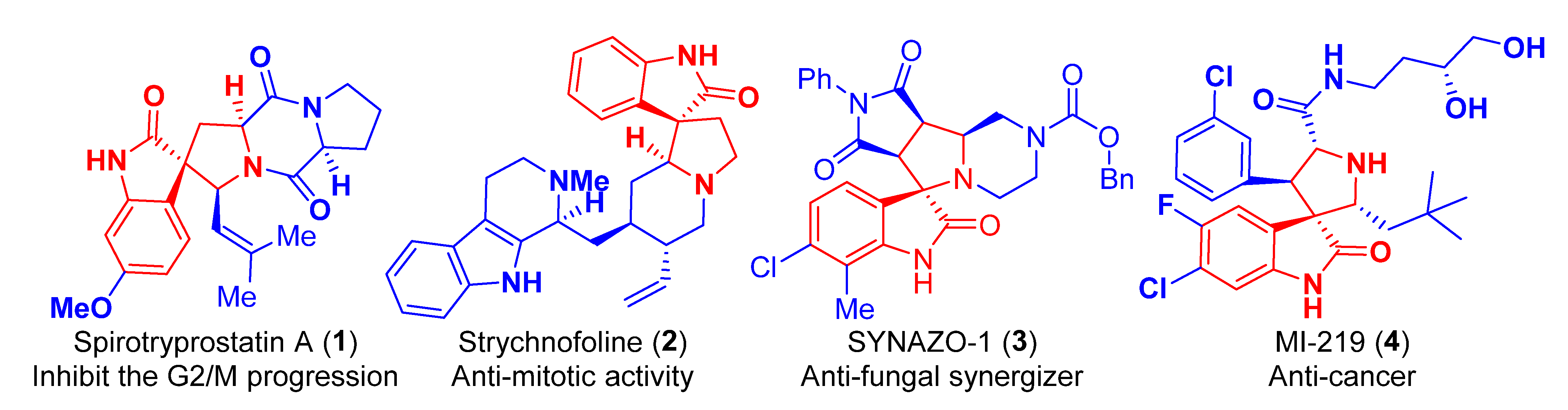
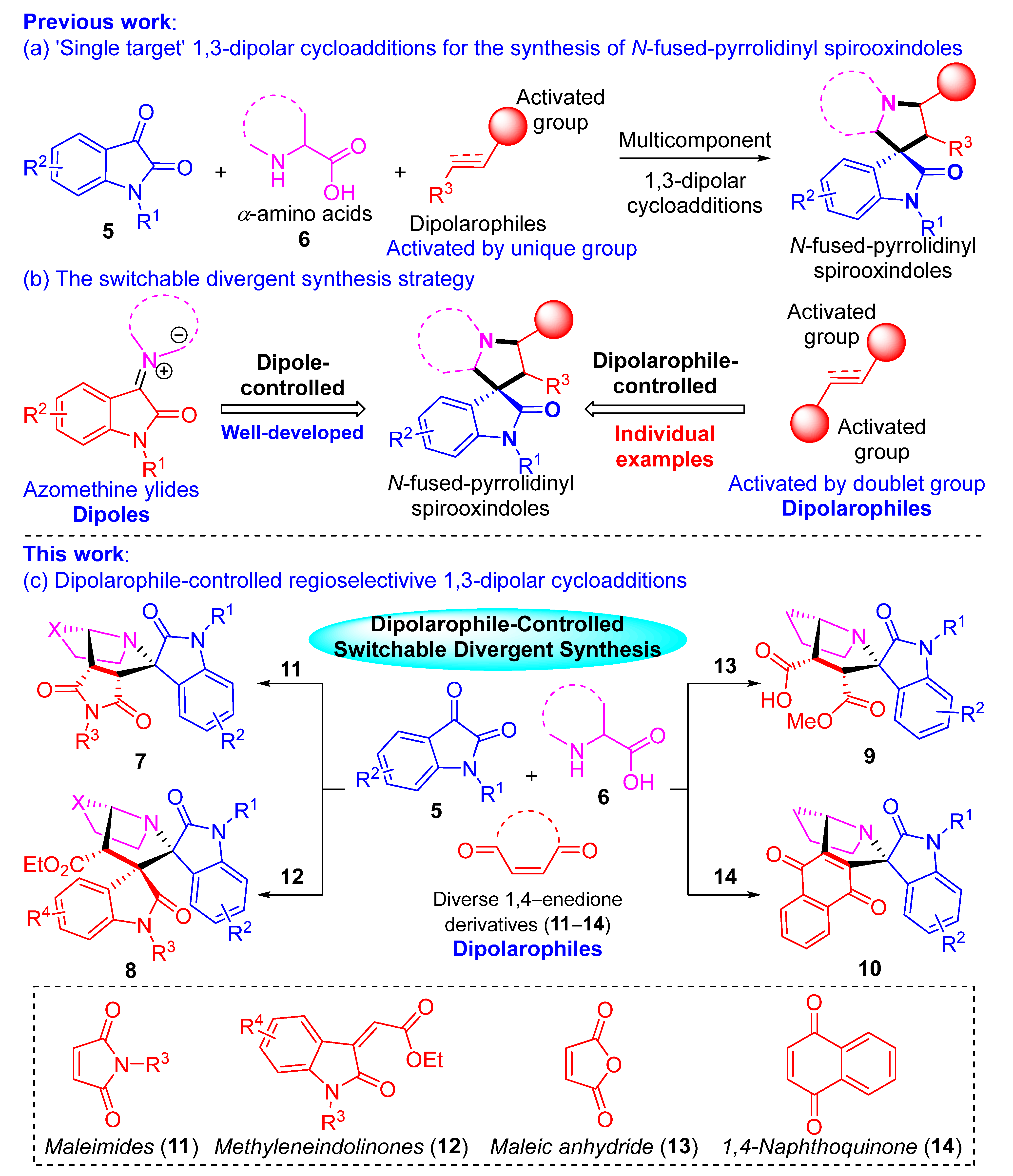
:1. Introduction
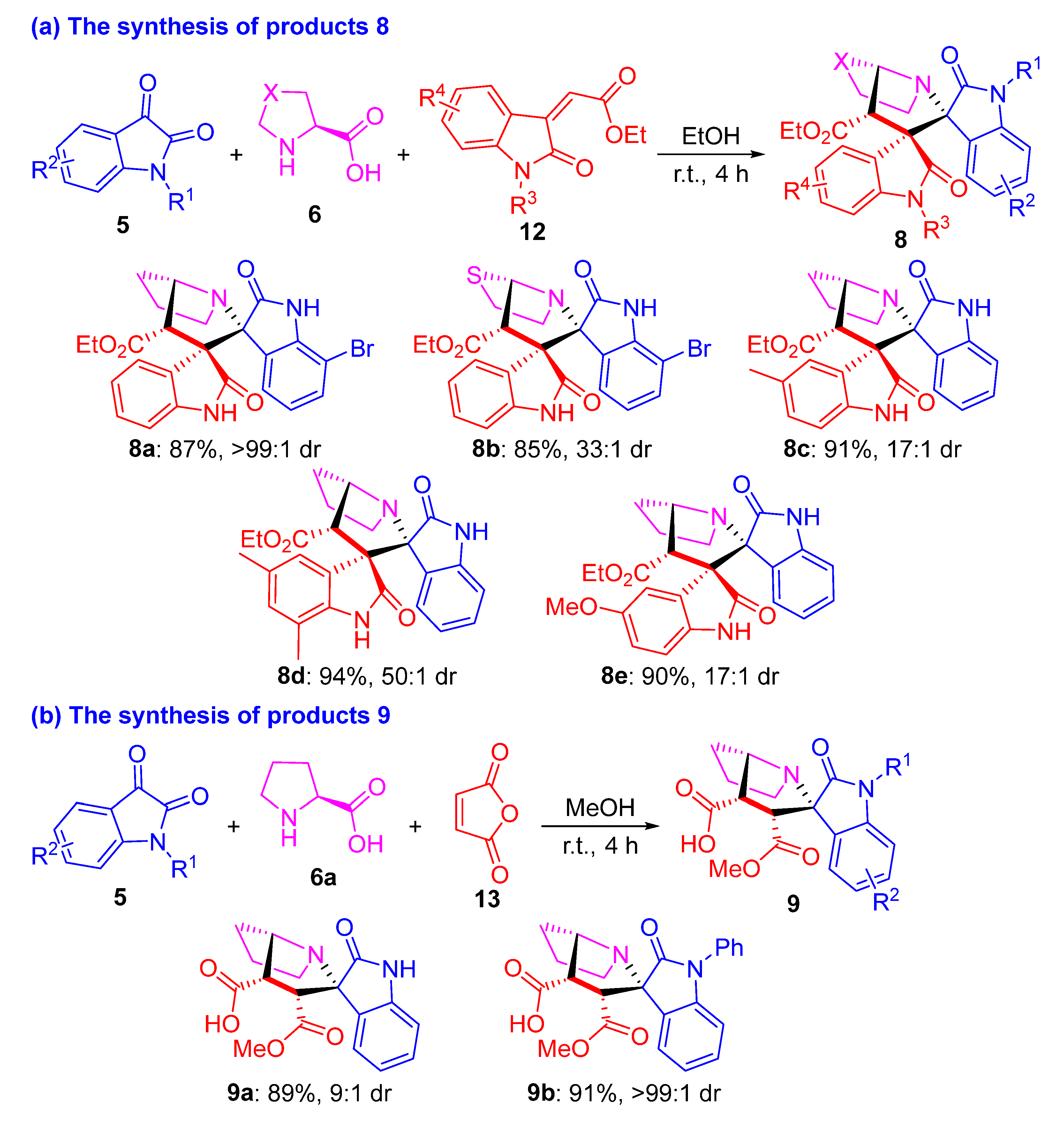
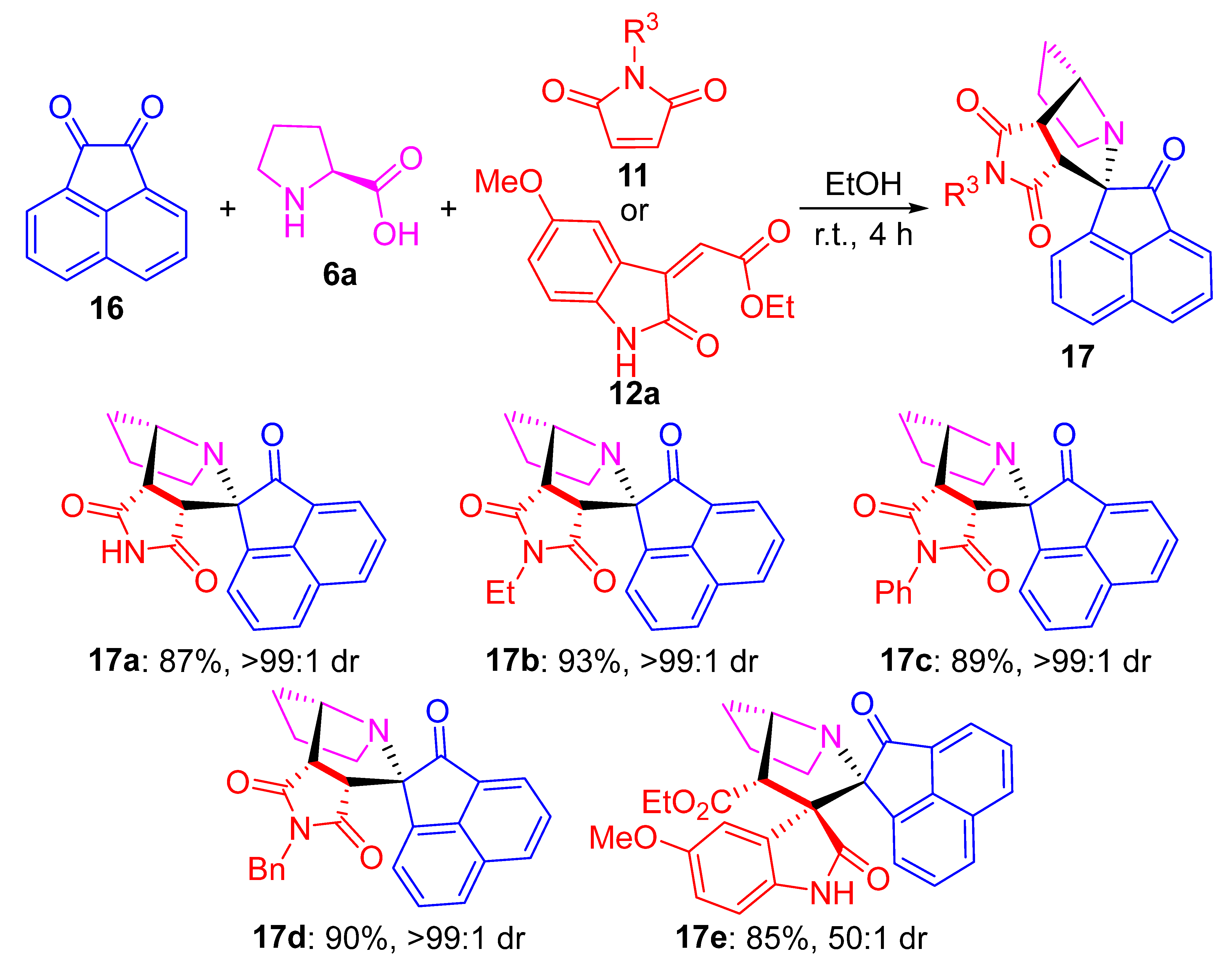
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compounds 7–10
3.3. General Procedure for the Synthesis of Compound 17
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem. Soc. Rev. 2012, 41, 7247–7290. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; Desta, Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.J.; Shi, F. Catalytic asymmetric synthesis of spirooxindoles: Recent developments. Chem. Commun. 2018, 54, 6607–6621. [Google Scholar] [CrossRef]
- Nasri, S.; Bayat, M.; Mirzaei, F. Recent strategies in the synthesis of spiroindole and spirooxindole scaffolds. Top. Curr. Chem. 2021, 379, 25–62. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Blowers, E.C.; Tebbe, C.; Contreras, J.I.; Radhakrishnan, P.; Kizhake, S.; Zhou, T.; Rajule, R.N.; Arnst, J.L.; Munkarah, A.R.; et al. Isatin derived spirocyclic analogues with α-Methylene-γ-butyrolactone as anticancer agents: A structure–activity relationship study. J. Med. Chem. 2016, 59, 5121–5127. [Google Scholar] [CrossRef]
- Nivetha, N.; Martiz, R.M.; Patil, S.M.; Ramu, R.; Sreenivasa, S.; Velmathi, S. Benzodioxole grafted spirooxindole pyrrolidinyl derivatives: Synthesis, characterization, molecular docking and anti-diabetic activity. RSC Adv. 2022, 12, 24192–24207. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashood, S.T.; Hamed, A.R.; Hassan, G.S.; Alkahtani, H.M.; Almehizia, A.A.; Alharbi, A.; Al-Sanea, M.M.; Eldehna, W.M. Antitumor properties of certain spirooxindoles towards hepatocellular carcinoma endowed with antioxidant activity. J. Enzyme Inhib. Med. Chem. 2020, 35, 831–839. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Sun, W.; Liu, L.; Lu, J.; McEachern, D.; Shargary, S.; Bernard, D.; Li, X.; Zhao, T.; et al. A Potent amall-molecule inhibitor of the MDM2–p53 interaction (MI-888) achieved complete and durable tumor regression in mice. J. Med. Chem. 2013, 56, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Dideberg, O.; Lamotte-Brasseur, J.; Dupont, L.; Campsteyn, H.; Vermeire, M.; Angenot, L. Structure cristalline et moléculaire d’un nouvel alcalöide bisindolique: Complexe moléculaire 1:2 strychnofoline-ethanol (C30H34N4O2·2C2H6O). Acta Crystallogr. B 1977, 33, 1796–1801. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, P.; Jian, J.; Chen, Y.; Xu, J. Nine-step total synthesis of (-)-strychnofoline. Chem. Commun. 2018, 54, 1125–1128. [Google Scholar] [CrossRef]
- Premachandra, I.D.U.A.; Scott, K.A.; Shen, C.T.; Wang, F.Q.; Lane, S.; Liu, H.Q.; Vranken, D.L.V. Potent synergy between spirocyclic pyrrolidinoindolinones and fluconazole against calbicans. ChemMedChem 2015, 10, 1672–1686. [Google Scholar] [CrossRef] [PubMed]
- Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R.S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 3933–3938. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, Y.-C.; Shi, X.-J.; Qi, P.-P.; Liu, H.-M. Natural product-derived spirooxindole fragments serve as privileged substructures for discovery of new anticancer agents. Anticancer Agents Med. Chem. 2016, 16, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, Z.; Qi, P.-P.; Yu, D.-Q.; Liu, H.-M. Discovery of orally active anticancer candidate CFI-400945 derived from biologically promising spirooxindoles: Success and challenges. Eur. J. Med. Chem. 2015, 95, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.-Y.; Zhou, J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016, 2, 382–392. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.-Z.; Wei, Y.; Shi, M. Recent advances in the construction of trifluoromethyl-containing spirooxindoles through cycloaddition reactions. Chem-Asian J. 2020, 15, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C.F. Organocatalytic asymmetric assembly reactions: Synthesis of spirooxindoles via organocascade strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Hong, L.; Wang, R. Recent advances in asymmetric organocatalytic construction of 3,3′-spirocyclic oxindoles. Adv. Synth. Catal. 2013, 355, 1023–1052. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.; Dharavath, R.; Madhavaram, M.; Adem, K.; Palithapu, M.; Maloth, G.; Parthasarathy, T. A conventional and solid-state synthesis, biological activity, and molecular docking studies of 6-arylbenzo[4,5]imidazo[1,2-a] [1,8]naphthyridin-10-ols. Synth. Commun. 2019, 49, 1–11. [Google Scholar] [CrossRef]
- Shen, P.; Guo, Y.; Wei, J.; Zhao, H.; Zhai, H.; Zhao, Y. Straightforward synthesis of succinimide-fused pyrrolizidines by a three-component reaction of α-diketone, amino acid, and maleimide. Synthesis 2020, 53, 1262–1270. [Google Scholar]
- Hashimoto, T.; Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef] [PubMed]
- Kissane, M.; Maguire, A.R. Asymmetric 1,3-dipolar cycloadditions of acrylamides. Chem. Soc. Rev. 2010, 39, 845–883. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.; Carretero, J.C. Recent advances in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Chem. Commun. 2014, 50, 12434–12446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Zhang, Y. Cycloaddition/annulation strategies for the construction of multisubstituted pyrrolidines and their applications in natural product synthesis. Org. Chem. Front. 2018, 5, 864–892. [Google Scholar] [CrossRef]
- Yan, L.-J.; Wang, Y.-C. Recent advances in green synthesis of 3,3′-spirooxindoles via isatin-based one-pot multicomponent cascade reactions in aqueous medium. ChemistrySelect 2016, 1, 6948–6960. [Google Scholar] [CrossRef]
- Karcev, D.D.; Efremova, M.M.; Molchanov, A.P.; Rostovskii, N.V.; Kryukova, M.A.; Bunev, A.S.; Khochenkov, D.A. Selective and reversible 1,3-dipolar cycloaddition of 2-(2-oxoindoline-3-ylidene)acetates with nitrones in the synthesis of functionalized spiroisoxazolidines. Int. J. Mol. Sci. 2022, 23, 12639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cobo, A.A.; Franz, A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021, 8, 4315–4348. [Google Scholar] [CrossRef]
- Haddad, S.; Boudriga, S.; Porzio, F.; Soldera, A.; Askri, M.; Knorr, M.; Rousselin, Y.; Kubicki, M.M.; Golz, C.; Strohmann, C. Regio- and stereoselective synthesis of spiropyrrolizidines and piperazines through azomethine ylide cycloaddition reaction. J. Org. Chem. 2015, 80, 9064–9075. [Google Scholar] [CrossRef]
- Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A.; Govindasami, P.; Altaf, M.; Menéndez, J.C. Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron 2018, 74, 5358–5366. [Google Scholar] [CrossRef]
- Mali, P.R.; Chandrasekhara Rao, L.; Bangade, V.M.; Shirsat, P.K.; George, S.A.; Jagadeesh Babu, N.; Meshram, H.M. A convenient and rapid microwave-assisted synthesis of spirooxindoles in aqueous medium and their antimicrobial activities. New J. Chem. 2016, 40, 2225–2232. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Regio- and stereoselective synthesis of spirooxindole 1′-nitro pyrrolizidines with five concurrent stereocenters under aqueous medium and their bioprospection using the zebrafish (Danio rerio) embryo model. Org. Biomol. Chem. 2013, 11, 7372–7386. [Google Scholar] [CrossRef] [PubMed]
- Barkov, A.Y.; Zimnitskiy, N.S.; Korotaev, V.Y.; Kutyashev, I.B.; Moshkin, V.S.; Sosnovskikh, V.Y. Highly regio- and stereoselective 1,3-dipolar cycloaddition of stabilised azomethine ylides to 3,3,3-trihalogeno-1-nitropropenes: Synthesis of trihalomethylated spiro[indoline-3,2′-pyrrolidin]-2-ones and spiro[indoline-3,3′-pyrrolizin]-2-ones. Tetrahedron 2016, 72, 6825–6836. [Google Scholar] [CrossRef]
- Alimohammadi, K.; Sarrafi, Y.; Tajbakhsh, M.; Yeganegi, S.; Hamzehloueian, M. An experimental and theoretical investigation of the regio- and stereoselectivity of the polar [3 + 2] cycloaddition of azomethine ylides to nitrostyrenes. Tetrahedron 2011, 67, 1589–1597. [Google Scholar] [CrossRef]
- Anusha Rani, M.; Vivek Kumar, S.; Malathi, K.; Muthu, M.; Almansour, A.I.; Suresh Kumar, R.; Ranjith Kumar, R. A One-pot multicomponent 1,3-dipolar cycloaddition strategy: Combinatorial synthesis of dihydrothiophenone-engrafted dispiro hybrid heterocycles. ACS Comb. Sci. 2017, 19, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhou, G.; Gong, Y.; Wei, Q.-D.; Tian, M.-Y.; Liu, X.-L.; Feng, T.-T.; Zhou, Y.; Yuan, W.-C. Molecular hybridization-guided one-pot multicomponent synthesis of turmerone motif-fused 3,3′-pyrrolidinyl-dispirooxindoles via a 1,3-dipolar cycloaddition reaction. Molecules 2017, 22, 645. [Google Scholar] [CrossRef] [PubMed]
- Revathy, K.; Lalitha, A. An efficient green chemistry protocol for the synthesis of novel spiropyrrolizidine compounds. RSC Adv. 2014, 4, 279–285. [Google Scholar] [CrossRef]
- Xiao, J.A.; Zhang, H.G.; Liang, S.; Ren, J.W.; Yang, H.; Chen, X.Q. Synthesis of pyrrolo(spiro-[2.3′]-oxindole)-spiro-[4.3″]-oxindole via 1,3-dipolar cycloaddition of azomethine ylides with 3-acetonylideneoxindole. J. Org. Chem. 2013, 78, 11577–11583. [Google Scholar] [CrossRef]
- Palomba, M.; De Monte, E.; Mambrini, A.; Bagnoli, L.; Santi, C.; Marini, F. A three-component [3 + 2]-cycloaddition/elimination cascade for the synthesis of spirooxindole-pyrrolizines. Org. Biomol. Chem. 2021, 19, 667–676. [Google Scholar] [CrossRef]
- Sankar, U.; Surya Kumar, C.V.; Subramanian, V.; Balasubramanian, K.K.; Mahalakshimi, S. Stereo-, regio-, and chemoselective [3 + 2]-cycloaddition of (2E,4E)-ethyl 5-(phenylsulfonyl)penta-2,4-dienoate with various azomethine ylides, nitrones, and nitrile oxides: Synthesis of pyrrolidine, isoxazolidine, and isoxazoline derivatives and a computational study. J. Org. Chem. 2016, 81, 2340–2354. [Google Scholar]
- Tiwari, K.N.; Pandurang, T.P.; Pant, S.; Kumar, R. Highly efficient and regioselective synthesis of spirooxindolo pyrrolizidines by reaction of isatin, proline and acrylonitrile/methyl acrylate in water. Tetrahedron Lett. 2016, 57, 2286–2289. [Google Scholar] [CrossRef]
- Cao, J.; Yang, F.; Sun, J.; Huang, Y.; Yan, C.-G. Construction of unique eight- or nine-membered polyheterocyclic systems via multicomponent reaction of l-proline, alkyl propiolate, and isatin. J. Org. Chem. 2019, 84, 622–635. [Google Scholar] [CrossRef]
- Mali, P.R.; Shirsat, P.K.; Khomane, N.; Nayak, L.; Nanubolu, J.B.; Meshram, H.M. 1,3-Dipolar cycloaddition reactions for the synthesis of novel oxindole derivatives and their cytotoxic properties. ACS Comb. Sci. 2017, 19, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; He, X.; Shang, Y.; Xie, M. Combinatorial synthesis of spiro[indoline-3,2′-pyrrole] derivatives via a three-component reaction under catalyst-free conditions. RSC Adv. 2016, 6, 10412–10418. [Google Scholar] [CrossRef]
- Kolle, S.; Barak, D.S.; Ghosh, A.; Jaiswal, V.; Kant, R.; Batra, S. Dehydrative transformation of spirooxindoles to pyrido[2,3-b]indoles via POCl3. ACS Omega 2019, 4, 5617–5629. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Zhu, R.-Y.; Zheng, J.; Shi, F.; Tu, S.-J. Enantioselective construction of spiro[indoline-3,2′-pyrrole] framework via catalytic asymmetric 1,3-dipolar cycloadditions using allenes as equivalents of alkynes. J. Org. Chem. 2015, 80, 512–520. [Google Scholar] [CrossRef]
- Qian, Y.-L.; Xia, P.-J.; Li, J.; Zhao, Q.-L.; Xiao, J.-A.; Xiang, H.-Y.; Yang, H. Diversity-driven and facile 1,3-dipolar cycloaddition to access dispirooxindole-imidazolidine scaffolds. Org. Biomol. Chem. 2017, 15, 8705–8708. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Harmat, V.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. 1,3-Dipolar cycloaddition of isatin-derived azomethine ylides with 2H-azirines: Stereoselective synthesis of 1,3-diazaspiro[bicyclo[3.1.0]hexane]oxindoles. J. Org. Chem. 2019, 84, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Filatov, A.S.; Knyazev, N.A.; Molchanov, A.P.; Panikorovsky, T.L.; Kostikov, R.R.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of functionalized 3-spiro[cyclopropa[a]pyrrolizine]- and 3-spiro[3-azabicyclo[3.1.0]hexane]oxindoles from cyclopropenes and azomethine ylides via [3 + 2]-cycloaddition. J. Org. Chem. 2017, 82, 959–975. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Nájera, C.; Yus, M. Chemodivergent reactions. Chem. Soc. Rev. 2020, 49, 7101–7166. [Google Scholar] [CrossRef]
- Chintawar, C.C.; Yadav, A.K.; Kumar, A.; Sancheti, S.P.; Patil, N.T. Divergent gold catalysis: Unlocking molecular diversity through catalyst control. Chem. Rev. 2021, 121, 8478–8558. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, M. Divergent synthesis of carbo- and heterocycles via gold-catalyzed reactions. ACS Catal. 2016, 6, 2515–2524. [Google Scholar] [CrossRef]
- Neufeldt, S.R.; Sanford, M.S. Controlling site selectivity in palladium-catalyzed C–H bond functionalization. Acc. Chem. Res. 2012, 45, 936–946. [Google Scholar] [CrossRef]
- Gong, P.; Ma, Y.; Wang, X.; Yu, L.; Zhu, S. A facile and diverse synthesis of coumarin substituted spirooxindole and dispiro oxindole-pyrrolizidine/pyrrolothiazole/pyrrolidine derivatives via 1, 3-dipolar cycloaddition. Tetrahedron 2021, 91, 132221–132231. [Google Scholar] [CrossRef]
- Rajesh, S.M.; Perumal, S.; Menéndez, J.C.; Yogeeswari, P.; Sriram, D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. MedChemComm 2011, 2, 626–630. [Google Scholar] [CrossRef]
- Wang, K.-K.; Li, Y.-L.; Chen, R.; Wang, Z.-Y.; Li, N.-B.; Zhang, L.L.; Gu, S. Substrate-controlled regioselectivity switchable [3 + 2] annulations to access spirooxindole skeletons. J. Org. Chem. 2022, 87, 8158–8169. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-K.; Li, Y.-L.; Chen, R.-X.; Sun, A.-L.; Wang, Z.-Y.; Zhao, Y.-C.; Wang, M.-Y.; Sheng, S. Substrate-controlled regioselectivity switch in a three-component 1,3-dipolar cycloaddition reaction to access 3,3′-pyrrolidinyl-spirooxindoles derivatives. Adv. Synth. Catal. 2022, 364, 2047–2052. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef]
- Ganesh, M.; Suraj, S. Expeditious entry into carbocyclic and heterocyclic spirooxindoles. Org. Biomol. Chem. 2022, 20, 5651–5693. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Lu, X.; Liu, Y.; Qiao, Y.; Qu, W.; Feng, F.; Sun, H. Recent research progress of Uncaria spp. based on alkaloids: Phytochemistry, pharmacology and structural chemistry. Eur. J. Med. Chem. 2021, 210, 112960. [Google Scholar] [CrossRef]
- Azizian, J.; Asadi, A.; Jadidi, K. One-pot highly diastereo-selective synthesis of new 2-substituted 8-(sprio-3′- indolino-2′-one)-pyrrolo[3,4-a]-pyrrolizine-1,3-diones mediated by azomethine ylide induced by microwave irradiation. Synth. Commun. 2001, 31, 2727–2733. [Google Scholar] [CrossRef]
- Rehn, S.; Bergman, J.; Stensland, B. The three-component reaction between isatin, α-amino acids, and dipolarophiles. Eur. J. Org. Chem. 2004, 2004, 413–418. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, D.; Wei, Y.; Shi, M. Highly efficient and stereoselective construction of bispirooxindole derivatives via a three-component 1,3-dipolar cycloaddition reaction. ChemistryOpen 2014, 3, 93–98. [Google Scholar] [CrossRef]
- Qian, Y.-L.; Li, B.; Xia, P.-J.; Wang, J.; Xiang, H.-Y.; Yang, H. Diastereospecific entry to pyrrolidinyldispirooxindole skeletons via three-component 1,3-dipolar cycloadditions. Tetrahedron 2018, 74, 6821–6828. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, W.-H.; Wang, D.-D.; Gong, Y.; Wei, Q.-D.; Liu, X.-L.; Feng, T.-T.; Zhou, Y.; Yuan, W.-C. 3-Methyl-4-nitro-5-isatylidenyl-isoxazoles as 1,3-dipolarophiles for synthesis of polycyclic 3,3′-pyrrolidinyl-dispirooxindoles and their biological evaluation for anticancer activities. Tetrahedron 2017, 73, 5176–5188. [Google Scholar] [CrossRef]
- Bhaskar, G.; Arun, Y.; Balachandran, C.; Saikumar, C.; Perumal, P.T. Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem. 2012, 51, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Jain, A.K.; Laxkar, A.K. Ethyl lactate as a promising bio based green solvent for the synthesis of spiro-oxindole derivatives via 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2013, 54, 3929–3932. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.-Y.; Xu, X.-P.; Ji, S.-J. Facile three-component synthesis of spirooxindolepyrrololine ring systems via 1,3-dipolar cycloaddition with 1,4-naphthoquinone. Synth. Commun. 2011, 41, 3280–3288. [Google Scholar] [CrossRef]
- Dandia, A.; Jain, A.K.; Sharma, S.; Singh, R. Task-specific ionic liquid mediated eco-compatible approach for the synthesis of spirooxindole derivatives and their DNA cleavage activity. J. Heterocycl. Chem. 2018, 55, 1419–1425. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J.P.; Zhang, W. One-pot synthesis of polycyclic spirooxindoles via montmorillonite K10-catalyzed C–H functionalization of cyclic amines. ACS Sustain. Chem. Eng. 2018, 6, 5574–5579. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wang, J.-L.; Burgess, K.S.; Zhang, J.-W.; Zheng, Q.-M.; Pu, Y.-D.; Yan, L.-J.; Chen, X.-B. Green synthesis of new pyrrolidine-fused spirooxindoles via three-component domino reaction in EtOH/H2O. RSC Adv. 2018, 8, 5702–5713. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Wang, J.-L.; Xu, D.; Burgess, K.S.; Zhu, A.-F.; Rao, Y.-Y.; Chen, X.-B.; Wang, Y.-C. Chemically sustainable and green one-pot multicomponent synthesis of highly functionalized polycyclic N-fused-pyrrolidine heterocycles. ChemistrySelect 2018, 3, 662–665. [Google Scholar] [CrossRef]


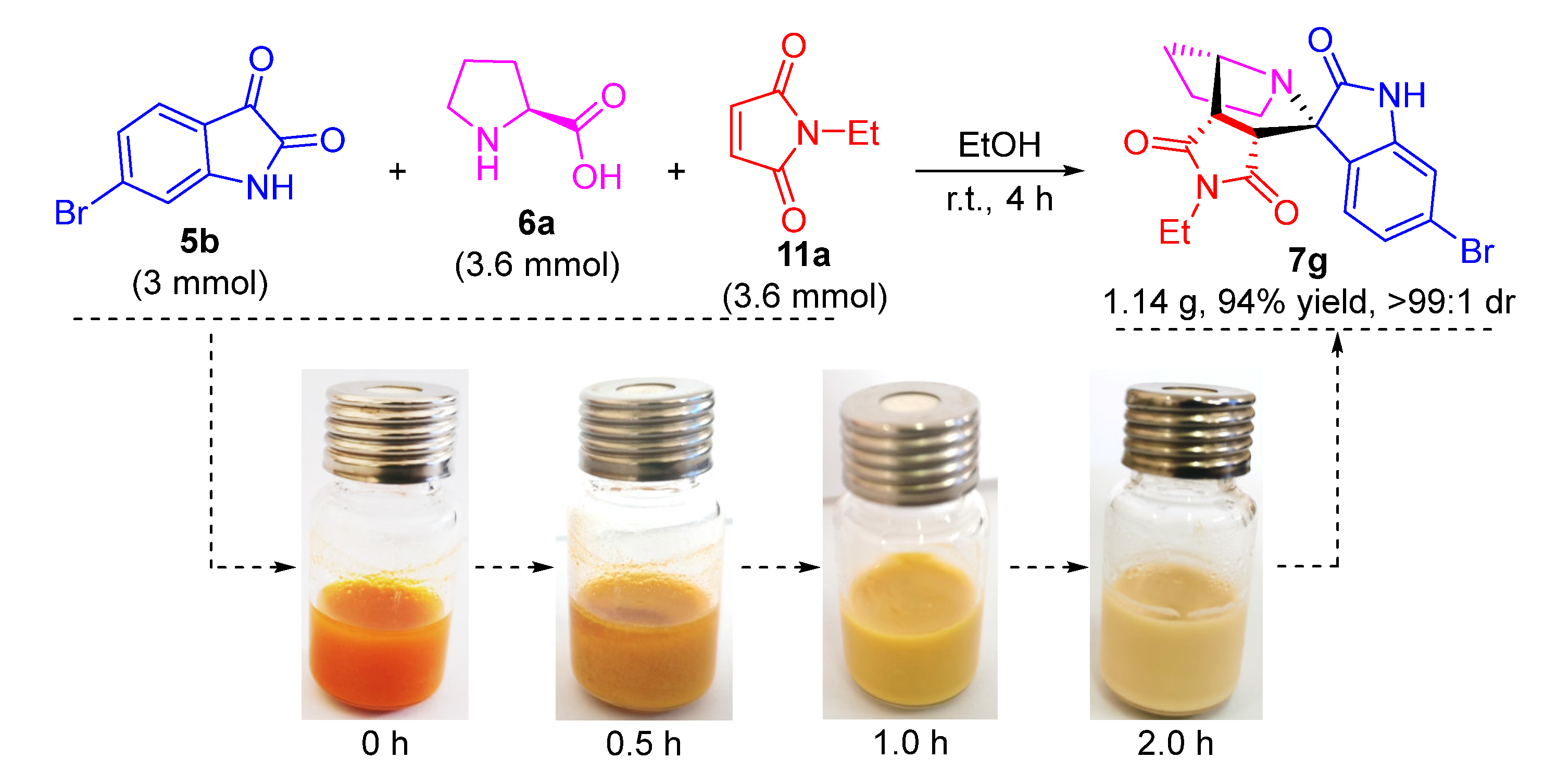
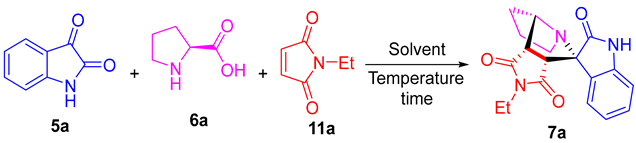 | ||||
|---|---|---|---|---|
| Entry | Solvent | Temperature (°C) | Time (h) | Yield b (%) |
| 1 | Toluene | r.t. c | 4 | 72 |
| 2 | Benzene | r.t. | 4 | 57 |
| 3 | Acetonitrile | r.t. | 4 | 49 |
| 4 | Ether | r.t. | 4 | 32 |
| 5 | DCE | r.t. | 4 | 67 |
| 6 | DCM | r.t. | 4 | 61 |
| 7 | CHCl3 | r.t. | 4 | 58 |
| 8 | THF | r.t. | 4 | 45 |
| 9 | Acetone | r.t. | 4 | 24 |
| 10 | 1,4-Dioxane | r.t. | 4 | 64 |
| 11 | DMSO | r.t. | 4 | 38 |
| 12 | DMF | r.t. | 4 | 25 |
| 13 | H2O | r.t. | 4 | 18 |
| 14 | iPrOH | r.t. | 4 | 81 |
| 15 | MeOH | r.t. | 4 | 94 |
| 16 | EtOH | r.t. | 4 | 93 |
| 17 | EtOH | 40 | 4 | 93 |
| 18 | EtOH | reflux | 4 | 94 |
| 19 | EtOH | r.t. | 2 | 78 |
| 20 | EtOH | r.t. | 8 | 93 |
| 21 | EtOH | r.t. | 24 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, L.; Yan, Y.; Li, S.; Lu, H.; Liu, J.; Dong, J. Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles. Int. J. Mol. Sci. 2023, 24, 3771. https://doi.org/10.3390/ijms24043771
Wang Y, Yan L, Yan Y, Li S, Lu H, Liu J, Dong J. Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles. International Journal of Molecular Sciences. 2023; 24(4):3771. https://doi.org/10.3390/ijms24043771
Chicago/Turabian StyleWang, Yongchao, Lijun Yan, Yuxin Yan, Sujin Li, Hongying Lu, Jia Liu, and Jianwei Dong. 2023. "Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles" International Journal of Molecular Sciences 24, no. 4: 3771. https://doi.org/10.3390/ijms24043771
APA StyleWang, Y., Yan, L., Yan, Y., Li, S., Lu, H., Liu, J., & Dong, J. (2023). Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles. International Journal of Molecular Sciences, 24(4), 3771. https://doi.org/10.3390/ijms24043771





