Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube
Abstract
:1. Introduction
2. Results
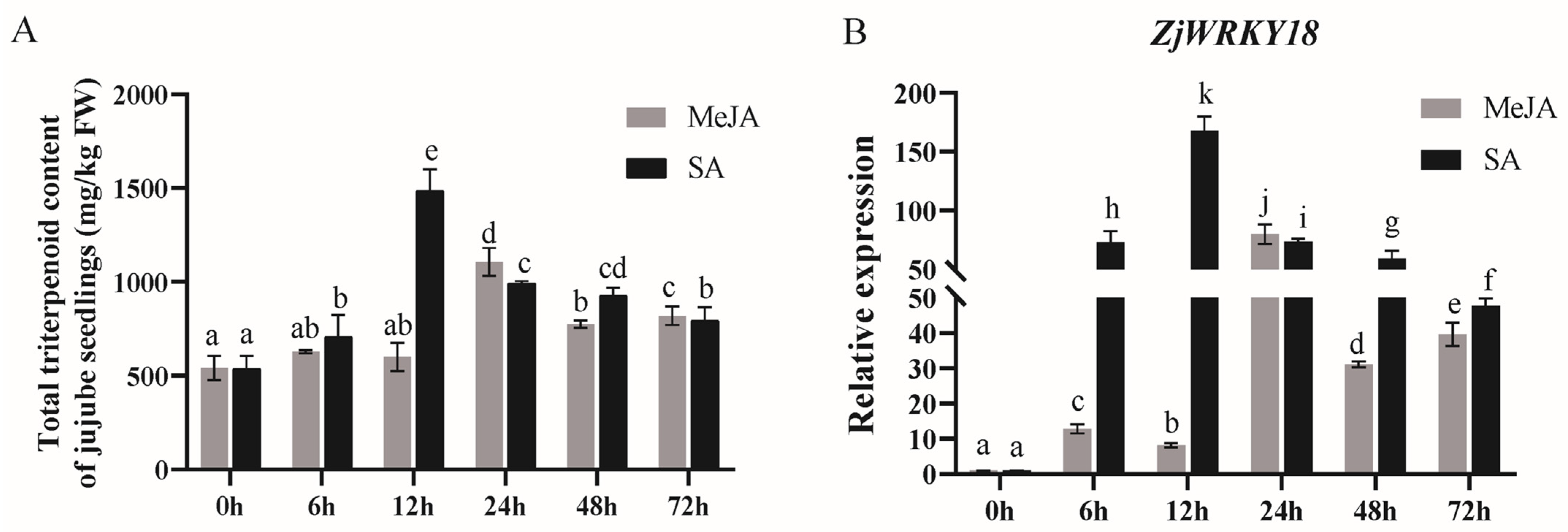
2.1. Identification and Expression Analysis of ZjWRKY18
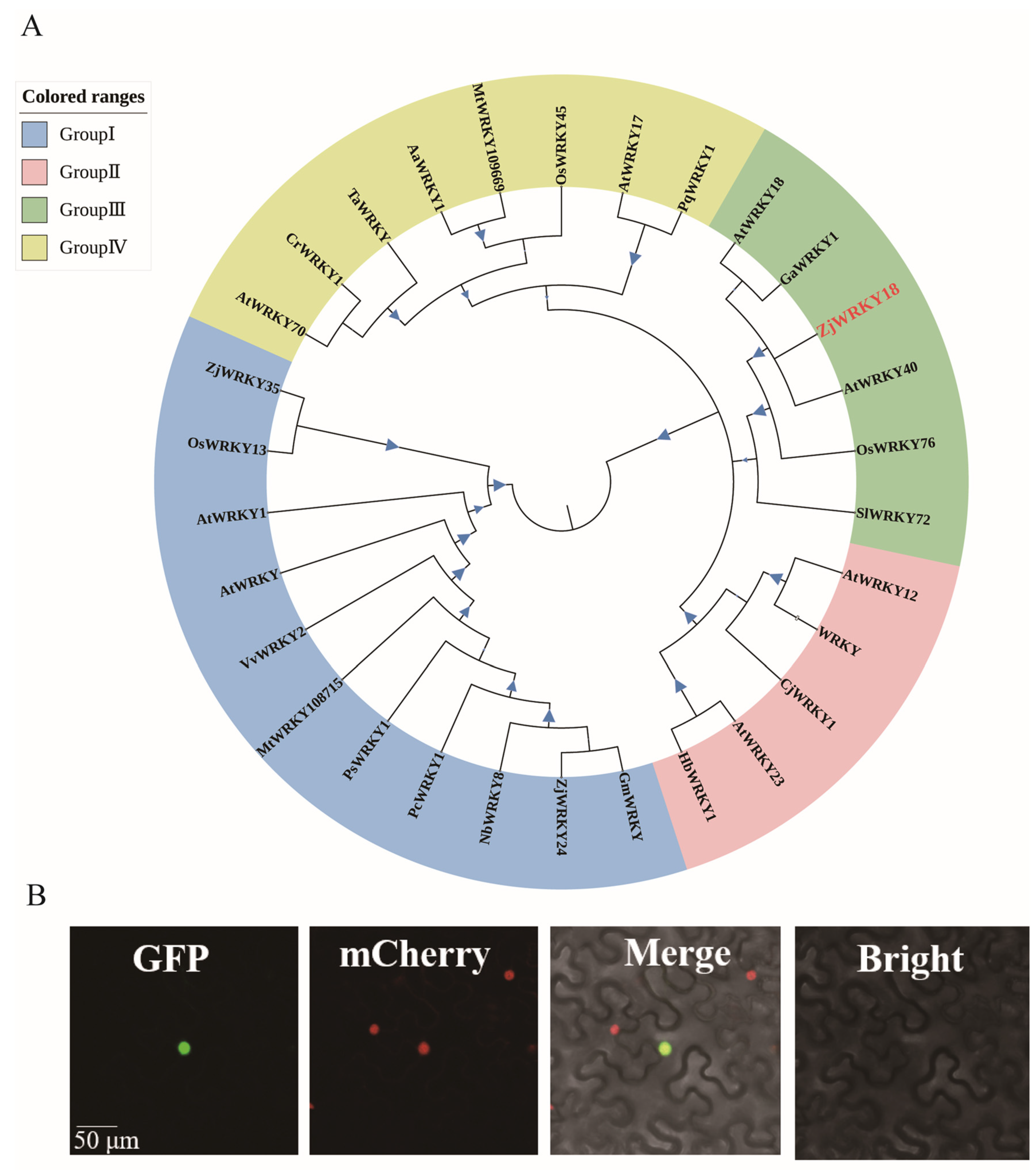
2.2. Phylogenetic and Subcellular Localization Analysis
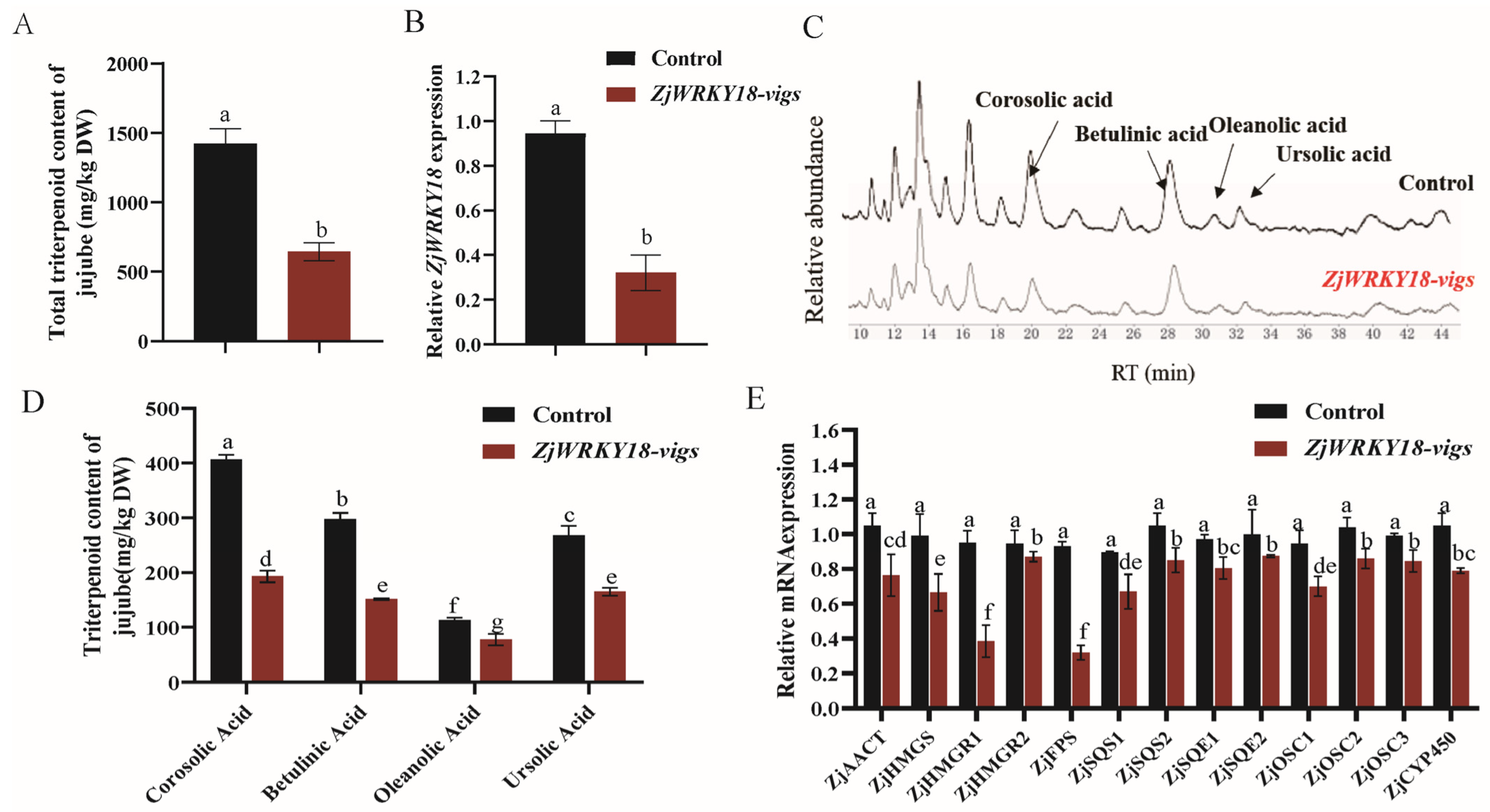
2.3. Suppression of ZjWRKY18 Expression Negatively Regulates Triterpenoid Pathway Genes and Reduces Triterpenoid Accumulation
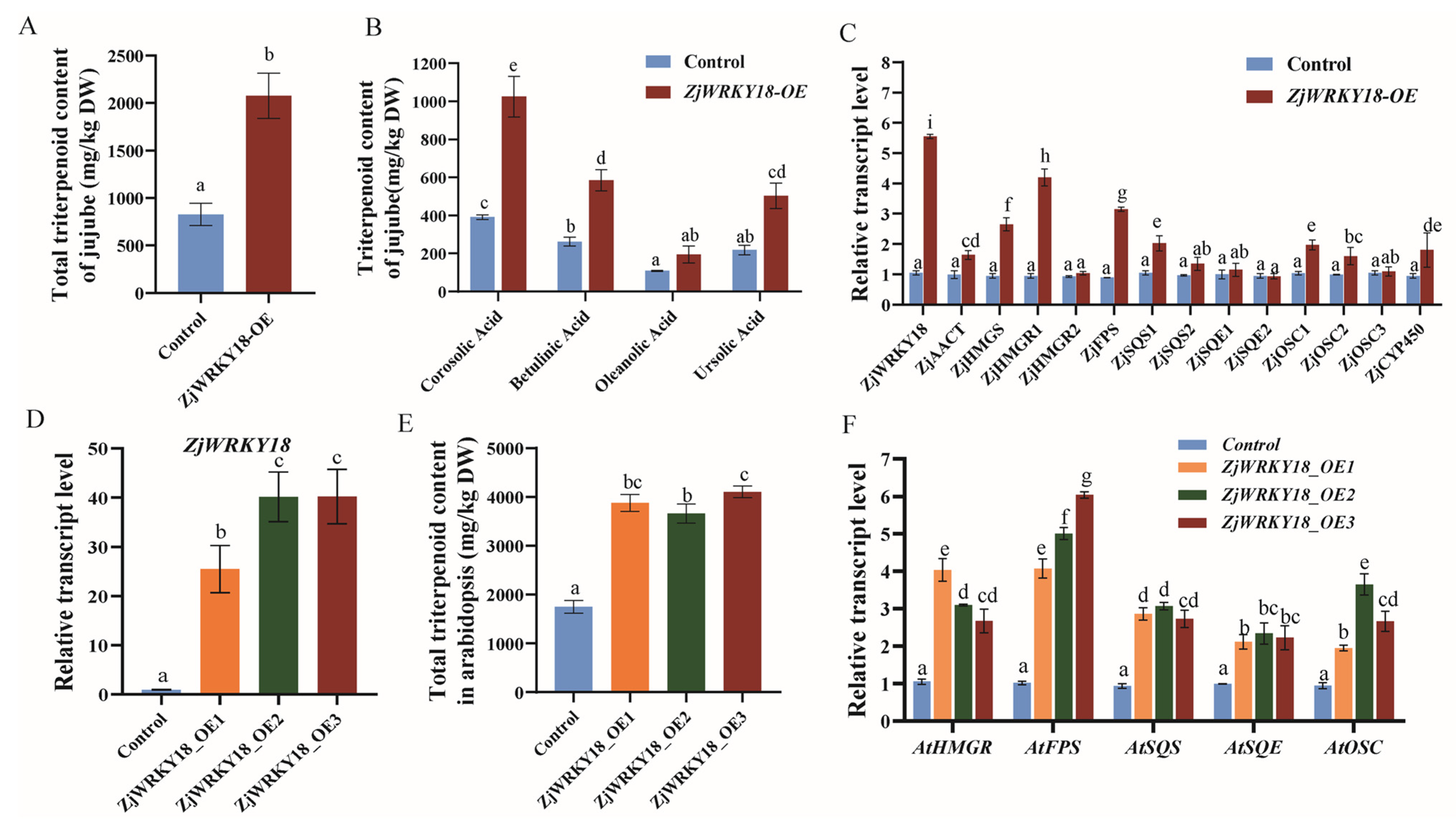
2.4. Overexpression of ZjWRKY18 Positively Regulates Triterpenoid Accumulation and Upregulates Triterpenoid Pathway Genes
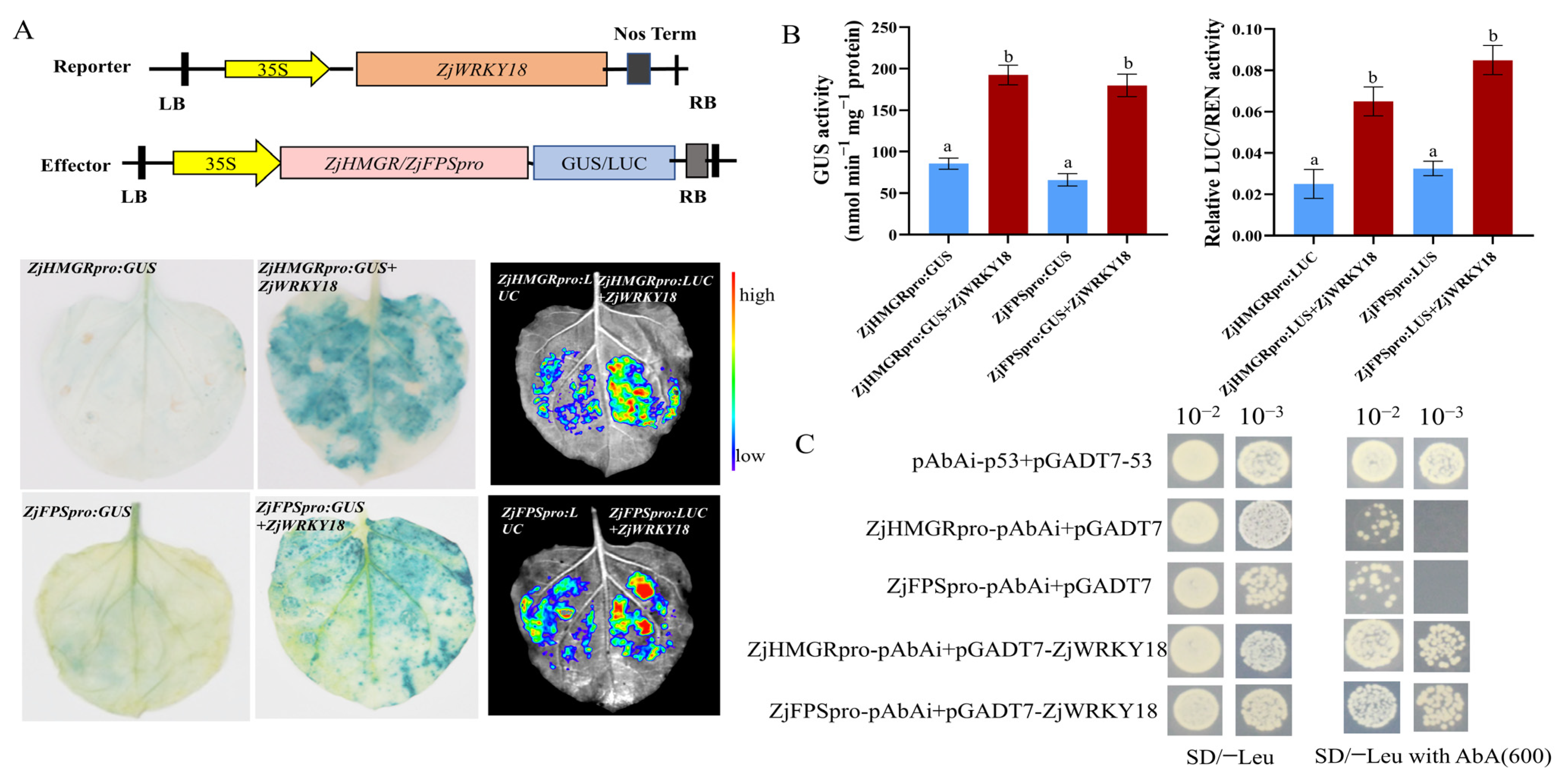
2.5. ZjWRKY18 Enhances HMGR and FPS Activities by Directly Binding to Their Promoters
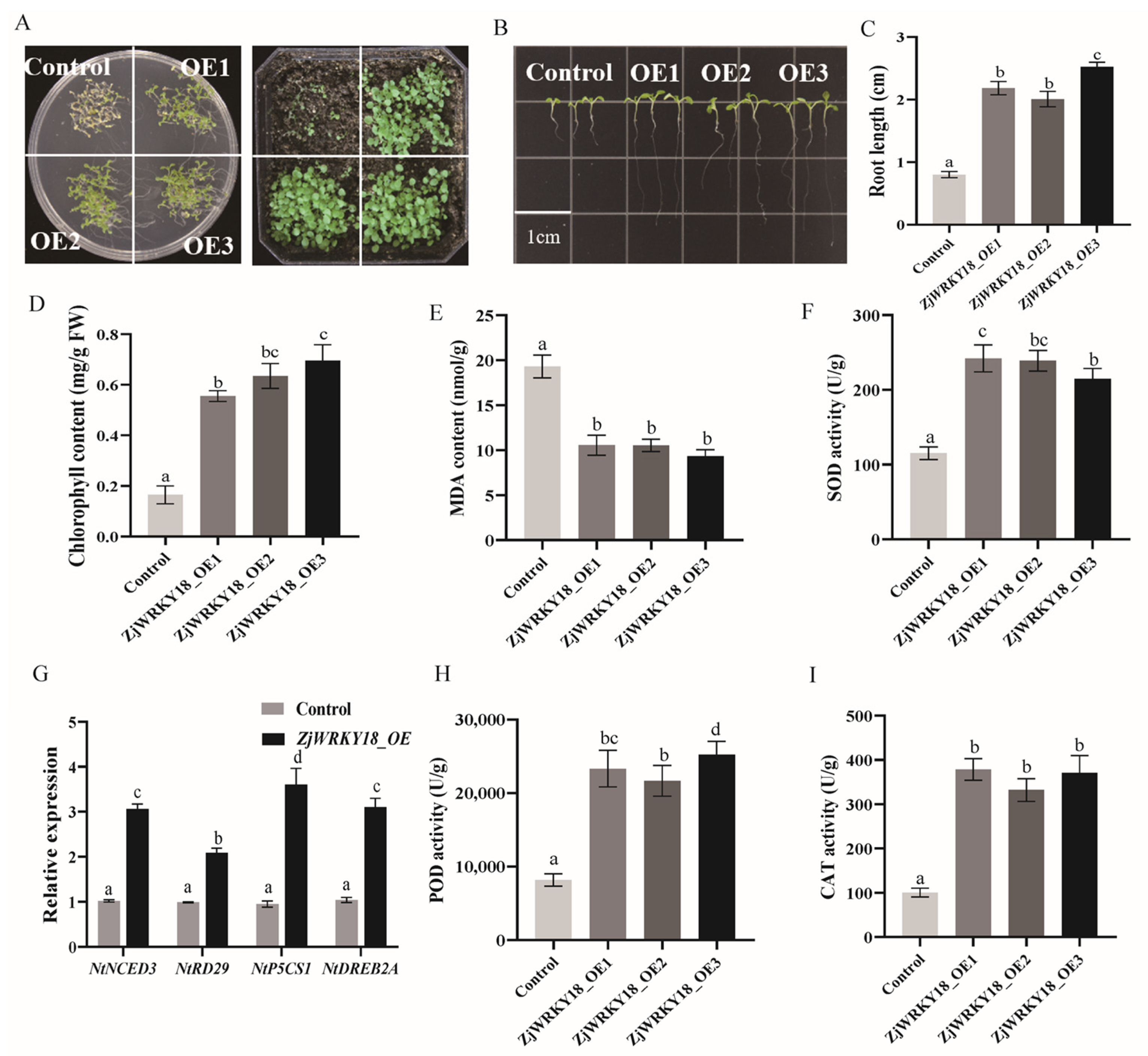
2.6. Overexpression of ZjWRKY18 Positively Regulates the Expression of Stress Genes, Leading to Increased Tolerance to Salt Stress
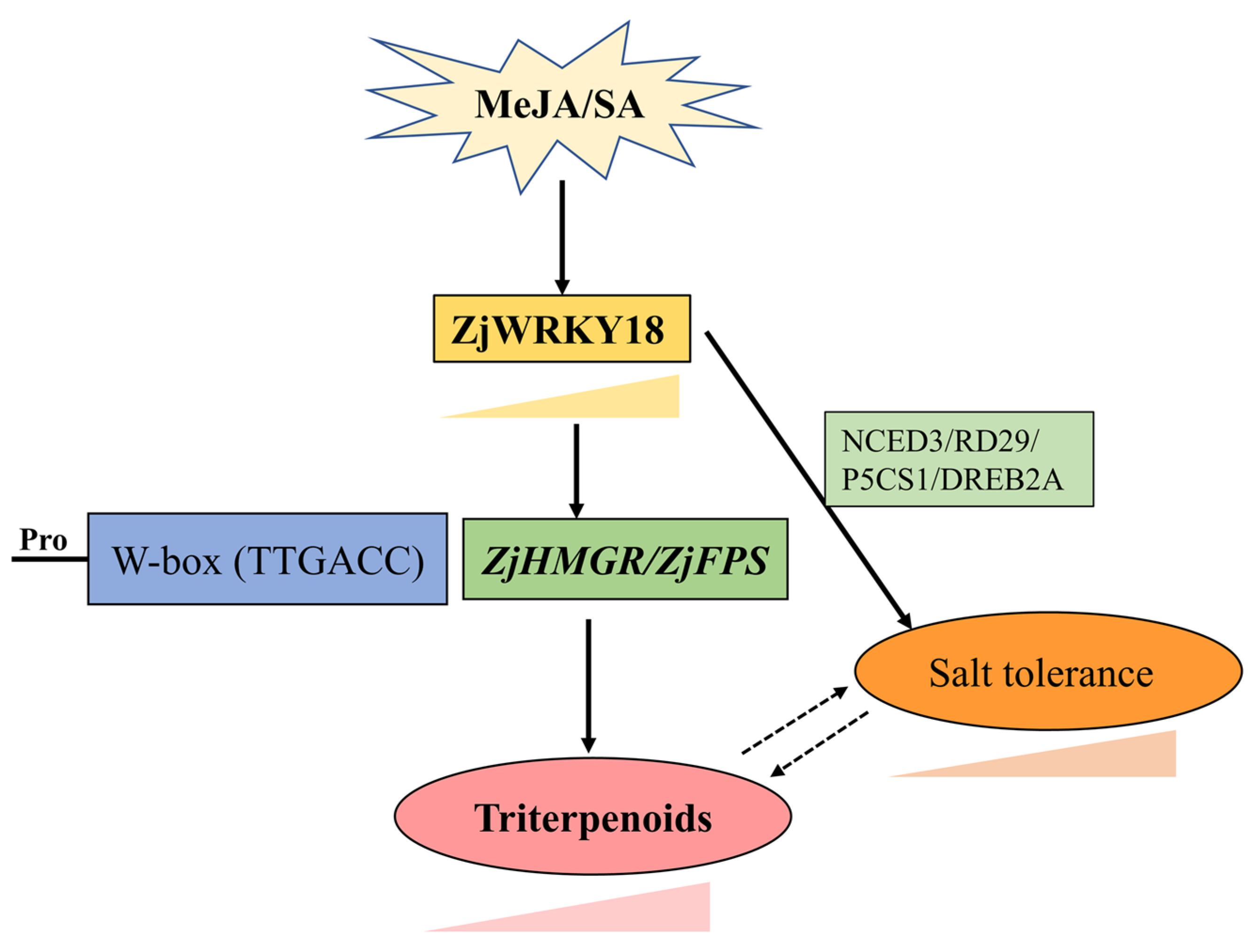
3. Discussion
4. Materials and Methods
4.1. Plant Material Culture and Collection
4.2. Phylogenetic Analysis
4.3. Subcellular Localization Analysis
4.4. Construction of VIGS and Overexpression Vectors and Generation of Transgenic Plants
4.5. Triterpenoids Extraction and Data Analysis
4.6. Glucuronidase and Luciferase Activity Assays
4.7. Yeast One-Hybrid (Y1H) Assay
4.8. Salt Stress Treatment Analysis
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, M.; Kushwaha, S.; Mishra, B.; Sangwan, N. Molecular and structural insights for the regulation of terpenoids in Ocimum basilicum and Ocimum tenuiflorum. Plant Growth Regul. 2022, 97, 61–75. [Google Scholar] [CrossRef]
- Kemen, A.C.; Honkanen, S.; Melton, R.E.; Findlay, K.C.; Mugford, S.T.; Hayashi, K.; Haralampidis, K.; Rosser, S.J.; Osbourn, A. Investigation of triterpene synthesis and regulation in oats reveals a role for beta-amyrin in determining root epidermal cell patterning. Proc. Natl. Acad. Sci. USA 2014, 111, 8679–8684. [Google Scholar] [CrossRef] [Green Version]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology invivo and invitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crop. Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Z.; Shi, Q.; Yue, R.; Li, X. Metabolite and Gene Expression Analysis Underlying Temporal and Spatial Accumulation of Pentacyclic Triterpenoids in Jujube. Genes 2022, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- De Costa, F.; Alves Yendo, A.C.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A.G. Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiol. Biochem. 2013, 66, 56–62. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Li, M.; Hou, L.; Liu, S.; Zhang, C.; Yang, W.; Pang, X.; Li, Y. Genome-wide identification and expression analysis of NAC transcription factors in Ziziphus jujuba Mill. reveal their putative regulatory effects on tissue senescence and abiotic stress responses. Ind. Crop. Prod. 2021, 173, 114093. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Singh, N.; Bansal, S.; Mishra, B.; Sangwan, R.S. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: Active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 2017, 159, 381–400. [Google Scholar] [CrossRef]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P.T. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Huang, J.-J.; Luo, Z.-Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Magedans, Y.V.; Phillips, M.A.; Fett-Neto, A.G. Production of plant bioactive triterpenoid saponins: From metabolites to genes and back. Phytochem. Rev. 2020, 20, 461–482. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhang, Y.; Jia, L.; Sun, L.; Wang, S.; Xiao, J.; Zhan, Y.; Yin, J. Functional identification of five CYP450 genes from birch responding to MeJA and SA in the synthesis of betulinic acid from lupitol. Ind. Crop. Prod. 2021, 167, 113513. [Google Scholar] [CrossRef]
- Tamura, K.; Yoshida, K.; Hiraoka, Y.; Sakaguchi, D.; Chikugo, A.; Mochida, K.; Kojoma, M.; Mitsuda, N.; Saito, K.; Muranaka, T.; et al. The Basic Helix-Loop-Helix Transcription Factor GubHLH3 Positively Regulates Soyasaponin Biosynthetic Genes in Glycyrrhiza uralensis. Plant Cell Physiol. 2018, 59, 783–796. [Google Scholar] [CrossRef]
- Yao, L.; Wang, J.; Sun, J.; He, J.; Paek, K.-Y.; Park, S.-Y.; Huang, L.; Gao, W. A WRKY transcription factor, PgWRKY4X, positively regulates ginsenoside biosynthesis by activating squalene epoxidase transcription in Panax ginseng. Ind. Crop. Prod. 2020, 154, 112671. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of Specialized Metabolism by WRKY Transcription Factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.Y.; Wu, Y.H.; Wang, K.; Bai, Z.Y.; Liu, Q.L.; Pan, Y.Z.; Zhang, L.; Jiang, B.B. Chrysanthemum WRKY gene DgWRKY5 enhances tolerance to salt stress in transgenic chrysanthemum. Sci. Rep. 2017, 7, 4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Shi, Q.; Wang, B.; Ma, A.; Wang, Y.; Xue, Q.; Shen, B.; Hamaila, H.; Tang, T.; Qi, X.; et al. Jujube metabolome selection determined the edible properties acquired during domestication. Plant J. 2022, 109, 1116–1133. [Google Scholar] [CrossRef]
- Yu, N.; Chen, Z.; Yang, J.; Li, R.; Zou, W. Integrated transcriptomic and metabolomic analyses reveal regulation of terpene biosynthesis in the stems of Sindora glabra. Tree Physiol. 2021, 41, 1087–1102. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Haring, M.A.; Schuurink, R.C. RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genom. 2014, 15, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, A.; Fukushima, S.; Okada, K.; Jiang, C.-J.; Yoshida, R.; Nakayama, A.; Shimono, M.; Sugano, S.; Yamane, H.; Takatsuji, H. WRKY45-dependent priming of diterpenoid phytoalexin biosynthesis in rice and the role of cytokinin in triggering the reaction. Plant Mol. Biol. 2014, 86, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Guo, D.; Li, H.-L.; Peng, S.-Q. Characterization of HbWRKY1, a WRKY transcription factor from Hevea brasiliensis that negatively regulates HbSRPP. Plant Physiol. Biochem. 2013, 71, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, Y.; Xu, J.; Li, Y.; Luo, H.; Zhu, Y.; Liu, M.; Wu, Q.; Song, J.; Sun, C.; et al. Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Cult. 2013, 114, 269–277. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-L.; Zhong, M.; Li, S.; Pan, Y.-Z.; Jiang, B.-B.; Jia, Y.; Zhang, H.-Q. Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol. Biochem. 2013, 69, 27–33. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The Cotton WRKY Transcription Factor GhWRKY17 Functions in Drought and Salt Stress in Transgenic Nicotiana benthamiana Through ABA Signaling and the Modulation of Reactive Oxygen Species Production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, R.; Wang, Y.; Wu, C.; Huang, J. Genome-Wide Identification of WRKY Transcription Factors in Chinese jujube (Ziziphus jujuba Mill.) and Their Involvement in Fruit Developing, Ripening, and Abiotic Stress. Genes 2019, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Li, Y.; Li, C.; Xiao, J.; Yang, J.; Li, X.; Sun, L.; Wang, S.; Tian, H.; Zhan, Y. Cloning, expression characteristics of a new FPS gene from birch (Betula platyphylla suk.) and functional identification in triterpenoid synthesis. Ind. Crop. Prod. 2020, 154, 112591. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Xiong, K.; Yan, X.; Ruan, S.; Wu, X. Identification of a Novel Metabolic Target for Bioactive Triterpenoids Biosynthesis in Ganoderma lucidum. Front. Microbiol. 2022, 13, 1376. [Google Scholar] [CrossRef]
- Shilpashree, H.B.; Sudharshan, S.J.; Shasany, A.K.; Nagegowda, D.A. Molecular characterization of three CYP450 genes reveals their role in withanolides formation and defense in Withania somnifera, the Indian Ginseng. Sci. Rep. 2022, 12, 1602. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, T.H.; Liang, Y.L.; Zhao, S.J.; Ren, L.Q.; Wang, Q.; Dou, B. Functional analysis of beta-amyrin synthase gene in ginsenoside biosynthesis by RNA interference. Plant Cell Rep. 2015, 34, 1307–1315. [Google Scholar] [CrossRef]
- Singh, A.K.; Dwivedi, V.; Rai, A.; Pal, S.; Reddy, S.G.E.; Rao, D.K.V.; Shasany, A.K.; Nagegowda, D.A. Virus-induced gene silencing of Withania somnifera squalene synthase negatively regulates sterol and defence-related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol. J. 2015, 13, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, F.; Sun, Y.; Liu, D.; Chen, C. Strengthening Triterpene Saponins Biosynthesis by Over-Expression of Farnesyl Pyrophosphate Synthase Gene and RNA Interference of Cycloartenol Synthase Gene in Panax notoginseng Cells. Molecules 2017, 22, 581. [Google Scholar] [CrossRef] [Green Version]
- Mertens, J.; Pollier, J.; Vanden Bossche, R.; Lopez-Vidriero, I.; Manuel Franco-Zorrilla, J.; Goossens, A. The bHLH Transcription Factors TSAR1 and TSAR2 Regulate Triterpene Saponin Biosynthesis in Medicago truncatula. Plant Physiol. 2016, 170, 194–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; Taylor, I.A.; Peng, Y.-L.; Wang, D.; Liu, J. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Guhling, O.; Yao, R.; Li, F.; Yeats, T.H.; Rose, J.K.C.; Jetter, R. Two Oxidosqualene Cyclases Responsible for Biosynthesis of Tomato Fruit Cuticular Triterpenoids. Plant Physiol. 2011, 155, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Grotewold, E. Transcription factors for predictive plant metabolic engineering: Are we there yet? Curr. Opin. Biotechnol. 2008, 19, 138–144. [Google Scholar] [CrossRef]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The Transcription Factor CrWRKY1 Positively Regulates the Terpenoid Indole Alkaloid Biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Wang, H.; Lundgren, A.; Brodelius, P.E. Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry 2014, 102, 89–96. [Google Scholar] [CrossRef]
- Basyuni, M.; Baba, S.; Inafuku, M.; Iwasaki, H.; Kinjo, K.; Oku, H. Expression of terpenoid synthase mRNA and terpenoid content in salt stressed mangrove. J. Plant Physiol. 2009, 166, 1786–1800. [Google Scholar] [CrossRef]
- Inafuku, M.; Basyuni, M.; Oku, H. Triterpenoid modulates the salt tolerance of lanosterol synthase deficient Saccharomyces cerevisiae, GIL77. Saudi J. Biol. Sci. 2018, 25, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta-Gene Regul. Mech. 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Dong, J.; Wang, T. Overexpression of MtWRKY76 increases both salt and drought tolerance in Medicago truncatula. Environ. Exp. Bot. 2016, 123, 50–58. [Google Scholar] [CrossRef]
- Du, C.; Zhao, P.; Zhang, H.; Li, N.; Zheng, L.; Wang, Y. The Reaumuria trigyna transcription factor RtWRKY1 confers tolerance to salt stress in transgenic Arabidopsis. J. Plant Physiol. 2017, 215, 48–58. [Google Scholar] [CrossRef]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178–E11187. [Google Scholar] [CrossRef] [Green Version]
- Ku, H.-M.; Hu, C.-C.; Chang, H.-J.; Lin, Y.-T.; Jan, F.-J.; Chen, C.-T. Analysis by virus induced gene silencing of the expression of two proline biosynthetic pathway genes in Nicotiana benthamiana under stress conditions. Plant Physiol. Biochem. 2011, 49, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wen, C.; Zhu, J.; Dai, H.; Zhang, Y. Apple Columnar Gene MdDMR6 Increases the Salt Stress Tolerance in Transgenic Tobacco Seedling and Apple Calli. J. Plant Growth Regul. 2020, 40, 187–196. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Xu, H.; Dai, Y.; Deng, D.; Chen, J. ZmWRKY33, a WRKY maize transcription factor conferring enhanced salt stress tolerances in Arabidopsis. Plant Growth Regul. 2013, 70, 207–216. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, N.-N.; Gong, S.-Y.; Lu, R.; Li, Y.; Li, X.-B. Overexpression of a cotton (Gossypium hirsutum) WRKY gene, GhWRKY34, in Arabidopsis enhances salt-tolerance of the transgenic plants. Plant Physiol. Biochem. 2015, 96, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Xu, X.; Wang, F.; Long, J.; Shen, W.; Jiang, D.; Zhao, X. The MYB transcription factor CiMYB42 regulates limonoids biosynthesis in citrus. BMC Plant Biol. 2020, 20, 254. [Google Scholar] [CrossRef]
- Paul, P.; Singh, S.K.; Patra, B.; Sui, X.; Pattanaik, S.; Yuan, L. A differentially regulated AP2/ERF transcription factor gene cluster acts downstream of a MAP kinase cascade to modulate terpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol. 2017, 213, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Du, J.; Zhu, D.; Li, X.; Li, X. Metabolomic and Transcriptomic Analyses of Anthocyanin Biosynthesis Mechanisms in the Color Mutant Ziziphus jujuba cv. Tailihong. J. Agric. Food Chem. 2020, 68, 15186–15198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, R.; Huo, Y.; Liu, S.; Yang, G.; Huang, J.; Zheng, C.; Wu, C. Expression of cotton PLATZ1 in transgenic Arabidopsis reduces sensitivity to osmotic and salt stress for germination and seedling establishment associated with modification of the abscisic acid, gibberellin, and ethylene signalling pathways. BMC Plant Biol. 2018, 18, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.J.; Nguyen, Q.A.; Lee, Y.G.; Song, Y.; Park, B.J.; Bae, H.-J. Enhanced Biomass Yield of and Saccharification in Transgenic Tobacco Over-Expressing beta-Glucosidase. Biomolecules 2020, 10, 806. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, W.; Yin, L.; Yan, F.; Xu, Y.; Chen, F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron. J. Biotechnol. 2015, 18, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wang, X.; Tang, Z.; Yuan, Y.; Xu, Y.; He, J.; Jiang, X.; Peng, S.-A.; Li, L.; Butelli, E.; et al. Subfunctionalization of the Ruby2-Ruby1 gene cluster during the domestication of citrus. Nat. Plants 2018, 4, 930–941. [Google Scholar] [CrossRef]
- Aktas, E.T.; Yildiz, H. Effects of electroplasmolysis treatment on chlorophyll and carotenoid extraction yield from spinach and tomato. J. Food Eng. 2011, 106, 339–346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Zhang, Z.; Shi, Q.; Duan, X.; Du, J.; Wu, C.; Li, X. Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube. Int. J. Mol. Sci. 2023, 24, 3899. https://doi.org/10.3390/ijms24043899
Wen C, Zhang Z, Shi Q, Duan X, Du J, Wu C, Li X. Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube. International Journal of Molecular Sciences. 2023; 24(4):3899. https://doi.org/10.3390/ijms24043899
Chicago/Turabian StyleWen, Cuiping, Zhong Zhang, Qianqian Shi, Xiaoshan Duan, Jiangtao Du, Cuiyun Wu, and Xingang Li. 2023. "Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube" International Journal of Molecular Sciences 24, no. 4: 3899. https://doi.org/10.3390/ijms24043899





