Transplantation of Adipose-Tissue-Engineered Constructs with CRISPR-Mediated UCP1 Activation
Abstract
:1. Introduction
2. Results
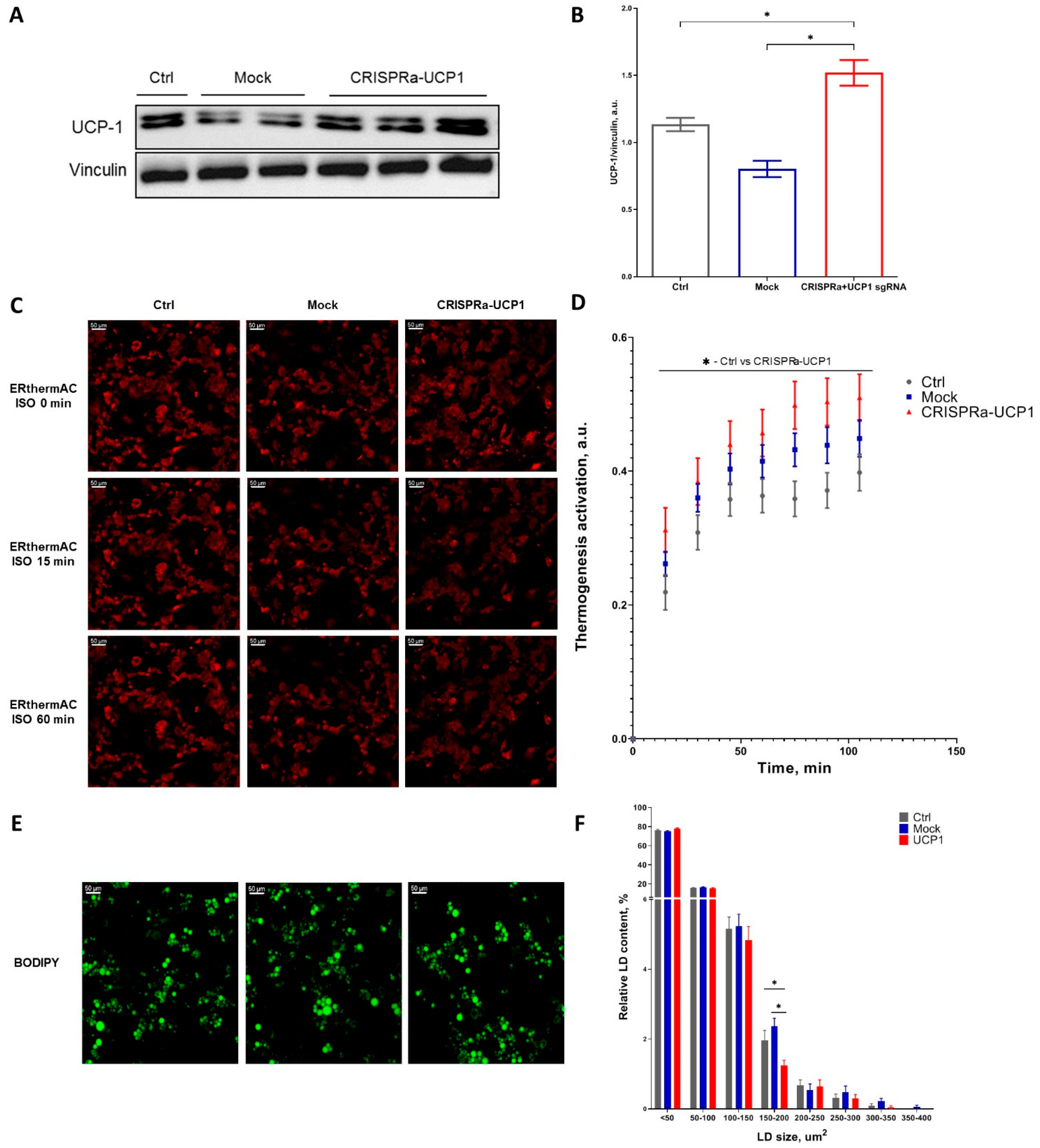
2.1. CRISPRa System Efficiently Increases UCP1 Expression and Activates Thermogenesis in Adipocytes
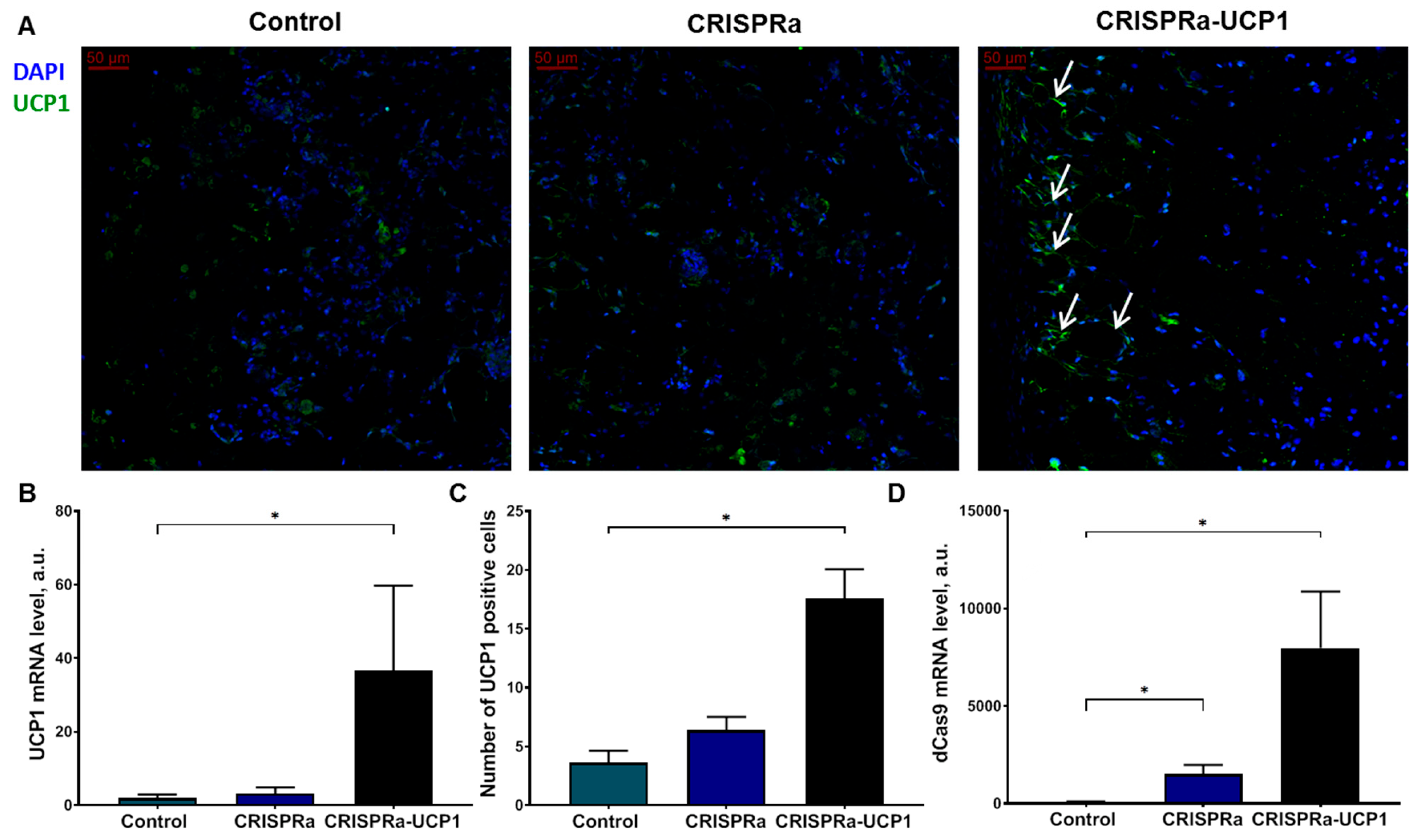
2.2. Transplanted Adipocytes Retain High UCP1 Expression
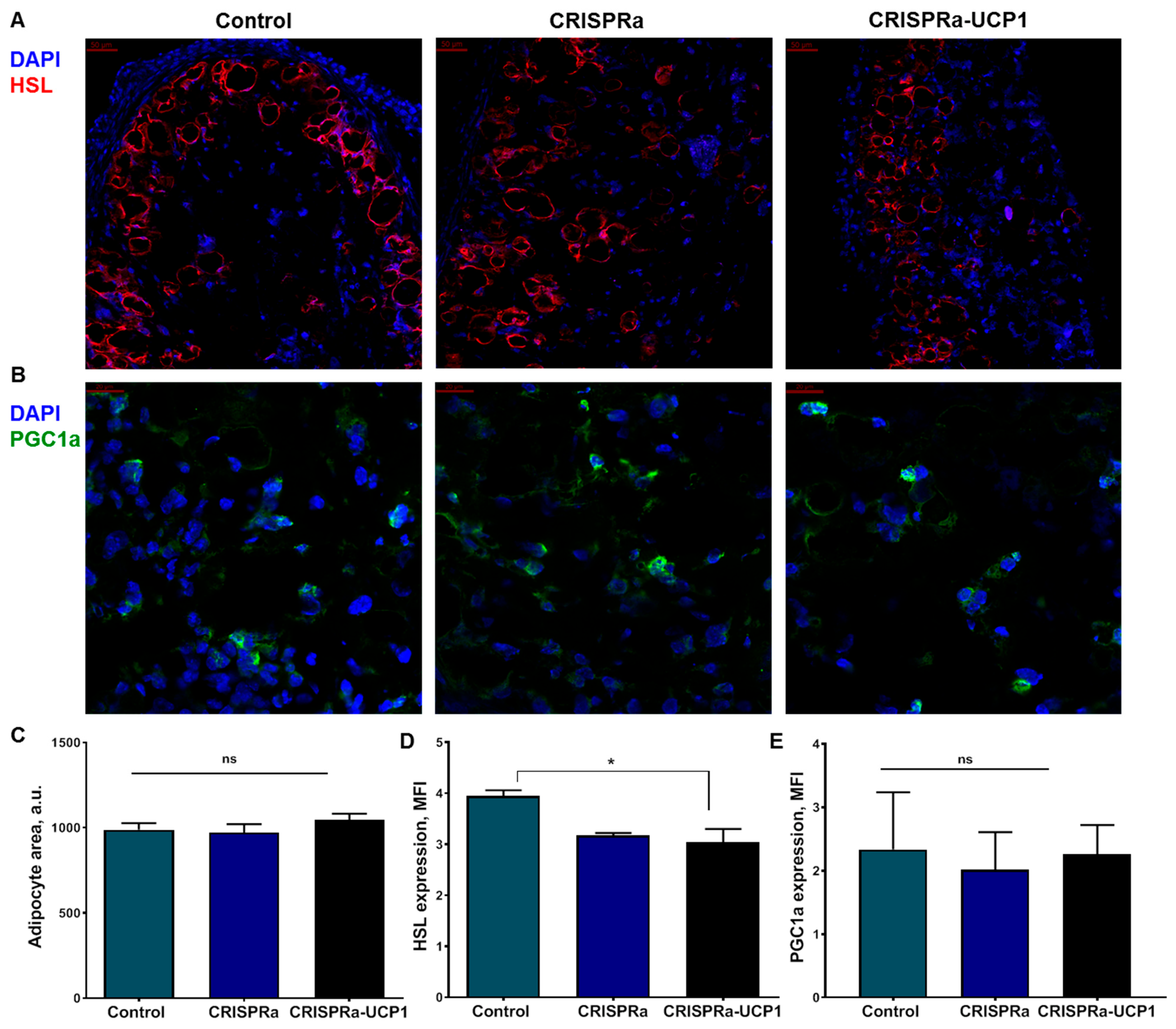
2.3. CRISPRa-UCP1 System Does Not Alter Morphology or Protein Expression in Transplanted Adipocytes
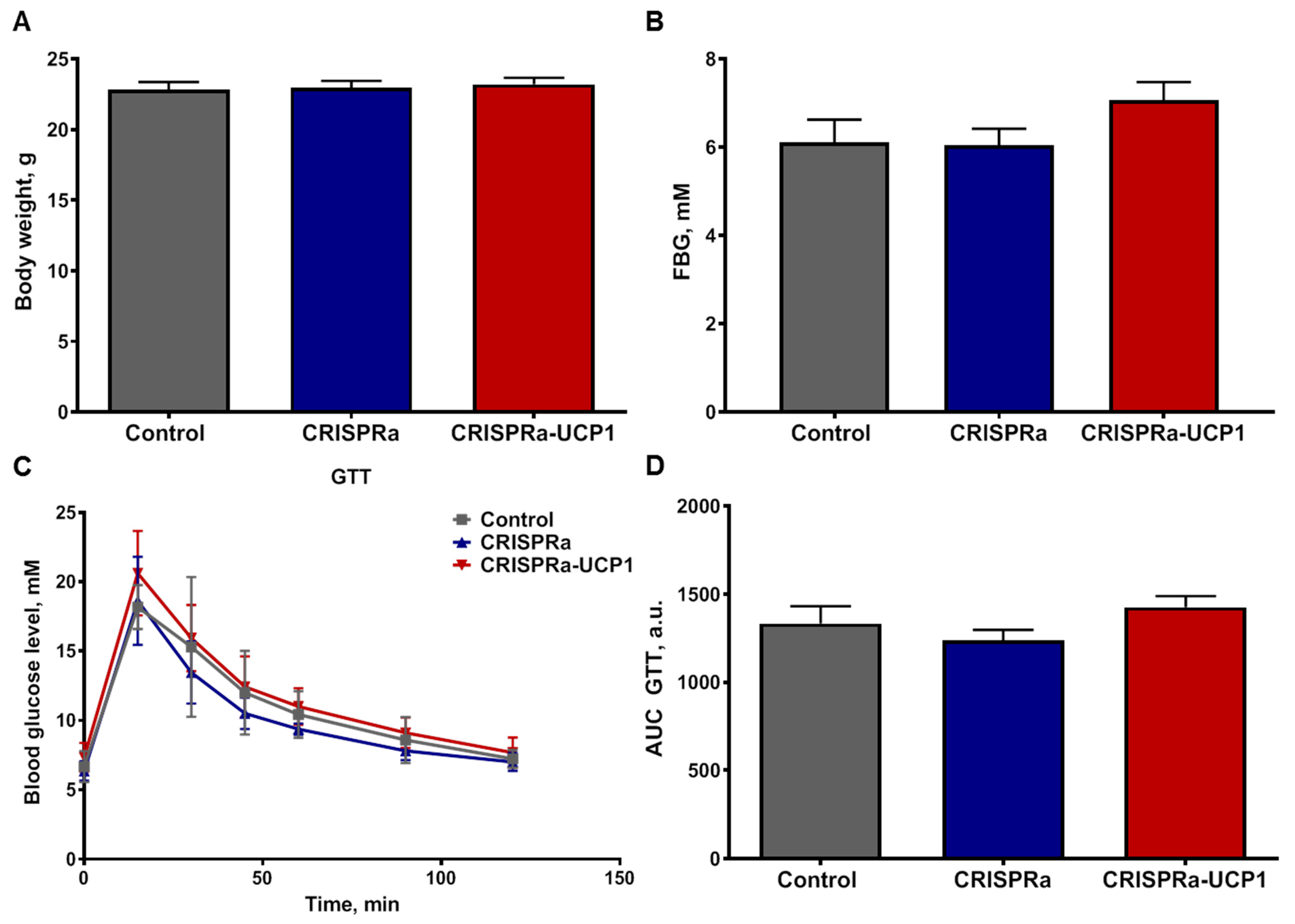
2.4. Transplantation of CRISPRa-Engineered Adipocytes Has No Effect on Systemic Glucose Metabolism
2.5. CRISPRa-UCP1-Modified Tissue Construct Does Not Induce Inflammation and Attracts Host Vasculature
3. Methods
3.1. Isolation, Culture and Adipogenic Differentiation of Murine ADSC
3.2. Generation of CRISPRa-Engineered Adipocytes
3.3. Western Blotting
3.4. Thermogenesis Assessment
3.5. Lipid Droplet Size Analysis
3.6. Animal Housing
3.7. CRISPRa-Engineered Adipocyte Transplantation in Animals
3.8. Immunohistochemistry of CRISPRa-Engineered Adipose Tissue Constructs
3.9. Gene Expression in CRISPRa-Engineered Adipose Tissue Constructs
3.10. Enzyme-Linked Immunosorbent Assay (ELISA)
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSC | adipose-derived stem cells; |
| ANOVA | analysis of variance; |
| AUC | area under curve; |
| CRISPR | clustered regularly interspaced short palindromic repeats; |
| CRISPRa | CRISPR activation technology; |
| CRP | C-reactive protein; |
| DAPI | 4′,6-diamidino-2-phenylindole; |
| DMEM | Dulbecco’s modified Eagle’s medium; |
| dCas9 | dead CRISPR associated protein 9; |
| ELISA | enzyme-linked immunosorbent assay; |
| FBG | fasting blood glucose; |
| GTT | glucose tolerance test; |
| HSF1 | heat shock factor 1; |
| HSL | hormone-sensitive lipase; |
| ISO | isoproterenol; |
| LD | lipid droplets; |
| MCP | MS2 coat protein; |
| MFI | mean fluorescence intensity; |
| PBS | phosphate-buffered saline; |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator-1 α; |
| SEM | standard error mean; |
| sgRNA | single guide RNA; |
| T2DM | type 2 diabetes mellitus; |
| TAG | triacylglycerides; |
| TNFα | tumor necrosis factor α; |
| UCP1 | uncoupling protein 1 |
References
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Tharp, K.M.; Jha, A.K.; Kraiczy, J.; Yesian, A.; Karateev, G.; Sinisi, R.; Dubikovskaya, E.A.; Healy, K.E.; Stahl, A. Matrix-Assisted Transplantation of Functional Beige Adipose Tissue. Diabetes 2015, 64, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Zhang, L.; Avery, J.; Yin, A.; Du, Y.; Wang, H.; Li, Z.; Fu, H.; Yin, H.; Dalton, S. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat. Commun. 2020, 11, 2758. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Abedi, M.; Foroughi Heravani, N.; Hadavandkhani, M.; Arabi, M.; Tayanloo-Beik, A.; Sheikh Hosseini, M.; Gerami, H.; Khatami, F.; Larijani, B.; et al. Brown adipose tissue transplantation as a novel alternative to obesity treatment: A systematic review. Int. J. Obes. 2021, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Dani, V.; Yao, X.; Dani, C. Transplantation of fat tissues and iPSC-derived energy expenditure adipocytes to counteract obesity-driven metabolic disorders: Current strategies and future perspectives. Rev. Endocr. Metab. Disord. 2022, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Kopecky, J.; Clarke, G.; Enerbäck, S.; Spiegelman, B.; Kozak, L.P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Investig. 1995, 96, 2914–2923. [Google Scholar] [CrossRef]
- Kopecký, J.; Hodný, Z.; Rossmeisl, M.; Syrový, I.; Kozak, L.P. Reduction of dietary obesity in aP2-Ucp transgenic mice: Physiology and adipose tissue distribution. Am. J. Physiol. 1996, 270, E768–E775. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, J.; Huang, J.; Zhang, H.; Zhang, R.; Zhang, X.; Cao, C.; Hambly, C.; Qin, G.; Yao, J.; et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E9474–E9482. [Google Scholar] [CrossRef]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; V, S.S.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Pan, R.; Zhu, X.; Maretich, P.; Chen, Y. Combating Obesity With Thermogenic Fat: Current Challenges and Advancements. Front. Endocrinol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Dewal, R.S.; Stanford, K.I. The beneficial effects of brown adipose tissue transplantation. Mol. Asp. Med. 2019, 68, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef] [PubMed]
- Tsagkaraki, E.; Nicoloro, S.M.; DeSouza, T.; Solivan-Rivera, J.; Desai, A.; Lifshitz, L.M.; Shen, Y.; Kelly, M.; Guilherme, A.; Henriques, F.; et al. CRISPR-enhanced human adipocyte browning as cell therapy for metabolic disease. Nat. Commun. 2021, 12, 6931. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Moiani, A.; Paleari, Y.; Sartori, D.; Mezzadra, R.; Miccio, A.; Cattoglio, C.; Cocchiarella, F.; Lidonnici, M.R.; Ferrari, G.; Mavilio, F. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J. Clin. Investig. 2012, 122, 1653–1666. [Google Scholar] [CrossRef]
- Park, J.; Yoon, J.; Kwon, D.; Han, M.-J.; Choi, S.; Park, S.; Lee, J.; Lee, K.; Lee, J.; Lee, S.; et al. Enhanced genome editing efficiency of CRISPR PLUS: Cas9 chimeric fusion proteins. Sci. Rep. 2021, 11, 16199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther.-Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, Y.; Nihongaki, Y.; Sato, M.; Yoshimoto, K. Control of Adipogenic Differentiation in Mesenchymal Stem Cells via Endogenous Gene Activation Using CRISPR-Cas9. ACS Synth. Biol. 2017, 6, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.N.; Chang, Y.H.; Truong, V.A.; Lai, P.L.; Nguyen, T.K.N.; Hu, Y.C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef]
- Becirovic, E. Maybe you can turn me on: CRISPRa-based strategies for therapeutic applications. Cell. Mol. Life Sci. 2022, 79, 130. [Google Scholar] [CrossRef]
- Airenne, K.J.; Hu, Y.C.; Kost, T.A.; Smith, R.H.; Kotin, R.M.; Ono, C.; Matsuura, Y.; Wang, S.; Ylä-Herttuala, S. Baculovirus: An insect-derived vector for diverse gene transfer applications. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 739–749. [Google Scholar] [CrossRef]
- Truong, V.A.; Hsu, M.N.; Kieu Nguyen, N.T.; Lin, M.W.; Shen, C.C.; Lin, C.Y.; Hu, Y.C. CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucleic Acids Res. 2019, 47, e74. [Google Scholar] [CrossRef]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? BioMed Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef]
- Munir, H.; Ward, L.S.C.; Sheriff, L.; Kemble, S.; Nayar, S.; Barone, F.; Nash, G.B.; McGettrick, H.M. Adipogenic Differentiation of Mesenchymal Stem Cells Alters Their Immunomodulatory Properties in a Tissue-Specific Manner. Stem Cells 2017, 35, 1636–1646. [Google Scholar] [CrossRef]
- Sztalryd, C.; Xu, G.; Dorward, H.; Tansey, J.T.; Contreras, J.A.; Kimmel, A.R.; Londos, C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 2003, 161, 1093–1103. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kasza, I.; Kühn, J.P.; Völzke, H.; Hernando, D.; Xu, Y.G.; Siebert, J.W.; Gibson, A.L.F.; Yen, C.E.; Nelson, D.W.; MacDougald, O.A.; et al. Contrasting recruitment of skin-associated adipose depots during cold challenge of mouse and human. J. Physiol. 2022, 600, 847–868. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.-Y.; Chen, C.-L.; Lin, S.-Y.; Li, K.-C.; Yeh, C.-L.; Chen, G.-Y.; Lin, C.-Y.; Hu, Y.-C. Efficient gene delivery into cell lines and stem cells using baculovirus. Nat. Protoc. 2014, 9, 1882–1899. [Google Scholar] [CrossRef] [PubMed]
- Kriszt, R.; Arai, S.; Itoh, H.; Lee, M.H.; Goralczyk, A.G.; Ang, X.M.; Cypess, A.M.; White, A.P.; Shamsi, F.; Xue, R.; et al. Optical visualisation of thermogenesis in stimulated single-cell brown adipocytes. Sci. Rep. 2017, 7, 1383. [Google Scholar] [CrossRef]
- Exner, T.; Beretta, C.A.; Gao, Q.; Afting, C.; Romero-Brey, I.; Bartenschlager, R.; Fehring, L.; Poppelreuther, M.; Füllekrug, J. Lipid droplet quantification based on iterative image processing. J. Lipid Res. 2019, 60, 1333–1344. [Google Scholar] [CrossRef]
- Gagnon, R.C.; Peterson, J.J. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J. Pharmacokinet. Biopharm. 1998, 26, 87–102. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Konermann, S.; Brigham, M.D.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.S.; Nishimasu, H.; et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2015, 517, 583–588. [Google Scholar] [CrossRef]
- Liao, H.K.; Hatanaka, F.; Araoka, T.; Reddy, P.; Wu, M.Z.; Sui, Y.; Yamauchi, T.; Sakurai, M.; O’Keefe, D.D.; Núñez-Delicado, E.; et al. In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-epigenetic Modulation. Cell 2017, 171, 1495–1507.e15. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. J. Am. Biol. Saf. Assoc. 2020, 25, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Counsell, J.R.; Asgarian, Z.; Meng, J.; Ferrer, V.; Vink, C.A.; Howe, S.J.; Waddington, S.N.; Thrasher, A.J.; Muntoni, F.; Morgan, J.E.; et al. Lentiviral vectors can be used for full-length dystrophin gene therapy. Sci. Rep. 2017, 7, 44775. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Kohn, D.B.; Ekert, J.E.; Tarantal, A.F. Morphological Analysis and Lentiviral Transduction of Fetal Monkey Bone Marrow-Derived Mesenchymal Stem Cells. Mol. Ther. 2004, 9, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Annoni, A.; Gregori, S.; Naldini, L.; Cantore, A. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell. Immunol. 2019, 342, 103802. [Google Scholar] [CrossRef] [PubMed]
- Michurina, S.S.; Stafeev, I.S.; Menshikov, M.Y.; Parfyonova, Y.V. Mitochondrial dynamics keep balance of nutrient combustion in thermogenic adipocytes. Mitochondrion 2021, 59, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef]
- Sponton, C.H.; de Lima-Junior, J.C.; Leiria, L.O. What puts the heat on thermogenic fat: Metabolism of fuel substrates. Trends Endocrinol. Metab. TEM 2022, 33, 587–599. [Google Scholar] [CrossRef]
- Steensels, S.; Ersoy, B.A. Fatty acid activation in thermogenic adipose tissue. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 79–90. [Google Scholar] [CrossRef]
- Guilherme, A.; Yenilmez, B.; Bedard, A.H.; Henriques, F.; Liu, D.; Lee, A.; Goldstein, L.; Kelly, M.; Nicoloro, S.M.; Chen, M.; et al. Control of Adipocyte Thermogenesis and Lipogenesis through β3-Adrenergic and Thyroid Hormone Signal Integration. Cell Rep. 2020, 31, 107598. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Ylä-Herttuala, S.; Airenne, K.J. How to avoid complement attack in baculovirus-mediated gene delivery. J. Invertebr. Pathol. 2011, 107, S71–S79. [Google Scholar] [CrossRef]
- Chang, M.C.; Eslami, Z.; Ennis, M.; Goodwin, P.J. Crown-like structures in breast adipose tissue of breast cancer patients: Associations with CD68 expression, obesity, metabolic factors and prognosis. npj Breast Cancer 2021, 7, 97. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chiu, S.-Y.; Huang, H.-W.; Cheng, B.-H.; Pan, H.-M.; Huang, W.-L.; Chang, H.-H.; Liao, C.-C.; Jiang, S.-T.; Su, Y.-C. A reporter mouse for non-invasive detection of toll-like receptor ligands induced acute phase responses. Sci. Rep. 2019, 9, 19065. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]

| sgRNA | Sequence | Bp Upstream of UCP1 Transcription Start Site |
|---|---|---|
| 1 | TCCACAGCTAGAATAGCTGT | 353 |
| 2 | ACAAAAGGCACCACGCTGCG | 252 |
| 3 | CAGTTCCTGATTATGCCCAG | 215 |
| 4 | AGGGCTTTGGGAGTGACGCG | 156 |
| mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| b-actin | AGACCTTCAACACCCCAGCCAT | GGATGGCGTGAGGGAGAGCATA |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG |
| dCas9 | GGCTACGCCGGATACATTGA | CTCTTGCCGCCTGAGGATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michurina, S.; Stafeev, I.; Boldyreva, M.; Truong, V.A.; Ratner, E.; Menshikov, M.; Hu, Y.-C.; Parfyonova, Y. Transplantation of Adipose-Tissue-Engineered Constructs with CRISPR-Mediated UCP1 Activation. Int. J. Mol. Sci. 2023, 24, 3844. https://doi.org/10.3390/ijms24043844
Michurina S, Stafeev I, Boldyreva M, Truong VA, Ratner E, Menshikov M, Hu Y-C, Parfyonova Y. Transplantation of Adipose-Tissue-Engineered Constructs with CRISPR-Mediated UCP1 Activation. International Journal of Molecular Sciences. 2023; 24(4):3844. https://doi.org/10.3390/ijms24043844
Chicago/Turabian StyleMichurina, Svetlana, Iurii Stafeev, Maria Boldyreva, Vu Anh Truong, Elizaveta Ratner, Mikhail Menshikov, Yu-Chen Hu, and Yelena Parfyonova. 2023. "Transplantation of Adipose-Tissue-Engineered Constructs with CRISPR-Mediated UCP1 Activation" International Journal of Molecular Sciences 24, no. 4: 3844. https://doi.org/10.3390/ijms24043844
APA StyleMichurina, S., Stafeev, I., Boldyreva, M., Truong, V. A., Ratner, E., Menshikov, M., Hu, Y.-C., & Parfyonova, Y. (2023). Transplantation of Adipose-Tissue-Engineered Constructs with CRISPR-Mediated UCP1 Activation. International Journal of Molecular Sciences, 24(4), 3844. https://doi.org/10.3390/ijms24043844









