Abstract
It is generally accepted that diet-derived polyphenols are bioactive compounds with several potentially beneficial effects on human health. In general, polyphenols have several chemical structures, and the most representative are flavonoids, phenolic acids, and stilbenes. It should be noted that the beneficial effects of polyphenols are closely related to their bioavailability and bioaccessibility, as many of them are rapidly metabolized after administration. Polyphenols—with a protective effect on the gastrointestinal tract—promote the maintenance of the eubiosis of the intestinal microbiota with protective effects against gastric and colon cancers. Thus, the benefits obtained from dietary supplementation of polyphenols would seem to be mediated by the gut microbiota. Taken at certain concentrations, polyphenols have been shown to positively modulate the bacterial component, increasing Lactiplantibacillus spp. and Bifidobacterium spp. involved in the protection of the intestinal barrier and decreasing Clostridium and Fusobacterium, which are negatively associated with human well-being. Based on the diet–microbiota–health axis, this review aims to describe the latest knowledge on the action of dietary polyphenols on human health through the activity of the gut microbiota and discusses micro-encapsulation of polyphenols as a strategy to improve the microbiota.
1. Introduction
1.1. Polyphenols and Their Therapeutic Application in Gastrointestinal Diseases
Polyphenols—organic chemical compounds with one or more phenolic rings with hydroxyl groups—are mainly present in foods such as fruits, vegetables, cereals, olives, pulses, chocolate, tea, coffee, wine, and grape pomace [,,]. It is well-known that a large variety of phenolic compounds with complex chemical structures perform various beneficial, antioxidant, and protective effects in human pathologies. In fact, polyphenols can protect cell constituents from oxidative damage limiting the risk of various degenerative diseases associated with oxidative stress. Research literature strongly supports the role of polyphenols in a range of biological functions including anti-inflammatory, immunomodulatory, anticancer, antidiabetic, cardioprotective, neuroprotective, and gastroprotective properties [,,,]. In humans, polyphenols activate the antioxidant system through the upregulation of various endogenous antioxidants and the elimination of excess free radicals. Specifically, polyphenols are involved in the reduction of atherosclerotic plaques at the endothelial level by inhibiting the oxidation of low-density lipoproteins [,] and neutralizing free radicals responsible for aging, with antitumor effects [,]. Polyphenols exhibit not only antioxidant but also pro-oxidant effects, inducing apoptosis through the activation of caspases [] and blocking cell proliferation [,]. Moreover, an important aspect concerns the chemo-preventive action of polyphenols consisting in affecting the tumor cells but not the normal cells []. Thus, polyphenols and their metabolites could be promising candidates for fighting various gastrointestinal diseases such as gastritis, gastric cancer, colorectal cancer, inflammatory bowel disease, and irritable bowel syndrome. Polyphenols and their active metabolites enhanced the production of short-chain fatty acids (SCFAs) and branched-chain amino acids (BCAAs) and could be useful in the treatment and prevention of various gastrointestinal disorders i.e., Crohn’s disease, ulcerative colitis, and colorectal cancer []. Results from in vitro and in vivo studies suggested that polyphenols, due to their antioxidant and anti-inflammatory effects, may modulate the colonic microbiota and contribute to the alleviation of symptoms of intestinal inflammation via the modulation of proinflammatory cytokines []. Moreover, a high intake of diet-derived polyphenols is associated with a lower risk of cancer, and new findings indicate that they also exhibit cancer-preventive effects []. In fact, it is well established that increased reactive oxygen species (ROS) production has been revealed in various types of cancers with numerous properties such as activating pro-tumorigenic signaling and enhancing cell survival and proliferation []. Experiments from a recent work highlighted that polyphenols from grape pomace are involved in antiproliferative and pro-apoptotic effects in HT29 and SW480 colorectal cancer cells []. Moreover, polyphenols modulate the immune system response and protect normal cells from free radical damage [].
Based on the previous evidence, there is a bidirectional interaction between polyphenols and gut microbiota []. Since gut microbiota is a topic that is emerging as a key player in the relationship between dietary habits and health, it is important to study the effects involving the polyphenol–gut microbiota axis. Although scientific evidence extensively discusses the importance of the dietary intake of polyphenols in various diseases [], this review focuses more on the modulation of polyphenols in dysbiosis conditions involved in gastrointestinal diseases through the regulation of gut microbiota. In addition, evidence from the main in vitro and in vivo studies on the beneficial microbial patterns of the gut microbiota in terms of the dose and format of phenolic compounds in specific foods were summarized. In particular, the potential physiological gastrointestinal effects of in vitro and in vivo studies focusing on phenolic compounds were reviewed. However, the beneficial effects of polyphenols are closely related to their bioavailability and bioaccessibility, thus it was assessed that gastrointestinal digestion of polyphenols improves their absorption and recovery index [,]. Considering the potential role of dietary polyphenols on human health, this review aims to describe the latest knowledge on the action of dietary polyphenols on human health through the activity of the gut microbiota and discusses micro-encapsulation of polyphenols as a new strategy to improve the microbiota. Indeed, polyphenols—with a protective effect on the gastrointestinal tract—promote the maintenance of eubiosis of the gut microbiota with protective effects against gastric and colon cancers. Therefore, the benefits obtained from dietary supplementation of polyphenols appear to be mediated by the gut microbiota.
1.2. Gut Microbiota and Diet-Derived Components
The human gastrointestinal tract harbors a unique and complex polymicrobial ecosystem made up of trillions of cells []. The gut microbiota is an additional organ that contributes to the nutrient metabolism of dietary components, influencing human health by producing harmful or beneficial metabolites, protecting against pathogens, modulating the immune system, and protecting against various diseases []. The composition of the intestinal microbiota is influenced by various factors such as genetics, age, stress conditions, medications, and diet. A strong correlation between microbiota composition and diet is demonstrated as a consequence of long-term dietary habits []. In this perspective, the macro- and micro-food components can influence the composition and metabolic pathways of the intestinal microbiota. Diet is the main carrier of microbes and, more importantly, acts as a source of nutrients for the oral and gastrointestinal microbiota. Thus, evidence has supported differences in the microbiome and metabolome based on dietary habits, particularly by comparing agrarian diets, such as the Mediterranean and Okinawan diets, with the Western diet, which is primarily characterized by high fat, refined sugars, and animal proteins and bottom-fermented fibers [].
Beneficial dietary effects derive from the intake of specific nutrients and are introduced in a sufficient quantity to improve the health of the host, acting as functional foods. Due to a not universally accepted definition of functional foods, various definitions have been given over time underling that the common factor is the health benefit on the host’s physiology [].
A prebiotic is a non-digestible product for the human body that positively influences the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon. A food ingredient, to be classified as a prebiotic, must not be hydrolyzed or absorbed in the upper gastrointestinal tract, must serve as a selective substrate for one or a limited number of potentially beneficial commensal bacteria in the colon, stimulating their growth or activating their metabolism, and finally, must be capable of altering the colonic microflora toward a presumably more favorable composition in terms of host health. Of all these characteristics, those specific to prebiotics are selectivity and fermentation in a mixed culture environment. The first compounds studied as prebiotics were those that promote the growth of lactic acid-producing microorganisms, such as lactulose, which is used in infant formula to increase lactobacilli in the gut []. Prebiotics include dietary fibers such as arabinoxylan—a nonstarch polysaccharide found in many cereals—some polysaccharides found in algae and microalgae, and oligosaccharides, although only fructans such as inulin and galacto-oligosaccharides fully meet the criteria established for classification as prebiotics. The benefits of prebiotics are mediated by their ability to modify the gut microbiota and especially to selectively modulate specific strains, stimulating the growth of beneficial species already residing in the colon. Because of their chemical structure and the resulting inability of the host to digest them, prebiotics are fermented directly in the colon by endogenous bacteria with SCFAs, resulting in a lower pH. Through this process, they can exert anti-inflammatory effects, such as stimulating an increase in regulatory T cells and a reduction in interferon []. Prebiotics can also inhibit the adhesion of pathogens to the intestinal epithelium, preventing their passage through the epithelium [,].
The most fermented dietary fibers by the gut microbiota are fructo-oligosaccharides, also known as FOSs, including inulin, galacto-oligosaccharides or GOSs, human milk oligosaccharides, isomalto-oligosaccharides, and xylans found in various foods, including asparagus, soy, artichoke, cucumber, wheat, honey, and breast milk []. Therefore, due to the high fiber content, the consumption of fruits and vegetables is associated with greater microbial richness, in particular, with beneficial saccharolytic bacterial patterns, such as lactobacilli and bifidobacteria, with known probiotic activity. In fact, probiotics are the main microbial patterns capable of metabolizing fibers into SCFAs. In turn, SCFAs have a broad spectrum of beneficial and healthful effects, such as energy substrates for colonocytes, improvement of the intestinal barrier function through mucin synthesis, and improvement of the immune system.
In specific pathologies, such as celiac disease and non-celiac gluten sensitivity, in which gluten causes adverse gastrointestinal symptoms, the gluten-free diet remains the only therapy adopted to date []. However, several trials have shown that strict adherence to a gluten-free diet leads to an unbalanced microbial composition []. The reason is linked to the absence of wheat in gluten-free baked goods which leads to a critical shortage of fructans. This class of fibers, similar to other prebiotics, plays a fundamental role in maintaining a eubiotic microbiota, in fact, previous studies have shown that even in healthy subjects, a gluten-free diet favored the onset of symptoms of intestinal relief only after switching to a normal diet again []. In recent years, a novel food considered a functional food has taken place, namely algae. Seaweeds—traditionally consumed as food or as medicinal herbs—have high nutritional values, due to their high protein content, and pharmaceutical values []. Therefore, among the different ways existing to manipulate the intestinal microbiota, the active components of algae are included. The consumption of seaweed applied to the physiopathology of type 2 diabetes mellitus can determine positive effects such as a reduction in hyperglycemia, hyperinsulinemia, and insulin resistance due to the high fiber content []. Furthermore, positive effects are also linked to the reduction in oxidative stress, due to the high quantity of antioxidants, and the energy intake from carbohydrates and fats, through the modulation of the microbiota. In addition to seaweed, other vegetables and fruits are also a source of micronutrients, such as vitamins and polyphenols. Among the main foods with a known high content of polyphenols are wine, green tea, grapes, red fruits, and coffee.
Since polyphenol-bioactive compounds from the diet and the health of the gut microbiota are closely related, maintaining a eubiotic state in the gut microbial ecosystem is essential to prevent a microbial imbalance related to various gastrointestinal diseases (Figure 1) [,,,,,].
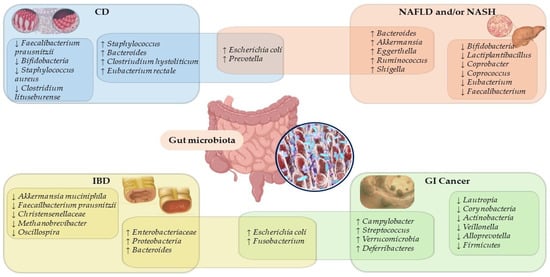
Figure 1.
Overlapping microbiota species and genera signatures in gastrointestinal disease. Abbreviation: CD, celiac disease; GI, gastrointestinal; IBD, inflammatory bowel disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; ↓, decrease; ↑, increase.
2. Diet-Derived Polyphenols: Healthy Outcomes
Polyphenols—constituents of plant-derived foods—include a wide variety of molecules divided into different classes based on their structure. Figure 2 illustrates the structures of the main polyphenolic compounds mentioned below belonging to flavonoids, phenolic acids, and stilbenes [,]. Flavonoids contain a common carbonaceous skeleton of diphenylpropanes, two benzene rings (A and B ring) joined by a three-carbon straight chain. The central chain can form a closed pyranic ring (C ring) with one of the benzene rings. Generally, flavonoids are divided into six different subclasses: flavonols, flavones, flavanones, isoflavones, anthocyanins, and flavanols.
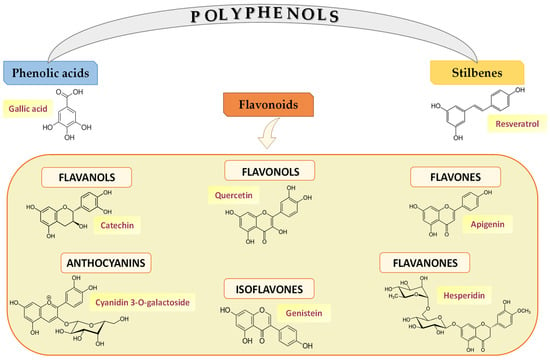
Figure 2.
Polyphenol classes and chemical structures of some of their main compounds.
Flavonols—including quercetin and kaempferol—are the most representative compounds of foods such as onions, cabbage, leeks, broccoli, blueberries, tea, and red wine. Studies in the literature have shown that kaempferol has strong cellular antioxidant capacity by eliminating ROS accumulation and shows antiproliferative activity against various human cancer cells and induces apoptosis of MCF-7 cells through the mitochondrial pathway []. Additionally, quercetin showed in vitro antioxidant activities and is involved in the removal of free radicals [] and the regulation of metal ions such as, for example, Cu2+ and Fe2+ ions. Tang et al. 2014 [] reported that C57BL/6J mice with alcohol-induced liver disease treated with quercetin showed inhibition in Fe2+-induced lipid peroxidation by going on to bind and inhibit the action of the ion itself. In addition, quercetin also demonstrated antitumor action by significantly modulating the cell cycle, promoting cell apoptosis, and inhibiting the generation of new blood vessels in several cancer cell lines [,,].
In contrast to flavonols, flavones represent the least representative class of flavonoids and are mainly found in parsley, celery, and the skin of some fruits. Research in the literature has highlighted the role of apigenin and its glycosides in influencing the state of intestinal health in the prevention of some types of cancer []. Moreover, flavanones are found in tomatoes and some aromatic plants, such as mint, and in very high concentrations in citrus fruits, such as in the case of hesperidin. In fact, orange juice contains up to 470–761 mg/L of hesperidin [], although most flavanones are concentrated in the whitish part of the fruit, i.e., the albedo. Studies on streptozotocin-induced diabetic rats showed that hesperidin has potential anti-hyperglycemic activity by reducing plasma glucose levels in a dose-dependent manner and improving glycogen content in the liver tissue through restoring the activities of the enzyme’s glycogen synthase and glycogen phosphorylase []. In addition, it was demonstrated that hesperidin significantly reduced HepG2 cell proliferation and induced apoptosis by activating the intrinsic caspase-3-dependent pathway through upregulation of the proapoptotic protein Bax []. The class of flavonoids also includes isoflavones, which are structurally similar to estrogens and classified as phytoestrogens capable of binding to their receptors []. Isoflavones are mainly found in legumes, especially soybeans []. Genistein, mostly found in these foods, exhibits antioxidant, anti-inflammatory, anti-angiogenic, pro-apoptotic, and antiproliferative activities, conferring its chemo-preventive and chemotherapeutic potential [].
Anthocyanins are water-soluble pigments that are responsible for the red, blue, and purple coloration of fruits, vegetables, and other plants; thus, they are mostly found in red wine, some varieties of cereals, and some vegetables (cabbage, beans, onions, radishes) and fruits. Anthocyanins are mainly found in the skin, except in some red fruits such as cherries and strawberries, where they are also present in the pulp. Importantly, among them stand out malvidin-3-galactoside (M3G) and Cyanidin 3-O-galactoside (Cy3Gal). M3G suppressed the proliferation, polarization, migration, and invasion of HepG2 cells in vitro by regulating the protein expression of cyclins D1, B, E and cleaved caspases-3 and caspase-3, Bax, p-JNK and p-p38, and by activating PTEN with decreased levels of p-AKT and MMP-2 and -9; while in vivo, M3G promoted apoptosis of liver cancer cells []. Cy3Gal, and its combination with other polyphenols, also had numerous effects on human health, including antioxidant, anti-inflammatory, antitumor, antidiabetic, antitoxic, cardiovascular, and neuronal-level properties []. So, these results suggest that both M3G and Cy3Gal, used as adjuvant ingredients or nutritional supplements, are involved in preventing cancer development with beneficial effects on human health.
Finally, flavonoids include flavanols, which are present in both monomers (catechins) and polymer (proanthocyanidins) forms. The main catechins found in fruit are catechin and epicatechin, while gallocatechin, epigallocatechin, and epigallocatechin gallate are mainly found in tea. Proanthocyanidins, also known as condensed tannins, are dimers, oligomers, and polymers of catechins and are responsible for the astringent character of fruits, drinks, and the bitterness of chocolate [].
Another prominent class of polyphenols consists of phenolic acids divided into two classes, namely benzoic acid derivatives and cinnamic acid derivatives. Phenolic acids are types of aromatic acid compounds including substances containing an aromatic ring and a benzene ring with one or more hydroxide groups including functional derivatives []. Hydroxybenzoic acids, such as gallic acid (GA) and protocatechuic acid, are present in low concentrations in a few plants consumed by humans, except for some red fruits such as, for example, blackberries. Among beverages high in GA is tea; in particular, tea leaves contain up to 4.5 g/kg fresh weight [], while protocatechuic acid is mostly found in raspberries and olive oil []. Moreover, hydroxycinnamic acids include coumaric, caffeic, and ferulic acids.
Stilbenes are characterized by the presence of a 1,2-diphenylethylene nucleus with hydroxyl substituted on the aromatic rings and exist in the form of monomers or oligomers []. The main compound representative of stilbenes is resveratrol, mainly concentrated in grapes, berries, and peanuts. Several data demonstrated the anticarcinogenic effects of resveratrol [] particularly colorectal and obesity-related effects [].
Polyphenols in green tea induced an increase in SCFAs-producing bacteria, such as Lachnospiraceae, Ruminococcaceae, and Bifidobacteriaceae, and a reduction in the abundance of oral and fecal Fusobacterium in healthy subjects []. Therefore, the production of SCFAs following polyphenol intake is associated with an improvement in some gastrointestinal diseases [,]. Indeed, scientific evidence has pointed out that the increase in SCFAs-producing bacterial taxa is involved in the reduction in intestinal inflammation in subjects with colorectal cancer [,].
3. Polyphenols: Bioaccessibility and Bioavailability
To evaluate the efficacy of polyphenols in disease prevention and the improvement of human health, it is important to understand the nature and distribution of these compounds in the diet in order to determine the most promising polyphenols in terms of bioavailability, which in turn depends on the relative content of compounds released from the food matrix along the digestive system (bioaccessibility), digestive stability, and efficiency of the transepithelial passage (intestinal absorption) [,,]. In fact, the biological function of each polyphenol is related to its metabolism (biotransformation), absorption (intestinal barrier), and utilization rate (bioavailability) and is influenced by various factors such as structure (binding with prosthetic groups), chemical interactions (with other macromolecules), and biotransformation process [].
Because polyphenols can have different chemical structures, it is not immediately possible to quantify their exact content in foods. Moreover, the beneficial action of polyphenols on human health depends not only on their content in foods but also on other factors such as their stability, microbiota, and digestive enzymes. Scientific evidence points out that among polyphenols, isoflavones are the most bioavailable followed by phenolic acids, flavanols, flavanones, and flavonols, and the last mentioned are anthocyanins and proanthocyanidins []. The bioavailability of flavonoids—which are hydrophilic compounds—is quite low because they are not well absorbed in the gut, so they cannot be used as nutraceuticals due to their low bioactivity. A viable strategy to improve their bioavailability is using microencapsulation and microemulsions of flavonoids. Therefore, it is important to consider that the bioaccessibility of polyphenols is not necessarily positively correlated with high concentrations of phenolic compounds in food matrices [,]. Important aspects concern the chemical structure of polyphenols and the diversity of species and genera in the gut microbiota, which allows for the production of certain enzymes involved in the biotransformation of polyphenols, such as deglycosylation [,]. In summary, bioavailability is profoundly influenced by the great diversity of polyphenol chemical structures, and a high polyphenol content in foods does not necessarily correlate with high bioavailability. In addition, biotransformations promoted by the gut microbiota affect bioavailability, which in turn is influenced by absorption and metabolism.
Small polyphenols can be directly adsorbed into the small intestine, while complex polyphenols remain undigested up to the large intestine. At this level, they are converted into low-molecular-weight metabolites by the gut microbial communities, which are bioavailable for host adsorption through methylation, sulfation, and glucuronidation reactions. Secondary metabolites derived from the microbial metabolism of polyphenols act as prebiotic-like molecules that, in turn, can modulate the growth of specific bacterial strains (Figure 3) []. Polyphenols interact with other food components in the intestine by binding to macromolecules such as fibers and forming chemical complexes and colloidal structures that reduce or enhance their bioavailability []. To the best knowledge, molecules absorbed from the small intestine are aglycones; however, most polyphenols are present in food in the form of esters, glycosides, or polymers that cannot be absorbed in their native form. In fact, these molecules must undergo hydrolysis by intestinal enzymes to facilitate their absorption. As shown in Figure 3, during absorption, polyphenols, through the processes of methylation, sulfation, and glucuronidation, are conjugated in the small intestine and liver, increasing their hydrophilicity and facilitating their urinary elimination []. Moreover, the impact of polyphenols on microbiota maintains a healthy microbiome that, in turn, shows suppressive effects on the progress of life-style related diseases, such as diabetes. Additionally, some classes of polyphenols, bio-transformed by microbiota metabolism, showed an anti-inflammatory effect delaying the onset and/or progression of different gastrointestinal pathologies, including ulcerative colitis. Thus, polyphenols are involved in the interaction with the gut microbiome and can operate as prebiotics and enhance the production of various beneficial microorganisms in the host’s gut, positively modulating the gut microbiota. In fact, these compounds not digested by human digestive enzymes are used as a substrate for selective microorganisms that confer human health benefits. The gut microbiota plays an important role in regulating the production of SCFAs, BCAAs, and vitamins and positively modulates lipid and glucose metabolism, thus promoting the overall health status of the host [,]. Moreover, it was confirmed that dietary supplementation with polyphenols can significantly enhance the production of health-beneficial bacterial species such as Bifidobacterium and Lactiplantibacillus, as well as suppress the production of damaging species such as Clostridium and Escherichia coli (E. coli) [].
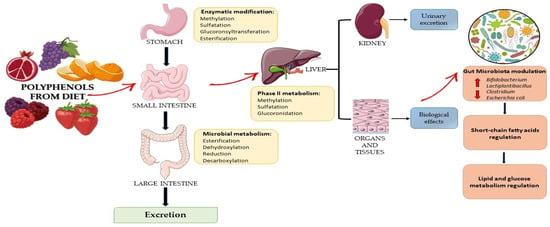
Figure 3.
Effects of dietary polyphenols on gut microbiota, their metabolites, and health benefits.
4. Polyphenols–Microbiota–Health Axis
It is well-known that gut microbiota dysbiosis—described as an altered microbial community with an increased population of pathogenic bacteria—can lead to pathological conditions. In fact, a change in terms of microbial diversity, bacterial functionality, and the presence of beneficial inhabitants often correlates with an increase in harmful ones, which seems to be implicated in cases of obesity, type 2 diabetes, inflammatory bowel disease (IBD), and colorectal cancer []. Diet is one of the factors that can positively modulate intestinal microbiota composition. Previous works showed how the production of fresh pasta using inulin—a soluble fiber—instead of durum wheat semolina significantly reduced E. coli cell density and increased prebiotic growth []. Even the polyphenols ingested through the diet can interact with intestinal microorganisms and determine a change toward a healthier profile. The mechanisms through which polyphenols modulate the microbiota are still unclear but in general, they can intervene both directly and indirectly. They can directly stimulate or inhibit the growth of some bacteria. In the first case, resistance is strongly associated with the ability of bacteria to metabolize these compounds, while in the second case, inhibition is related to the antimicrobial capabilities of these compounds [,]. In this sense, several works report the selective bactericidal effect of polyphenols against specific bacteria []. Recently, the antimicrobial effect of grape pomace polyphenols in combination with a probiotic, Lactiplantibacillus plantarum, on the growth of pathogenic microorganisms such as E. coli, Bacillus megaterium, and Listeria monocytogenes has been demonstrated []. Different polyphenols introduced with the diet such as gallic acid, hesperedin, and naringin can modulate the intestinal microbiota through antimicrobial and prebiotic actions (Table 1). Regarding GA, previous studies demonstrated many biological properties, including antioxidant, antitumor, anti-inflammatory, and antimicrobial properties, and recent studies have shown how GA and its metabolites improve the activity of the gut microbiome by modulating immune responses []. GA inhibited the development of metastasis in gastric adenocarcinoma cells by inhibiting the Ras/PI3K/AKT signaling pathway [], reduced the viability of human colon cancer cells by suppressing cell proliferation, and was involved in the regulation of the NF-κB, AP-1, STAT-1, and OCT-1 signaling pathways []. In addition, recent studies have evaluated the effects of GA on the gut microbiota. In general, dietary intake components are used by the gut microbiota to produce energy and metabolites. These metabolites enter the bloodstream and influence gut activity and the immune system []. A recent study evaluated the effects of GA, in a mouse model with ulcerative colitis, on reducing dysbiosis of the gut microbiota. Indeed, following GA intake, an increase in the Lactobacillaceae and Prevotellaceae families and a reduction in some pathogenic bacteria such as Firmicutes and Proteobacteria were recorded [,,]. Furthermore, GA is able to alleviate the environmental stress of beagle puppies, which is considered an excellent model for the study of the human microbiota due to its high similarities with the human one [], rebalancing the state of intestinal health through an increase in Lactiplantibacillus and Faecalibaculum and a reduction in Escherichia, Shigella, and Clostridium. Indeed, exposure to environmental stress is known to cause a disruption in the intestinal barrier, increased inflammatory responses, and intestinal dysbiosis [].
Other polyphenols with positive activities on the gut microbiota are those contained in orange juice. Hesperidin and naringin, in healthy women, not only improved blood biochemical parameters (glucose, low-density lipoprotein cholesterol, and insulin sensitivity) but more importantly, positively regulated the composition and metabolic activity of the microbiota by increasing the population of Bifidobacterium spp. and Lactiplantibacillus spp. and increasing SCFA production. These results suggest that orange consumption improves the gut microbiota and its metabolites []. The composition and activity of the microbiota were also tested on an in vitro model of the colon using the administration of a citrus extract with a high content of hesperedin and naringin, which observed an increase in beneficial bacteria and a reduction in harmful ones []. A study conducted on a model of hepatic steatosis in C57BL/6J mice fed a high-fat diet (HFD) in addition to resveratrol, rich in red grape skin [], resulted in a change in the composition of the gut microbiota by going on to reverse HFD-induced dysbiosis with an increase in the abundance of Bacteroidetes and a decrease in Firmicutes and Proteobacteria, and by going on to reduce the parameters related to hepatic steatosis. Therefore, this study showed that resveratrol improves hepatic steatosis in HFD mice by positively modulating the gut microbiota [].
A study conducted on rats with streptozotocin-induced diabetic peripheral neuropathy (DPN) showed how quercetin intake improved both neuropathy status and intestinal dysbiosis in DPN rats by decreasing four potential pathogenic species (f_Porphyromonadaceae, f_Oxalobacteraceae, g_Oxalobacter and g_Klebsiella) associated with DPN phenotypes and increased ROS and by enriching two prebiotic species (p_Actinobacteria and c_Actinobacteria) []. Thus, polyphenols exhibit prebiotic effects by increasing the beneficial bacteria abundance and inhibiting harmful ones, thus improving the status of some diseases [,]. For example, polyphenols contained in grape pomace extracts and seeds positively altered the gut microbiota of mice fed a high-fat diet by significantly increasing the relative abundance of Prevotella and reducing the relative abundance of Streptococcus [], while proanthocyanidin extract from grape seeds in the same mouse model has been shown to have a potential prebiotic effect by positively regulating the growth of Roseburia, Prevotella, and Clostridium XIVa [].
Red wine polyphenols in patients with obesity-associated metabolic syndrome also resulted in a significant increase in the number of fecal bifidobacteria and lactobacilli that are important in protecting the intestinal barrier at the expense of harmful LPS-producing bacteria such as E. coli and Enterobacter cloacae []. Furthermore, in healthy adults, red wine intake resulted in increased fecal concentrations of Bifidobacterium, Enterococcus, and Eggerthella lenta [], although in vitro studies have shown inhibition of Enterococcus and Eggerthella lenta bacterial groups by GA and resveratrol metabolites [].
At the basis of this direct antimicrobial action, there could be several molecular mechanisms: it has been demonstrated that subjects with IBD had an increase in E. coli caused in turn by an increase in adhesins, which facilitate the adhesion of bacteria to the surface of the intestinal epithelium []. Some phenolic compounds, such as resveratrol, have been able to inhibit the growth of E. coli and other bacteria harmful to human health, reducing the cell adhesion between these and the intestinal epithelium [,]. Furthermore, some polyphenols can exert their antimicrobial action by interacting with bacterial proteins and inhibiting bacterial nucleic acid synthesis, modifying the integrity and synthesis of the cell wall, altering the functionality and fluidity of the bacterial cell membrane, modulating the metabolism cell, inhibiting biofilm formation and quorum sensing, and chelated metals such as iron, copper, and zinc that are important for bacterial metabolism [,,]. Cranberry polyphenol extract was implicated in the downregulation of genes coding for outer membrane proteins in E. coli O157:H7, with bactericidal effects against Salmonella strains, by reducing the expression of virulence genes and of those implicated in the cell wall and membrane biogenesis and acting as inhibitors of quorum sensing [,,,]. In addition to direct antimicrobial actions, polyphenols can also act indirectly: some scientific evidence has reported how some phenolic metabolites are able to influence the growth of some bacteria which, in turn, modulate the development of others [,,]. Furthermore, polyphenols can also have a prebiotic effect by stimulating the development of beneficial bacteria through various mechanisms of action, such as providing the microbiota with carbon sources, acting as electronic acceptors, and generating proton motive forces during their metabolization [].

Table 1.
Main studies on the impacts of polyphenols on the gut microbiota.
Table 1.
Main studies on the impacts of polyphenols on the gut microbiota.
| Polyphenols | Foods | Dose | Model | Main Findings | References |
| GA | Plants, black-berries, tea | 10 mg/kg BD for 7 days | DSS-induced colitis in mice | ↑ Lactobacillaceae, Prevotellaceae ↓ Firmicutes, Proteobacteria | [] |
| 500 mg/kg for 1 week | Dog puppies exposed to multiple environmental stressors | ↑ Lactiplantibacillus, Faecalibaculum ↓ Escherichia, Shigella, Clostridium | [] | ||
| HSP, NAR | Citrus fruits | 300 mL/day OJ for 2 months | Healthy female | ↑ Bifidobacterium spp., Lactiplantibacillus spp. | [] |
| 250–350 mg/day CFE for 3 days | In vitro model of colon | ↑ Roseburia, Eubacterium ramulus, Bacteroides eggerthii ↓ Firmicutes | [] | ||
| RES | Grapes, berries, peanuts | 4 g/kg for 12 weeks | HFD-induced hepatic steatosis in C57BL/6J mice | ↑ Bacteroidetes ↓ Firmicutes, Proteobacteria | [] |
| 60 mg/kg for 5 weeks | HFD-induced hyperglycemia in C57BL/6J mice | ↓ Alistipes, Clostridium | [] | ||
| QRC | Onions, cabbage, leeks, broccoli, blue-berries, tea, red wine | 50 mg/kg BD for 12 weeks | STZ-induced diabetic peripheral neuropathy in rats | ↑ f_Porphyromonadaceae, f_ Oxalobacteraceae, g_ Oxalobacter, g_ Klebsiella ↓ p_Actinobacteria, c_ Actinobacteria | [] |
| 100 μL/10 g BD for 6 weeks | MSG-induced abdominal obese C57BL/6J mice | ↓ Firmicutes, Lachnospiraceae, Ruminicoccaceae ↑ Bacteroidetes | [] | ||
| GPE, GSE | Grape pomace | 200 mg/kg BD for 7 days | HDF in C57BL/6J mice | ↑ Prevotella ↓ Streptococcus | [] |
| 300 mg/kg BD/day for 7 weeks | HFD-induced obesity in mice | ↑ Roseburia, Prevotella, Clostridium XIVa | [] | ||
| RWPs | Red wine | 272 mL/day | Obesity-associated metabolic syndrome in adult | ↑ Bifidobacteria and lactobacilli ↓ Escherichia coli, Enterobacter cloacae | [] |
| 272 mL/day | Healthy adults | ↑ Bifidobacterium, Enterococcus, Eggerthella lenta | [] | ||
| GTP | Green tea | 400 mL/day for 2 weeks | Healthy adults | ↑ Lachnospiraceae, Ruminococcaceae, Bifidobacteriacea ↓ Fusobacterium | [] |
| 0.67 mg/mL for 10 days | In vitro model of colon | ↓ Firmicutes ↑ Ruminococcaceae | [] |
Abbreviations: BD, body weight; CFE, citrus fruit extract; DSS, dextran sodium sulphate; HFD, high fat diet; HSP, hesperidin; GA, gallic acid; GPE, grape pomace extracts polyphenols; GSE seed extracts polyphenols; GTP, green tea polyphenols; NAR, naringine; OJ, orange juice; QRC, quercetin RES, resveratrol; RWPs, red wine polyphenols; STZ, streptozotocin. ↓, decrease; ↑, increase.
5. Micro-Encapsulation of Polyphenols and New Strategies for Microbiota Improvement
Bioactive components of the diet, such as polyphenols, play a key role in modulating the gut microbiota. Today, growing evidence has shown that the gut microbiota can use these components to produce bioactive metabolites of phenolic acids. In fact, several metabolites show promising effects in the prevention and treatment of several diseases. In addition, scientific research has shown that polyphenol supplementation can modulate the growth and virulence properties of the gut microbiota to prevent and manage chronic diseases [].
However, some polyphenols are insoluble in water and unstable, with low oral bioavailability, limiting their biological activities and hindering the process of studying the association between polyphenols and gut microbiota []. To better understand their impact on human health, encapsulation technologies have emerged rapidly in the gut microbiota scenario. Microencapsulation allows the protection of the biochemical functionality of a wide range of compounds by incorporating them into a protective matrix. Indeed, encapsulation increases the water solubility of polyphenols, protects them from unfavorable conditions during the digestive process, and releases them into targeted areas by modulating the gut microbiota [].
To achieve good encapsulation efficiency, it is of primary importance to define encapsulating agents. There is a well-known and huge range of different materials used as encapsulating agents, which can be natural, semisynthetic, or synthetic polymers []. Various wall materials have been used over the years, including yeast cells, β-cyclodextrins, mixtures of alginate and chitosan, gelatin, maltodextrins, proteins, gum arabic, mesquite gum, xanthan, and inulin [,,].
In particular, starch—composed of amylose and amylopectin—is safe, cheap, and easy to obtain, and it is suitable for encapsulation because of its biocompatibility and nontoxicity []. For these reasons, it can be modified with physical, chemical, and/or enzymatic approaches to achieve desired properties and encapsulate a range of hydrophilic and hydrophobic bioactive compounds such as polyphenols []. Accordingly, the encapsulation of polyphenols with starch could promote positive effects on gastrointestinal diseases playing an important role in modulating the gut microbiota. Several food ingredients have been successfully encapsulated in starch-based systems with optimal results []. Resistant starch could also efficiently deliver and release polyphenols at specific sites such as local treatment of colonic dysfunction to control the right dose of polyphenols to be administered []. Various resistant starch systems suitable for the encapsulation of polyphenolic substances, such as native starch [], are available. In general, starch is widely processed using chemical approaches such as physicochemical methods and enzymatic hydrolysis []. Acetylation, esterification, and phosphorylation are common chemical modifications in which hydrophilic native starch is converted to hydrophobic starch derivatives. Recently, starch modified with octenyl succinic anhydride (OSA) in the amorphous form has been used in the encapsulation of hydrophobic polyphenols []. OSA-modified waxy corn starch could interact with tea polyphenols and tea catechins released gradually during in vivo digestion []. Enzymes including α-amylase, pullulanase, glucanotransferase, and others are frequently used for the preparation of branched starch or type III resistant starch []. Pullulanase-treated starch nanoparticles improved the stability and antioxidant activity of curcumin, and the encapsulation efficiency achieved was up to 92.49% []. Rice starch modified with 4-α-glucanotransferase, used to encapsulate curcumin, greatly improved its solubility, stability, and bioaccessibility.
Therefore, recent studies on polyphenols encapsulation using starch have shown that it controls the number of polyphenols released in the upper digestive tract potentially preventing the polyphenols from reaching the lower digestive tract. Thus, the encapsulation of polyphenols using starch can positively influence the abundance and composition of the gut microbiota with a significant role in human health.
6. Conclusions and Future Perspectives
Diet is a major driver of the composition and, especially, metabolic activities of the human gut microbiota. Particularly, polyphenols may exert anti-inflammatory, antioxidant, anti-cancer, and anti-diabetic activities by positively modulating the gut microbiota. Dietary macro- and micronutrients seem to be the main drivers of the metabolic pathways of the oral and intestinal microbiota affecting, in turn, the fecal, urinary, and blood metabolomes. Once normalized, the resilience of the microbiome, defined as the tolerance to perturbation, and the predisposition to a disease state could be weakened. Understanding bioactive molecules from the diet such as polyphenols, that underlie compositional and functional changes, allows us to design personalized therapies that target the gut microbiota. Nowadays, increasingly sophisticated and innovative strategies are available, such as the encapsulation of starch, to convey polyphenols, keeping the effectiveness of the molecules intact and increasing their absorption capacity in the body. Thus, resistant starch not only serves as a promising transporter but also as a prebiotic by influencing the abundance and composition of the gut microbiota. However, further studies are necessary to fully describe the complex food–gut human axis, and considering the key role of gut microbiota on human health, emerging dietary treatments are not only economical but also offer a targetable and non-invasive approach for treating chronic diseases.
Author Contributions
Conceptualization, G.R.C.; methodology, T.L., M.C., G.R.C., V.D.N. and M.N.; data curation, T.L., M.C., G.R.C. and V.D.N.; writing—original draft preparation, T.L., M.C. and G.R.C.; writing—review and editing, G.R.C. and M.N.; supervision, G.R.C. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by RC 2023, Prog. No. 15 (D.D.G. n. 661/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caponio, G.R.; Lorusso, M.P.; Sorrenti, G.T.; Marcotrigiano, V.; Difonzo, G.; De Angelis, E.; Guagnano, R.; Ciaula, A.D.; Diella, G.; Logrieco, A.F.; et al. Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health. Nutrients 2020, 12, 939. [Google Scholar] [CrossRef]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, "Food of the Gods": History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A brief review on emerging trends in global polyphenol research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Cisternino, A.M.; Gigante, I.; Lanzilotta, E.; Iacovazzi, P.A.; Lippolis, A.; Lippolis, T.; et al. Impact of Fresh Table Grape Intake on Circulating microRNAs Levels in Healthy Subjects: A Significant Modulation of Gastrointestinal Cancer-Related Pathways. Mol. Nutr. Food Res. 2021, 65, 2100428. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; Covas, M.I.; Fito, M.; Schroder, H.; Miro-Casas, E.; Gimeno, E.; Lopez-Sabater, M.C.; de la Torre, R.; Farre, M.; Investigators, S. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—A randomized controlled trial. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors—A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Khalil, M.; Caponio, G.R.; Diab, F.; Shanmugam, H.; Di Ciaula, A.; Khalifeh, H.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Unraveling the beneficial effects of herbal Lebanese mixture “Za’atar”. History, studies, and properties of a potential healthy food ingredient. J. Funct. Foods 2022, 90, 104993. [Google Scholar] [CrossRef]
- Elbling, L.; Weiss, R.M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Mickshe, M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb. J. 2005, 19, 807. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284s–291s. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, T.; Yaacob, N.S. The flavonoid fisetin as an anticancer agent targeting the growth signaling pathways. Eur. J. Pharmacol. 2016, 789, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastro. Hepat. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotech. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R. Dietary Supplements and Mortality Rate in Older Women The Iowa Women’s Health Study. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell. Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Hu, Y.L.; Chen, D.W.; Zheng, P.; Yu, J.; He, J.; Mao, X.B.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed. Res. Int. 2019, 2019, 5403761. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; Mesquita, L.M.D.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroentero. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroentero. 2016, 22, 7186–7202. [Google Scholar] [CrossRef]
- Catalioto, R.M.; Maggi, C.A.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Probiotic-induced changes in the intestinal epithelium: Implications in gastrointestinal disease. Trop. Gastroenterol. 2009, 30, 76–85. [Google Scholar]
- Tandon, D.; Haque, M.M.; Gote, M.; Jain, M.; Bhaduri, A.; Dubey, A.K.; Mande, S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 2019, 9, 5473. [Google Scholar] [CrossRef]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroentero. 2015, 21, 7110–7119. [Google Scholar] [CrossRef]
- Palmieri, O.; Castellana, S.; Bevilacqua, A.; Latiano, A.; Latiano, T.; Panza, A.; Fontana, R.; Ippolito, A.M.; Biscaglia, G.; Gentile, A.; et al. Adherence to Gluten-Free Diet Restores Alpha Diversity in Celiac People but the Microbiome Composition Is Different to Healthy People. Nutrients 2022, 14, 2452. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastro. Hepat. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, H.; Kang, C.S.; Shin, T.S.; Kim, J.W.; Park, J.M.; Kim, J.G.; Kim, Y.K. Dysbiotic change in gastric microbiome and its functional implication in gastric carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Smet, A.; Kupcinskas, J.; Link, A.; Hold, G.L.; Bornschein, J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? Cell Mol. Gastroenter. 2022, 13, 857–874. [Google Scholar] [CrossRef]
- Marasco, G.; Di Biase, A.R.; Schiumerini, R.; Eusebi, L.H.; Iughetti, L.; Ravaioli, F.; Scaioli, E.; Colecchia, A.; Festi, D. Gut Microbiota and Celiac Disease. Digest. Dis. Sci. 2016, 61, 1461–1472. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroentero. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastro. Hepat. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidant 2020, 9, 61. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 19, 273. [Google Scholar] [CrossRef]
- Liao, W.Z.; Chen, L.Y.; Ma, X.; Jiao, R.; Li, X.F.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Oh, W.Y.; Ambigaipalan, P.; Shahidi, F. Preparation of Quercetin Esters and Their Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 10653–10659. [Google Scholar] [CrossRef]
- Tang, Y.H.; Li, Y.Y.; Yu, H.Y.; Gao, C.; Liu, L.; Xing, M.Y.; Liu, L.G.; Yao, P. Quercetin attenuates chronic ethanol hepatotoxicity: Implication of "free" iron uptake and release. Food Chem. Toxicol. 2014, 67, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Wang, T.C.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Özcan, Ö.; Aldemir, O.; Karabulut, B. Flavones (Apigenin, Luteolin, Chrysin) and Their Importance for Health. Mellifera 2020, 20, 16–27. [Google Scholar]
- Leuzzi, U.; Caristi, C.; Panzera, V.; Licandro, G. Flavonoids in pigmented orange juice and second-pressure extracts. J. Agric. Food Chem. 2000, 48, 5501–5506. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Banu, H.H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Method 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Naz, H.; Tarique, M.; Ahamad, S.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Luqman, S.; Hassan, M.I. Hesperidin-CAMKIV interaction and its impact on cell proliferation and apoptosis in the human hepatic carcinoma and neuroblastoma cells. J. Cell. Biochem. 2019, 120, 15119–15130. [Google Scholar] [CrossRef]
- Ziaei, S.; Halaby, R. Dietary Isoflavones and Breast Cancer Risk. Medicine 2017, 4, 18. [Google Scholar] [CrossRef]
- Caponio, G.R.; Wang, D.Q.H.; Di Ciaula, A.; De Angelis, M.; Portincasa, P. Regulation of Cholesterol Metabolism by Bioactive Components of Soy Proteins: Novel Translational Evidence. Int. J. Mol. Sci. 2021, 22, 227. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. J. Food Biochem. 2021, 45, 13972. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lin, J.; Tian, J.L.; Si, X.; Jiao, X.Y.; Zhang, W.J.; Gong, E.S.; Li, B. Blueberry Malvidin-3-galactoside Suppresses Hepatocellular Carcinoma by Regulating Apoptosis, Proliferation, and Metastasis Pathways In Vivo and In Vitro. J. Agric. Food Chem. 2019, 67, 625–636. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liang, H.R.; Guo, Y.Z.; Yang, D. Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 20, e00370. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological Activities of Protocatechuic Acid. Acta. Pol. Pharm. 2015, 72, 643–650. [Google Scholar]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.R.; Wang, X.J.; Tang, X.W. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.X.; Kwah, M.X.Y.; Liu, C.L.; Ma, Z.W.; Shanmugam, M.K.; Ding, L.W.; Xiang, X.Q.; Ho, P.C.L.; Wang, L.Z.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Yuan, X.J.; Long, Y.; Ji, Z.H.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.X.; Zhang, W.L.; Wen, X.H.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef]
- Miao, H.; Wu, N.; Luan, C.; Yang, X.; Zhang, R.; Lv, N.; Zhu, B. Quantitation of intestinal Fusobacterium and butyrate- producing bacteria in patients with colorectal adenomas and colorectal cancer. Wei Sheng Wu Xue Bao 2014, 54, 1228–1234. [Google Scholar]
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q.; Yang, L.; Liu, Z.J.; Yuan, Y.Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.D.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of simulated digestion on the phenolic components of red grapes and their corresponding wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Shivashankara, K.S.; Acharya, S.N. Bioavailability of Dietary Polyphenols and the Cardiovascular Diseases. Open Nutraceuticals J. 2010, 3, 227–241. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Ma, G.L.; Chen, Y.T. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; Dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Ho, H.H.; Chang, C.S.; Ho, W.C.; Liao, S.Y.; Wu, C.H.; Wang, C.J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappa B activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Gao, T.; Li, L.; Chen, Y.; Xiao, D.; Liu, W.; Zou, B.; Lu, B.; Tian, X.; et al. A holistic view of gallic acid-induced attenuation in colitis based on microbiome-metabolomics analysis. Food Funct. 2019, 10, 4046–4061. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P.; et al. Gallic Acid Alleviates Gut Dysfunction and Boosts Immune and Antioxidant Activities in Puppies Under Environmental Stress Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2022, 12, 2021. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidelix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Sost, M.M.; Ahles, S.; Verhoeven, J.; Verbruggen, S.; Stevens, Y.; Venema, K. A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients 2021, 13, 3915. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Meleth, S.; Kadiyar, S.K. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems (Publication with Expression of Concern. See vol. 24, pg. 6103, 2018). Clin. Cancer Res. 2005, 11, 1918–1927. [Google Scholar] [CrossRef]
- Yin, X.H.; Liao, W.Y.; Li, Q.R.; Zhang, H.M.; Liu, Z.H.; Zheng, X.J.; Zheng, L.; Feng, X. Interactions between resveratrol and gut microbiota affect the development of hepatic steatosis: A fecal microbiota transplantation study in high-fat diet mice. J. Funct. Foods 2020, 67, 103883. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.C.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X.H. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharm. 2020, 127, 110147. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Xu, S.; Fang, J.; Jiang, H.M. The Protective Effect of Polyphenols for Colorectal Cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Wang, Y.H.; Lin, Y.; Lang, Y.X.; Li, E.H.; Zhang, X.Y.; Zhang, Q.; Feng, Y.; Meng, X.J.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Lu, F.; Liu, F.J.; Zhou, Q.; Hu, X.S.; Zhang, Y. Effects of grape pomace and seed polyphenol extracts on the recovery of gut microbiota after antibiotic treatment in high-fat diet-fed mice. Food Sci. Nutr. 2019, 7, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Boto-Ordonez, M.; Urpi-Sarda, M.; Queipo-Ortuno, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef]
- Jung, C.M.; Heinze, T.M.; Schnackenberg, L.K.; Mullis, L.B.; Elkins, S.A.; Elkins, C.A.; Steele, R.S.; Sutherland, J.B. Interaction of dietary resveratrol with animal-associated bacteria. Fems. Microbiol. Lett. 2009, 297, 266–273. [Google Scholar] [CrossRef]
- Kotlowski, R.; Bernstein, C.N.; Sepehri, S.; Krause, D.O. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 2007, 56, 669–675. [Google Scholar] [CrossRef]
- Bustos, I.; García-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yañéz -Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of low-dose dietary resveratrol on colonic microbiota, inflammation, and tissue damage in a rat model of DSS-induced colitis. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Wu, V.C.; Qiu, X.; de los Reyes, B.G.; Lin, C.S.; Pan, Y. Application of cranberry concentrate (Vaccinium macrocarpon) to control Escherichia coli O157:H7 in ground beef and its antimicrobial mechanism related to the downregulated slp, hdeA and cfa. Food Microbiol. 2009, 26, 32–38. [Google Scholar] [CrossRef]
- Das, Q.; Lepp, D.; Yin, X.; Ross, K.; McCallum, J.L.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Transcriptional profiling of Salmonella enterica serovar Enteritidis exposed to organic blueberry pomace ethanolic extract. PloS ONE 2019, 6, e0219163. [Google Scholar] [CrossRef]
- Vadekeetil, A.; Alexandar, V.; Chhibber, S.; Harjai, K. Adjuvant effect of blueberry proan-thocyanidin active fraction on the antivirulent property of ciprofloxacin against Pseu-domonas aeruginosa. Microb. Pathog. 2016, 90, 98–103. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Hütt, P.; Shchepetova, J.; Lõivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated modulation of the gut microbiota: Towards prebiotics and beyond. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Sreng, N.; Champion, S.; Martin, J.C.; Khelaifia, S.; Christensen, J.E.; Padmanabhan, R.; Azalbert, V.; Blasco-Baque, V.; Loubieres, P.; Pechere, L.; et al. Resveratrol-mediated glycemic regulation is blunted by curcumin and is associated to modulation of gut microbiota. J. Nutr. Biochem. 2019, 72, 108218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, K.; Zhu, J. Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules 2020, 25, 5101. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.A.R.; Pinheiro-Castro, N.; Novaes, G.M.; Pascoal, G.D.L.; Ong, T.P. Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Res. Int. 2019, 125, 108646. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.B.; Tao, Y.; Li, D.D.; Xie, G.J.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Microencapsulation of polyphenols—The specific case of the microencapsulation of Sambucus nigra L. extracts—A review. Trends Food Sci. Tech. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Nunes, M.R.; Benvenutti, E.V.; da Luz, S.R.; D’Avila, R.F.; Rutz, J.K. Microencapsulation of gallic acid in chitosan, beta-cyclodextrin and xanthan. Ind. Crop. Prod. 2013, 46, 138–146. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Arozarena, I.; Marin-Arroyo, M.R. Optimization of a Wall Material Formulation to Microencapsulate a Grape Seed Extract Using a Mixture Design of Experiments. Food Bioprocess Tech. 2013, 6, 941–951. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.Y.; Yu, L.; Zhu, P.T.; Zhou, X.P.; Liu, H.S.; Yang, Y.Y.; Zhou, J.Q.; Gao, C.C.; Bao, X.Y.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohyd. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Gamage, N.H.; Jing, L.; Worsham, M.J.; Ali, M.M. Targeted Theranostic Approach for Glioma Using Dendrimer-Based Curcumin Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 393. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Lu, K.Y.; Jiang, B.; Li, X.F.; Cui, S.W. Structure and physicochemical properties for modified starch-based nanoparticle from different maize varieties. Food Hydrocoll. 2017, 67, 37–44. [Google Scholar] [CrossRef]
- Guo, L.; Tao, H.T.; Cui, B.; Janaswamy, S. The effects of sequential enzyme modifications on structural and physicochemical properties of sweet potato starch granules. Food Chem. 2019, 277, 504–514. [Google Scholar] [CrossRef]
- Abbas, S.; Karangwa, E.; Bashari, M.; Hayat, K.; Hong, X.; Sharif, H.R.; Zhang, X.M. Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly. Ultrason. Sonochem. 2015, 23, 81–92. [Google Scholar] [CrossRef]
- Peng, S.L.; Xue, L.; Leng, X.; Yang, R.B.; Zhang, G.Y.; Hamaker, B.R. Slow Digestion Property of Octenyl Succinic Anhydride Modified Waxy Maize Starch in the Presence of Tea Polyphenols. J. Agric. Food Chem. 2015, 63, 2820–2829. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotech. 2020, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, J.P.; Qin, C.; Hu, Y.; Xu, X.M.; Jin, Z.Y. Effects of Degree of Polymerization on Size, Crystal Structure, and Digestibility of Debranched Starch Nanoparticles and Their Enhanced Antioxidant and Antibacterial Activities of Curcumin. ACS Sustain. Chem. Eng. 2019, 7, 8499–8511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).