Abstract
Spinal cord injury (SCI) often causes loss of sensory and motor function resulting in a significant reduction in quality of life for patients. Currently, no therapies are available that can repair spinal cord tissue. After the primary SCI, an acute inflammatory response induces further tissue damage in a process known as secondary injury. Targeting secondary injury to prevent additional tissue damage during the acute and subacute phases of SCI represents a promising strategy to improve patient outcomes. Here, we review clinical trials of neuroprotective therapeutics expected to mitigate secondary injury, focusing primarily on those in the last decade. The strategies discussed are broadly categorized as acute-phase procedural/surgical interventions, systemically delivered pharmacological agents, and cell-based therapies. In addition, we summarize the potential for combinatorial therapies and considerations.
1. Introduction
The World Health Organization estimates that 250,000–500,000 people suffer a new spinal cord injury (SCI) each year [1]. Historically, there have been about 17,810 new cases every year in the United States (US) alone [1]. The leading causes of SCIs are vehicular accidents, falls, violence, sports accidents, and medical/surgical complications [1]. Of SCIs in the US, almost 50% are incomplete tetraplegia, followed by complete paraplegia, incomplete paraplegia, and complete tetraplegia [1]. Paraplegia refers to neurological deficits, ranging from minor to complete loss of sensory and motor functions of the lower extremities only, while tetraplegia affects all four extremities. In 2020, the lifetime cost of healthcare for SCI patients was estimated to be USD 1.7–5.1 million for an injury occurring at 25 years of age and USD 1.2–2.8 million for an injury at 50 years of age [2]. While no therapies exist that can reliably restore lost spinal cord functions, extensive research efforts have led to clinical trials of a number of promising treatment options. This review summarizes SCI therapies evaluated in clinical trials in the last decade and some preclinical studies of therapies with a high potential for clinical translation. We focus on therapies administered with the goals of reducing secondary injury and increasing neuroprotection.
Primary injury to the spinal cord is typically caused by a mechanical insult and most often presents as an incomplete injury rather than total severance of the cord. Secondary injury, during which progressive losses of tissue and function often occur, includes acute (within 2 days of primary SCI), subacute (2–30 days after SCI), and intermediate (30 days to 6 months after SCI) phases. Secondary injury is marked by swelling and edema at the site of injury, additional hemorrhage, and an active inflammatory response that leads to extensive death and demyelination of neurons [3]. Around 6 months after primary injury, the SCI is typically considered to have stabilized and be in the chronic phase [4]. Chronic injuries often include fluid-filled cysts surrounded by fibrotic and gliotic scar tissue at the lesion core. Although injury-induced tissue reorganization and remodeling reaches a relatively steady state by the chronic phase, unresolved inflammation is thought to continue for many years [5,6].
Clinical therapies that successfully modulate the inflammatory response to mitigate the detrimental effects of secondary injury after SCI are expected to preserve neurological functions, condition the injury site to support regeneration, and improve patient outcomes. Therapeutic strategies aimed at neuroprotection include (1) early procedural interventions for acute management, (2) pharmacological therapies, (3) cell-based therapies, and (4) combinatorial therapies. Therapeutic agents are being developed to address multiple concurrent processes that occur during secondary SCI, for example, by limiting intraspinal pressure (ISP), modulating the inflammatory response, promoting survival of neurons and their circuitry, myelinating oligodendrocytes, and/or mitigating formation of chronic barriers to tissue regeneration (e.g., scar and cyst formation). Overall, evidence suggests that better clinical outcomes could be achieved with a combinatorial therapy that simultaneously addresses many, if not all, of the issues listed above. Development of therapies that effectively attenuate secondary injury and reduce the barriers to spinal cord regeneration will set the stage for later interventions in intermediate or chronic phases designed to actively promote regeneration of lost tissue. Thus, this review focuses on clinical innovations aimed at limiting secondary injury and promoting neuroprotection.
2. Spinal Cord Injury
2.1. Intact Spinal Cord
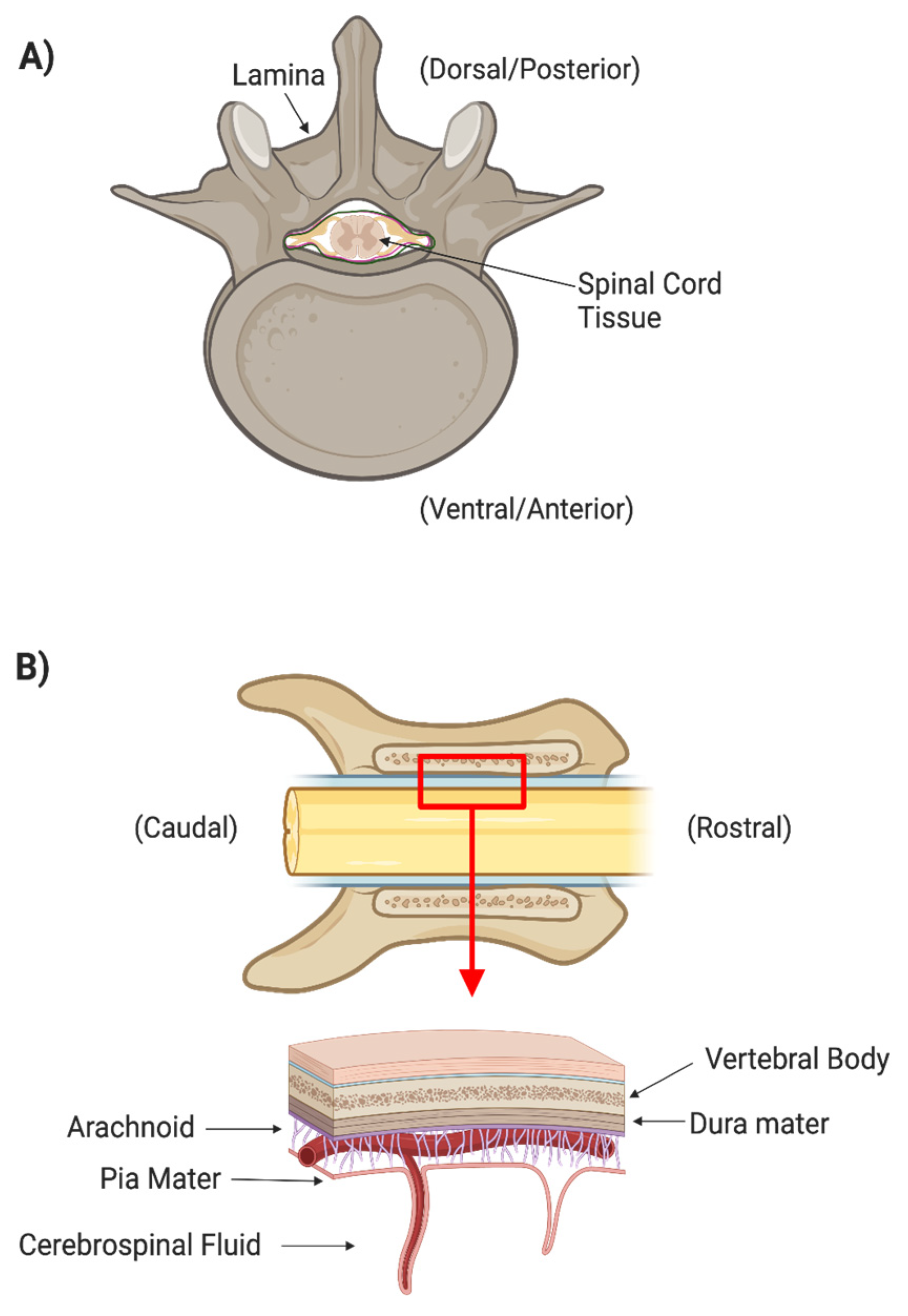
There are many layers of protection that have evolved in the central nervous system (CNS). The spinal cord is protected by the spinal column, which consists of osseous vertebrae. The epidural space between the vertebrae and the outermost meningeal layer contains fat and vasculature to cushion the cord itself from outside mechanical forces (Figure 1). Three meningeal layers provide additional physical protection and separate peripheral extracellular fluids from the cerebrospinal fluid (CSF). The dura mater is the thickest and outermost meningeal layer. Below the dura mater are the relatively thin arachnoid membrane and the innermost meningeal layer, the pia mater. The CSF between the arachnoid and pia mater allows the spinal cord to freely float within the dura mater. The arachnoid is covered in finger-like villi, or arachnoid granulations, which maintain the CSF’s osmotic balance and filter waste from the CSF into the bloodstream. CSF is secreted by specialized ependymal cells in the choroid plexus of the four cerebral ventricles and flows freely through the ventricular-system and central canal of the spinal cord.
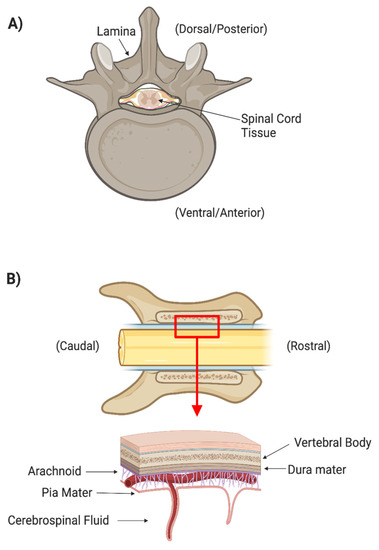
Figure 1.
(A) Axial cross section of spinal cord surrounded by the boney spinal column. (B) Coronal cross section of spinal cord and column showing the different meningeal layers that protect the spinal tissues.
Neurons are the primary information carriers of the CNS. All neurons have three main compartments: (1) a cell body (or soma) containing the nucleus and organelles, (2) dendrites that receive inputs from other neurons, and (3) an axon that relays outputs in the form of action potentials. Likewise, glial cells are crucial to CNS function. Oligodendrocytes are glia cells that form insulating, myelin sheaths around axons to facilitate long-range conduction. Areas of dense axonal tracts with insulating myelin appear white in gross CNS tissue and, thus, these areas are commonly referred to as white matter. In contrast, unmyelinated neurons dominate the gray matter of the spinal cord. Astrocytes are glia with diverse roles in the CNS; for example, they provide metabolic support for neurons and form the blood–spinal cord barrier (BSCB) [7,8,9]. Microglia are the resident immune cells of the CNS and are also involved in processes such as synaptic pruning during development [10,11]. Both astrocytes and microglia play important roles in neuroinflammation during the secondary injury response after SCI, as discussed in the following section.
2.2. Pathophysiology of SCI
SCI consists of two phases: the primary injury and the secondary injury. The primary injury is the physical force applied to the spinal cord that results in tissue damage. These injuries can be caused by laceration, stretch, compression, and shearing [2]. One of the most immediate consequences of primary injury is the retraction of damaged axons, resulting in loss of these synaptic connections and eventually leading to apoptosis of the damaged neuron and, often, its post-synaptic partner(s) [12,13]. The primary injury also causes local bleeding, loss of physiological blood flow, and edema near the lesion [13]. As components of blood are cytotoxic in the CNS [14,15], limiting parenchymal bleeding in acute SCI is an important strategy to limit secondary injury. Limiting edema and restoring local blood flow through intact vasculature are also important, as ischemia is another major cause of cell death after SCI [13,16].
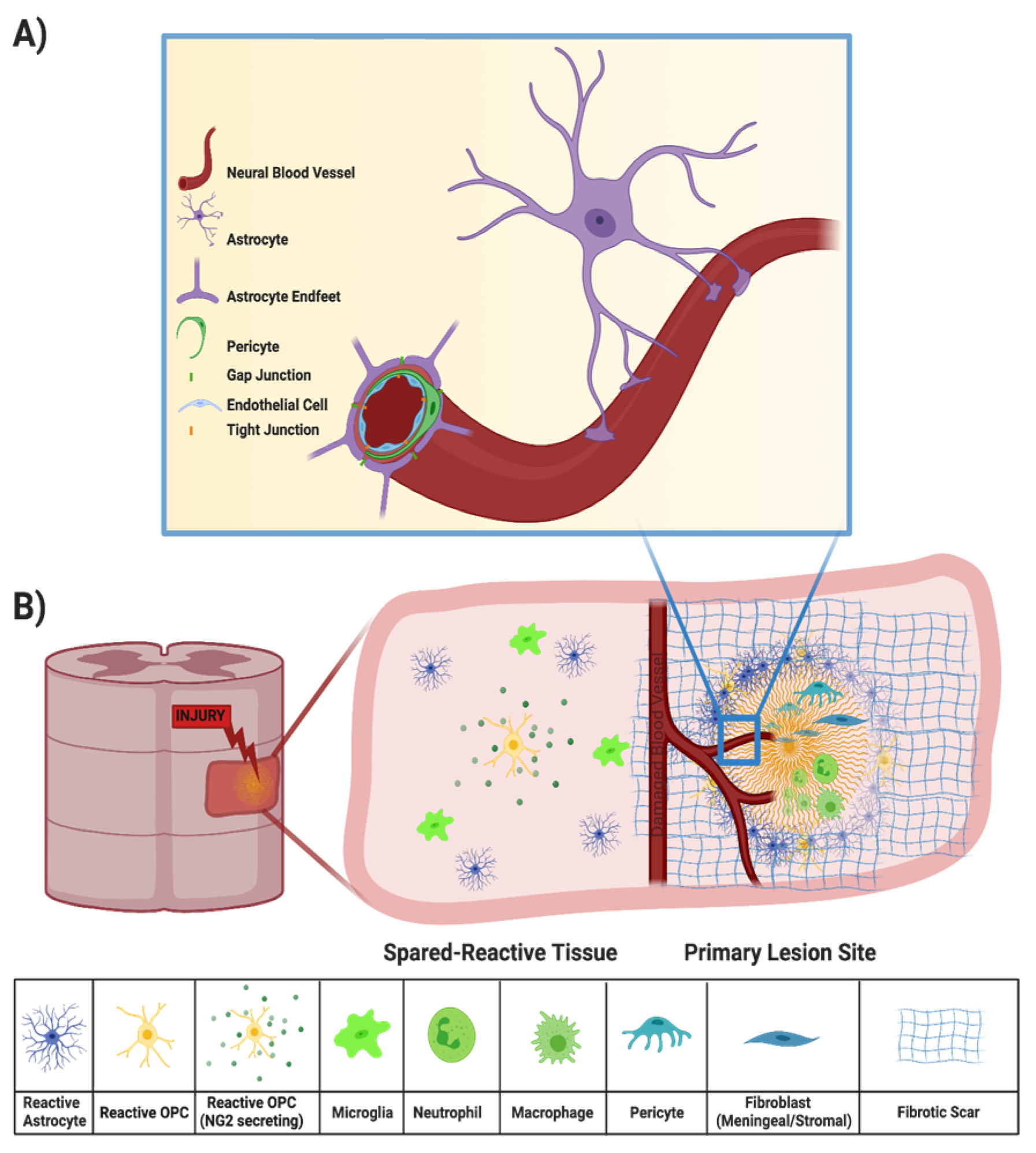
Injury to the spinal cord may also disrupt the BSCB, which is created by tight junctions between the end feet of specialized astrocytes and endothelial cells surrounding the blood vessels that supply the CNS (Figure 2A) [9]. All incoming metabolites are shuttled through the BSCB astrocytes, protecting the CNS from chemical compounds and infectious organisms that may otherwise compromise neural functions [9,17]. Peripheral inflammatory cells, such as macrophages and neutrophils, can enter the spinal cord parenchyma from the bloodstream through the compromised BSCB (Figure 2B). Within minutes of the primary SCI, both peripheral inflammatory cells and microglia initiate the secondary injury cascade [18]. While peripheral macrophages and resident microglia both phagocytose cellular debris and potentially infectious agents after injury [19,20], they also secrete a cocktail of pro-inflammatory cytokines that propagate an oxidative inflammatory response that results in additional neuronal death [21,22,23]. Local cytokine concentrations peak during the acute phase of SCI (about 6–12 h after primary injury) but may persist for several days [24,25].
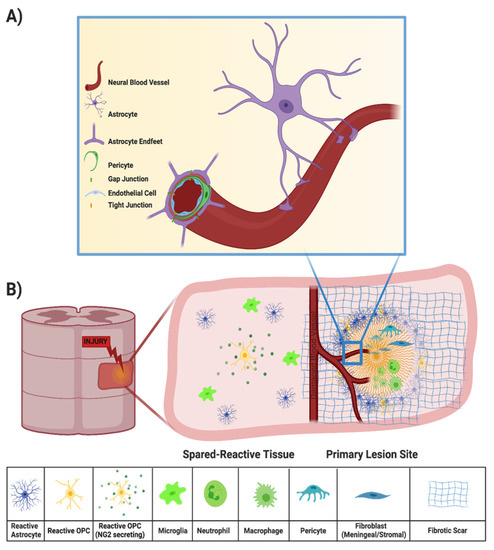
Figure 2.
(A) Components of the BSCB, in which the neural blood vessel is composed of endothelial cells surrounded by pericytes and end feet of astrocytes that tightly regulate entry of drugs, solutes, and cells into the CNS. (B) Components of the secondary injury response, in which a compromised BSCB results in entry of peripheral immune cells, recruitment of microglia, reactive astrocytes, OPCs, and fibroblasts, and fibrotic scar deposition.
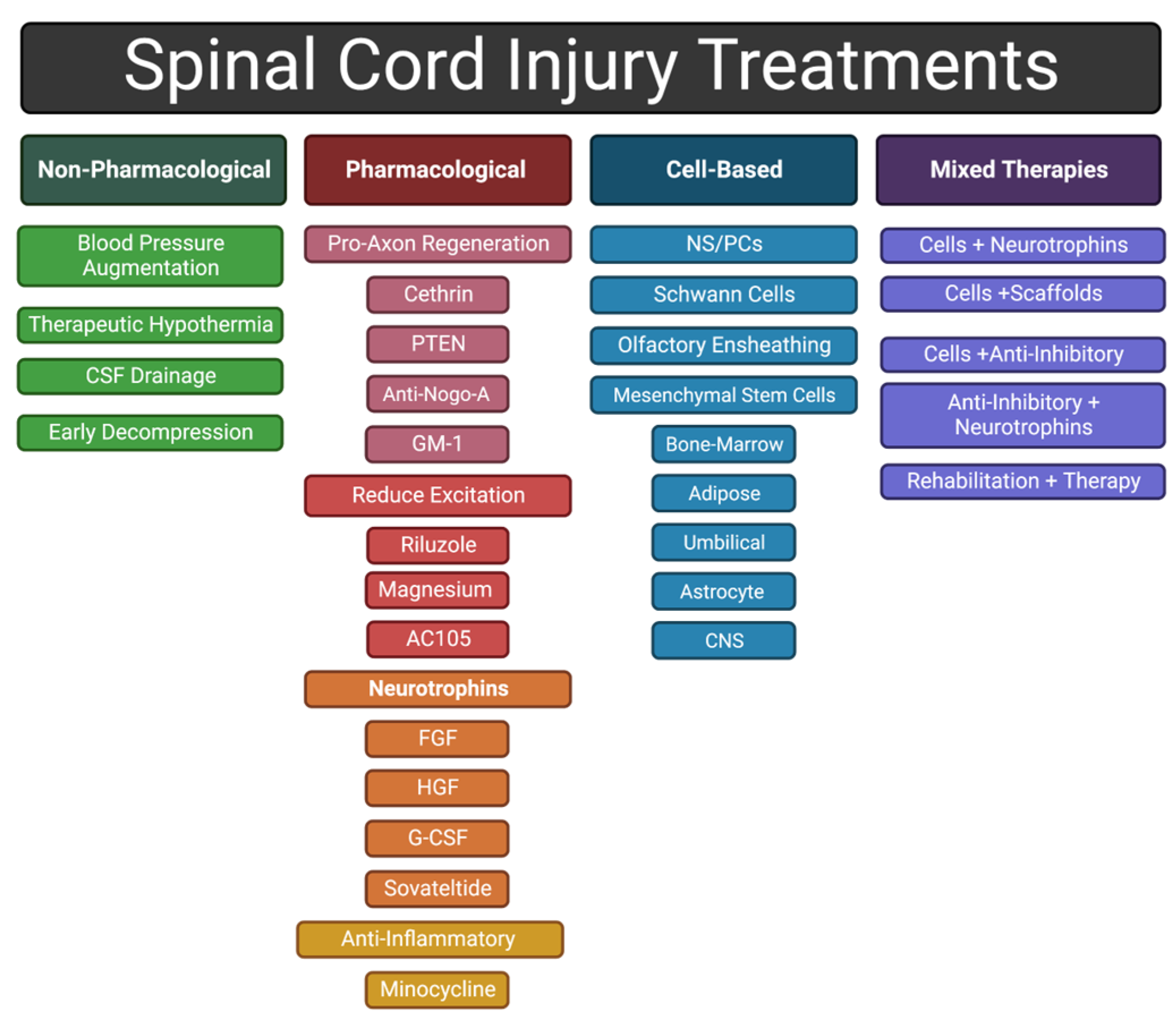
Debris from damaged axons and myelin sheaths, necrotic/apoptotic cells, and the disrupted extracellular matrix (ECM) also contributes to secondary injury [13]. For example, release of excessive amounts of excitatory neurotransmitters from damaged neurons cause neighboring neurons to fire excessively, leading to excitotoxicity [26]. In another example, high-molecular-weight hyaluronic acid in the ECM is degraded by hyaluronidases after SCI, and loss of interactions between hyaluronic acid and resident astrocytes induces their activation, a fundamental step in glial scar formation [27,28]. Typically, by the end of the subacute phase, the injury center becomes a fluid-formed cyst surrounded by scar tissue and the BSCB has been re-established [4]. Scar tissue includes a glial scar, at the border between injury and intact tissue, and a fibrotic scar, lying between the glial scar and the cyst [29,30]. In this review, we discuss a variety of therapeutic approaches to address the different mechanisms that contribute to the injury response (Figure 3).
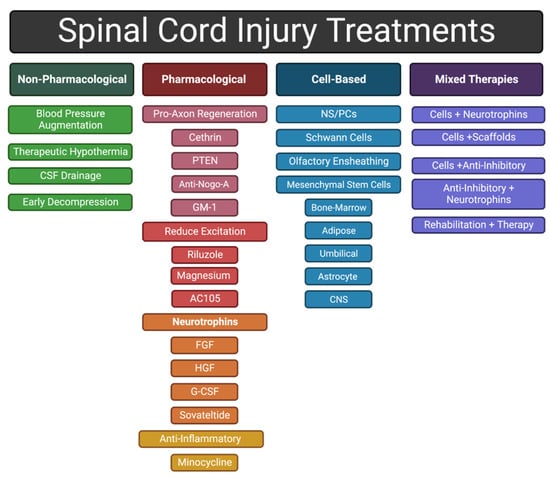
Figure 3.
General categories of approaches to treating an SCI discussed in this review.
The standard clinical assessment of the extent of an individual’s sensory and motor impairments following SCI is the American Spinal Injury Association Impairment Scale (AIS) [31]. AIS A indicates complete disruption of all ascending and descending pathways at a certain spinal segment, such as after complete transection of the spinal cord. AIS B–E indicate increasingly better neurological functions, with AIS E being essentially normal function.
3. Managing Acute Pathophysiology after Traumatic SCI
The primary goal of early interventions, typically during the acute phase of SCI, is to reduce tissue loss caused by secondary damage and, thus, preserve more neurological functions for patients. This section discusses the mechanisms of the following investigational, early procedural interventions in greater detail, as well as preclinical and clinical studies evaluating their safety and efficacy in acute SCI with a focus on: (1) elevation of mean arterial pressure (MAP), (2) decompression surgery (DS), (3) CSF drainage, and (4) therapeutic hypothermia (TH). A summary of promising studies for early stage interventions can be found in Table 1.

Table 1.
Clinical Studies of Early Stage Interventions.
3.1. Augmentation of Mean Arterial Blood Pressure
The most immediate complication in the acute phase of cervical and high thoracic SCI is systemic hypotension, resulting from the disruption of the autonomic nervous system’s regulation of blood pressure [4]. Since 2002, the American Association of Neurological Surgeons (AANS) has recommended that the MAP of traumatic SCI patients be maintained between 85 and 90 mmHg for seven days post-injury [44,45]. In clinical settings, MAP is manipulated with vasopressors such as norepinephrine (NE), phenylephrine (PE), and dopamine [46,47,48,49]. A preclinical study in a porcine model supported the hypothesis that increasing MAP improves blood flow to the injured spinal cord [46]. Specifically, investigators compared the abilities of NE and PE to improve blood flow into an acutely contused thoracic SCI. While both NE and PE increased MAP and improved blood flow and oxygenation at the lesion for a brief period immediately after DS, only PE was associated with a greater possibility of hemorrhage at the SCI site. While these preclinical results suggest that MAP elevation via NE, combined with DS, can safely increase perfusion of the injury site, it remains unclear whether this transient increase in perfusion can affect secondary injury or functional outcomes.
While we have not found any reports from controlled clinical studies, several retrospective studies have investigated whether maintenance of MAP above 85 mmHg during the acute phase of SCI, as recommended by the AANS, had any effects on patient outcomes [50,51]. A 2015 study analyzed San Francisco General Hospital intensive care unit data collected from 100 acute SCIs at all injury levels. MAP elevation was achieved with one or two administrations of NE, PE, and/or dopamine, and some of the patients also underwent DS [50]. Interestingly, the duration of MAP augmentation, defined as the proportion of time that MAP remains elevated, above 85 mmHg was better correlated with neurological recovery than the average MAP over 7 days, a metric that does not account for episodes of hypotension, thereby supporting the need to maintain MAP above a certain threshold for optimal clinical outcomes.
A separate, 2017 retrospective study analyzed the data of 94 traumatic SCI patients who underwent DS early (<24 h after SCI) or late (>24 h after SCI) and were administered NE, as needed, to increase MAP to at least 85 mmHg [52]. Patients for whom MAP was maintained at or above 85 mmHg for at least 2 consecutive hours within 5 days post-injury were at least 10 times more likely to experience improvements in AIS motor grade by 26 days post-injury, independent of when DS was performed. Additionally, a larger proportion of patients who received early DS experienced AIS improvements at later time points (up to 252 days post-injury).
Another smaller retrospective study of 25 traumatic SCI patients, treated at Santa Clara Valley Medical Center in the US, investigated the influence of MAP, measured by an inline arterial sensor during DS, on recovery of motor function [53]. Patients whose MAP remained within the range 70–94 mmHg for longer total periods of time during hospitalization, DS, post-surgery, and acute rehabilitation, experienced greater recovery of motor functions. In addition to supporting the idea that maintaining a normotensive state through pharmacological MAP elevation during the acute phase of SCI increases the likelihood of neurological recovery, this study also suggested that hypertension (MAP >94 mmHg) may have negative effects. Logistic regression analysis in acute cervical SCI patients suggest that increased MAP to the goal of >85 mmHg for 7 days post-injury did not increase the risk for hemorrhagic contusion expansion [54].
In summary, the optimal MAP range may be between 85 mmHg and 94 mmHg. While the duration of MAP elevation during the acute phase of SCI appears to be important, the mechanisms of action for its effects of neurological recovery remain unclear. The clinical use of MAP elevation to treat acute SCI has been rationalized by the idea of a corresponding increase in blood perfusion at the injury site, which is expected to reduce ischemic injury. In a porcine SCI model, MAP augmentation was reported to lead to around a 25% increase in spinal cord blood flow [55]. However, in contrast to this porcine study, there is emerging evidence that impairment in local tissue auto-regulation effectively prevents the expected increase in blood flow, which calls into question whether treatment with drugs intended to augment MAP may benefit acute SCI patients by some alternative mechanism [56]. In the future, it will be important to determine the local spinal vascular mechanisms that can influence adequate tissue perfusion to maximize tissue sparing after SCI.
3.2. Therapeutic Hypothermia
TH aims to lower the spinal cord temperature to approximately 33 °C, with the goal of reducing inflammation to limit secondary damage after acute trauma. Systemic TH has been shown to benefit patients in several different clinical situations, including cardiac arrest [57,58], stroke [59,60], traumatic brain injury [61,62], and cerebral aneurysm [63,64]. During acute SCI, there are several potential mechanisms by which TH may be neuroprotective. On the cellular level, lowering temperature reduces basal metabolic rate, reducing energy demands and prolonging survival in ischemic conditions [65,66]. Global hypothermia may also upregulate neuroprotective factors and downregulate inflammatory factors, effectively reducing secondary injury [67,68,69]. Clinically, TH has been realized after SCI either by locally cooling the cord, by administering cold saline to the exposed spinal cord during surgery, or by systemic cooling using a variety of techniques such as cooling blankets, ice baths, or inserting a cooling catheter placed in the inferior vena cava through a femoral vein [65,70].
Several preclinical studies in rodents have found that administering systemic or local epidural TH within 30 min of SCI and prior to DS reduces inflammation, excitotoxicity-mediated secondary injury, lesion volume, loss of neuronal tracts, and locomotor deficits [65,66,70,71,72,73,74,75,76,77,78]. One preclinical study evaluated for how long systemic TH, induced through surface cooling, could effectively mitigate secondary injury from cord compression prior to performing DS [78]. Rodents received TH starting at 30 min and continuing up until 7.5 h after a compression SCI, followed by DS. Rodents who received TH for 7.5 h before DS had significantly better histological and functional outcomes than animals that received only DS 8 h after SCI. Furthermore, rodents receiving TH for 7.5 h before DS compression displayed similar outcomes to those whose cords were compressed for only 2 h prior to DS. Therefore, as TH can be administered en route to the hospital, it can be used to mitigate neurological deterioration at the earliest timepoints after SCI before treatments such as DS and CSF drainage are possible.
TH gained widespread attention in 2007 when cold saline was induced intravenously to an NFL player within 15 min of a complete C3/C4 SCI (AIS A) suffered during a televised game. A case report published in 2010 described the details of how systemic TH was applied, the performance of DS approximately 3 h after SCI, and how the patient made an impressive recovery, converting from AIS A to AIS D four months after the initial injury [79]. At least three unique clinical trials since 2010, including around 70 patients, have applied systemic TH administration within an average of 8 h after SCI for durations of 12–48 h and reported that around half of the patients improved by at least one AIS grade within several days of SCI [32,33,34,80]. However, due to the low number of patients and injury heterogeneity, it is not clear whether this level of recovery truly represents improvement compared to the untreated SCI population [80].
Despite its potential benefits for SCI patients, some serious risks are associated with systemic TH, including pneumonia, temperature-related cardiac complications, thromboembolism, infection, and potential insult of the femoral artery [34,35,70]. While application of TH by local cooling in the epidural space above the injury may decrease some of these risks, such as cardiac issues and pneumonia, local and surface cooling raises the risk of infection and variability in cooling. Additionally, some studies have suggested that systemic TH may be more effective at suppressing apoptosis in animal models of SCI [81,82]. One clinical trial investigated effects of local epidural cooling; however, the trial was terminated as three out of five total patients developed infections, a clear risk given the more invasive nature of local cooling [35].
While many side effects of TH remain to be resolved, the benefits are clear. In 2019, the University of Miami completed a prospective, observational study on 41 patients who received systemic TH for 48 h [36]. While results are still pending publication, this same group initiated a larger, multi-center, randomized clinical trial in 2017 [37]. Trial enrollment is still ongoing, and 120 patients are expected by the estimated completion date in 2024.
3.3. Cerebrospinal Fluid Drainage
Another approach to reduce mounting ISP in acute SCI is drainage of excess CSF, typically by removing fluid at a constant rate using a syringe inserted into the intrathecal space to steadily lower ISP to about 10 mmHg [83]. In SCI patients, persistent extradural compression can reduce or occlude the subarachnoid space resulting in a CSF pressure differential across the injury site [84]. In a porcine acute contusion model, the cranial and caudal CSF pressures were found to have an increasing pressure differential (0.39 mm Hg/h) during the compression [84]. After decompression, cranial ISP decreased but caudal ISP increased, resulting in no net change in CSF pressure differential. Thus, recording CSF pressure in the lumbar spinal cord will likely not be sufficient to estimate pressures rostral to the SCI.
In acute SCI, it is generally thought that CSF drainage should allow for increased perfusion pressure within the spinal cord. However, a porcine study reported that, unlike MAP augmentation that led to short-term improvements in perfusion, CSF drainage alone did not substantially improve blood flow in the injured spinal cord [55]. Interestingly, performing CSF drainage in combination with MAP augmentation did result in longer term improvements in spinal cord perfusion when compared to MAP augmentation alone.
In human subjects with acute SCI, a phase 1/2 clinical trial in the US, including 22 patients with acute cervical or thoracic AIS A-C SCIs, investigated the safety and efficacy of CSF drainage [38,39]. Around half of the patients underwent CSF drainage within 48 h of SCI followed by ISP recording during the next 72 h. While the average ISP for patients who underwent CSF drainage did not fluctuate significantly, there was a significant rise in the average ISP for patients who did not receive CSF drainage. While no significant behavioral or histological changes were reported as a direct result of CSF drainage, this trial demonstrated procedure safety and motivated a phase 2b clinical trial that included patients with acute (≤24 h of injury) cervical (C4-C8) AIS A-C SCIs [40]. Patients were divided into two cohorts who received either CSF drainage (achieving ISP of 10 mmHg) and MAP elevation using an NE drip (achieving 100–110 mmHg) or MAP maintenance only. This study concluded in August 2019 with 15 patients and the results are pending. It is not clear to the authors of this review why MAP was maintained above the AANS recommended range for acute SCI in this study. While CSF drainage was found to be safe in these trials, it is possible that excessive removal of CSF may cause the spinal cord and brain to lose the cushioning provided by the CSF and, in turn, contact surrounding bony structures, leading to temporary neurological deficits [85,86,87]. Other potential complications of the drainage procedure include leakage of CSF at the site of dural puncture, subdural hematoma, cerebral herniation, and infection [83,88,89].
3.4. Decompression Surgery
Following primary SCI, inflammation leads to increased ISP, resulting in compression of injured tissue, occlusion of local vasculature, and ischemia [90,91,92,93,94]. ISP ranges from 4 to 18 mmHg for healthy individuals to 30–35 mmHg for patients with acute contusive SCIs [95,96]. DS aims to alleviate excessive ISP by increasing the volume available for spinal cord tissue swelling or to remove bony fragments infringing into the spinal cord [97,98]. DS almost always involves a laminectomy, which is the removal of the vertebral lamina, and is often coupled with vertebral instrumentation to restore stability of the spinal column. Several clinical studies have found that performing DS within 24 h of the primary SCI is associated with shorter hospital stays and increased probability of post-operative neurological recovery, while other studies of surgeries within 72 days or later of injury did not report any benefits [41,98,99,100,101,102,103,104,105,106]. In general, studies have found benefits of early DS only for patients with incomplete injuries and AIS B–D, but not with more severe, complete injuries, and AIS A [42,43,107]. However, a systematic meta-analysis of clinical trials did not find statistically significant long-term advantages of early over late DS, which the authors suggested could be due to variability in the level of SCI, follow-up timing, and specific outcomes being measured [107].
Furthermore, cases of extensive edema and swelling may result in deformation of the dura-bound spinal cord [108,109], in which additional decompression may be achieved through opening the meningeal membranes, including the dura membrane (durotomy) alone or in addition to the pia mater (piotomy) [43]. Typically, a duraplasty is performed after durotomy to re-seal the meningeal membrane using synthetic, allogenic, or autologous grafts [110]. Preclinical animal studies demonstrated that meningeal decompression after laminectomy, including durotomy and piotomy, was more effective than laminectomy alone, in terms of reducing ISP, and resulted in improved functional outcomes [94,111]. A human cadaveric study compared the ability of three DS approaches (laminectomy only, laminectomy with durotomy, and laminectomy with durotomy and piotomy) to reduce ISP, measured at maximal kyphosis by sensors placed within the cord throughout the cervical and thoracic segments [112]. On average, this study found that laminectomy alone reduced ISP by around 20%, while midsagittal durotomy or durotomy with piotomy additionally reduced ISP by around 66% and 99%, respectively. Although this study used a cadaveric model that does not account for the dynamic edema of a living subject, these findings provide evidence that meningeal incision reduces ISP to a greater extent than laminectomy alone. However, drastic reductions in ISP following durotomy and piotomy may be detrimental in cases of moderate edema, as ISP could drop below physiological levels.
A small-scale clinical study performed in China measured changes in ISP before and after durotomy in three patients with excessive edema and reported that durotomy followed by duraplasty using a polymeric synthetic graft lowered ISP [43]. While this prior study unfortunately did not have a durotomy-only control group, results from another small-scale study performed in China did observe better recovery of neurological function in patents receiving durotomy with duraplasty when compared to durotomy alone [42,43].
The duraplasty graft used may also have significant effects. Preclinical studies, performed in rodent compressive SCI models, have reported that durotomy followed by allographic duraplasty leads to reduced cystic cavity volumes, inflammation, scar tissue formation, and secondary injury overall [113,114,115,116]. Conversely, in rodent compressive SCI models where synthetic grafts were used, no histological changes were observed, some animals displayed increased lesion sizes, and others had decreased hindlimb coordination as well as the development of neuropathic pain [117,118]. Differences in the effects of allographic and synthetic duraplasty may be due to the presence of specific bioactive factors present only in allografts or biocompatibility issues inherent to the synthetic graft materials used. For example, duraplasty using a hydrogel-based dural sealant, DuraSeal, led to additional compression of the spinal cord in several subjects after the material expanded in vivo [117].
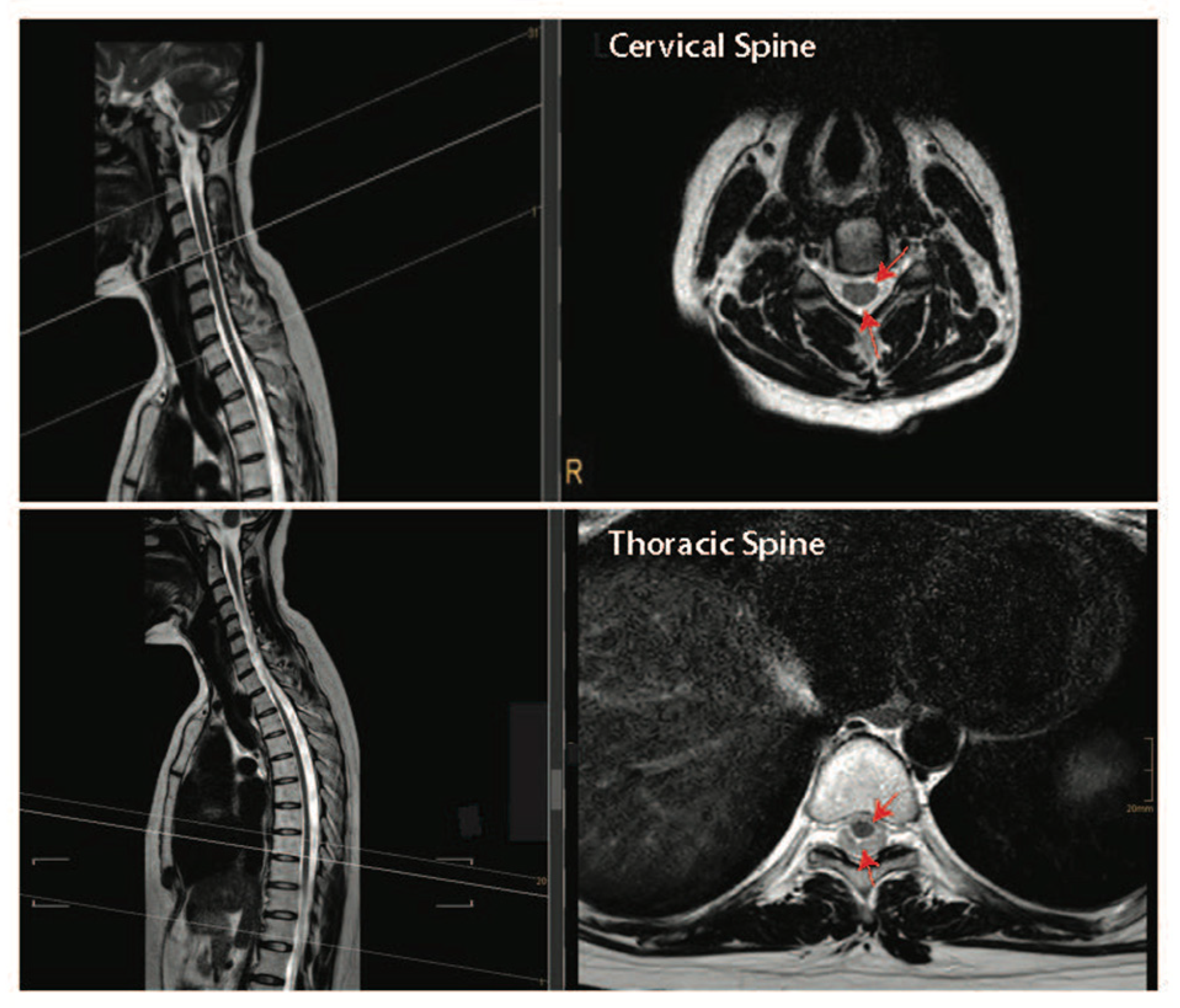
Together, the pre-clinical and clinical data presented above suggest that performing both a durotomy with a piotomy may be more effective than a durotomy alone at reducing the raised ISP after acute SCI. However, the highly invasive nature of durotomies and piotomies introduces risks, including further mechanical damage to the spinal cord, infections, excessive ISP reduction, CSF leaks and fistulas, hemorrhage, and pseudo-meningoceles, thereby necessitating a thorough risk–benefit analysis that accounts for patient-specific injury pathologies [119]. For example, the risk–benefit analysis for durotomy and piotomy likely depends on the SCI level [112]. Specifically, the human spinal cord tissue occupies only 50% of the subdural space within the thoracic spinal cord, while it occupies about 90% of the subdural space within the cervical spinal cord (Figure 4). Given the larger space in which the spinal cord can expand without dural compression at the thoracic level [120], benefits associated with a durotomy and/or piotomy may be more pronounced for cervical level injuries, where more severe swelling would lead to stronger cord compression. Furthermore, as discussed above, performing DS within the acute phase of SCI (within 72 h) appears to facilitate significant functional recovery [97,100,115,121].
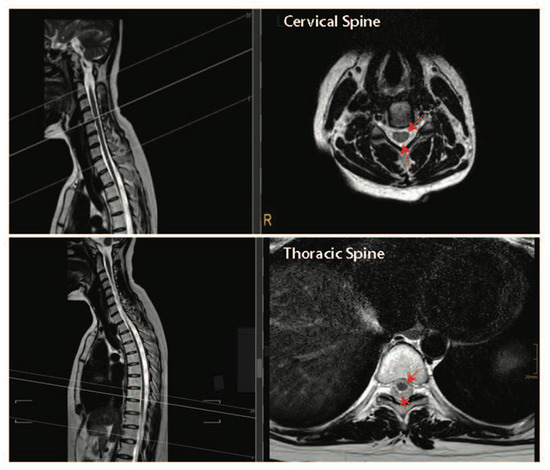
Figure 4.
Intact cervical and thoracic spinal cord tissue can float freely within the spinal column in humans. Top panel—T2-weighed sagittal and cross-sectional images of the cervical spine. Bottom panel—T2-weighed sagittal and cross-sectional images of the thoracic spine. Dark structures where the red arrows are pointing are the spinal cord tissue and the lighter areas depict cerebral spinal fluid-filled subarachnoid space. Images are courtesy of Dr. Tobias Prasse.
In the future, it will be important to identify specific patients who would benefit from both bony and/or meningeal decompression using intraoperative imaging that can detect local changes in blood flow and tissue perfusion. One such intraoperative imaging technique is in development by Khaing et al., using an ultrafast, contrast-enhanced ultrasound technique to image and quantify acute hemodynamic changes in rat traumatic SCI models [122,123]. Development and use of such intraoperative imaging modalities that can assess local blood flow changes will be essential for effective identification of patients who would likely benefit from invasive DS.
4. Pharmacological Therapies
The following sub-sections discuss several pharmacological agents that hold promise for treatment of acute SCI with the goal of mitigating secondary injury by reducing bleeding, inflammation, and excitotoxicity, as well as improving angiogenesis and/or neurogenesis. A summary of promising pharmacologic therapies can be seen in Table 2. While the pharmacological agents targeting barriers of axonal regeneration also hold promise as treatments for acute and chronic SCI, they are beyond the scope of this manuscript. Thus, readers are referred to a recent review of this area by Uyeda and Muramatsu (2020) [124].

Table 2.
Clinical Studies of Pharmacological Therapeutics.
4.1. Reducing Intra-Spinal Bleeding
Intraspinal bleeding in acute SCI is thought to promote secondary damage and compromise functional recovery [137,138,139,140]. Glibenclamide (also known as glyburide) is a potent blocker of sulfonylurea receptor 1-regulated, calcium-activated, [ATP]-sensitive, nonspecific cation channels, which are regulated in endothelial cells during acute SCI [141]. Systemic treatment with glibenclamide at 200 ng/h delivered via a subcutaneously implanted osmotic minipump may aid in reducing the effects of secondary hemorrhage and progressive hemorrhagic necrosis that follow SCI [142,143]. In pre-clinical studies, multiple laboratories have shown that early treatment with glibenclamide after a cervical-level contusion in rats can prevent capillary fragmentation, reduce progressive hemorrhagic necrosis, reduce lesion volumes, and improve functional outcomes [141,142,143]. As glibenclamide is already an FDA-approved treatment for type 2 diabetes, it is an attractive and low-risk molecule for translation in SCI therapeutics. A phase 1 clinical trial began in 2017 to evaluate the safety of oral glibenclamide treatment of acute cervical SCI patients (AIS A-C) [125]. However, this trial was terminated in 2021 due to low enrollment and the principal investigator leaving the institution [125]. Given evidence from preclinical studies, glibenclamide remains a promising treatment for limiting secondary cell death due to progressive hemorrhage after acute SCI.
4.2. Reducing Inflammation
A variety of studies have shown that post-SCI inflammation further exacerbates neuronal damage following the initial mechanical trauma and that reducing inflammation can encourage cell survival, increase regeneration, and decrease muscle denervation [144,145,146,147,148]. While many studies are aiming to better understand how inflammation drives secondary injury, they have relied on experimental techniques that have not yet been readily translated to a clinically setting (e.g., rodent genetic techniques, gene therapy, biomaterials carriers for stem cell transplants) and these studies have overwhelmingly reported that decreasing inflammation benefits functional outcomes [149]. This section discusses several pharmacotherapies that, while approved by the FDA for non-SCI uses, act by decreasing inflammation and, thus, may provide some benefits to SCI patients. In addition, these compounds have been previously evaluated by the FDA for other applications and could potentially be translated to clinical use at a relatively accelerated pace.
Previously, methylprednisolone sodium succinate (MPSS), a synthetic corticosteroid, was used off-label for management of acute SCI. It was thought that, since MPSS and other steroids are known to cause immunosuppression and therefore limit inflammation, these drugs may be useful in the setting of an acute SCI. In recent years, MPSS use in SCI has fallen out of favor, as there is increasing evidence that it can result in serious complications in polytrauma patients, while clinical evidence that MPSS effectively prevents secondary damage is minor [150,151]. While approved for multiple other indications, MPSS never received FDA approval for SCIs.
Minocycline is a semisynthetic, lipophilic, tetracycline-derived antibiotic that can penetrate the BSCB, enabling systemically delivered drugs to reach the CNS. In vitro models suggest that minocycline inhibits the expression of pro-inflammatory cytokines to prevent neurotoxicity and apoptosis [152]. Several preclinical studies in rodent models of acute SCI have collectively demonstrated that minocycline induces increased expression of anti-inflammatory cytokines (i.e., IL-10), decreased expression of several proinflammatory cytokines (i.e., NO, TNF-α, IL-1β, and IL-6), while inhibiting recruitment and activation of microglia [153,154,155,156]. Together, these actions effectively dampen the inflammatory response, resulting in markedly improved functional outcomes and smaller lesion sizes.
A phase 2 clinical trial in Canada evaluated the efficacy of intravenously delivered minocycline to patients with cervical or thoracic SCI within 12 h of injury and continued for 7 days [126,127]. Patients additionally underwent DS within 24 h of primary injury. Strikingly, patients with cervical injuries given minocycline experienced an average 14-point improvement in motor score, while patients with thoracic injuries given minocycline experienced no significant difference in functional recovery when compared to patients in the control group with DS only. While the study had a relatively small sample size, results suggest that minocycline therapy may be beneficial to SCI patients suffering from cervical SCI. These findings motivated a phase 3 trial, initiated in 2013, which used the same paradigm for treatment as the phase 2 trial, but focused on patients with acute cervical SCI (AIS A-D) [128]. The study was expected to reach completion in 2018, but its status has not been updated since 2014 and the current status is unknown.
Like minocycline, azithromycin has been approved by the FDA for use as an antibiotic drug. Beyond its antibiotic function, azithromycin can induce peripheral macrophages, which readily infiltrate lesions in acute SCI, to adopt a pro-regenerative phenotype, sometimes characterized as M2 macrophages [157,158]. Studies in vitro show that azithromycin reduces the expression of pro-inflammatory cytokines (i.e., IL-12, IL-6, IL-1β, TNF-α, and NO) [90,159,160,161,162,163]. Preclinical studies using mouse models of thoracic contusion have demonstrated that azithromycin treatment for 7 days, starting within 30 min of SCI, increased the presence of M2-type macrophages near lesions in a dose-dependent manner and led to improved tissue sparing and recovery of locomotor function [159,164].
More recently, studies in a rat thoracic contusion model showed that azithromycin delivered at 30 min post-SCI and continued daily for 3 days results in significantly improved locomotion, decreased mechanical sensitivity, and decreased thermal allodynia [165]. In addition, this study corroborated previous in vitro and in vivo results [157,158,159,160,162,164], demonstrating that azithromycin treatment significantly altered the lesion microenvironment, as evidenced by decreased levels of TNF-α, increased levels of IL-10, increased numbers of M2 macrophages, and decreased presence of M1 macrophages. Although no clinical trial data are available, this shift from a pro-inflammatory to a pro-regenerative microenvironment has been associated with improved outcomes in SCI patients [159,164,165]. Given the strong preclinical evidence and previous FDA approval, azithromycin is ripe for translation to clinical trials for treatment of acute SCI.
4.3. Reducing Neuroexcitation Toxicity
Following an SCI, excitatory neurotransmitters released from damaged axons increase electrical activation of adjacent neurons, flooding their intracellular compartments with ions such as Na+ and Ca2+ [166]. This influx of excitatory species induces rapid firing and ultimately leads to neuronal cell death, in a process referred to as excitotoxicity [166,167,168]. Another mode of excitotoxicity stems from the injury causing metabolic changes and membrane damage, such that a neuron’s membrane potential can no longer be appropriately regulated. As a result, excess intracellular Ca2+, which broadly activates calcium-dependent enzymes with widespread downstream activities, then leads to structural and functional damages within the neuron [169]. This section discusses small-molecule drugs thought to confer neuroprotection through reduction in excitatory neurotoxicity, one of the major drivers of secondary injury following SCI.
Riluzole is a benzothiazole currently FDA-approved for the treatment of amyotrophic lateral sclerosis (ALS). Riluzole has many potential mechanisms of action, but generally functions to decrease neuroexcitation [170,171,172,173]. In a rodent model of compressive cervical SCI, daily riluzole administration for 7 weeks after injury improved motor recovery and attenuated neuropathic pain at 8 weeks after injury when compared to control animals [174]. Further analysis revealed decreased activation of the NMDA (N-methyl-D-aspartic acid) receptor for the neurotransmitter glutamine in astrocytes near lesions in riluzole-treated animals, suggesting that astrocytes experienced less glutamatergic overexcitation. Furthermore, riluzole treatment diminished microglia activation, which the authors posit may be a result of protection of neurons and glia from excitotoxicity and apoptosis. Despite these positive results, a separate study compared therapeutic effects of riluzole, TH, and glibenclamide, each administered 4 h after cervical contusion in rodents, and found that, while riluzole was beneficial, both TH and glibenclamide were more effective at decreasing lesion volume and mitigating the extent of motor deficits [175].
Given the relatively large differences in responses to SCI and the immune systems between rodents and humans, effects in human trials may be very different [176]. A prospective multicenter phase 1 trial completed in April 2012 was the first in the US to administer riluzole to SCI patients, including both cervical and thoracic injuries [131]. Patients with cervical SCI who received riluzole orally every 12 h for 14 days after SCI experienced robust improvements in functional grades that exceeded historical patient data [129]. While these benefits were not observed in thoracic SCI patients, this may be due to the low enrollment rate of these patients. Interestingly, when compared to data from ALS patients, SCI patients had substantially lower effective plasma concentrations of riluzole, indicating that the administered dosage may need to be increased to maintain an effective dose [177]. If benefits for SCI are dose-dependent, treatment efficacy may be improved with higher doses of riluzole [130]. These exciting findings led to a multicenter, randomized, placebo-controlled, double-blinded, phase 2/3 clinical trial of riluzole for acute cervical SCI in the US, initiated in October 2013 [132,133]. Unfortunately, the trial was terminated early due to poor enrollment and no results have been published.
4.4. Biologics Promoting Angiogenesis and Neurogenesis
Many researchers have posited that an effective treatment for SCI will require a combination of actions, including promoting vascularization and inflammatory resolution [149,178]. Blood vessels in the spinal cord, compromised by the initial physical SCI and subsequent edema, create an ischemic environment, and contribute to secondary injury. Sovateltide, also known as IRL-1620, SPI-1620, or PMZ-1620, is a synthetic endothelin-B receptor agonist used to combat ischemic injury. Activation of endothelin-B receptors, expressed by vascular endothelium, induces secretion of nitric oxide, which causes vasodilation [179,180]. In a rodent model of cerebral stroke (middle cerebral artery occlusion), sovateltide administered in the acute phase of injury enhances angiogenesis, neuronal survival, and neurogenesis, while reducing oxidative stress [180,181,182]. Together, these effects are likely responsible for the observed reductions in infarct size and loss of neurological functions [181].
Promising results in preclinical studies have motivated human trials. An interim report of a recently completed prospective, multicentric, randomized, double-blinded, placebo-controlled clinical trial run by Pharmazz, Inc. in India found that sovateltide, administered as an intravenous bolus at 1, 3, and 6 days after an acute cerebral ischemic stroke, was well tolerated and significantly improved patient outcomes [183,184]. Pharmazz, Inc. is now recruiting for a phase 2 trial in India evaluating effects of an equivalent dosing regimen of sovateltide after acute injury, specifically for partial SCI (C5-S5) where no vertebral fracture has occurred [134].
Granulocyte colony stimulating factor (G-CSF), also known as filgrastim, is a growth factor. It is used clinically as a pegylated version of recombinant human G-CSF, produced in a mammalian cell system, to stimulate white blood cell production. In vitro, G-CSF has been shown to protect primary cortical neurons against excitotoxicity induced by glutamate [185]. In various animal models of CNS injury, G-CSF has been found to stimulate angiogenesis and neurogenesis, reduce inflammation and scar formation, and improve tissue sparing, presumably leading to functional recovery [186,187,188,189,190]. A series of clinical trials in Japan determined that an intravenous dosage of 10 μg/kg/day of G-CSF within 48 h of cervical or thoracic SCI and continuing for 5 consecutive days was safe [191]. At a one-year follow-up, 15 out of the 17 patients who received G-CSF experienced improvements to their overall AIS, compared to only 9 out of 24 control patients [192]. However, patient cohorts were determined based on the institute in which the patients were treated, rather than in a random manner, and control patients did not receive placebo injections [192]. More recently, the same research group published results of a prospective, multicenter, randomized, double-blinded, placebo-controlled comparative study of G-CSF treatment for acute cervical (C4-C7) SCI (AIS B and C) in 26 patients, reporting no significant differences in functional improvement between patients in the G-CSF and control groups 3 months after SCI [136]. However, they did report a trend towards better outcomes in patients who received G-CSF 6 months after SCI, indicating that G-CSF may have some late-term benefits [135,193].
5. Cell-Based Therapies
Many studies have focused on developing cell-based therapies for SCI, several of which have been investigated in clinical trials of patients with acute and chronic SCI. Among these are multipotent stem cells and differentiated glia. While cell therapies may benefit SCI repair in several ways, the expected beneficial mechanisms can be categorized into two broad categories: (1) mitigation of secondary injury through secretion of neurotrophic and anti-inflammatory factors, which improves tissue sparing and establishes an environment conducive to regeneration, and (2) remyelination and functional engraftment into host neural circuitry, which represents true tissue regeneration. While the latter goal of tissue regeneration is obviously highly desirable and may be necessary to adequately treat chronic SCI, many barriers remain for translation of this strategy, including poor engraftment, extensive apoptosis, and inappropriate differentiation with potential for tumorigenesis of transplanted cells [194,195]. In contrast, leveraging the anti-inflammatory properties of the secretome of cells transplanted into an acute SCI environment likely will be easier to translate to clinical practice, given that these cells only need to live for a relatively short period of time and do not need to differentiate or form functional synaptic connections to be therapeutically effective. The latter goal is the focus of this section.
Researchers have explored transplantation of cells sourced from various tissues, at various stages of differentiation, and treated with various ex vivo protocols to identify cell sources and biomanufacturing methods that produce effective therapeutics for acute SCI. While autologous sources, such as from induced pluripotent stem cells (iPSCs), Schwann cells (SCs), or mesenchymal stem cells (MSCs), would avoid potential complications from immune-rejection, variation of these cells across patients and the economic costs of personalized biomanufacturing are difficulties that may be avoided by development of a single, therapeutic cell line from an allogeneic source, such as neural stem/progenitor cells (NS/PCs) derived from an established embryonic stem cell line. Furthermore, autologous cell sources may not be feasible for older individuals, whose cells are likely less proliferative and may not retain sufficient plasticity. Culture, expansion, and maintenance of therapeutic cells also present challenges. For example, culture methods must eliminate animal products, such as serum, to avoid immunogenicity after cell transplantation [196,197,198].
Additional challenges include the timing, injection location relative to the injury, and method of stem cell delivery. For example, earlier administration (e.g., within 48 h of primary SCI) may be more effective, but the preparation of autologous cells is time intensive. The location and method of transplantation are both key determinants of how well transplanted cells survive. Cells injected into the spinal cord tissue adjacent to, rather than directly into, lesions or within biomaterial carriers [199,200,201] had improved survival rates. This section focuses on therapeutic stem cells, derived from a variety of sources, that have been investigated in clinical trials for their ability to reduce secondary injury when used to treat acute SCI. A summary of promising cell-based therapies can be seen in Table 3.

Table 3.
Clinical Studies of Cell-Based Therapies.
5.1. Mesenchymal Stem Cells
MSCs are self-renewing, multipotent cells that can be derived from multiple sources, including umbilical cord (UC-MSCs) or bone marrow (BM-MSCs). MSCs are typically associated with their capacity to generate cells comprising connective tissues, fat, skeletal and cardiac muscle, and cells of the immune system, such as macrophages [233,234]. However, given the proper cues, they can also readily differentiate into neurons and/or glial cells in vitro [235,236] and in vivo [237,238]. MSC administration in cases of acute or subacute SCI has been found in phase 1 clinical trials to be well tolerated in both the short and long terms [239,240,241,242]. In general, MSCs are given in three or four doses over 1–12 weeks in clinical trials. With regard to treatment efficacy, several studies have reported notable benefits to individual patients. However, two recent meta-analyses of human trials found modest improvements in AIS sensory scores, but not AIS motor scores [242,243].
The therapeutic benefits of MSCs have been attributed primarily to secretion of anti-inflammatory and proregenerative proteins and extracellular vesicles near the injured tissue, rather than to the differentiation of transplanted MSCs into new, functional tissues [244,245]. Therapeutic MSCs have been administered via intrathecal, intraspinal, and intravenous injection [246,247]. While intrathecal and intraspinal injections likely result in a greater number of transplanted cells reaching the injury site, intravenous delivery is less risky and invasive for the patient. However, intravenous delivery requires MSCs to travel from the systemic circulation, through the BSCB, into the injury site. While there is abundant evidence that MSCs respond to factors secreted in injured tissue and will travel towards the injury in response, intravenous administration may be limited by the BSCB. As the BSCB heals, it may prevent MSC entry into the CNS [248].
MSCs can be used for autologous (isolated directly from the patient) or allogeneic (isolated from a separate individual) transplantation. For autologous use, UC-MSCs would need to be banked at birth for later in life, while BM-MSCs can be isolated from adult patients at the time of need. MSCs can also be derived from adipose tissues (AD-MSCs) for autologous transplantation. While we have not found any clinical trial results evaluating administration of AD-MSCs in patients with subacute SCI, there are ongoing phase 1 [203] and phase 2 [204] clinical trials in the US for chronic SCI with estimated completion dates in 2023 and 2024, respectively. Initial reports of the patients from the phase 1 trial showed no severe adverse events associated with AD-MSC delivery at 3-, 6-, 12-, and 18 months post-injection [202,203]. In a rat model of contusive, thoracic-level SCI, AD-MSCs or UC-MSCs were transplanted intrathecally during the subacute phase of injury. Both types of MSCs provided similar benefits by modulating the immune response to be more amenable to axon regrowth [249]. However, AD-MSCs and UC-MSCs had unique cytokine and gene expression profiles, suggesting they differ in their mechanisms of action.
5.1.1. UC-MSCs
UC-MSCs are non-invasively and readily accessible immediately after birth in the Wharton’s jelly, a gelatinous tissue within the umbilical cord [250]. Autologous transplantation may be a viable strategy in the future if UC-MSC isolation and banking become standard practice. Allogeneic sources are also reasonable, given that UC-MSCs exhibit limited immunogenicity through low expression of MHC type II and co-stimulatory molecules [251,252,253]. While previously the umbilical cord was considered as waste tissue after birth, ethical concerns with allogenic transplantation eventually may require a similar infrastructure to organ donation. Notably, UC-MSCs have secretomes whose composition is unique from that of MSCs sourced from other tissues. UC-MSCs have a higher proliferation capacity and potentially lower immunogenicity when compared to other MSC sources [251,253,254] and are thought to induce a shift from a proinflammatory tissue microenvironment to a pro-regenerative one [255,256,257,258]. Together, the immunomodulatory properties of UC-MSCs make them an attractive therapeutic candidate for acute SCI, where they would be expected to reduce the negative effects of secondary injury.
A handful of small-scale clinical trials in China have established the safety and, tentatively, efficacy of UC-MSC transplantation into the acutely injured spinal cord, e.g., intrathecally or under the arachnoid membrane [205,206,259]. No adverse events have been reported, and several patients experienced sensory or motor improvements after treatment. A recently completed randomized, double-blinded, placebo-controlled, phase 1/2a clinical trial [209] in Spain showed that intrathecal UC-MSCs can improve pinprick sensations in dermatomes below the injury level when compared to controls [208]. While most completed clinical trials have focused on patients with chronic SCI and do not provide any information on how UC-MSCs may provide immunomodulation in acute SCI to mediate recovery, one phase 1/2 clinical trial giving UC-MSC injections monthly for 4 months in subacute or chronic cervical, thoracic, or thoracolumbar SCI patients resulted in dramatic improvements in motor control, bowel/bladder function, and light touch sensation that had plateaued by 12 months after final administration, with only mild adverse events such as fevers and headaches after injection [206,207]. Another phase 2 clinical trial to confirm these prior results in subacute SCI is currently recruiting, although the status of this trial has not been updated since April 2019 [210].
Recently, Xiao et al. (2021) demonstrated that injection of extracellular vesicles secreted by UC-MSCs into a T10 clip compression SCI in a rodent model decreased astrocyte activation, increased neuronal sparing, and improved recovery of motor functions [244]. They further found that repair was mediated by miR-29b-3p released from extracellular vesicles, which inhibited PTEN activation to upregulate the Akt/mTOR pathway. Use of extracellular vesicles, rather than the original UC-MSC source, is an attractive route towards cell-free therapies that could replace cell transplantation.
5.1.2. BM-MSCs
Bone marrow is a rich source of MSCs in adults. Bone marrow transplantations have been standard clinical practice for decades for the treatment of immunological diseases, including leukemia, establishing that BM-MSCs are safe for human transplantation. However, bone marrow harvesting is an invasive procedure, especially if compared to procedures such as UC-MSC isolation. A phase 1 clinical trial in Pakistan has demonstrated safe repeated administration (typically two to three doses within a one-month span) of autologous BM-MSCs through intrathecal injections in subacute and chronic SCI patients [213]. While the study sizes were small and without placebo controls, several individual patients showed slight-to-modest improvements in motor and sensory functions in follow-up examinations, which typically took place 6–12 months after treatment without any treatment-related adverse events [212]. Currently, there are two phase 1 clinical trials in Jordan, which compared effectiveness for SCI treatment of intrathecal injection of BM-MSCs to that of AD-MSCs [214] or UC-MSCs [215] with results pending.
Despite promising results, efficacy of BM-MSC therapy has not been widely demonstrated. However, it is apparent from the pilot and phase 1/2 clinical data available that BM-MSC administration may be most beneficial when performed during acute and subacute, rather than chronic, SCI cases. In this study, most subacute SCI patients showed motor and sensory improvements 3 months after cell delivery, while only 1 out of 13 chronic SCI patients showed improvements [211,260]. Thus, BM-MSC secretion of immunomodulatory and neurotrophic factors, which can reduce effects of secondary injury, may be responsible for these benefits rather than by true regeneration. In the future, timing and method of BM-MSC administration will likely require further optimization to yield greater and more consistent benefits for SCI patients.
Athersys, Inc. has developed a BM-MSC-derived cell product of multipotent adult progenitor cells (MAPCs) for allogenic transplantation, termed MultiStem® MAPCs, which represent the non-hematopoietic stem cells present in bone marrow stroma. Compared to BM-MSCs, MAPCs have a broader differentiation potential and are less susceptible to senescence in culture [261,262,263]. Phase 1 and 2 clinical trials have shown intravenous infusion of MultiStem® to be both safe and effective in cases of ischemic stroke [264,265]. Therapeutic effects were greater when cells were administered closer to the time of ischemic injury, indicating these effects were due to mitigation of secondary injury. While there have yet to be clinical trials exploring MAPCs for SCI, Depaul et al. investigated intravenous administration of MAPCs immediately following a moderate, contusive SCI at T8 in rats and reported significant gains in motor function with MAPC treatment [266]. However, they also reported that few MAPCs were found in the spinal cord and that most actually trafficked to the spleen. Thus, it is possible that intravenously delivered cells induce a peripheral immune response that limits inflammation in the CNS, similar to the mechanism by which acutely and intravenously administered synthetic nanoparticles have been shown to improve recovery after SCI [267].
5.2. Neural Stem/Progenitor Cells
NS/PCs, which have the potential to differentiate into all cell types present in the adult CNS, are often categorized by their degree of plasticity. NS/PCs include multipotent cells, which can become neurons or glia, and lineage-restricted neural or oligodendroglial progenitors. Therapeutic NS/PCs can be obtained from both allogenic sources, as with cell lines generated from fetal brain or spinal cord tissue or in vitro differentiation of an established ESC line, and autologous sources, as with iPSC-derived NS/PCs. Several researchers have explored the ability of NS/PCs to regenerate tissues in chronic SCI in animal models [268,269,270,271,272]. These studies aimed to replace lost neural tissue or develop relay circuits by transplanting NS/PCs. However, this section focuses on the use of NS/PCs to modulate the neuroinflammatory environment during acute SCI to improve functional recovery. Like MSCs, NS/PCs secrete an assortment of factors with immunomodulatory and neuroprotective effects [273,274,275], making NS/PC administration during acute SCI an attractive strategy to reduce secondary injury [276,277].
5.2.1. Multipotent Neural Stem/Progenitor Cells and Neuronal Progenitor Cells
Several preclinical animal studies have reported that NS/PCs transplanted during subacute SCI reduces inflammatory activation of glial and immune cells [205,274,278,279]. A 2020 study found that exosomes derived from NS/PCs (isolated from fetal mouse cortex) improved functional recovery after SCI in rodents, presumably due to enhanced angiogenesis near the injury site [273]. Another preclinical study found that human iPSC-derived NS/PCs promoted functional recovery in mice with acute SCI [280]. Similarly, allogeneic NS/PCs, derived from ESCs, have been found to promote functional recovery in nonhuman primates after SCI [281].
However, results from preclinical studies have been largely inconsistent and not all studies found that reduced inflammation after acute/subacute NS/PC transplantation corresponded to gains in motor functions [282]. Differences in the NS/PC source, timing of injections, and location of injections may all affect outcomes [283,284]. For example, administration directly into, rather than adjacent to, the injury site, may result in extensive death of transplanted NS/PCs and reduced therapeutic benefit. However, one study suggested that proximity of administration and injury sites does not have an effect on transplanted NS/PC survival, as long as a threshold number of cells were delivered [284]. Thus, NS/PCs could feasibly be grafted into the lesion epicenter to avoid further damaging adjacent rostral and caudal tissues at the time of injection. Injection location concerns are at least partially mitigated by the observed migration of NS/PCs from an administration site into the injury site a few millimeters away [194,285,286].
In a chronic SCI model, transplanted human iPSC-derived NS/PCs differentiated into neurons and glia, but did not restore function [287], further indicating that one major therapeutic benefit of NS/PC transplantation is through early immunomodulation rather than through delayed regeneration. Currently, a few phase 1/2 clinical trials using ESC-derived NS/PCs are recruiting or completed with results yet to be published [216,217,218]. For example, there is an ongoing study led by Yonsei University Health System in Korea to evaluate effects of human ESC-derived NCAM+ NS/PCs, transplanted during subacute, cervical SCI [218]. There is currently one iPSC-derived NS/PC trial in Japan for subacute complete SCI that is recruiting [219]. As iPSC technology is relatively new and mostly in preclinical trials currently, we expect many more iPSC-derived NS/PCs to enter clinical trials in the near future, once production, safety, and efficacy are standardized in the preclinical setting.
5.2.2. Oligodendrocyte Progenitor Cells
In contrast to multipotent NS/PCs and NS/PCs, OPCs are currently being evaluated in clinical trials as a therapy for acute SCI [221,223,288,289]. Preclinical studies in thoracic and cervical SCI models in rodents have reported that acutely administered OPCs secrete neuroprotective factors [290,291], reduce cavitation [289,292], and mediate functional recovery [289,293]. Endogenous NS/PCs in the spinal cord, specifically characterized as olig2+ OPCs, promote repair after SCI [294,295]. Furthermore, in rodent models, impressive motor recovery has been observed when transplanted NS/PCs differentiate into myelinating oligodendrocytes [293,296,297]. Together, these observations and the lower potential for OPCs to form tumors when compared to multipotent NS/PCs, presumably because they can be prepared as a more mature and pure population, have justified a push towards clinical translation of therapeutic OPCs [288,289].
Recently, this charge has been led by Lineage Cell Therapeutics, Inc. (Carlsbad, CA, USA), which developed a therapeutic cell line for allogeneic transplantation into the spinal cord derived from human ESCs, known as AST-OPC1. Phase 1 and 2 clinical trials conducted during the past decade in the US have aimed to evaluate the safety and efficacy of AST-OPC1 administration to patients with subacute (21–42 days following SCI) SCI (AIS A or B) [221,223]. Patients were given low level immunosuppressive therapy for 60 days after cell transplantation to prevent immunogenic rejection. GRNOPC1s, an earlier name for the AST-OPC1 lineage, showed no unanticipated serious adverse effects in 49 of 50 annual visits for the first 10 years following transplantation in thoracic patients [220], whereas AST-OPC1s resulted in 21 of 22 cervical patients recovering one or more levels of neurological function at 1-year follow up [222]. Earlier in 2021, Lineage Cell Therapeutics, Inc. announced its intention to submit an Investigational New Drug application to the FDA to set the stage for a new phase 1 clinical trial investigating AST-OPC1 delivery using Neurgain Technologies’ Parenchymal Spinal Delivery System [298], a compact device consisting of a micromanipulator and a disposable magnetic needle assembly. Neurgain’s system is expected to enable delivery of cells to the cervical spinal cord without the need to stop the patient’s respiration, provide a more accurate dosing and location of injected cells, and facilitate off-the-shelf availability of freshly thawed AST-OPC1 cells [299]. The hope is that a more precise delivery strategy may improve therapeutic efficacy to a point at which instigating a phase 3 clinical trial would be justified.
5.3. Glial Cells
Glia cells provide extensive trophic support to neurons in the healthy nervous system, making them good candidates for a neuroprotective therapy if transplanted during acute or subacute SCI. While we have not found any clinical trials evaluating astrocyte transplantation to date, preclinical trials have worked to identify specific populations of non-reactive astrocytes that can improve functional outcomes after SCI [300]. However, as glia originating in the CNS, which include astrocytes and oligodendrocytes, are more difficult to isolate and culture, development of glial cell therapies has been largely focused on peripherally derived glia, including olfactory ensheathing cells (OECs) and SCs that myelinate axons in the olfactory bulb and peripheral nerves, respectively. OECs have the advantage of being relatively non-invasive to isolate from a patient for autologous transplantation. OECs can also be isolated from fetal or donor organ tissues for allogenic transplantation [301,302]. While SCs can be harvested from a peripheral nerve, a neurological defect is left at the site of harvest. In addition, obtaining enough autologous SCs for transplantation from older individuals presents a significant limitation to clinical translation [303]. Alternatively, allogenic SCs can be obtained from organ donors or cadaveric tissues and cryopreserved [304,305].
5.3.1. Olfactory Ensheathing Cells
OECs insulate axons in the nasal mucosa and secrete factors that support neuronal function [306]. In rodent models of SCI, acute transplantation of OECs near or into lesions has been reported to reduce inflammation, improve tissue sparing, and enhance behavioral recovery, indicating that OECs can mitigate some effects of secondary injury [307,308,309]. Alternatively, intravenously delivered OECs can traffic to SCI lesions in rats within 10 min of the injection, resulting in a reduced SCI inflammatory response, increased neurogenesis and remyelination, and improved motor function [310]. The first human trial of OEC administration for SCI evaluated patients with chronic injuries [311]. Over the past two decades, several other phase 1/2 clinical trials have evaluated transplantation of OECs from various sources, including cells derived from the SCI patient and human fetal tissues, in chronic SCI [224,225,226]. However, a meta-analysis of clinical trials in 2015 determined, while OEC transplantation was safe overall, there was no evidence to support therapeutic efficacy [312]. An ongoing clinical study is evaluating isolation of OECs from both deceased and living donors and their effects on SCI after allogenic transplantation [227]. Still, clinical trials of OEC transplantation in acute SCI have not been performed to date, possibly due to the time-intensive nature of retrieving and preparing these cells for transplantation.
5.3.2. Schwann Cells
SCs are the glia responsible for myelination of axons in the peripheral nervous system (PNS) and are not normally present in the CNS. After SCI, endogenous SCs cross the compromised BSCB and enter the spinal cord, where they have been observed to remyelinate CNS axons, at least in rodents and dogs [313,314]. Preclinical studies in rodents have shown that transplantation of SCs into acute and subacute SCI reduces inflammation, improves tissue sparing, and increases axon myelination [309,315,316]. There is evidence from rodent studies that SC transplants outperform OEC and mixed SC/OEC transplants [315]. In contrast, a small clinical study reported no differences between patients receiving SCs, OECs, or SC/OEC mixtures [317]. However, all patients receiving cell transplants had improved functional recovery over patients in control groups.
As with other cell-based therapies, SCs secrete trophic factors that can promote survival and prevent “die back” of axons [318]. Neuroprotection during secondary injury has been widely proposed as a mechanism for beneficial effects of SC transplantation in SCI [315,319]. Additionally, SCs secrete ECM that is supportive of axonal growth, which may be key to their benefits. For example, human SCs transplanted into injured rodent spinal cords modify the glial scar, creating a tissue microenvironment conducive to axonal crossing [305]. As with other cell-based therapies, it is possible that acellular biological products, such as SC-derived exosomes, may provide comparable benefits to transplantation of living cells, while avoiding many of the complications associated with therapeutic cells, including reproducibility, plasticity, and immunogenicity [320].
Most clinical studies to date have transplanted autologous SCs isolated from the patient’s own sural nerve (an easily accessible sensory nerve in the lower leg) and expanded in vitro [231,321]. While allogenic cells potentially can be isolated from cadaveric tissue or potentially live donors or obtained from cultured clinical grade cell lines, use of autologous cells has the advantages of a less burdensome regulatory pathway and decreased costs [303,322]. Human trials have shown that a previous SCI does not hinder the isolation of high quality SCs from their sural nerve for sub-culture, cryopreservation, and processing for transplantation [198,323]. However, minimal rounds of sub-culturing are desirable, as expansion may reduce the myelination potential of transplanted SCs [324,325]. In a previous clinical trial, the average time from sural nerve harvest to SC transplantation in patients was around 26 days, but only yielded enough cells to allow for one round of SC transplantation [231,232].
Of note, it has been suggested that patient age may be a significant factor in determining efficacy, as SCs isolated from older individuals may exhibit variable phenotypes [303]. While SCs can be isolated from patients of all ages with similar yields of viable cells, proliferation is slower in SCs from older individuals, which may increase the time needed for expansion prior to transplantation [304,305]. A trial evaluating SC transplantation in humans was performed in Iran and published in 2008 [229]. Four patients with chronic, mid-thoracic SCI (AIS A-C) received transplants of autologous SCs isolated from sural nerve. While only one patient showed functional improvement one year after treatment, no adverse effects were found providing initial confidence in safety. In 2012, a 5-year follow-up study of six patients with chronic injuries (AIS A-C) in China confirmed the safety of transplantation of SCs, again from autologous sural nerves, and reported at least some functional improvements in all patients [230].
As with the other cell types discussed, a prevailing theory is that a major barrier to therapeutic efficacy is excessive loss of SCs after transplantation, likely through necrosis due to inflammatory or immunogenic processes [195,305,326]. Thus, researchers at The Miami Project conducted phase 1 clinical trials in the US investigating isolation of autologous SCs from sural nerves followed by transplantation into the injury epicenter using a cell injection system designed to gently deliver cells to the spinal cord [327]. Autologous SCs were transplanted in patients with subacute (within 30 days of injury) [231,232] or chronic [328,329] thoracic SCI. In the subacute study of six patients and the chronic study of eight patients, no post-transplantation adverse events or serious complications were reported at 1 year or 6–24 months, respectively. However, no strong evidence of efficacy was reported either [231,328].
6. Combinatorial Strategies
The consensus is that a multi-pronged approach is needed to prevent tissue loss and promote tissue regeneration in the injured spinal cord. For example, in rodent models, delivery of an immunomodulatory factor (e.g., MSSP) in combination with a neuroprotective factor (e.g., DS, growth factors, stem cells) provided additional benefits over either factor alone [330]. Moreover, several research groups have reported summative benefits of combining multiple therapeutic modalities after SCI, for example by transplanting stem cells in conjunction with small molecule drugs or biomaterial scaffolds to improve survival and promote differentiation of stem cell grafts [331]. A few small-scale clinical trials have begun to explore various combinatorial strategies to treat acute SCI. A summary of promising combinatorial strategies can be seen in Table 4.
A relatively straight-forward combinatorial approach is to perform DS at the same time as cell transplantation since the neurosurgeon may already have to open the dural/pial membranes to perform the dural DS. A phase 1/2 clinical trial in Vietnam divided acute and early subacute SCI patients within 2 weeks of injury, into two study arms: (1) patients receiving DS only and (2) patients receiving DS followed immediately by injection of autologous AD-MSCs into the intrathecal space just above and below lesions [332,333]. For patients in the treatment group, additional and escalating dosages of AD-MSCs, such that more cells were delivered with the fourth than the second injection, were administered via lumbar puncture at 30, 45, and 75 days after DS. Patients who received AD-MSCs showed improvements in all measures at the 3- and 6-month follow-up examinations when compared to before treatment. The only metric that was compared between treatment and control groups was AIS scores, which showed that AD-MSC transplantation with DS resulted in almost double the AIS improvement when compared to DS only patients. In an ongoing clinical trial in Spain, a therapeutic cell line derived from allogenic AD-MSCs (FAB117-HC) is being administered to patients within 96 h of SCI through intramedullary injection at the same time as DS [334]. A second and larger dose of FAB177-HC is then administered up to 120 h post-SCI during stabilization surgery. Importantly, this trial includes both dose escalation and efficacy studies, the latter of which have been designed to compare patients receiving therapeutic cells to those in a control group. The primary outcome measures are expected to be completed in July 2022.
Combining stem cell transplants with rehabilitation strategies, such as use of an exoskeleton, virtual reality training, or other locomotive training, has also been explored in clinical trials in the US [335,336]. Theoretically, secreted factors from therapeutic cells would act to preserve tissue during secondary injury, while rehabilitation encourages both regenerating axons and, potentially, induces new neurons differentiated from transplanted cells to make functional connections and networks. Systemic administration of drug therapies in conjunction with stem cell transplantation may also provide additive or synergistic clinical benefits. For example, phase 1/2 clinical trials in Korea have investigated treatment with both BM-MSC transplants and G-CSF [260,337]. As discussed earlier, G-CSF has positive effects on SCI, potentially including its ability to stimulate endogenous stem cells [338,339]. Clinical trials found that co-treatment with autologous BM-MSCs and G-CSF was safe [337] and led to functional benefits when administered within 2 weeks or within 3–8, but not greater than 8, weeks after SCI, indicating that these benefits arise from attenuation of secondary injury and neuroprotection rather than actual tissue regeneration [260].
Beyond co-delivery of cells and various small molecule drugs, cells can be genetically modified prior to transplantation to overproduce therapeutic biomolecules or otherwise direct cell behavior. For example, several preclinical studies have shown that transplantation of genetically engineered cells to overexpress neurotrophic factors can improve SCI outcomes over non-modified cells [340,341,342,343]. This is advantageous over direct protein or exosome delivery, which have a much shorter half-life after delivery [344]. Alternatively, stem cells can be genetically engineered to drive differentiation into a particular cell type. A recent study reported that, when transplanted acutely after SCI in a rat model, NS/PCs (isolated from E14 rat cortices) transduced to overexpress Wnt5a resulted in significant gains in functional improvement over non-transduced cells [345]. The authors suggest that these additional benefits were a result of increased neuronal differentiation of transplanted NS/PCs with Wnt5a overexpression.

Table 4.
Clinical Studies of Combinatorial Therapeutics.
Table 4.
Clinical Studies of Combinatorial Therapeutics.
| Intervention | Study Size/Type | Results | Reference |
|---|---|---|---|
| DS + Cell Transplant | N = 47 Phase 1/2 | In acute and early subacute thoracic SCI patients, combination of DS and local intrathecal AD-MSCs transplantation showed improvement in all measures (AIS motor grade, electrophysiological parameters, SCI site edema, and urinary and bowel function) at 3- and 6-month follow-up examinations. AIS scores doubled in the combination group when compared to DS-only. | Tien et al. (2019) [333] NCT02034669 [332] |
| Phase 1/2 | Recruiting. Study ongoing. | NCT02917291 [334] | |
| Cell Transplant + Rehabilitation | Phase 2 | Recruiting. Study ongoing. | NCT03979742 [336] |
| Cell Transplant + Drug Therapies | N = 6 Cohort | No serious complications were found with co-treatment with autologous BM-MSCs and G-CSF in six patients with complete subacute cervical SCI. | H. C. Park et al. (2005) [337] |
| N = 35 Phase 1/2 | Co-treatment with autologous BM-MSCs and G-CSF led to functional benefits seemingly through attenuation of secondary injury. Administration within 2 weeks or within 3–8 weeks, but not >8 weeks showed benefits when compared to decompression alone. | Yoon et al. (2007) [260] | |
| Biomaterial-Mediated Cell Transplant | N = 8 Phase 1 | Combined application of NeuroRegen collagen scaffold and UC-MSCs is safe and feasible for clinical therapy in complete chronic cervical or thoracic SCI patients. Additionally, promising sensory-motor improvements measured at 1 year follow-up of the treatment group suggest the potential efficacy of this therapy combination. | Zhao et al. (2017) [346] NCT02352077 [347] |
| N = 7 Phase 1 | BM-MSC loaded NeuroRegen collagen scaffold is a safe therapy for acute thoracic SCI patients. Minimal sensory and no motor improvements were measured over a 3-year follow-up period. | W. Chen et al. (2020) [348] | |
| N = 40 Phase 1 | Acute administration of bovine collagen scaffold loaded UC-MSCs within 21 days of injury led to improvements in AIS scores, bowel/urinary function, and injury-site electrophysiological activity over the 12-month post-operative period when compared to an intervention-free control group in cervical SCI patients. | W.S. Deng et al. (2020) [252] NCT02510365 [349] | |
| Phase 1/2 | Recruiting. Study ongoing. | NCT03933072 [350] |
Cell transplants can be genetically engineered to express multiple factors with the potential to enhance therapeutic benefits. For example, rat SCs can be engineered to secrete bifunctional neurotrophin (D15A) and chondroitinase ABC (ChABC) [351,352,353,354]. While D15A mimics effects of the neurotrophins BDNF and NT3 [355,356], ChABC is an enzyme that degrades chondroitin sulfate proteoglycans and can thus reduce glial scar deposition. In a preclinical study, transplantation of genetically engineered SCs to overexpress ChABC and D15A directly into the injury site of subacute, thoracic contusions in rats resulted in increased numbers of myelinated axons projecting into descending motor tract fibers, with corresponding improvements in motor function and reductions in thermal and mechanical allodynia [357].
As discussed in Section 5, low transplant survival rates and loss of desired phenotypes are major limitations to cell-based therapies. In preclinical studies, biomaterial scaffolds have been widely investigated as carriers for cell transplants, as detailed in a recent review article [358]. In general, biomaterials have been demonstrated to increase cell survival during [199,359] and after [200,360] injection into the spinal cord, which correlated with improved functional outcomes [361,362,363]. Furthermore, biomaterial scaffolds have been developed that can better maintain cell phenotypes of adhered cells, including MSCs [364], SCs [365], and NS/PCs [366]. Such promising preclinical results have led researchers to initiate clinical trials investigating the safety and efficacy of biomaterial-mediated cell transplantation after SCI.
Several phase 1/2 clinical trials in China have investigated transplantation of therapeutic cells, including UC-MSCs, in scaffolds derived from bovine collagen I [252,346,347,348]. One of the largest of these studies, in which treatment was administered within 21 days of cervical SCI, included two patient cohorts: (1) individuals receiving UC-MSCs on collagen-based scaffolds (N = 20) and (2) individuals receiving no additional intervention (negative control, N = 20) [252,349]. The authors found that patients who received the combinatorial treatment had improvements in AIS scores, bowel/urinary function, and injury-site electrophysiological activity at 12 months post-operation over the control group. Unfortunately, the control group did not receive DS, but they did receive other standard supportive therapies, such as infection control and rehabilitation. Additionally, this study did not provide any evidence as to whether the combinatorial therapy had benefits over either scaffolds or cell-based therapies alone.
Autologous nerve grafts, often derived from the peripheral sural nerve, represent another scaffold with potential to enhance the therapeutic effects of cell transplants after SCI. While not yet approved for clinical use, therapeutic strategies based on providing SCs or SC-laden autologous nerve grafts have been largely effective at treating PNS injuries [365,367]. In a study with a small number of patients with chronic SCI, safety and modest functional improvements were reported with transplantation of autologous sural nerve grafts and OECs [228] or BM-MSCs [368]. Clinical trials investigating transplantation of autologous OECs with sural nerve grafts are currently recruiting in Poland [350]. Alternatively, allogenic nerve grafts can be decellularized to preserve the native ECM content and structure, including aligned tubes that can guide and bundle regenerating axons, but allow for evasion of the immune response to allogenic cells [369,370]. However, while cadaver-derived, decellularized nerve grafts have been used for decades clinically in PNS repair, we have not found any reports of their transplantation into humans after SCI.
7. Conclusions and Future Perspectives
In summarizing clinical trials aimed at limiting secondary damage and maximizing functional recovery for patients with acute/subacute SCI, we have discussed promising therapeutic strategies in four broad categories: (1) early procedural and/or surgical management, (2) pharmacological therapies, (3) cell-based therapies, and (4) combinatorial therapies. While there are specific challenges associated with each treatment being tested (e.g., half-life limitations and off-target effects of pharmacological therapies, or time needed to expand autologous SCs within a time frame appropriate for use in acute SCI), there are also several common challenges. In particular, while several phase 2 clinical trials have been performed, only a few treatments have advanced to phase 3 clinical trials. Specific barriers to clinical translation of therapeutics discussed in this review article are highlighted below.
- (1)
- Recruitment of acute and subacute SCI patients. On average, the rate of recruitment for patients with acute SCI is one patient per center per month [371,372]. This estimate can even be lower depending on the inclusion/exclusion criteria of the study and center location. Several clinical trials discussed in this study were terminated due to low enrollment. Establishing multicenter trials maybe one way to increase the number of eligible patients to be enrolled. However, multicenter trials add logistical and financial challenges. Additionally, for acute SCI patients dealing with a life changing condition, participating in a clinical trial at early timepoints after injury may be a difficult decision. Therefore, it is important that the literature provided to patients is well-developed and that designated clinical research professionals speak to patients and their families to recruit acute SCI patients into clinical trials.
- (2)
- Patient selection. Patient selection is one of the most important factors that can affect the risk–benefit analysis of an intervention and efficacy of measured outcomes, but it presents a constant challenge in clinical trials. Clinical SCI is, by nature, heterogenous in its severity, location, and mode of injury. Therefore, it is essential to determine which patient populations are most likely to benefit from any therapeutic intervention. In the clinical trials reviewed here, therapeutic benefits were usually reported in around 20–30% of the patients. However, many trials included patients with AIS A–D, often with injuries at all levels of the spinal cord lumped together into a single dataset, which makes it difficult to discern whether specific injury characteristics correlated to treatment efficacy. However, this practice is not uncommon as overly restrictive patient selection criteria leads to low recruitment numbers. Therefore, care should be taken to consider all the trade-offs when making inclusion criteria for a clinical study with SCI patients. It will also be important to develop new biomarkers that can be used to stratify SCI patients, such as by evaluating the anatomical and functional deficits within the injured cord using new imaging modalities (e.g., MRI, perfusion CT, ultrasound imaging), instead of relying on neurological grading alone.
- (3)
- Scaling up cell production. Challenges remain regarding the industrial scaling-up and reproducibility of bio manufactured cells for transplantation into humans [373,374]. For example, use of iPSC-derived NS/PCs would require rapid expansion and differentiation to enable administration during the acute or subacute phases of SCI. However, established culture methods used to expand and convert iPSCs to NS/PCs are primarily performed manually by highly trained technicians and, therefore, the ability to generate the large number of cells required for use in clinical therapy is limited. Furthermore, new strategies for quality control during the biomanufacturing process are needed. While previous studies have relied on a handful of biomarkers at a single time point, stem cell populations are often highly heterogenous and dynamically plastic. Thus, the field would benefit from innovative strategies to monitor the regenerative potential of therapeutic cells through dynamic measurements that better indicate their functional activity during biomanufacturing. Additionally, a better understanding of which cells within a heterogeneous population are responsible for the therapeutic benefits, identification of biomarkers for these cells, and methods for enriching populations for cells with the most therapeutic potential will all likely be required for cell-based therapies to become a standard SCI treatment.
- (4)
- Funding and infrastructure. Financial and infrastructural support required to sustain large and long-term clinical trials for SCI patients are limited, in part because the population of patients suffering from SCI is relatively small when compared to patients suffering from conditions such as strokes, though both result in long-lasting neurological deficits. Therefore, more creative and non-traditional approaches, such as working with patient advocacy groups, as well as obtaining support from private foundations in addition to public institutions, are needed to support clinical studies evaluating new therapeutic strategies for patients with SCI.
- (5)
- Low reporting. The lack of published results for many of the already completed clinical trials makes it difficult to draw clear conclusions about therapeutic efficacy. Of the 27 total clinical trials regarding SCIs referenced in this review, only eight were completed with publications. Five are completed with results yet to be published, nine are still recruiting, two have unknown statuses, two were terminated due to slow enrollment, and one was terminated by a business decision. Additionally, most clinical trials with published results were phase 1, and thus designed primarily to investigate treatment safety rather than efficacy.
In summary, early neuroprotective therapies offer exciting opportunities to improve the preservation and recovery of sensory and motor functions after traumatic SCI. However, logistical (patient recruiting, scaling up production of high-quality stem cells for transplantation, funding, low reporting) and pathophysiological (patient heterogeneity and lack of pathophysiological-based biomarker for recruitment) factors remain significant barriers to clinical translation of new spinal cord injury therapies. Key future steps will be to identify new biomarkers based on the pathophysiology of the injured cord to aid in the stratification of patients and to develop strategies for efficient recruiting of patients with spinal cord injuries.
Author Contributions
Z.Z.K., S.K.S., J.Y.C., G.S., S.E., N.P. and R.D.D. wrote the manuscript and T.P., Z.Z.K. and S.K.S. reviewed and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by funding from the NIH R01NS121191 (ZZK), NIH R03AG073929 (ZZK), Craig H. Neilsen Foundation #725788 (ZZK), and NIH R01CA241927 (SKS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spinal Cord Injury. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 31 July 2022).
- Spinal Cord Injury Facts and Figures at a Glance 2020 SCI Data Sheet. Available online: www.msktc.org/sci/model-system-centers (accessed on 1 August 2022).
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular Events after Spinal Cord Injury: Contribution to Secondary Pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Keough, M.B.; Duncan, G.J.; Sparling, J.S.; Yong, V.W.; Stys, P.K.; Tetzlaff, W. Remyelination after spinal cord injury: Is it a target for repair? Prog. Neurobiol. 2014, 117, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Pukos, N.; Goodus, M.; Sahinkaya, F.R.; McTigue, D.M. Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 2019, 67, 2178–2202. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-Astrocyte Metabolic Interactions: Understanding the Transition into Abnormal Astrocytoma Metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Liu, B.; Teschemacher, A.G.; Kasparov, S. Neuroprotective potential of astroglia. J. Neurosci. Res. 2017, 95, 2126–2139. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Li, J.; Wang, K.-F.; Xia, W.-W.; Zhu, Z.-Q.; Wang, C.-R.; Li, X.-F.; Liu, H.-Y. Blood–Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Yip, P.K.; Malaspina, A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012, 7, 6. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Tsering, C.; Grunfeld, S.; Welch, K. Further studies on platelet-mediated neurotoxicity. Brain Res. 1992, 577, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, S.; Kijima, K.; Hara, M.; Saito, T.; Tamaru, T.; Tanaka, M.; Konno, D.-J.; Nakashima, Y.; Okada, S. Tranexamic acid reduces heme cytotoxicity via the TLR4/TNF axis and ameliorates functional recovery after spinal cord injury. J. Neuroinflamm. 2019, 16, 160. [Google Scholar] [CrossRef]
- Hawkins, L.A.; Devitt, A. Current Understanding of the Mechanisms for Clearance of Apoptotic Cells—A Fine Balance. J. Cell Death 2013, 6, JCD-S11037. [Google Scholar] [CrossRef]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.; Franklin, R. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Lee, Y.; Shih, K.; Bao, P.; Ghirnikar, R.; Eng, L. Cytokine chemokine expression in contused rat spinal cord. Neurochem. Int. 2000, 36, 417–425. [Google Scholar] [CrossRef]
- Zendedel, A.; Johann, S.; Mehrabi, S.; Joghataei, M.T.; Hassanzadeh, G.; Kipp, M.; Beyer, C. Activation and Regulation of NLRP3 Inflammasome by Intrathecal Application of SDF-1a in a Spinal Cord Injury Model. Mol. Neurobiol. 2016, 53, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Houghtling, R.A.; MacArthur, L.; Bayer, B.M.; Bregman, B.S. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp. Neurol. 2003, 184, 313–325. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Velumian, A.A.; Fehlings, M.G.; Datto, J.P.; Bastidas, J.C.; Miller, N.L.; Shah, A.K.; Arheart, K.L.; Marcillo, A.E.; Dietrich, W.D.; et al. The Role of Excitotoxicity in Secondary Mechanisms of Spinal Cord Injury: A Review with an Emphasis on the Implications for White Matter Degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]
- Struve, J.; Maher, P.C.; Li, Y.-Q.; Kinney, S.; Fehlings, M.; Kuntz, C.; Sherman, L.S. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia 2005, 52, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 2011, 8, 046033. [Google Scholar] [CrossRef]
- Li, Z.; Yu, S.; Hu, X.; Li, Y.; You, X.; Tian, D.; Cheng, L.; Zheng, M.; Jing, J. Fibrotic Scar After Spinal Cord Injury: Crosstalk with Other Cells, Cellular Origin, Function, and Mechanism. Front. Cell. Neurosci. 2021, 15, 720938. [Google Scholar] [CrossRef]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Dididze, M.; Green, B.A.; Dietrich, W.D.; Vanni, S.; Wang, M.Y.; Levi, A.D. Systemic hypothermia in acute cervical spinal cord injury: A case-controlled study. Spinal Cord 2013, 51, 395–400. [Google Scholar] [CrossRef]
- Hansebout, R.R.; Hansebout, C.R. Local cooling for traumatic spinal cord injury: Outcomes in 20 patients and review of the literature. J. Neurosurg. Spine 2014, 20, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Casella, G.; Green, B.A.; Dietrich, W.D.; Vanni, S.; Jagid, J.; Wang, M.Y. Clinical Outcomes Using Modest Intravascular Hypothermia After Acute Cervical Spinal Cord Injury. Neurosurgery 2010, 66, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.J.; Hogg, F.R.A.; Kearney, S.; Kopp, M.A.; Blex, C.; Serdani, L.; Sherwood, O.; Schwab, J.M.; Zoumprouli, A.; Papadopoulos, M.C.; et al. Effects of local hypothermia–rewarming on physiology, metabolism and inflammation of acutely injured human spinal cord. Sci. Rep. 2020, 10, 8125. [Google Scholar] [CrossRef] [PubMed]
- Hypothermia Following Acute Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01739010 (accessed on 31 July 2022).
- Systemic Hypothermia in Acute Cervical Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02991690 (accessed on 31 July 2022).
- Kwon, B.K.; Curt, A.; Belanger, L.M.; Bernardo, A.; Chan, D.; Markez, J.A.; Gorelik, S.; Slobogean, G.P.; Umedaly, H.; Giffin, M.; et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: A prospective randomized trial—Clinical Article. J. Neurosurg. Spine 2009, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cerebrospinal Fluid (CSF) Drainage and Cytokine Profiling in the Treatment of Acute Spinal Cord Injury (SCI)—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00135278 (accessed on 25 December 2022).
- Cerebrospinal Fluid Drainage (CSFD) in Acute Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02495545 (accessed on 31 July 2022).
- Haghnegahdar, A.; Behjat, R.; Saadat, S.; Badhiwala, J.; Farrokhi, M.R.; Niakan, A.; Eghbal, K.; Barzideh, E.; Shahlaee, A.; Ghaffarpasand, F.; et al. A Randomized Controlled Trial of Early versus Late Surgical Decompression for Thoracic and Thoracolumbar Spinal Cord Injury in 73 Patients. Neurotrauma Rep. 2020, 1, 78–87. [Google Scholar] [CrossRef]
- Telemacque, D.; Zhu, F.; Chen, K.; Chen, L.; Ren, Z.; Yao, S.; Qu, Y.; Sun, T.; Guo, X. Method of Decompression by durotomy and duroplasty for cervical spinal cord injury in patients without fracture or dislocation. J. Neurorestoratol. 2018, 6, 158–164. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, S.; Ren, Z.; Telemacque, D.; Qu, Y.; Chen, K.; Yang, F.; Zeng, L.; Guo, X. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: A novel concept and method of surgical decompression. Eur. Spine J. 2019, 28, 2275–2282. [Google Scholar] [CrossRef]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. Can. Med. Assoc. J. 2013, 185, 485–492. [Google Scholar] [CrossRef]
- Hadley, M.N.; Walters, B.C.; Grabb, P.A.; Oyesiku, N.M.; Przybylski, G.J.; Resnick, D.K.; Ryken, T.C. Blood Pressure Management after Acute Spinal Cord Injury. Neurosurgery 2002, 50, S58–S62. [Google Scholar] [CrossRef]
- Streijger, F.; So, K.; Manouchehri, N.; Gheorghe, A.; Okon, E.B.; Chan, R.M.; Ng, B.; Shortt, K.; Sekhon, M.S.; Griesdale, D.E.; et al. A Direct Comparison between Norepinephrine and Phenylephrine for Augmenting Spinal Cord Perfusion in a Porcine Model of Spinal Cord Injury. J. Neurotrauma 2018, 35, 1345–1357. [Google Scholar] [CrossRef]
- Muzevich, K.M.; Voils, S.A. Role of Vasopressor Administration in Patients with Acute Neurologic Injury. Neurocritical Care 2009, 11, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Strebel, S.P.; Steiner, L.A. Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur. J. Anaesthesiol. 2008, 25, 98–103. [Google Scholar] [CrossRef]
- Steiner, L.A.; Johnston, A.J.; Czosnyka, M.; Chatfield, D.A.; Salvador, R.; Coles, J.P.; Gupta, A.K.; Pickard, J.D.; Menon, D.K. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit. Care Med. 2004, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.; Whetstone, W.; Saigal, R.; Ferguson, A.; Talbott, J.; Bresnahan, J.; Dhall, S.; Pan, J.; Beattie, M.; Manley, G. Mean Arterial Blood Pressure Correlates with Neurological Recovery after Human Spinal Cord Injury: Analysis of High Frequency Physiologic Data. J. Neurotrauma 2015, 32, 1958–1967. [Google Scholar] [CrossRef]
- Inoue, T.; Manley, G.T.; Patel, N.; Whetstone, W.D. Medical and Surgical Management after Spinal Cord Injury: Vasopressor Usage, Early Surgerys, and Complications. J. Neurotrauma 2014, 31, 284–291. [Google Scholar] [CrossRef]
- Dakson, A.; Brandman, D.; Thibault-Halman, G.; Christie, S.D. Optimization of the mean arterial pressure and timing of surgical decompression in traumatic spinal cord injury: A retrospective study. Spinal Cord 2017, 55, 1033–1038. [Google Scholar] [CrossRef]
- Ehsanian, R.; Haefeli, J.; Quach, N.; Kosarchuk, J.; Torres, D.; Stuck, E.D.; Endo, J.; Crew, J.D.; Dirlikov, B.; Bresnahan, J.C.; et al. Exploration of surgical blood pressure management and expected motor recovery in individuals with traumatic spinal cord injury. Spinal Cord 2020, 58, 377–386. [Google Scholar] [CrossRef]
- Mushlin, H.M.; Lessing, N.; Wessell, A.P.; Chryssikos, T.; Pratt, N.; Caffes, N.; Oliver, J.; Aarabi, B.; Schwartzbauer, G. The Effect of Elevated Mean Arterial Blood Pressure in Cervical Traumatic Spinal Cord Injury with Hemorrhagic Contusion. World Neurosurg. 2020, 144, e405–e413. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Kalani, M.Y.S.; Bichard, W.D.; Baaj, A.A.; Gonzalez, L.F.; Preul, M.C.; Theodore, N. Cerebrospinal Fluid Drainage and Induced Hypertension Improve Spinal Cord Perfusion After Acute Spinal Cord Injury in Pigs. Neurosurgery 2015, 76, 461–469. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C. Targeted Perfusion Therapy in Spinal Cord Trauma. Neurotherapeutics 2020, 17, 511–521. [Google Scholar] [CrossRef]
- Green, R.S.; Howes, D.W. Stock your emergency department with ice packs: A practical guide to therapeutic hypothermia for survivors of cardiac arrest. CMAJ Can. Med. Assoc. J. 2007, 176, 759–762. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Neugebauer, H.; Schneider, H.; Bösel, J.; Hobohm, C.; Poli, S.; Kollmar, R.; Sobesky, J.; Wolf, S.; Bauer, M.; Tittel, S.; et al. Outcomes of Hypothermia in Addition to Decompressive Hemicraniectomy in Treatment of Malignant Middle Cerebral Artery Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 571–579. [Google Scholar] [CrossRef]
- Lyden, P.D.; Hemmen, T.M.; Grotta, J.; Rapp, K.; Raman, R. Endovascular Therapeutic Hypothermia for Acute Ischemic Stroke: ICTuS 2/3 Protocol. Int. J. Stroke 2013, 9, 117–125. [Google Scholar] [CrossRef]
- Adelson, P.D.; Wisniewski, S.R.; Beca, J.; Brown, S.D.; Bell, M.; Muizelaar, J.P.; Okada, P.; Beers, S.R.; Balasubramani, G.K.; Hirtz, D. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): A phase 3, randomised controlled trial. Lancet Neurol. 2013, 12, 546–553. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. The Effects of Posttraumatic Hypothermia on Diffuse Axonal Injury Following Parasagittal Fluid Percussion Brain Injury in Rats. Ther. Hypothermia Temp. Manag. 2012, 2, 14–23. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Ogilvy, C.S.; Shuer, L.M.; Connolly, E.S.; Solomon, R.A.; Lam, A.; Kassell, N.F.; Baker, C.J.; Giannotta, S.L.; Cockroft, K.M.; et al. Comparison of Endovascular and Surface Cooling during Unruptured Cerebral Aneurysm Repair. Neurosurgery 2004, 55, 307–315. [Google Scholar] [CrossRef]
- Hindman, B.J.; Todd, M.M.; Gelb, A.W.; Loftus, C.M.; Craen, R.A.; Schubert, A.; Mahla, M.E.; Tomer, J.C. Mild Hypothermia as a Protective Therapy during Intracranial Aneurysm Surgery: A Randomized Prospective Pilot Trial. Neurosurgery 1999, 44, 23–32. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Clin. Neurosurg. 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Kwon, B.K.; Mann, C.; Sohn, H.M.; Hilibrand, A.S.; Phillips, F.M.; Wang, J.C.; Fehlings, M.G. Hypothermia for spinal cord injury. Spine J. 2008, 8, 859–874. [Google Scholar] [CrossRef]
- Lee, S.M.; Zhao, H.; Maier, C.M.; Steinberg, G.K. The Protective Effect of Early Hypothermia on PTEN Phosphorylation Correlates with Free Radical Inhibition in Rat Stroke. J. Cereb. Blood Flow Metab. 2009, 29, 1589–1600. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sekizuka, E.; Sato, S.; Yamaguchi, N.; Inamasu, J.; Bertalanffy, H.; Kawase, T. Effects of Moderate Hypothermia on Leukocyte- Endothelium Interaction in the Rat Pial Microvasculature After Transient Middle Cerebral Artery Occlusion. Stroke 1999, 30, 1679–1686. [Google Scholar] [CrossRef]
- Yokobori, S.; Frantzen, J.; Bullock, R.; Gajavelli, S.; Burks, S.; Bramlett, H.; Dietrich, W.D. The Use of Hypothermia Therapy in Traumatic Ischemic/Reperfusional Brain Injury: Review of the Literatures. Ther. Hypotherm. Temp. Manag. 2011, 1, 185–192. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Levi, A.D.; Wang, M.; Green, B.A. Hypothermic Treatment for Acute Spinal Cord Injury. Neurotherapeutics 2011, 8, 229–239. [Google Scholar] [CrossRef]
- Wakamatsu, H.; Matsumoto, M.; Nakakimura, K.; Sakabe, T. The Effects of Moderate Hypothermia and Intrathecal Tetracaine on Glutamate Concentrations of Intrathecal Dialysate and Neurologic and Histopathologic Outcome in Transient Spinal Cord Ischemia in Rabbits. Anesth. Analg. 1999, 88, 56–62. [Google Scholar] [CrossRef]
- Ishikawa, T.; Marsala, M. Hypothermia prevents biphasic glutamate release and corresponding neuronal degeneration after transient spinal cord ischemia in the rat. Cell. Mol. Neurobiol. 1999, 19, 199–208. [Google Scholar] [CrossRef]
- Friedman, L.; Ginsberg, M.; Belayev, L.; Busto, R.; Alonso, O.; Lin, B.; Globus, M.-T. Intraischemic but not postischemic hypothermia prevents non-selective hippocampal downregulation of AMPA and NMDA receptor gene expression after global ischemia. Mol. Brain Res. 2001, 86, 34–47. [Google Scholar] [CrossRef]
- Goss, J.R.; Styren, S.D.; Miller, P.D.; Kochanek, P.; Palmer, A.M.; Marion, D.W.; DeKosky, S. Hypothermia Attenuates the Normal Increase in Interleukin 1β RNA and Nerve Growth Factor Following Traumatic Brain Injury in the Rat. J. Neurotrauma 1995, 12, 159–167. [Google Scholar] [CrossRef]
- Kinoshita, K.; Chatzipanteli, K.; Vitarbo, E.; Truettner, J.S.; Alonso, O.F.; Dietrich, W.D.; Selman, W.R.; Lee, S.M.; Kelly, D.F. Interleukin-1β Messenger Ribonucleic Acid and Protein Levels after Fluid-Percussion Brain Injury in Rats: Importance of Injury Severity and Brain Temperature. Neurosurgery 2002, 51, 195–203. [Google Scholar] [CrossRef]
- Yu, C.G.; Jimenez, O.; Marcillo, A.E.; Weider, B.; Bangerter, K.; Dietrich, W.D.; Castro, S.; Yezierski, R.P. Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. J. Neurosurg. Spine 2000, 93, 85–93. [Google Scholar] [CrossRef]
- Jr, T.P.L.; Cho, K.-S.; Garg, M.S.; Lynch, M.P.; Marcillo, A.E.; Koivisto, D.L.; Stagg, M.; Abril, R.M.; Patel, S.; Dietrich, W.D.; et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J. Comp. Neurol. 2009, 514, 433–448. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Kerr, N.F.; Gatt, A.M.; Aleksoska, E.; Cox, S.F.; Ghasem-Zadeh, A.; Wills, T.E.; Howells, D.W. Hypothermia Prior to Decompression: Buying Time for Treatment of Acute Spinal Cord Injury. J. Neurotrauma 2010, 27, 1357–1368. [Google Scholar] [CrossRef]
- Cappuccino, A.; Bisson, L.J.; Carpenter, B.; Marzo, J.; Dietrich, W.D.; Cappuccino, H. The Use of Systemic Hypothermia for the Treatment of an Acute Cervical Spinal Cord Injury in a Professional Football Player. Spine 2010, 35, E57–E62. [Google Scholar] [CrossRef] [PubMed]
- Ransom, S.C.; Brown, N.J.; Pennington, Z.A.; Lakomkin, N.; Mikula, A.L.; Bydon, M.; Elder, B.D. Hypothermia Therapy for Traumatic Spinal Cord Injury: An Updated Review. J. Clin. Med. 2022, 11, 1585. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Chua, S.M.; Prasad, A.; Kakkos, I.; Jiang, W.; Yue, M.; Liu, X.; All, A.H. Neuroprotective assessment of prolonged local hypothermia post contusive spinal cord injury in rodent model. Spine J. 2018, 18, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Kim, Y.-H.; Kim, J.-W.; Kim, S.-I.; Ha, K.-Y. Effects of Therapeutic Hypothermia on Apoptosis and Autophagy After Spinal Cord Injury in Rats. Spine 2015, 40, 883–890. [Google Scholar] [CrossRef]
- Murakami, H.; Yoshida, K.; Hino, Y.; Matsuda, H.; Tsukube, T.; Okita, Y. Complications of cerebrospinal fluid drainage in thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2004, 39, 243–245. [Google Scholar] [CrossRef]
- Jones, C.F.; Newell, R.S.; Lee, J.H.T.; Cripton, P.A.; Kwon, B.K. The Pressure Distribution of Cerebrospinal Fluid Responds to Residual Compression and Decompression in an Animal Model of Acute Spinal Cord Injury. Spine 2012, 37, E1422–E1431. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.H.-B.; Matthias, M.; Bernays, R.-L.; Krayenbuhl, N.; Kollias, S. Cervical myelopathy due to chronic overshunting in a pediatric patient: Case report and review of the literature. Turk. Neurosurg. 2013, 23, 410–414. [Google Scholar] [CrossRef]
- Agarwal, N.; Hansberry, D.R.; Goldstein, I.M. Tethered Cord as a Complication of Chronic Cerebral Spinal Fluid Diversion. Int. J. Spine Surg. 2017, 11, 26. [Google Scholar] [CrossRef]
- Várallyay, P.; Nagy, Z.; Szűcs, A.; Czigléczki, G.; Markia, B.; Nagy, G.; Osztie, É.; Vajda, J.; Vitanovics, D. Miyazaki syndrome: Cervical myelo/radiculopathy caused by overshunting. A systematic review. Clin. Neurol. Neurosurg. 2019, 186, 105531. [Google Scholar] [CrossRef] [PubMed]
- McHardy, F.E.; Bayly, P.J.M.; Wyatt, M.G. Fatal subdural haemorrhage following lumbar spinal drainage during repair of thoraco-abdominal aneurysm. Anaesthesia 2001, 56, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kitpanit, N.; Ellozy, S.H.; Connolly, P.H.; Agrusa, C.J.; Lichtman, A.D.; Schneider, D.B. Risk factors for spinal cord injury and complications of cerebrospinal fluid drainage in patients undergoing fenestrated and branched endovascular aneurysm repair. J. Vasc. Surg. 2021, 73, 399–409.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Li, J.-J.; Zhang, X.; Liu, C.-B.; Yang, D.-G.; Qin, C.; Dong, X.-C.; Li, D.-P.; Zhang, C.; Guo, Y.; et al. Dynamic changes in intramedullary pressure 72 hours after spinal cord injury. Neural Regen. Res. 2019, 14, 886–895. [Google Scholar] [CrossRef]
- Saadoun, S.; Bell, B.A.; Verkman, A.S.; Papadopoulos, M.C. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 2008, 131, 1087–1098. [Google Scholar] [CrossRef]
- Werndle, M.C.; Saadoun, S.; Phang, I.; Czosnyka, M.; Varsos, G.V.; Czosnyka, Z.H.; Smielewski, P.; Jamous, A.; Bell, B.A.; Zoumprouli, A.; et al. Monitoring of Spinal Cord Perfusion Pressure in Acute Spinal Cord Injury: Initial Findings of the Injured Spinal Cord Pressure Evaluation Study. Crit. Care Med. 2014, 42, 646–655. [Google Scholar] [CrossRef]
- Noussitou, F.L.; Gorgas, D.; Rohrbach, H.; Henke, D.; Howard, J.; Forterre, F. Assessment of Intramedullary Spinal Pressure in Small Breed Dogs with Thoracolumbar Disk Extrusion Undergoing Hemilaminectomy. Vet. Surg. 2015, 44, 944–948. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Cates, L.N.; Fischedick, A.E.; McClintic, A.M.; Mourad, P.D.; Hofstetter, C.P.; Kwon, B.K.; Streijger, F.; Fallah, N.; Noonan, V.K.; et al. Temporal and Spatial Evolution of Raised Intraspinal Pressure after Traumatic Spinal Cord Injury. J. Neurotrauma 2017, 34, 645–651. [Google Scholar] [CrossRef]
- Lee, S.C.M.; Lueck, C.J. Cerebrospinal Fluid Pressure in Adults. J. Neuro-Ophthalmol. 2014, 34, 278–283. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Kerr, N.F.; Gatt, A.M.; Cox, S.F.; Ghasem-Zadeh, A.; Wills, T.E.; Sidon, T.K.; Howells, D.W. Intracanal Pressure in Compressive Spinal Cord Injury: Reduction with Hypothermia. J. Neurotrauma 2011, 28, 809–820. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Wills, T.E.; Skeers, P.; Battistuzzo, C.R.; Macleod, M.R.; Howells, D.W.; Sena, E.S. Meta-Analysis of Pre-Clinical Studies of Early Decompression in Acute Spinal Cord Injury: A Battle of Time and Pressure. PLoS ONE 2013, 8, e72659. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, M.F.; Noonan, V.K.; Fallah, N.; Fisher, C.G.; Finkelstein, J.; Kwon, B.K.; Rivers, C.S.; Ahn, H.; Paquet, J.; Tsai, E.C.; et al. The Influence of Time from Injury to Surgery on Motor Recovery and Length of Hospital Stay in Acute Traumatic Spinal Cord Injury: An Observational Canadian Cohort Study. J. Neurotrauma 2015, 32, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Levi, L.; Wolf, A.; Rigamonti, D.; Ragheb, J.; Mirvis, S.; Robinson, W.L. Anterior Decompression in Cervical Spine Trauma: Does the Timing of Surgery Affect the Outcome?—PubMed. Neurosurgery 1991, 29, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Clohisy, J.C.; Akbarnia, B.A.; Bucholz, R.D.; Burkus, J.K.; Backer, R.J. Neurologic Recovery Associated with Anterior Decompression of Spine Fractures at the Thoracolumbar Junction (T12–L1). Spine 1992, 17, 325–330. [Google Scholar] [CrossRef]
- Furlan, J.C.; Noonan, V.K.; Cadotte, D.W.; Fehlings, M.G.; Speidel, J.; Mattucci, S.; Liu, J.; Kwon, B.K.; Tetzlaff, W.; Oxland, T.R.; et al. Timing of Decompressive Surgery of Spinal Cord after Traumatic Spinal Cord Injury: An Evidence-Based Examination of Pre-Clinical and Clinical Studies. J. Neurotrauma 2011, 28, 1371–1399. [Google Scholar] [CrossRef] [PubMed]
- Duh, M.-S.; Shepard, M.J.; Wilberger, J.E.; Bracken, M.B. The Effectiveness of Surgery on the Treatment of Acute Spinal Cord Injury and Its Relation to Pharmacological Treatment. Neurosurgery 1994, 35, 240–249. [Google Scholar] [CrossRef]
- Campagnolo, D.; Esquieres, R.; Kopacz, K. Effect of Timing of Stabilization on Length of Stay and Medical Complications Following Spinal Cord Injury. J. Spinal Cord Med. 1997, 20, 331–334. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Daugherty, R.J.; Sheehan, T.P.; Dante, S.J.; Cotler, J.M.; Balderston, R.A.; Herbison, G.J.; Northrup, B.E. Neurologic Outcome of Early Versus Late Surgery for Cervical Spinal Cord Injury. Spine 1997, 22, 2609–2613. [Google Scholar] [CrossRef]
- McKinley, W.; Meade, M.A.; Kirshblum, S.; Barnard, B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch. Phys. Med. Rehabil. 2004, 85, 1818–1825. [Google Scholar] [CrossRef]
- McLain, R.F.; Benson, D.R. Urgent Surgical Stabilization of Spinal Fractures in Polytrauma Patients. Spine 1999, 24, 1646–1654. [Google Scholar] [CrossRef]
- Wilson, J.R.; Tetreault, L.A.; Kwon, B.K.; Arnold, P.M.; Mroz, T.E.; Shaffrey, C.; Harrop, J.S.; Chapman, J.R.; Casha, S.; Skelly, A.C.; et al. Timing of Decompression in Patients with Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 95S–115S. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Guo, X. Durotomy and Dural Grafting to Treat Lower Cervical Spine Injuries with Extensive Spinal Cord Edema. Neural Regen. Res. 2015, 10, 1969–1970. [Google Scholar]
- Qu, Y.; Luo, Z.; Guo, X.; Cui, W.; Sun, T.; Huang, Z.; Wu, Y. The Durotomy or Myelotomy for the Spinal Cord Extensive Swelling with/without Intramedullary Hemorrhage. Zhonghua Guke Zazhi 2015, 35, 710–713. [Google Scholar]
- Wang, B.; Shi, W.; Zhang, Y.; Wang, Y.; Yang, C.; Huang, T.; Tian, Q.-L.; Qu, Y.; Wang, J.-L. Duraplasty with autologous nuchal ligament fascia to reduce postoperative complications in pediatric patients undergoing neoplasia resection with a suboccipital midline approach. J. Neurosurg. Pediatr. 2022, 30, 538–546. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Cates, L.N.; Dewees, D.M.; Hyde, J.E.; Gaing, A.; Birjandian, Z.; Hofstetter, C.P. Effect of Durotomy versus Myelotomy on Tissue Sparing and Functional Outcome after Spinal Cord Injury. J. Neurotrauma 2021, 38, 746–755. [Google Scholar] [CrossRef]
- Winestone, J.S.; Farley, C.W.; Curt, B.A.; Chavanne, A.; Dollin, N.; Pettigrew, D.B.; Kuntz, C. Laminectomy, durotomy, and piotomy effects on spinal cord intramedullary pressure in severe cervical and thoracic kyphotic deformity: A cadaveric study. J. Neurosurg. Spine 2012, 16, 195–200. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Zhang, C.; Li, W. Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: A randomized, controlled preclinical research. J. Orthop. Surg. Res. 2016, 11, 34. [Google Scholar] [CrossRef]
- Iannotti, C.; Zhang, Y.P.; Shields, L.B.; Han, Y.; Burke, D.A.; Xu, X.-M.; Shields, C.B. Dural Repair Reduces Connective Tissue Scar Invasion and Cystic Cavity Formation after Acute Spinal Cord Laceration Injury in Adult Rats. J. Neurotrauma 2006, 23, 853–865. [Google Scholar] [CrossRef]
- Smith, J.S.; Anderson, R.; Pham, T.; Bhatia, N.; Steward, O.; Gupta, R. Role of Early Surgical Decompression of the Intradural Space After Cervical Spinal Cord Injury in an Animal Model. J. Bone Jt. Surg. Ser. A 2010, 92, 1206–1214. [Google Scholar] [CrossRef]
- Saadoun, S.; Werndle, M.C.; de Heredia, L.L.; Papadopoulos, M.C. The dura causes spinal cord compression after spinal cord injury. Br. J. Neurosurg. 2016, 30, 582–584. [Google Scholar] [CrossRef]
- Jalan, D.; Saini, N.; Zaidi, M.; Pallottie, A.; Elkabes, S.; Heary, R.F. Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J. Neurosurg. Spine 2017, 26, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Camlar, M.; Turk, Ç.; Buhur, A.; Oltulu, F.; Ören, M.; Senoglu, M.; Ozer, F. Does Decompressive Duraplasty Have a Neuroprotective Effect on Spinal Trauma?: An Experimental Study. World Neurosurg. 2019, 126, e288–e294. [Google Scholar] [CrossRef]
- Guerin, P.; El Fegoun, A.B.; Obeid, I.; Gille, O.; Lelong, L.; Luc, S.; Bourghli, A.; Cursolle, J.C.; Pointillart, V.; Vital, J.-M. Incidental durotomy during spine surgery: Incidence, management and complications. A retrospective review. Injury 2012, 43, 397–401. [Google Scholar] [CrossRef]
- Frostell, A.; Hakim, R.; Thelin, E.; Mattsson, P.; Svensson, M. A Review of the Segmental Diameter of the Healthy Human Spinal Cord. Front. Neurol. 2016, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Perrin, R.G. The role and timing of early decompression for cervical spinal cord injury: Update with a review of recent clinical evidence. Injury 2005, 36, S13–S26. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Cates, L.N.; Hyde, J.; DeWees, D.M.; Hammond, R.; Bruce, M.; Hofstetter, C.P. Contrast-Enhanced Ultrasound for Assessment of Local Hemodynamic Changes Following a Rodent Contusion Spinal Cord Injury. Mil. Med. 2020, 185, 470–475. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Cates, L.N.; DeWees, D.M.; Hannah, A.; Mourad, P.; Bruce, M.; Hofstetter, C.P. Contrast-enhanced ultrasound to visualize hemodynamic changes after rodent spinal cord injury. J. Neurosurg. Spine 2018, 29, 306–313. [Google Scholar] [CrossRef]
- Uyeda, A.; Muramatsu, R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int. J. Mol. Sci. 2020, 21, 8116. [Google Scholar] [CrossRef]
- Spinal Cord Injury Neuroprotection with Glyburide—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02524379 (accessed on 31 July 2022).
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236. [Google Scholar] [CrossRef]
- Minocycline and Perfusion Pressure Augmentation in Acute Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00559494 (accessed on 25 December 2022).
- Minocycline in Acute Spinal Cord Injury (MASC)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01828203 (accessed on 31 July 2022).
- Fehlings, M.G.; Wilson, J.R.; Frankowski, R.F.; Toups, E.G.; Aarabi, B.; Harrop, J.S.; Shaffrey, C.I.; Harkema, S.J.; Guest, J.D.; Tator, C.H.; et al. Riluzole for the treatment of acute traumatic spinal cord injury: Rationale for and design of the NACTN Phase I clinical trial. J. Neurosurg. Spine 2012, 17, 151–156. [Google Scholar] [CrossRef]
- Grossman, R.G.; Fehlings, M.G.; Frankowski, R.F.; Burau, K.D.; Chow, D.S.-L.; Tator, C.; Teng, A.; Toups, E.G.; Harrop, J.S.; Aarabi, B.; et al. A Prospective, Multicenter, Phase I Matched-Comparison Group Trial of Safety, Pharmacokinetics, and Preliminary Efficacy of Riluzole in Patients with Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Safety of Riluzole in Patients with Acute Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00876889 (accessed on 31 July 2022).
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.L.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2016, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Riluzole in Spinal Cord Injury Study—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01597518 (accessed on 31 July 2022).
- PMZ-1620 (Sovateltide) in Patients of Acute Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04054414 (accessed on 31 July 2022).
- Koda, M.; Hanaoka, H.; Fujii, Y.; Hanawa, M.; Kawasaki, Y.; Ozawa, Y.; Fujiwara, T.; Furuya, T.; Ijima, Y.; Saito, J.; et al. Randomized trial of granulocyte colony-stimulating factor for spinal cord injury. Brain 2021, 144, 789–799. [Google Scholar] [CrossRef]
- Koda, M.; Hanaoka, H.; Sato, T.; Fujii, Y.; Hanawa, M.; Takahashi, S.; Furuya, T.; Ijima, Y.; Saito, J.; Kitamura, M.; et al. Study protocol for the G-SPIRIT trial: A randomised, placebo-controlled, double-blinded phase III trial of granulocyte colony-stimulating factor-mediated neuroprotection for acute spinal cord injury. BMJ Open 2018, 8, e019083. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, K.; Duan, L.; Wang, Y.-Z.; Ju, G. Beneficial effects of early hemostasis on spinal cord injury in the rat. Spinal Cord 2016, 54, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Ju, B.G.; Yune, T.Y. Mithramycin A Improves Functional Recovery by Inhibiting BSCB Disruption and Hemorrhage after Spinal Cord Injury. J. Neurotrauma 2018, 35, 508–520. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Ju, B.G.; Youn, I.; Yune, T.Y. Protocatechuic acid improves functional recovery after spinal cord injury by attenuating blood-spinal cord barrier disruption and hemorrhage in rats. Neurochem. Int. 2019, 124, 181–192. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, J.; Yu, T.; Chen, Z.; Xiao, Z.; Wang, J.; Hu, Y.; Wu, Y.; Zhu, D. Flufenamic acid inhibits secondary hemorrhage and BSCB disruption after spinal cord injury. Theranostics 2018, 8, 4181–4198. [Google Scholar] [CrossRef]
- Simard, J.M.; Tsymbalyuk, O.; Ivanov, A.; Ivanova, S.; Bhatta, S.; Geng, Z.; Woo, S.K.; Gerzanich, V. Endothelial sulfonylurea receptor 1–regulated NCCa-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J. Clin. Investig. 2007, 117, 2105–2113. [Google Scholar] [CrossRef]
- Popovich, P.G.; Lemeshow, S.; Gensel, J.C.; Tovar, C.A. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp. Neurol. 2012, 233, 615–622. [Google Scholar] [CrossRef]
- Simard, J.M.; Tsymbalyuk, O.; Keledjian, K.; Ivanov, A.; Ivanova, S.; Gerzanich, V. Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp. Neurol. 2012, 233, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Vajn, K.; Plunkett, J.A.; Tapanes-Castillo, A.; Oudega, M. Axonal regeneration after spinal cord injury in zebrafish and mammals: Differences, similarities, translation. Neurosci. Bull. 2013, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. M1 Means Kill; M2 Means Heal. J. Immunol. 2017, 199, 2191–2193. [Google Scholar] [CrossRef]
- Chen, J.; Fu, E.J.; Patel, P.R.; Hostetler, A.J.; Sawan, H.A.; Moss, K.A.; Hocevar, S.E.; Anderson, A.; Chestek, C.A.; Shea, L.D. Lentiviral Interleukin-10 Gene Therapy Preserves Fine Motor Circuitry and Function After a Cervical Spinal Cord Injury in Male and Female Mice. Neurotherapeutics 2021, 18, 503–514. [Google Scholar] [CrossRef] [PubMed]
- David, S.; López-Vales, R.; Yong, V.W. Harmful and beneficial effects of inflammation after spinal cord injury: Potential Therapeutic Implications. In Handbook of Clinical Neurology; North-Holland Publishing Company: Amsterdam, The Netherlands, 2012; Volume 109, pp. 485–502. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Wu, Y.-P.; Chen, W.-S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wilson, J.R.; Harrop, J.S.; Kwon, B.K.; Tetreault, L.A.; Arnold, P.M.; Singh, J.; Hawryluk, G.; Dettori, J.R. Efficacy and Safety of Methylprednisolone Sodium Succinate in Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 116S–137S. [Google Scholar] [CrossRef] [PubMed]
- Alderson, P.; Roberts, I. Corticosteroids for acute traumatic brain injury. Cochrane Database Syst. Rev. 2005, 2005, CD000196. [Google Scholar] [CrossRef]
- Lee, S.M.; Yune, T.Y.; Kim, S.J.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J. Neurochem. 2004, 91, 568–578. [Google Scholar] [CrossRef]
- Afshary, K.; Chamanara, M.; Talari, B.; Rezaei, P.; Nassireslami, E. Therapeutic Effects of Minocycline Pretreatment in the Locomotor and Sensory Complications of Spinal Cord Injury in an Animal Model. J. Mol. Neurosci. 2020, 70, 1064–1072. [Google Scholar] [CrossRef]
- Seabrook, T.J.; Jiang, L.; Maier, M.; Lemere, C.A. Minocycline affects microglia activation, Aβ deposition, and behavior in APP-tg mice. Glia 2006, 53, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Aceves, M.; Terminel, M.N.; Okoreeh, A.; Aceves, A.R.; Gong, Y.M.; Polanco, A.; Sohrabji, F.; Hook, M.A. Morphine increases macrophages at the lesion site following spinal cord injury: Protective effects of minocycline. Brain Behav. Immun. 2019, 79, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Yune, T.Y.; Kim, S.J.; Park, D.W.; Lee, Y.K.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline Reduces Cell Death and Improves Functional Recovery after Traumatic Spinal Cord Injury in the Rat. J. Neurotrauma 2003, 20, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.S.; Sundareshan, V.; Cory, T.J.; Hayes, D.; Anstead, M.I.; Feola, D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008, 61, 554–560. [Google Scholar] [CrossRef]
- Haydar, D.; Cory, T.J.; Birket, S.E.; Murphy, B.S.; Pennypacker, K.R.; Sinai, A.P.; Feola, D.J. Azithromycin Polarizes Macrophages to an M2 Phenotype via Inhibition of the STAT1 and NF-κB Signaling Pathways. J. Immunol. 2019, 203, 1021–1030. [Google Scholar] [CrossRef]
- Gensel, J.C.; Kopper, T.J.; Zhang, B.; Orr, M.B.; Bailey, W.M. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 2017, 7, 40144. [Google Scholar] [CrossRef]
- Vrančić, M.; Banjanac, M.; Nujić, K.; Bosnar, M.; Murati, T.; Munić, V.; Polančec, D.S.; Belamarić, D.; Parnham, M.; Haber, V.E. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br. J. Pharmacol. 2012, 165, 1348–1360. [Google Scholar] [CrossRef]
- Banjanac, M.; Kos, V.M.; Nujić, K.; Vrančić, M.; Belamarić, D.; Crnković, S.; Hlevnjak, M.; Haber, V.E. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 2012, 66, 357–362. [Google Scholar] [CrossRef]
- Sugiyama, K.; Shirai, R.; Mukae, H.; Ishimoto, H.; Nagata, T.; Sakamoto, N.; Ishii, H.; Nakayama, S.; Yanagihara, K.; Mizuta, Y.; et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin. Exp. Immunol. 2007, 147, 540–546. [Google Scholar] [CrossRef]
- Yamauchi, K.; Shibata, Y.; Kimura, T.; Abe, S.; Inoue, S.; Osaka, D.; Sato, M.; Igarashi, A.; Kubota, I. Azithromycin suppresses interleukin-12p40 expression in lipopolysaccharide and interferon-γ stimulated macrophages. Int. J. Biol. Sci. 2009, 5, 667–678. [Google Scholar] [CrossRef]
- Zhang, B.; Bailey, W.M.; Kopper, T.J.; Orr, M.B.; Feola, D.J.; Gensel, J.C. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J. Neuroinflamm. 2015, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Rismanbaf, A.; Afshari, K.; Ghasemi, M.; Badripour, A.; Haj-Mirzaian, A.; Dehpour, A.R.; Shafaroodi, H. Therapeutic Effects of Azithromycin on Spinal Cord Injury in Male Wistar Rats: A Role for Inflammatory Pathways. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 83, 411–419. [Google Scholar] [CrossRef]
- Schwartz, G.; Fehlings, M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. Spine 2001, 94, 245–256. [Google Scholar] [CrossRef]
- Li, S.; Stys, P.K. Mechanisms of Ionotropic Glutamate Receptor-Mediated Excitotoxicity in Isolated Spinal Cord White Matter. J. Neurosci. 2000, 20, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef]
- Stys, P.K. General Mechanisms of Axonal Damage and Its Prevention. J. Neurol. Sci. 2005, 233, 3–13. [Google Scholar] [CrossRef]
- Debono, M.-W.; Le Guern, J.; Canton, T.; Doble, A.; Pradier, L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 1993, 235, 283–289. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Benz, A.; Fu, T.; Wang, M.; Covey, D.; Zorumski, C.; Mennerick, S. Neuroprotective agent riluzole potentiates postsynaptic GABAA receptor function. Neuropharmacology 2002, 42, 199–209. [Google Scholar] [CrossRef]
- Azbill, R.; Mu, X.; Springer, J. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000, 871, 175–180. [Google Scholar] [CrossRef]
- Fumagalli, E.; Funicello, M.; Rauen, T.; Gobbi, M.; Mennini, T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008, 578, 171–176. [Google Scholar] [CrossRef]
- Moon, E.S.; Karadimas, S.K.; Yu, W.-R.; Austin, J.W.; Fehlings, M.G. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol. Dis. 2014, 62, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Hosier, H.; Peterson, D.; Tsymbalyuk, O.; Keledjian, K.; Smith, B.R.; Ivanova, S.; Gerzanich, V.; Popovich, P.G.; Simard, J.M.; Farhadi, H.F.; et al. A Direct Comparison of Three Clinically Relevant Treatments in a Rat Model of Cervical Spinal Cord Injury. J. Neurotrauma 2015, 32, 1633–1644. [Google Scholar] [CrossRef]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.A. Impacts of Spinal Cord Injury and Formulation on Riluzole Pharmacokinetics and Pharmacokinetics/Pharmacodynamics (PK/PD) Correlation. Ph.D. Dissertation, University of Houston, Houston, TX, USA, 2012. [Google Scholar]
- Yao, C.; Cao, X.; Yu, B. Revascularization After Traumatic Spinal Cord Injury. Front. Physiol. 2021, 12, 631500. [Google Scholar] [CrossRef] [PubMed]
- Kobari, M.; Fukuuchi, Y.; Tomita, M.; Tanahashi, N.; Konno, S.; Takeda, H. Dilatation of cerebral microvessels mediated by endothelin ETB receptor and nitric oxide in cats. Neurosci. Lett. 1994, 176, 157–160. [Google Scholar] [CrossRef]
- Leonard, M.G.; Gulati, A. Endothelin B receptor agonist, IRL-1620, enhances angiogenesis and neurogenesis following cerebral ischemia in rats. Brain Res. 2013, 1528, 28–41. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Briyal, S.; Gulati, A. Sovateltide (IRL-1620) activates neuronal differentiation and prevents mitochondrial dysfunction in adult mammalian brains following stroke. Sci. Rep. 2020, 10, 12737. [Google Scholar] [CrossRef]
- Briyal, S.; Ranjan, A.K.; Hornick, M.G.; Puppala, A.K.; Luu, T.; Gulati, A. Anti-apoptotic activity of ETB receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia. Sci. Rep. 2019, 9, 10439. [Google Scholar] [CrossRef]
- Gulati, A.; Hornick, M.G.; Briyal, S.; Lavhale, M.S. A Novel Neuroregenerative Approach Using ETB Receptor Agonist, IRL-1620, to Treat CNS Disorders. Physiol. Res. 2018, 67, S95–S113. [Google Scholar] [CrossRef]
- Gulati, A.; Agrawal, N.; Vibha, D.; Misra, U.K.; Paul, B.; Jain, D.; Pandian, J.; Borgohain, R. Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. CNS Drugs 2021, 35, 85–104. [Google Scholar] [CrossRef]
- Pan, C.; Gupta, A.; Prentice, H.; Wu, J.-Y. Protection of taurine and granulocyte colony-stimulating factor against excitotoxicity induced by glutamate in primary cortical neurons. J. Biomed. Sci. 2010, 17, S18. [Google Scholar] [CrossRef]
- Kawabe, J.; Koda, M.; Hashimoto, M.; Fujiyoshi, T.; Furuya, T.; Endo, T.; Okawa, A.; Yamazaki, M. Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats: Laboratory Investigation. J. Neurosurg. Spine 2011, 15, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Koda, M.; Kamada, T.; Someya, Y.; Kadota, R.; Mannoji, C.; Miyashita, T.; Okada, S.; Okawa, A.; Moriya, H.; et al. Granulocyte Colony-Stimulating Factor Attenuates Neuronal Death and Promotes Functional Recovery After Spinal Cord Injury in Mice. J. Neuropathol. Exp. Neurol. 2007, 66, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Nishio, Y.; Kamada, T.; Someya, Y.; Okawa, A.; Mori, C.; Yoshinaga, K.; Okada, S.; Moriya, H.; Yamazaki, M. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007, 1149, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kadota, R.; Koda, M.; Kawabe, J.; Hashimoto, M.; Nishio, Y.; Mannoji, C.; Miyashita, T.; Furuya, T.; Okawa, A.; Takahashi, K.; et al. Granulocyte Colony-Stimulating Factor (G-CSF) Protects Oligpdendrocyte and Promotes Hindlimb Functional Recovery after Spinal Cord Injury in Rats. PLoS ONE 2012, 7, e50391. [Google Scholar] [CrossRef] [PubMed]
- Dittgen, T.; Pitzer, C.; Plaas, C.; Kirsch, F.; Vogt, G.; Laage, R.; Schneider, A. Granulocyte-Colony Stimulating Factor (G-CSF) Improves Motor Recovery in the Rat Impactor Model for Spinal Cord Injury. PLoS ONE 2012, 7, e29880. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yamazaki, M.; Okawa, A.; Sakuma, T.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; Kawabe, J.; et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur. Spine J. 2012, 21, 2580–2587. [Google Scholar] [CrossRef]
- Inada, T.; Takahashi, H.; Yamazaki, M.; Okawa, A.; Sakuma, T.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; et al. Multicenter Prospective Nonrandomized Controlled Clinical Trial to Prove Neurotherapeutic Effects of Granulocyte Colony-Stimulating Factor for Acute Spinal Cord Injury: Analyses of Follow-up Cases after at Least 1 Year. Spine 2014, 39, 213–219. [Google Scholar] [CrossRef]
- NIPH Clinical Trials Search. Available online: https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000018752 (accessed on 1 August 2022).
- Sontag, C.J.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Injury to the Spinal Cord Niche Alters the Engraftment Dynamics of Human Neural Stem Cells. Stem Cell Rep. 2014, 2, 620–632. [Google Scholar] [CrossRef]
- Hill, C.E.; Hurtado, A.; Blits, B.; Bahr, B.A.; Wood, P.M.; Bunge, M.B.; Oudega, M. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur. J. Neurosci. 2007, 26, 1433–1445. [Google Scholar] [CrossRef]
- Mirfeizi, L.; Stratton, J.A.; Kumar, R.; Shah, P.; Agabalyan, N.; Stykel, M.G.; Midha, R.; Biernaskie, J.; Kallos, M.S. Serum-free bioprocessing of adult human and rodent skin-derived Schwann cells: Implications for cell therapy in nervous system injury. J. Tissue Eng. Regen. Med. 2017, 11, 3385–3397. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nekanti, U.; Haus, D.L.; Funes, G.; Moreno, D.; Kamei, N.; Cummings, B.J.; Anderson, A.J. Induction of early neural precursors and derivation of tripotent neural stem cells from human pluripotent stem cells under xeno-free conditions. J. Comp. Neurol. 2014, 522, 2767–2783. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, H.-R.; Arjmand, B.; Javidan, A.N.; Saberi, H.; Soleimani, M.; Tavakoli, S.A.-H.; Khodadadi, A.; Tirgar, N.; Mohammadi-Jahani, F. Clinical grade cultivation of human Schwann cell, by the using of human autologous serum instead of fetal bovine serum and without growth factors. Cell Tissue Bank. 2011, 13, 281–285. [Google Scholar] [CrossRef]
- McCreedy, D.A.; Wilems, T.S.; Xu, H.; Butts, J.C.; Brown, C.R.; Smith, A.W.; Sakiyama-Elbert, S.E. Survival, differentiation, and migration of high-purity mouse embryonic stem cell-derived progenitor motor neurons in fibrin scaffolds after sub-acute spinal cord injury. Biomater. Sci. 2014, 2, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef]
- Johnson, P.; Tatara, A.; McCreedy, D.A.; Shiu, A.; Sakiyama-Elbert, S.E. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter 2010, 6, 5127–5137. [Google Scholar] [CrossRef]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report from a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Adipose Stem Cells for Traumatic Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03308565 (accessed on 1 August 2022).
- Autologous Adipose Derived Mesenchymal Stem Cells for Spinal Cord Injury Patients—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04520373 (accessed on 1 August 2022).
- Liu, J.; Han, D.; Wang, Z.; Xue, M.; Zhu, L.; Yan, H.; Zheng, X.; Guo, Z.; Wang, H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 2013, 15, 185–191. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.Y.; Chen, Z.H.; Wang, N.X.; Zhang, L.M.; Chen, Y.Y.; Mo, J.; Dong, J.W.; et al. Repeated Subarachnoid Administrations of Allogeneic Human Umbilical Cord Mesenchymal Stem Cells for Spinal Cord Injury: A Phase 1/2 Pilot Study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef]
- Repeated Subarachnoid Administrations of HUC-MSCs in Treating SCI—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02481440 (accessed on 1 August 2022).
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2020, 23, 146–156. [Google Scholar] [CrossRef]
- Intrathecal Administration of Expanded Wharton’s Jelly Mesenchymal Stem Cells in Chronic Traumatic Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03003364 (accessed on 1 August 2022).
- Intrathecal Transplantation of UC-MSC in Patients with Sub-Acute Spinal Cord Injury—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03521336 (accessed on 1 December 2021).
- Syková, E.; Homola, A.; Mazanec, R.; Lachmann, H.; Konrádová, Š.L.; Kobylka, P.; Pádr, R.; Neuwirth, J.; Komrska, V.; Vávra, V.; et al. Autologous Bone Marrow Transplantation in Patients with Subacute and Chronic Spinal Cord Injury. Cell Transplant. 2006, 15, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Autologous Mesenchymal Stem Cells Transplantation for Spinal Cord Injury- A Phase I Clinical Study—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02482194 (accessed on 1 December 2021).
- Safety and Effectiveness of BM-MSC vs AT-MSC in the Treatment of SCI Patients—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02981576 (accessed on 1 August 2022).
- Treatment of Spinal Cord Injuries with (AutoBM-MSCs)vs (WJ-MSCs).—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04288934 (accessed on 1 August 2022).
- Study of Human Central Nervous System Stem Cells (HuCNS-SC) in Patients with Thoracic Spinal Cord Injury—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01321333 (accessed on 1 December 2021).
- Study of Human Central Nervous System (CNS) Stem Cell Transplantation in Cervical Spinal Cord Injury—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02163876 (accessed on 1 December 2021).
- Safety and Exploratory Efficacy of Transplantation Therapy Using PSA-NCAM(+) NPC in AIS-A Level of Sub-Acute SCI—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04812431 (accessed on 1 August 2022).
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.L.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.; Fessler, R.G. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J. Neurosurg. Spine 2022, 37, 321–330. [Google Scholar] [CrossRef]
- Safety Study of GRNOPC1 in Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01217008 (accessed on 1 August 2022).
- Fessler, R.G.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.D.; McKenna, S.L. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine 2022, 37, 812–820. [Google Scholar] [CrossRef]
- Dose Escalation Study of AST-OPC1 in Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02302157 (accessed on 1 August 2022).
- Mackay-Sim, A.; Feron, F.; Cochrane, J.; Bassingthwaighte, L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.; Licina, P.; et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 2008, 131, 2376–2386. [Google Scholar] [CrossRef]
- Wu, J.; Sun, T.; Ye, C.; Yao, J.; Zhu, B.; He, H. Clinical Observation of Fetal Olfactory Ensheathing Glia Transplantation (OEGT) in Patients with Complete Chronic Spinal Cord Injury. Cell Transplant. 2012, 21, 33–37. [Google Scholar] [CrossRef]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Miedzybrodzki, R.; Czyz, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of Autologous Olfactory Ensheathing Cells in Complete Human Spinal Cord Injury. Cell Transplant. 2013, 22, 1591–1612. [Google Scholar] [CrossRef]
- Collection and Characterisation of Human Olfactory Ensheathing Cells—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02870426 (accessed on 1 December 2021).
- Amr, S.M.; Gouda, A.; Koptan, W.T.; Galal, A.A.; Abdel-Fattah, D.S.; Rashed, L.A.; Atta, H.M.; Abdel-Aziz, M.T. Bridging defects in chronic spinal cord injury using peripheral nerve grafts combined with a chitosan-laminin scaffold and enhancing regeneration through them by co-transplantation with bone-marrow-derived mesenchymal stem cells: Case series of 14 patients. J. Spinal Cord Med. 2013, 37, 54–71. [Google Scholar] [CrossRef]
- Saberi, H.; Moshayedi, P.; Aghayan, H.-R.; Arjmand, B.; Hosseini, S.-K.; Emami-Razavi, S.-H.; Rahimi-Movaghar, V.; Raza, M.; Firouzi, M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008, 443, 46–50. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Ning, G.-Z.; Feng, S.-Q.; Kong, X.-H.; Chen, J.-T.; Zheng, Y.-F.; Ban, D.-X.; Liu, T.; Li, H.; Wang, P. Transplantation of Autologous Activated Schwann Cells in the Treatment of Spinal Cord Injury: Six Cases, more than Five Years of Follow-up. Cell Transplant. 2012, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bunge, M.B.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Safety of Autologous Human Schwann Cells (AhSC) in Subjects with Subacute SCI—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01739023 (accessed on 25 December 2022).
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells 2014, 6, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-Like Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Wang, L.-W.; Hu, Z.-B.; Guo, W.-T.; Wei, J.-S.; Lin, H.; Sun, X.; Chen, L.-X.; Yang, L.-J. Differentiation of Human Bone Marrow Mesenchymal Stem Cells into Neuron-Like Cells In Vitro. Spine 2011, 36, 997–1005. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Yu, Z.; Yu, Y.; Sun, B.; Xiao, C.; Luo, S.; Li, L. Effects of Edaravone on Functional Recovery of a Rat Model with Spinal Cord Injury Through Induced Differentiation of Bone Marrow Mesenchymal Stem Cells into Neuron-Like Cells. Cell. Reprogram. 2021, 23, 47–56. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Ma, J.; Sun, Y.; Yu, B.-Q.; Jiao, Z.-M.; Wang, D.; Yu, M.-Y.; Li, J.-Y.; Fu, J. Tanshinone IIA promotes the differentiation of bone marrow mesenchymal stem cells into neuronal-like cells in a spinal cord injury model. J. Transl. Med. 2018, 16, 193. [Google Scholar] [CrossRef]
- Prockop, D.J.; Brenner, M.; Fibbe, W.E.; Horwitz, E.; Le Blanc, K.; Phinney, D.G.; Simmons, P.J.; Sensebe, L.; Keating, A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010, 12, 576–578. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Gulati, A.; Arora, A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: Systematic review and meta-analysis. Cytotherapy 2021, 23, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019, 28, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, W.; Rong, D.; Xu, Z.; Zhang, Z.; Ye, H.; Xie, L.; Wu, Y.; Zhang, Y.; Wang, X. Human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate the repair of spinal cord injury via the miR-29b-3p/PTEN/Akt/mTOR axis. Cell Death Discov. 2021, 7, 212. [Google Scholar] [CrossRef]
- Huang, W.; Suggs, L.J.; Farrar, R.P. The Therapeutic Potential of Apoptotic Mesenchymal Stem Cells in Muscle Regeneration. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, H. Roles of Mesenchymal Stem Cells in Spinal Cord Injury. Stem Cells Int. 2017, 2017, 5251313. [Google Scholar] [CrossRef]
- So, K.-F.; Zhou, L.-B.; Liu, A.-M.; Chen, B.-L.; Yu, L.-T.; Liu, T.; Shi, L.-L.; Yu, P.-P.; Qu, Y.-B. Human adipose tissue- and umbilical cord-derived stem cells: Which is a better alternative to treat spinal cord injury? Neural Regen. Res. 2020, 15, 2306–2317. [Google Scholar] [CrossRef]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; NI, P.; Qiu, X.; Lin, X.; Wu, W.; Xie, L.; Lin, L.; Min, J.; Lai, X.; et al. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int. J. Mol. Med. 2014, 33, 263–270. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhang, S.; Deng, W.-S.; Ma, K.; Liang, B.; Liu, X.-Y.; Xu, H.-Y.; Zhang, J.; Shi, H.-Y.; Sun, H.-T. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700. [Google Scholar] [CrossRef]
- Thaweesapphithak, S.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Roytrakul, S.; Manochantr, S. Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Res. Ther. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Wright, K.; Bhattacharjee, A.; Balain, B.; Richardson, J.; Roberts, S. Isolation and Characterisation of Mesenchymal Stem Cells from Different Regions of the Human Umbilical Cord. BioMed. Res. Int. 2013, 2013, 916136. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.; Zhang, S.; Qiu, Y.; Chen, X.; Zhang, X.; Cai, C.; Peng, Y.; Mao, R.; Pevsner-Fischer, M.; Ben-Horin, S.; et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5+ B regulatory cells. Stem Cell Res. Ther. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chou, H.-L.; Chang, Y.-H.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Ye, L.; Zhang, T.; Cheng, J.; Chen, G.; Zhang, Q.; Yang, Y. Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Sci. Rep. 2016, 6, 37566. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, S.; Wang, G.; Cheng, J.; Zhang, Y.; Chen, W.; Tai, Y.; Chen, S.; Chen, G.; Liu, W.; et al. Umbilical Cord-Derived Mesenchymal Stem Cells Instruct Dendritic Cells to Acquire Tolerogenic Phenotypes Through the IL-6-Mediated Upregulation of SOCS1. Stem Cells Dev. 2014, 23, 2080–2092. [Google Scholar] [CrossRef]
- Guo, G.; Shen, L.; Li, Z. Clinical Studies of Umbilical Cord Blood Mesenchymal Stem Cells Transplantation on Spinal Cord Injury. Chin. J. Pract. Med. 2012, 39, 58–60. [Google Scholar] [CrossRef]
- Yoon, S.H.; Shim, Y.S.; Park, Y.H.; Chung, J.K.; Nam, J.H.; Kim, M.O.; Park, H.C.; Park, S.R.; Min, B.-H.; Kim, E.Y.; et al. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells 2007, 25, 2066–2073. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999, 96, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Montoya, F.; Kidder, B.L.; Pauwelyn, K.A.; Chase, L.G.; Luttun, A.; Crabbe, A.; Geraerts, M.; Sharov, A.A.; Piao, Y.; Ko, M.S.; et al. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007, 8, R163. [Google Scholar] [CrossRef] [PubMed]
- MultiStem® Administration for Stroke Treatment and Enhanced Recovery Study—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03545607 (accessed on 3 August 2022).
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- DePaul, M.; Palmer, M.; Lang, B.T.; Cutrone, R.; Tran, A.P.; Madalena, K.M.; Bogaerts, A.; Hamilton, J.A.; Deans, R.J.; Mays, R.W.; et al. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci. Rep. 2015, 5, 16795. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Saito, E.; Gurczynski, S.J.; Moore, B.B.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2019, 116, 14947–14954. [Google Scholar] [CrossRef]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440.e5. [Google Scholar] [CrossRef]
- Lu, P.; Gomes-Leal, W.; Anil, S.; Dobkins, G.; Huie, J.R.; Ferguson, A.; Graham, L.; Tuszynski, M. Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Rep. 2019, 13, 105–114. [Google Scholar] [CrossRef]
- White, T.E.; Lane, M.A.; Sandhu, M.S.; O’Steen, B.E.; Fuller, D.D.; Reier, P.J. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp. Neurol. 2010, 225, 231–236. [Google Scholar] [CrossRef]
- Zholudeva, L.; Iyer, N.; Qiang, L.; Spruance, V.M.; Randelman, M.L.; White, N.W.; Bezdudnaya, T.; Fischer, I.; Sakiyama-Elbert, S.E.; Lane, M. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2883–2903. [Google Scholar] [CrossRef]
- Dulin, J.N.; Adler, A.F.; Kumamaru, H.; Poplawski, G.H.D.; Lee-Kubli, C.; Strobl, H.; Gibbs, D.; Kadoya, K.; Fawcett, J.W.; Lu, P.; et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun. 2018, 9, 84. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Y.; Li, C.-J.; Li, M.; Rong, Z.-J.; Jiang, L.; Guo, Z.; Lu, H.-B.; Hu, J.-Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020, 245, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Karova, K.; Wainwright, J.V.; Machova-Urdzikova, L.; Pisal, R.V.; Schmidt, M.; Jendelova, P.; Jhanwar-Uniyal, M. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-κB pathway inhibition. J. Neuroinflamm. 2019, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Mayle, R.E.; Cox, C.A.; Park, D.Y.; Smith, R.L.; Corcoran-Schwartz, I.; Ponnusamy, K.E.; Oshtory, R.; Smuck, M.W.; Mitra, R.; et al. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012, 12, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.; Biziato, D.; Brambilla, E.; Donegà, M.; Alfaro-Cervello, C.; Snider, S.; Salani, G.; Pucci, F.; Comi, G.; García-Verdugo, J.M.; et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 2012, 135, 447–460. [Google Scholar] [CrossRef]
- Emgård, M.; Piao, J.; Aineskog, H.; Liu, J.; Calzarossa, C.; Odeberg, J.; Holmberg, L.; Samuelsson, E.-B.; Bezubik, B.; Vincent, P.H.; et al. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp. Neurol. 2014, 253, 138–145. [Google Scholar] [CrossRef]
- Cheng, I.; Park, D.Y.; Mayle, R.E.; Githens, M.; Smith, R.L.; Park, H.Y.; Hu, S.S.; Alamin, T.F.; Wood, K.B.; Kharazi, A.I. Does timing of transplantation of neural stem cells following spinal cord injury affect outcomes in an animal model? J. Spine Surg. 2017, 3, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Githens, M.; Smith, R.L.; Johnston, T.R.; Park, D.Y.; Stauff, M.P.; Salari, N.; Tileston, K.R.; Kharazi, A.I. Local versus distal transplantation of human neural stem cells following chronic spinal cord injury. Spine J. 2016, 16, 764–769. [Google Scholar] [CrossRef]
- Kong, D.; Feng, B.; Amponsah, A.E.; He, J.; Guo, R.; Liu, B.; Du, X.; Liu, X.; Zhang, S.; Lv, F.; et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res. Ther. 2021, 12, 172. [Google Scholar] [CrossRef]
- Iwai, H.; Shimada, H.; Nishimura, S.; Kobayashi, Y.; Itakura, G.; Hori, K.; Hikishima, K.; Ebise, H.; Negishi, N.; Shibata, S.; et al. Allogeneic Neural Stem/Progenitor Cells Derived from Embryonic Stem Cells Promote Functional Recovery After Transplantation into Injured Spinal Cord of Nonhuman Primates. Stem Cells Transl. Med. 2015, 4, 708–719. [Google Scholar] [CrossRef]
- Pomeshchik, Y.; Puttonen, K.A.; Kidin, I.; Ruponen, M.; Lehtonen, S.; Malm, T.; Åkesson, E.; Hovatta, O.; Koistinaho, J. Transplanted Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Do Not Promote Functional Recovery of Pharmacologically Immunosuppressed Mice with Contusion Spinal Cord Injury. Cell Transplant. 2015, 24, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.-M.; Wang, T.-H. Optimal Location and Time for Neural Stem Cell Transplantation into Transected Rat Spinal Cord. Cell. Mol. Neurobiol. 2011, 31, 407–414. [Google Scholar] [CrossRef]
- Iwai, H.; Nori, S.; Nishimura, S.; Yasuda, A.; Takano, M.; Tsuji, O.; Fujiyoshi, K.; Toyama, Y.; Okano, H.; Nakamura, M. Transplantation of Neural Stem/Progenitor Cells at Different Locations in Mice with Spinal Cord Injury. Cell Transplant. 2014, 23, 1451–1464. [Google Scholar] [CrossRef]
- McMahon, S.S.; Albermann, S.; Rooney, G.E.; Shaw, G.; Garcia, Y.; Sweeney, E.; Hynes, J.; Dockery, P.; O’Brien, T.; Windebank, A.J.; et al. Engraftment, migration and differentiation of neural stem cells in the rat spinal cord following contusion injury. Cytotherapy 2010, 12, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Piltti, K.M.; Avakian, S.N.; Funes, G.M.; Hu, A.; Uchida, N.; Anderson, A.J.; Cummings, B.J. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res. 2015, 15, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.E.; Chang, E.-A.; Suhr, S.T.; Schlosser, L.O.; Mondello, S.E.; Moritz, C.T.; Cibelli, J.B.; Horner, P.J. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp. Neurol. 2013, 248, 491–503. [Google Scholar] [CrossRef]
- Priest, C.A.; Manley, N.C.; Denham, J.; Wirth, E.D.; Lebkowski, J.S. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen. Med. 2015, 10, 939–958. [Google Scholar] [CrossRef]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D.; Lebkowski, J.S. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Denham, J.; Thies, R.S.; Li, Y.; Gautam, A.; Yang, J.; Qiu, L.; Melkoumian, Z.; Weber, J.; Telukuntla, L.; et al. Oligodendrocyte Progenitor Cells Derived from Human Embryonic Stem Cells Express Neurotrophic Factors. Stem Cells Dev. 2006, 15, 943–952. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.-C.; Li, J.; Guan, X.-Y.; Gao, L.; Ma, L.-X.; Li, R.-X.; Peng, Y.-W.; Zhu, G.-P. Transplantation of Oligodendrocyte Precursor Cells Improves Locomotion Deficits in Rats with Spinal Cord Irradiation Injury. PLoS ONE 2013, 8, e57534. [Google Scholar] [CrossRef]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [PubMed]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-W.; Oh, S.; Lee, K.H.; Lee, B.H.; Chang, M.-S. Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury. Int. J. Stem Cells 2018, 11, 177–186. [Google Scholar] [CrossRef]
- Salewski, R.P.; Mitchell, R.A.; Li, L.; Shen, C.; Milekovskaia, M.; Nagy, A.; Fehlings, M.G. Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl. Med. 2015, 4, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-T.; Jiang, L.; Liu, L.; Yin, Y.; Luo, Z.-R.; Long, Z.-Y.; Li, S.; Yu, L.-H.; Wu, Y.-M.; Liu, Y. Local injection of Lenti-Olig2 at lesion site promotes functional recovery of spinal cord injury in rats. CNS Neurosci. Ther. 2017, 23, 475–487. [Google Scholar] [CrossRef]
- Lineage Set for Clinical Testing with OPC Transplant Therapy for Spinal Cord Injuries—Spinal News International. Available online: https://spinalnewsinternational.com/lineage-set-for-clinical-testing-with-opc-transplant-therapy-for-spinal-cord-injuries/ (accessed on 2 August 2022).
- Lineage Enters into Exclusive Agreement with Neurgain Technologies to Evaluate Novel Delivery System for OPC1 to Treat Spinal Cord Injury | Business Wire. Available online: https://www.businesswire.com/news/home/20210208005187/en/Lineage-Enters-Into-Exclusive-Agreement-with-Neurgain-Technologies-to-Evaluate-Novel-Delivery-System-for-OPC1-to-Treat-Spinal-Cord-Injury (accessed on 2 December 2021).
- Davies, S.J.A.; Shih, C.-H.; Noble, M.; Mayer-Proschel, M.; Davies, J.E.; Proschel, C. Transplantation of Specific Human Astrocytes Promotes Functional Recovery after Spinal Cord Injury. PLoS ONE 2011, 6, e17328. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Wang, H.; Xi, H.; Gou, C.; Zhang, J.; Zhang, F.; Liu, Y. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up with MRI. Chin. J. Repar. Reconstr. Surg. 2006, 20, 439–443. [Google Scholar]
- Guest, J.; Herrera, L.P.; Qian, T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord 2005, 44, 135–142. [Google Scholar] [CrossRef]
- Monje, P.V.; Deng, L.; Xu, X.-M. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front. Cell. Neurosci. 2021, 15, 690894. [Google Scholar] [CrossRef]
- Boyer, P.J.; Tuite, G.F.; Dauser, R.C.; Muraszko, K.M.; Tennekoon, G.I.; Rutkowski, J. Sources of Human Schwann Cells and the Influence of Donor Age. Exp. Neurol. 1994, 130, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.; Athauda, G.; De La Cruz, G.; Chan, W.; Golshani, R.; Berrocal, Y.; Henao, M.; Lalwani, A.; Mannoji, C.; Assi, M.; et al. Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia 2017, 65, 1278–1301. [Google Scholar] [CrossRef] [PubMed]
- Ursavas, S.; Darici, H.; Karaoz, E. Olfactory ensheathing cells: Unique glial cells promising for treatments of spinal cord injury. J. Neurosci. Res. 2021, 99, 1579–1597. [Google Scholar] [CrossRef]
- Khankan, R.R.; Griffis, K.G.; Haggerty-Skeans, J.R.; Zhong, H.; Roy, R.R.; Edgerton, V.R.; Phelps, P.E. Olfactory Ensheathing Cell Transplantation after a Complete Spinal Cord Transection Mediates Neuroprotective and Immunomodulatory Mechanisms to Facilitate Regeneration. J. Neurosci. 2016, 36, 6269–6286. [Google Scholar] [CrossRef] [PubMed]
- Keyvan-Fouladi, N.; Raisman, G.; Li, Y. Functional Repair of the Corticospinal Tract by Delayed Transplantation of Olfactory Ensheathing Cells in Adult Rats. J. Neurosci. 2003, 23, 9428–9434. [Google Scholar] [CrossRef] [PubMed]
- Barbour, H.R.; Plant, C.D.; Harvey, A.R.; Plant, G.W. Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion. BMC Neurosci. 2013, 14, 106. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, X.; Chen, Y.; Xia, H. Intravenous transplantation of olfactory bulb ensheathing cells for a spinal cord hemisection injury rat model. Cell Transplant. 2019, 28, 1585–1602. [Google Scholar] [CrossRef]
- Senior, K. Olfactory ensheathing cells to be used in spinal-cord repair trial. Lancet Neurol. 2002, 1, 269. [Google Scholar] [CrossRef]
- Li, L.; Adnan, H.; Xu, B.; Wang, J.; Wang, C.; Li, F.; Tang, K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2015, 24, 919–930. [Google Scholar] [CrossRef]
- Thomas, A.M.; Seidlits, S.K.; Goodman, A.G.; Kukushliev, T.V.; Hassani, D.M.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr. Biol. 2014, 6, 694–705. [Google Scholar] [CrossRef]
- Smith, P.M.; Jeffery, N. Histological and Ultrastructural Analysis of White Matter Damage after Naturally-occurring Spinal Cord Injury. Brain Pathol. 2006, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Oudega, M.; Bates, M.L.; Wood, P.M.; Kleitman, N.; Bunge, M.B. Schwann Cell but Not Olfactory Ensheathing Glia Transplants Improve Hindlimb Locomotor Performance in the Moderately Contused Adult Rat Thoracic Spinal Cord. J. Neurosci. 2002, 22, 6670–6681. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Bastidas, J.; Izabel, S.S.; Ghosh, M. Schwann Cell Transplantation Subdues the Pro-Inflammatory Innate Immune Cell Response after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, H.; Xi, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. A Prospective Randomized Double-Blind Clinical Trial Using a Combination of Olfactory Ensheathing Cells and Schwann Cells for the Treatment of Chronic Complete Spinal Cord Injuries. Cell Transplant. 2014, 23 (Suppl. S1), 35–44. [Google Scholar] [CrossRef]
- Schira, J.; Heinen, A.; Poschmann, G.; Ziegler, B.; Hartung, H.; Stühler, K.; Küry, P. Secretome analysis of nerve repair mediating Schwann cells reveals Smad-dependent trophism. FASEB J. 2019, 33, 4703–4715. [Google Scholar] [CrossRef]
- Xu, X.M.; Guénard, V.; Kleitman, N.; Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995, 351, 145–160. [Google Scholar] [CrossRef]
- Lopez-Leal, R.; Díaz-Viraqué, F.; Catalan, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann Cell Reprogramming into Repair Cells Increases MiRNA-21 Expression in Exosomes Promot-ing Axonal Growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef]
- Khan, A.; Diaz, A.; Brooks, A.E.; Burks, S.S.; Athauda, G.; Wood, P.; Lee, Y.-S.; Silvera, R.; Donaldson, M.; Pressman, Y.; et al. Scalable culture techniques to generate large numbers of purified human Schwann cells for clinical trials in human spinal cord and peripheral nerve injuries. J. Neurosurg. Spine 2022, 36, 135–144. [Google Scholar] [CrossRef]
- El Seblani, N.; Welleford, A.S.; Quintero, J.E.; van Horne, C.G.; Gerhardt, G.A. Invited review: Utilizing peripheral nerve regenerative elements to repair damage in the CNS. J. Neurosci. Methods 2020, 335, 108623. [Google Scholar] [CrossRef]
- Saberi, H.; Firouzi, M.; Habibi, Z.; Moshayedi, P.; Aghayan, H.R.; Arjmand, B.; Hosseini, K.; Razavi, H.E.; Yekaninejad, M.S. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases: Clinical Article. J. Neurosurg. Spine 2011, 15, 515–525. [Google Scholar] [CrossRef]
- Levi, A.D.; Bunge, R.P. Studies of Myelin Formation after Transplantation of Human Schwann Cells into the Severe Combined Immunodeficient Mouse. Exp. Neurol. 1994, 130, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Guenard, V.; Aebischer, P.; Bunge, R. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J. Neurosci. 1994, 14, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, F.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.-H.; et al. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.J.; Solano, J.P.; Benavides, F.D.; Guest, J.D. Intraspinal Delivery of Schwann Cells for Spinal Cord Injury. Methods Mol. Biol. 2018, 1739, 467–484. [Google Scholar] [CrossRef]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef]
- The Safety of AhSC in Chronic SCI with Rehabilitation—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02354625 (accessed on 1 August 2022).
- Ibarra, A.; Silva-García, R.; Parra-Villamar, D.; Blancas-Espinoza, L.; Garcia-Vences, E.; Herrera-García, J.; Flores-Romero, A.; Toscano-Zapien, A.; Villa, J.V.; Barrera-Roxana, R.; et al. Neuroprotective effect of immunomodulatory peptides in rats with traumatic spinal cord injury. Neural Regen. Res. 2021, 16, 1273–1280. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Zhang, H.; Zhang, Q.; Zhao, Y.; Xiao, Z.; Liu, W.; Chen, B.; Gao, L.; Sun, Z.; et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 2021, 269, 120479. [Google Scholar] [CrossRef]
- Transplantation of Autologous Adipose Derived Stem Cells (ADSCs) in Spinal Cord Injury Treatment—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02034669 (accessed on 1 December 2021).
- Tien, N.L.B.; Hoa, N.D.; Van Thanh, V.; Van Thach, N.; Ngoc, V.T.N.; Dinh, T.C.; Phuong, T.N.T.; Toi, P.L.; Chu, D.T. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced. J. Med. Sci. 2019, 7, 4399–4405. [Google Scholar] [CrossRef]
- Safety and Preliminary Efficacy of FAB117-HC in Patients with Acute Traumatic Spinal Cord Injury—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02917291 (accessed on 1 December 2021).
- Stem Cell Spinal Cord Injury Exoskeleton and Virtual Reality Treatment Study—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03225625 (accessed on 2 December 2021).
- Umbilical Cord Blood Cell Transplant into Injured Spinal Cord with Lithium Carbonate or Placebo Followed by Locomotor Training—Tabular View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03979742 (accessed on 1 December 2021).
- Park, H.C.; Shim, Y.S.; Ha, Y.; Yoon, S.H.; Park, S.R.; Choi, B.H.; Park, H.S. Treatment of Complete Spinal Cord Injury Patients by Autologous Bone Marrow Cell Transplantation and Administration of Granulocyte-Macrophage Colony Stimulating Factor. Tissue Eng. 2005, 11, 913–922. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Eskian, M.; Vaccaro, A.R.; Rahimi-Movaghar, V. Granulocyte Colony-Stimulating Factor (G-CSF) for the Treatment of Spinal Cord Injury. CNS Drugs 2017, 31, 911–937. [Google Scholar] [CrossRef]
- Pang, C.-Y.; Yang, K.-L.; Fu, C.-H.; Sun, L.-Y.; Chen, S.-Y.; Liao, C.-H. G-CSF enhances the therapeutic potency of stem cells transplantation in spinal cord-injured rats. Regen. Med. 2019, 14, 571–583. [Google Scholar] [CrossRef]
- Talifu, Z.; Qin, C.; Xin, Z.; Chen, Y.; Liu, J.; Dangol, S.; Ma, X.; Gong, H.; Pei, Z.; Yu, Y.; et al. The Overexpression of Insulin-Like Growth Factor-1 and Neurotrophin-3 Promote Functional Recovery and Alleviate Spasticity After Spinal Cord Injury. Front. Neurosci. 2022, 16, 863793. [Google Scholar] [CrossRef]
- Allahdadi, K.J.; De Santana, T.A.; Santos, G.C.; Azevedo, C.M.; Mota, R.A.; Nonaka, C.K.; Silva, D.N.; Valim, C.X.R.; Figueira, C.P.; Dos Santos, W.L.C.; et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res. Ther. 2019, 10, 146. [Google Scholar] [CrossRef]
- Zeng, X.; Qiu, X.-C.; Ma, Y.-H.; Duan, J.-J.; Chen, Y.-F.; Gu, H.-Y.; Wang, J.-M.; Ling, E.-A.; Wu, J.-L.; Wu, W.; et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 2015, 53, 184–201. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, R.-P.; Li, J.-D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. 2014, 156, 1409–1418. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Zhu, Z.; Gu, C.; Waqas, A.; Chen, L. Emerging Exosomes and Exosomal MiRNAs in Spinal Cord Injury. Front. Cell Dev. Biol. 2021, 9, 703989. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Long, L.; Lu, X.; Zhu, K.; Tuo, Y.; Chen, N.; Zhao, X.; Wang, L.; Wan, Y. Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury. Exp. Mol. Med. 2020, 52, 2020–2033. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined with Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- NeuroRegen ScaffoldTM with Stem Cells for Chronic Spinal Cord Injury Repair—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02352077 (accessed on 10 January 2023).
- Chen, W.; Zhang, Y.; Yang, S.; Sun, J.; Qiu, H.; Hu, X.; Niu, X.; Xiao, Z.; Zhao, Y.; Zhou, Y.; et al. NeuroRegen Scaffolds Combined with Autologous Bone Marrow Mononuclear Cells for the Repair of Acute Complete Spinal Cord Injury: A 3-Year Clinical Study. Cell Transplant. 2020, 29, 1–11. [Google Scholar] [CrossRef]
- Functional Neural Regeneration Collagen Scaffold Transplantation in Acute Spinal Cord Injury Patients—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02510365 (accessed on 28 December 2022).
- Autologous Bulbar Olfactory Ensheathing Cells and Nerve Grafts for Treatment of Patients with Spinal Cord Transection—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03933072 (accessed on 2 August 2022).
- James, N.D.; Shea, J.; Muir, E.M.; Verhaagen, J.; Schneider, B.L.; Bradbury, E.J. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp. Neurol. 2015, 271, 131–135. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Miller, J.H.; Coulson-Thomas, Y.M.; Horn, K.; Alilain, W.J.; Silver, J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 2010, 70, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.M.; Amps, J.; Viapiano, M.S.; Matthews, R.T.; Wagoner, M.R.; Whitaker, C.M.; Alilain, W.; Yonkof, A.L.; Khalyfa, A.; Cooper, N.G.; et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp. Neurol. 2008, 209, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Xu, X.-M.; Devries, W.H.; Enzmann, G.U.; Ping, P.; Tsoulfas, P.; Wood, P.M.; Bunge, M.B.; Whittemore, S.R. Functional Recovery in Traumatic Spinal Cord Injury after Transplantation of Multineurotrophin-Expressing Glial-Restricted Precursor Cells. J. Neurosci. 2005, 25, 6947–6957. [Google Scholar] [CrossRef] [PubMed]
- Urfer, R.; Tsoulfas, P.; Soppet, D.; Escandón, E.; Parada, L.; Presta, L. The binding epitopes of neurotrophin-3 to its receptors trkC and gp75 and the design of a multifunctional human neurotrophin. EMBO J. 1994, 13, 5896–5909. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of Engineered Schwann Cell Grafts to Secrete Neurotrophin and Chondroitinase Promotes Axonal Regeneration and Locomotion after Spinal Cord Injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef]
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef]
- Xue, F.; Wu, E.J.; Zhang, P.X.; Li-Ya, A.; Kou, Y.H.; Yin, X.F.; Han, N. Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen. Res. 2015, 10, 104–111. [Google Scholar] [CrossRef]
- Johnson, P.; Tatara, A.; Shiu, A.; Sakiyama-Elbert, S.E. Controlled Release of Neurotrophin-3 and Platelet-Derived Growth Factor from Fibrin Scaffolds Containing Neural Progenitor Cells Enhances Survival and Differentiation into Neurons in a Subacute Model of SCI. Cell Transplant. 2010, 19, 89–101. [Google Scholar] [CrossRef]
- Park, S.-S.; Lee, Y.J.; Lee, S.H.; Lee, D.; Choi, K.; Kim, W.-H.; Kweon, O.-K.; Han, H.J. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 2012, 14, 584–597. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-Distance Growth and Connectivity of Neural Stem Cells after Severe Spinal Cord Injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Hejčl, A.; Šedý, J.; Kapcalová, M.; Toro, D.A.; Amemori, T.; Lesný, P.; Likavčanová-Mašínová, K.; Krumbholcová, E.; Přádný, M.; Michálek, J.; et al. HPMA-RGD Hydrogels Seeded with Mesenchymal Stem Cells Improve Functional Outcome in Chronic Spinal Cord Injury. Stem Cells Dev. 2010, 19, 1535–1546. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 1115–1127. [Google Scholar] [CrossRef]
- Rao, Z.; Lin, Z.; Song, P.; Quan, D.; Bai, Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front. Cell. Neurosci. 2022, 16, 926222. [Google Scholar] [CrossRef]
- Kang, P.H.; Kumar, S.; Schaffer, D.V. Novel biomaterials to study neural stem cell mechanobiology and improve cell-replacement therapies. Curr. Opin. Biomed. Eng. 2017, 4, 13–20. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B.; et al. Functional Regeneration of Supraspinal Connections in a Patient with Transected Spinal Cord following Transplantation of Bulbar Olfactory Ensheathing Cells with Peripheral Nerve Bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized Acellular Nerve Graft Is Immunologically Tolerated and Supports Regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef]
- DePaul, M.A.; Lin, C.-Y.; Silver, J.; Lee, Y.-S. Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci. Rep. 2017, 7, 9018. [Google Scholar] [CrossRef]
- Furlan, J.C.; Rademeyer, H.J.; Gastle, N.; Walden, K.; Lemay, J.-F.; Ho, C.; Musselman, K.; Brady, J.; Mouneimne, M.; Knox, J.; et al. The Potential Effects of Concomitant Traumatic Brain Injury (TBI) on the Survival, and Neurological and Functional Recovery after Traumatic Spinal Cord Injury (SCI): An Analysis of a Cohort of 499 Cases. J. Spinal Cord Med. 2021, 44, S278–S324. [Google Scholar] [CrossRef]
- Craven, B.C.; Brisbois, L.; Pelletier, C.; Rybkina, J.; Heesters, A.; Verrier, M.C. Central Recruitment: A process for engaging and recruiting individuals with spinal cord injury/disease in research at Toronto Rehabilitation Institute. J. Spinal Cord Med. 2021, 44, S240–S249. [Google Scholar] [CrossRef]
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling Up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654. [Google Scholar] [CrossRef]
- Reisman, H.B. Problems in Scale-Up of Biotechnology Production Processes. Crit. Rev. Biotechnol. 1993, 13, 195–253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).