Mechanical Ventilation-Related High Stretch Mainly Induces Endoplasmic Reticulum Stress and Thus Mediates Inflammation Response in Cultured Human Primary Airway Smooth Muscle Cells
Abstract
1. Introduction
2. Results
2.1. High Stretch Induces Differential Expression of mRNAs in ASMCs
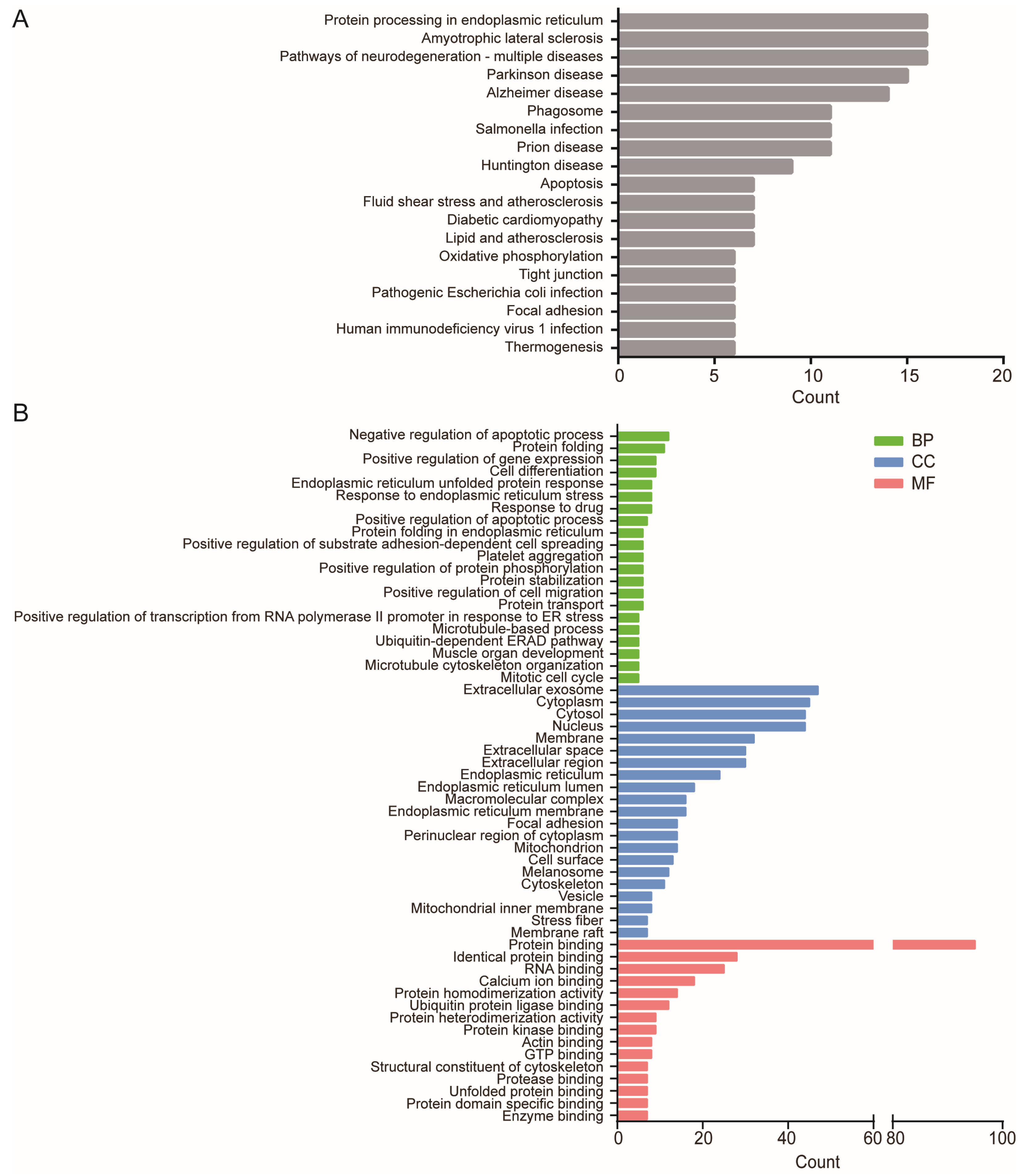
2.2. High-Stretch-Induced DE-mRNAs Are Related to Specific Functional Enrichment
2.3. High-Stretch-Induced DE-mRNAs Forms Distinct Protein–Protein Interaction Network
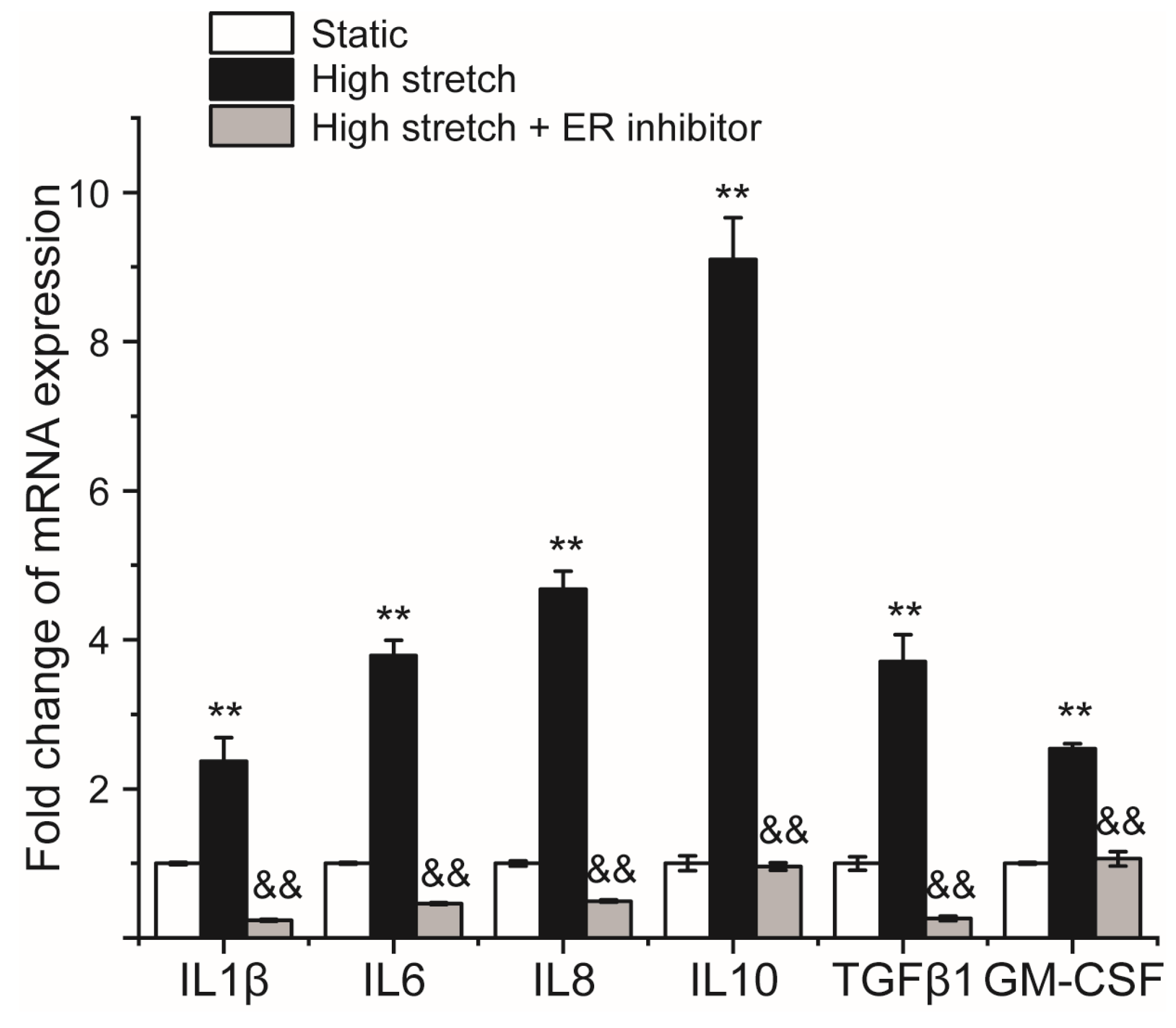
2.4. Inhibition of ER Stress Prevents High-Stretch-Enhanced Expression of Hub Genes Related to ER Stress and Downstream Inflammation Signaling
2.5. Inhibition of ER Stress Prevents High-Stretch-Enhanced mRNA Expression of Airway Inflammatory Factors
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Culture of ASMCs with/out High Stretch
4.3. Whole Genome-Wide mRNA Sequencing in Cultured ASMCs
4.4. Principal Component Analysis
4.5. Differential Expression Analysis of mRNAs in Cultured ASMCs
4.6. Bioinformatics Analysis
4.7. Protein–Protein Interaction Analysis and Hub Gene Identification
4.8. Quantitative PCR Analysis of mRNA Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanchal, R.S.; Truwit, J.D. Recent advances in understanding and treating acute respiratory distress syndrome. F1000Research 2018, 7, F1000 Faculty Rev-725. [Google Scholar] [CrossRef]
- Petrucci, N.; De Feo, C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2013, 2013, CD003844. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Barbas, C.S.V.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A.; Network“, T.A.R.D.S. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am. J Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef]
- Gattinoni, L.; Marini, J.J.; Collino, F.; Maiolo, G.; Rapetti, F.; Tonetti, T.; Vasques, F.; Quintel, M. The future of mechanical ventilation: Lessons from the present and the past. Crit. care 2017, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Madahar, P.; Beitler, J.R. Emerging concepts in ventilation-induced lung injury. F1000Research 2020, 9, F1000 Faculty Rev-1222. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.C.; Slutsky, A.S. Invited review: Mechanisms of ventilator-induced lung injury: A perspective. J. Appl. Physiol. 2000, 89, 1645–1655. [Google Scholar] [CrossRef]
- Edibam, C. Ventilator-induced lung injury and implications for clinical management. Crit. care Resusc.J Australas. Acad. Crit. Care Med. 2000, 2, 269–277. [Google Scholar]
- Luo, M.; Ni, K.; Sun, Y.; Guo, J.; Wen, K.; Deng, L. Toward an optimized strategy of using various airway mucus clearance techniques to treat critically ill COVID-19 patients. Biocell 2022, 46, 855–871. [Google Scholar] [CrossRef]
- Garfield, B.; Handslip, R.; Patel, B.V. Ventilator-associated lung injury. Encycl. Respir. Med. 2021, 361, 406–417. [Google Scholar]
- Wilson, M.R.; Takata, M. Inflammatory mechanisms of ventilator-induced lung injury: A time to stop and think? Anaesthesia 2013, 68, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Uhlig, U. Pharmacological interventions in ventilator-induced lung injury. Trends Pharmacol. Sci. 2004, 25, 592–600. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Chen, L.; Xiong, W.; Song, L.; Li, B.; Zhou, T.; Pei, L.; Yuan, S.; Yao, S.; et al. Death-associated protein kinase 1 mediates ventilator-induced lung injury in mice by promoting alveolar epithelial cell Apoptosis. Anesthesiology 2020, 133, 905–918. [Google Scholar] [CrossRef]
- Wan, B.; Xu, W.J.; Zhan, P.; Jin, J.J.; Xi, G.M.; Chen, M.Z.; Hu, Y.B.; Zhu, S.H.; Liu, H.B.; Wang, X.X.; et al. Topotecan alleviates ventilator-induced lung injury via NF-kappaB pathway inhibition. Cytokine 2018, 110, 381–388. [Google Scholar] [CrossRef]
- Ma, H.; Feng, X.; Ding, S. Hesperetin attenuates ventilator-induced acute lung injury through inhibition of NF-κB-mediated inflammation. Eur. J Pharmacol. 2015, 769, 333–341. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-Induced Lung Injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Cabrera-Benitez, N.E.; Laffey, J.G.; Parotto, M.; Spieth, P.M.; Villar, J.; Zhang, H.; Slutsky, A.S. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: A significant contributor to poor outcome. Anesthesiology 2014, 121, 189–198. [Google Scholar] [CrossRef]
- Farooqi, F.I.; Morgan, R.C.; Dhawan, N.; Dinh, J.; Yatzkan, G.; Michel, G. Airway hygiene in COVID-19 pneumonia: Treatment responses of 3 critically ill cruise ship employees. Am. J. Case. Rep. 2020, 21, e926596. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Ni, K.; Guo, J.; Bu, B.; Liu, L.; Pan, Y.; Li, J.; Luo, M.; Deng, L. MircroRNA Let-7a-5p in airway smooth muscle cells is most responsive to high stretch in association with cell mechanics modulation. Front. Physiol. 2022, 13, 830406. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Ito, S.; Morosawa, M.; Furuya, K.; Naruse, K.; Sokabe, M.; Yamaguchi, E.; Hasegawa, Y. Cyclic stretch enhances reorientation and differentiation of 3-D culture model of human airway smooth muscle. Biochem. Biophys. Rep. 2018, 16, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, A. The role of stretch-activated ion channels in acute respiratory distress syndrome: Finally a new target? Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L639–L652. [Google Scholar] [CrossRef] [PubMed]
- Bartolak-Suki, E.; LaPrad, A.S.; Harvey, B.C.; Suki, B.; Lutchen, K.R. Tidal stretches differently regulate the contractile and cytoskeletal elements in intact airways. PLoS ONE 2014, 9, e94828. [Google Scholar] [CrossRef]
- Lin, T.Y.; Venkatesan, N.; Nishioka, M.; Kyoh, S.; Al-Alwan, L.; Baglole, C.J.; Eidelman, D.H.; Ludwig, M.S.; Hamid, Q. Monocyte-derived fibrocytes induce an inflammatory phenotype in airway smooth muscle cells. Clin. Exp. Allergy 2014, 44, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Pairet, N.; Mang, S.; Fois, G.; Keck, M.; Kuhnbach, M.; Gindele, J.; Frick, M.; Dietl, P.; Lamb, D.J. TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS ONE 2018, 13, e0196055. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Knox, A.J.; Boriek, A.M. CCAAT/enhancer-binding protein and activator protein-1 transcription factors regulate the expression of interleukin-8 through the mitogen-activated protein kinase pathways in response to mechanical stretch of human airway smooth muscle cells. J. Biol. Chem. 2003, 278, 18868–18876. [Google Scholar] [CrossRef]
- Fang, X.; Ni, K.; Guo, J.; Li, Y.; Zhou, Y.; Sheng, H.; Bu, B.; Luo, M.; Ouyang, M.; Deng, L. FRET visualization of cyclic stretch-activated erk via calcium channels mechanosensation while not Integrin β1 in airway smooth muscle cells. Front. Cell Dev. Biol. 2022, 10, 847852. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Ergin, S.; Kherad, N.; Alagoz, M. RNA sequencing and its applications in cancer and rare diseases. Mol. Biol. Rep. 2022, 49, 2325–2333. [Google Scholar] [CrossRef]
- Ketkar, S.; Burrage, L.C.; Lee, B. RNA sequencing as a diagnostic tool. JAMA 2023, 329, 85–86. [Google Scholar] [CrossRef]
- Shih, W.; Chai, S. Data-Driven vs. Hypothesis-Driven Research: Making sense of big data. Acad. Manag. Proc. 2016, 2016, 14843. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3β. J. Biol. Chem. 2010, 285, 29336–29347. [Google Scholar] [CrossRef]
- Kanefsky, J.; Lenburg, M.; Hai, C.M. Cholinergic receptor and cyclic stretch-mediated inflammatory gene expression in intact ASM. Am. J. Respir. Cell Mol. Biol. 2006, 34, 417–425. [Google Scholar] [CrossRef]
- Chen, S.; Xia, J.; Zhan, Q.; Zhang, Y. Microarray analysis reveals the changes in circular RNA expression and molecular mechanisms in mice with ventilator-induced lung injury. Front. Physiol. 2022, 13, 838196. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Tanjore, H.; Blackwell, T.S.; Lawson, W.E. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L721–L729. [Google Scholar] [CrossRef]
- Leonard, A.; Rahman, A.; Grose, V.; Slavin, S.A.; Fazal, F.J.T.F.J. ER stress-BiP axis regulates endothelial barrier dysfunction and inflammation in acute lung injury. FASEB J. 2016, 30. [Google Scholar]
- Pathinayake, P.S.; Waters, D.W.; Nichol, K.S.; Brown, A.C.; Reid, A.T.; Hsu, A.Y.; Horvat, J.C.; Wood, L.G.; Baines, K.J.; Simpson, J.L.; et al. Endoplasmic reticulum-unfolded protein response signalling is altered in severe eosinophilic and neutrophilic asthma. Thorax 2022, 77, 443–451. [Google Scholar] [CrossRef]
- Cirone, M. Could UPR manipulation help to tune the inflammatory response in the course of COVID-19? Virol. Immunol. J. 2020, 4, 000245. [Google Scholar]
- You, K.; Wang, L.; Chou, C.H.; Liu, K.; Nakata, T.; Jaiswal, A.; Yao, J.; Lefkovith, A.; Omar, A.; Perrigoue, J.G.; et al. QRICH1 dictates the outcome of ER stress through transcriptional control of proteostasis. Science 2021, 371, eabb6896. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Balch, W.E.; Sznajder, J.I.; Budinger, S.; Finley, D.; Laposky, A.D.; Cuervo, A.M.; Benjamin, I.J.; Barreiro, E.; Morimoto, R.I.; Postow, L.; et al. Malfolded Protein Structure and Proteostasis in Lung Diseases. Am. J Respir. Crit. Care Med. 2013, 189, 96–103. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO reports 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef]
- Zhang, H.; He, L.; Cai, L. Transcriptome Sequencing: RNA-Seq. Methods Mol. Biol. 2018, 1754, 15–27. [Google Scholar]
- Damuth, E.; Mitchell, J.A.; Bartock, J.L.; Roberts, B.W.; Trzeciak, S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: A systematic review and meta-analysis. Lancet. Respir. Med. 2015, 3, 544–553. [Google Scholar] [CrossRef]
- Grootjans, J.; Kaser, A.; Kaufman, R.J.; Blumberg, R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016, 16, 469–484. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Quinn, D.A.; Moufarrej, R.K.; Volokhov, A.; Hales, C.A. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J. Appl. Physiol. 2002, 93, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Keane, M.P.; Burdick, M.D.; Londhe, V.; Xue, Y.Y.; Li, K.; Phillips, R.J.; Strieter, R.M. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J. Clin. Invest. 2002, 110, 1703–1716. [Google Scholar] [CrossRef]
- Imai, Y.; Kawano, T.; Iwamoto, S.; Nakagawa, S.; Takata, M.; Miyasaka, K. Intratracheal anti-tumor necrosis factor-alpha antibody attenuates ventilator-induced lung injury in rabbits. J. Appl. Physiol. 1999, 87, 510–515. [Google Scholar] [CrossRef]
- Imai, Y.; Parodo, J.; Kajikawa, O.; de Perrot, M.; Fischer, S.; Edwards, V.; Cutz, E.; Liu, M.; Keshavjee, S.; Martin, T.R.; et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003, 289, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Amado-Rodríguez, L.; Albaiceta, G.M. Towards prevention of ventilator-induced lung injury: Is mechanotransduction the answer? Minerva Anestesiol 2014, 80, 874–876. [Google Scholar] [PubMed]
- Uhlig, U.; Uhlig, S. Ventilation-induced lung injury. Compr. Physiol. 2011, 1, 635–661. [Google Scholar] [PubMed]
- Dolinay, T.; Himes, B.E.; Shumyatcher, M.; Lawrence, G.G.; Margulies, S.S. Integrated stress response mediates epithelial injury in mechanical ventilation. Am. J. Respir. Cell Mol. Biol. 2017, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zeng, Q.; Dai, H.; Zhang, W.; Wang, X.; Ma, R.; Hong, X.; Zhao, C.; Pan, L. Endoplasmic reticulum stress is involved in ventilator-induced lung injury in mice via the IRE1α-TRAF2-NF-κB pathway. Int. Immunopharmacol. 2020, 78, 106069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Ye, L.; Ling, M.; Ma, R.; Li, J.; Chen, H.; Pan, L. TLR4/TRAF6/NOX2 signaling pathway is involved in ventilation-induced lung injury via endoplasmic reticulum stress in murine model. Int. Immunopharmacol. 2021, 96, 107774. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Yousof, T.; Sharma, H.; Austin, R.C.; Fox-Robichaud, A.E. Stressing the endoplasmic reticulum response as a diagnostic tool for sepsis. Ann. Transl. Med. 2022, 10, 812. [Google Scholar] [CrossRef]
- Li, F.; Lin, Q.; Shen, L.; Zhang, Z.; Wang, P.; Zhang, S.; Xing, Q.; Xia, Z.; Zhao, Z.; Zhang, Y.; et al. The diagnostic value of endoplasmic reticulum stress-related specific proteins GRP78 and CHOP in patients with sepsis: A diagnostic cohort study. Ann. Transl. Med. 2022, 10, 470. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; He, J.; Zhang, L.; Zhou, J.; Balaji, K.S.; Shen, S.; Peng, J.; Sharma, A.; Fu, J. Evaluation and characterization of HSPA5 (GRP78) expression profiles in normal individuals and cancer patients with COVID-19. Int. J. Biol. Sci. 2021, 17, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Dvela-Levitt, M.; Kost-Alimova, M.; Emani, M.; Kohnert, E.; Thompson, R.; Sidhom, E.-H.; Rivadeneira, A.; Sahakian, N.; Roignot, J.; Papagregoriou, G.; et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell 2019, 178, 521–535.e23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, M.; Wang, Q.; Zhu, M.; Chen, S.; Li, H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch. Oral Biol. 2017, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, S.; Huang, Z.; Wen, J.; Li, H. Ca2+-Dependent Endoplasmic Reticulum Stress Regulates Mechanical Stress-Mediated Cartilage Thinning. J. Dent. Res. 2016, 95, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Castillero, E.; Akashi, H.; Pendrak, K.; Yerebakan, H.; Najjar, M.; Wang, C.; Naka, Y.; Mancini, D.; Sweeney, H.L.; D′ Armiento, J.; et al. Attenuation of the unfolded protein response and endoplasmic reticulum stress after mechanical unloading in dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H459–H470. [Google Scholar] [CrossRef]
- Di Stasio, E.; De Cristofaro, R. The effect of shear stress on protein conformation: Physical forces operating on biochemical systems: The case of von Willebrand factor. Biophys. Chem. 2010, 153, 1–8. [Google Scholar] [CrossRef]
- Puchner, E.M.; Alexandrovich, A.; Kho, A.L.; Hensen, U.; Schäfer, L.V.; Brandmeier, B.; Gräter, F.; Grubmüller, H.; Gaub, H.E.; Gautel, M. Mechanoenzymatics of titin kinase. Proc. Natl. Acad. Sci. USA 2008, 105, 13385–13390. [Google Scholar] [CrossRef]
- Hachiya, N.S.; Kozuka, Y.; Kaneko, K. Mechanical stress and formation of protein aggregates in neurodegenerative disorders. Med. Hypotheses 2008, 70, 1034–1037. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Zhang, M.; Zeng, H.; Lu, Y.; Liu, L.; Li, J.; Deng, L. Artesunate attenuates airway resistance in vivo and relaxes airway smooth muscle cells in vitro via bitter taste receptor-dependent calcium signalling. Exp. Physiol. 2019, 104, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ni, K.; Gu, R.; Qin, Y.; Guo, J.; Che, B.; Pan, Y.; Li, J.; Liu, L.; Deng, L. Chemical activation of Piezo1 alters biomechanical behaviors toward relaxation of cultured airway smooth muscle cells. Biol. Pharm. Bull. 2023, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Fairbank, N.J.; Fabry, B.; Smith, P.G.; Maksym, G.N. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004, 287, C440–C448. [Google Scholar] [CrossRef]
- Deng, L.; Bosse, Y.; Brown, N.; Chin, L.Y.M.; Connolly, S.C.; Fairbank, N.J.; King, G.G.; Maksym, G.N.; Paré, P.D.; Seow, C.Y.; et al. Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle. Pulm. Pharmacol. Ther. 2009, 22, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, A.; Vidal Melo, M.F. Follow the Voxel-A new method for the analysis of regional strain in lung injury. Crit. Care Med. 2018, 46, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Retamal, J.; Hurtado, D.; Villarroel, N.; Bruhn, A.; Bugedo, G.; Amato, M.B.P.; Costa, E.L.V.; Hedenstierna, G.; Larsson, A.; Borges, J.B. Does regional lung strain correlate with regional inflammation in acute respiratory distress syndrome during nonprotective ventilation? An experimental porcine study. Crit. Care Med. 2018, 46, e591–e599. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Jhaveri, D.J.; Marshall, V.M.; Bauer, D.C.; Edson, J.; Narayanan, R.K.; Robinson, G.J.; Lundberg, A.E.; Bartlett, P.F.; Wray, N.R.; et al. A comparative study of techniques for differential expression analysis on RNA-Seq data. PLoS ONE 2014, 9, e103207. [Google Scholar] [CrossRef]
- Costa-Silva, J.; Domingues, D.; Lopes, F.M. RNA-Seq differential expression analysis: An extended review and a software tool. PLoS ONE 2017, 12, e0190152. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, R.; Lv, Y.; Zhu, L.; Lin, Y. Altered expression of miRNAs and mRNAs reveals the potential regulatory role of miRNAs in the developmental process of early weaned goats. PLoS ONE 2019, 14, e0220907. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jiang, C.; Wang, F.; Yan, H.; Long, D.; Zhao, J.; Wang, J.; Zhang, C.; Li, Y.; Tian, X.; et al. Integrative analysis of miRNA and mRNA expression profiles associated with human atrial aging. Front. Physiol. 2019, 10, 1226. [Google Scholar] [CrossRef]
- Huang, P.; Li, F.; Mo, Z.; Geng, C.; Wen, F.; Zhang, C.; Guo, J.; Wu, S.; Li, L.; Brünner, N.; et al. A comprehensive RNA study to identify circrna and mirna biomarkers for docetaxel resistance in breast cancer. Front. Oncol. 2021, 11, 669270. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Vai, S.; Caramelli, D.; Lari, M. The Illumina Sequencing Protocol and the NovaSeq 6000 System. Methods Mol. Biol. 2021, 2242, 15–42. [Google Scholar] [PubMed]
- Attoff, K.; Gliga, A.; Lundqvist, J.; Norinder, U.; Forsby, A. Whole genome microarray analysis of neural progenitor C17.2 cells during differentiation and validation of 30 neural mRNA biomarkers for estimation of developmental neurotoxicity. PLoS ONE 2017, 12, e0190066. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, X.; Chintagari, N.R.; Breshears, M.; Wang, Y.; Liu, L. MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Med. Genomics 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, M.; Wang, S.; Wen, X.; Zhang, S. Identification of key candidate genes and biological pathways in bladder cancer. PeerJ 2018, 6, e6036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Rajamanikham, V.; Baron, M.; Patel, S.; Mathur, S.K.; Schwantes, E.A.; Ober, C.; Jackson, D.J.; Gern, J.E.; Lemanske, R.F., Jr.; et al. Association of ORMDL3 with rhinovirus-induced endoplasmic reticulum stress and type I Interferon responses in human leucocytes. Clin. Exp. Allergy 2017, 47, 371–382. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, P.; Fernandez, D.; Gutierrez, D.; Parra-Cordero, M.; Osorio, F. Human cDC1s display constitutive activation of the UPR sensor IRE1. Eur. J. Immunol. 2022, 52, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Q.; Ma, Z.; Jin, T.; Lin, J.; Lv, Q.; Wang, M.; Fu, G.; Xu, S. Downregulation of activating transcription factor 4 attenuates lysophosphatidycholine-induced inflammation via the NF-kappaB pathway. Eur. J. Pharmacol. 2021, 911, 174457. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.; Su, H.; Shi, H.; Xiong, Q.; Su, Z. Overexpression of miR-1283 Inhibits Cell Proliferation and Invasion of Glioma Cells by Targeting ATF4. Oncol. Res. 2019, 27, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.M.; Gallego, P.; An, X.; Best, S.E.; Thomas, G.; Wells, C.; Harris, M.; Cotterill, A.; Thomas, R. Peripheral blood monocyte gene expression profile clinically stratifies patients with recent-onset type 1 diabetes. Diabetes 2012, 61, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Zhang, X.; Li, L.R.; Zhang, J.Y.; Chen, Y.P. MiR-200a and miR-200b restrain inflammation by targeting ORMDL3 to regulate the ERK/MMP-9 pathway in asthma. Experimental lung research 2020, 46, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, Z.; Li, H.; Li, C.; Yang, Z.; Li, Y.; She, D.; Cao, L.; Wang, W.; Liu, C.; et al. Perfluorocarbon reduces cell damage from blast injury by inhibiting signal paths of NF-kappaB, MAPK and Bcl-2/Bax signaling pathway in A549 cells. PLoS ONE 2017, 12, e0173884. [Google Scholar]
- Yanzhang, P. Stretch-induced expression of CYR61 increases the secretion of IL-8 in A549 Cells via the NF-κβ lκβ pathway. Curr. Med. Sci. 2018, 38, 672–678. [Google Scholar]
- Huang, Q.; Hua, H.; Li, W.; Chen, X.; Cheng, L. Simple hypertrophic tonsils have more active innate immune and inflammatory responses than hypertrophic tonsils with recurrent inflammation in children. J. Otolaryngol. Head Neck Surg. 2020, 49, 35. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Luo, X.; Cai, C.; Xu, J.; Zhuang, G. Airborne fine particle decreases the cell viability and induces inflammation in human bronchial epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017, 42, 1042–1047. [Google Scholar] [PubMed]
- Risse, P.A.; Jo, T.; Suarez, F.; Hirota, N.; Tolloczko, B.; Ferraro, P.; Grutter, P.; Martin, J.G. Interleukin-13 inhibits proliferation and enhances contractility of human airway smooth muscle cells without change in contractile phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L958–L966. [Google Scholar] [CrossRef] [PubMed]
- Tassignon, J.; Burny, W.; Dahmani, S.; Zhou, L.; Stordeur, P.; Byl, B.; De Groote, D. Monitoring of cellular responses after vaccination against tetanus toxoid: Comparison of the measurement of IFN-gamma production by ELISA, ELISPOT, flow cytometry and real-time PCR. J. Immunol. Methods. 2005, 305, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xing, W.; Hong, J.; Wang, M.; Huang, Y.; Zhu, C.; Yuan, Y.; Zeng, W. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann. Surg. Oncol. 2012, 19, 3556–3565. [Google Scholar] [CrossRef] [PubMed]

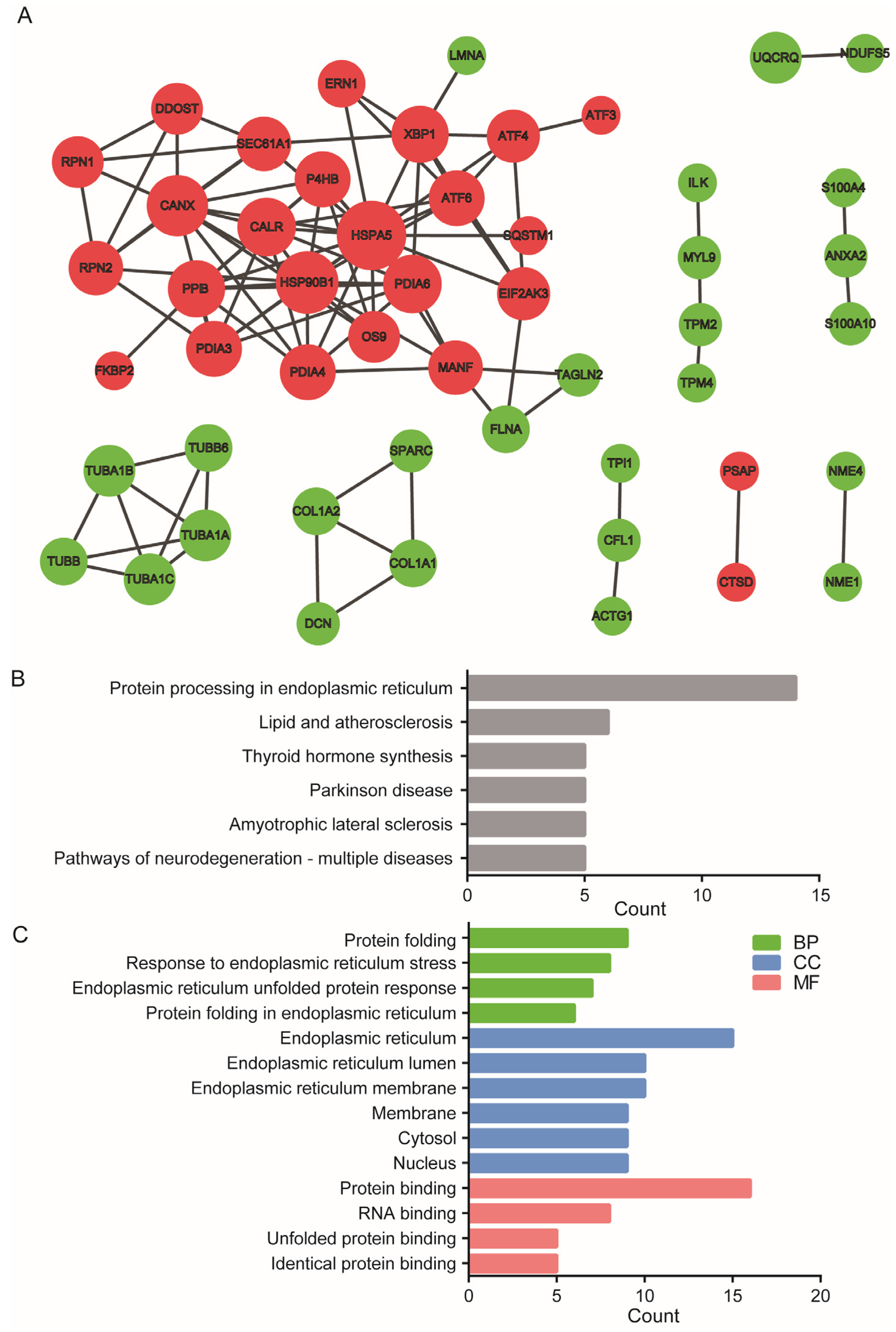


| Node | HSPA5 | HSP90B1 | CANX | PDIA6 | CALR | PPIB | XBP1 | P4HB | PDIA3 | PDIA4 | ATF6 | MANF | SEC61A1 | RPN2 | EIF2AK3 | ATF4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Degree | 12 | 12 | 11 | 9 | 9 | 8 | 8 | 7 | 7 | 6 | 6 | 6 | 6 | 6 | 5 | 5 |
| Expression change | up | up | up | up | up | up | up | up | up | up | up | up | up | up | up | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Guo, J.; Ni, K.; Wen, K.; Qin, Y.; Gu, R.; Wang, C.; Liu, L.; Pan, Y.; Li, J.; et al. Mechanical Ventilation-Related High Stretch Mainly Induces Endoplasmic Reticulum Stress and Thus Mediates Inflammation Response in Cultured Human Primary Airway Smooth Muscle Cells. Int. J. Mol. Sci. 2023, 24, 3811. https://doi.org/10.3390/ijms24043811
Yang C, Guo J, Ni K, Wen K, Qin Y, Gu R, Wang C, Liu L, Pan Y, Li J, et al. Mechanical Ventilation-Related High Stretch Mainly Induces Endoplasmic Reticulum Stress and Thus Mediates Inflammation Response in Cultured Human Primary Airway Smooth Muscle Cells. International Journal of Molecular Sciences. 2023; 24(4):3811. https://doi.org/10.3390/ijms24043811
Chicago/Turabian StyleYang, Chongxin, Jia Guo, Kai Ni, Kang Wen, Youyuan Qin, Rong Gu, Chunhong Wang, Lei Liu, Yan Pan, Jingjing Li, and et al. 2023. "Mechanical Ventilation-Related High Stretch Mainly Induces Endoplasmic Reticulum Stress and Thus Mediates Inflammation Response in Cultured Human Primary Airway Smooth Muscle Cells" International Journal of Molecular Sciences 24, no. 4: 3811. https://doi.org/10.3390/ijms24043811
APA StyleYang, C., Guo, J., Ni, K., Wen, K., Qin, Y., Gu, R., Wang, C., Liu, L., Pan, Y., Li, J., Luo, M., & Deng, L. (2023). Mechanical Ventilation-Related High Stretch Mainly Induces Endoplasmic Reticulum Stress and Thus Mediates Inflammation Response in Cultured Human Primary Airway Smooth Muscle Cells. International Journal of Molecular Sciences, 24(4), 3811. https://doi.org/10.3390/ijms24043811





