A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification
Abstract
1. Introduction
2. Results and Discussion
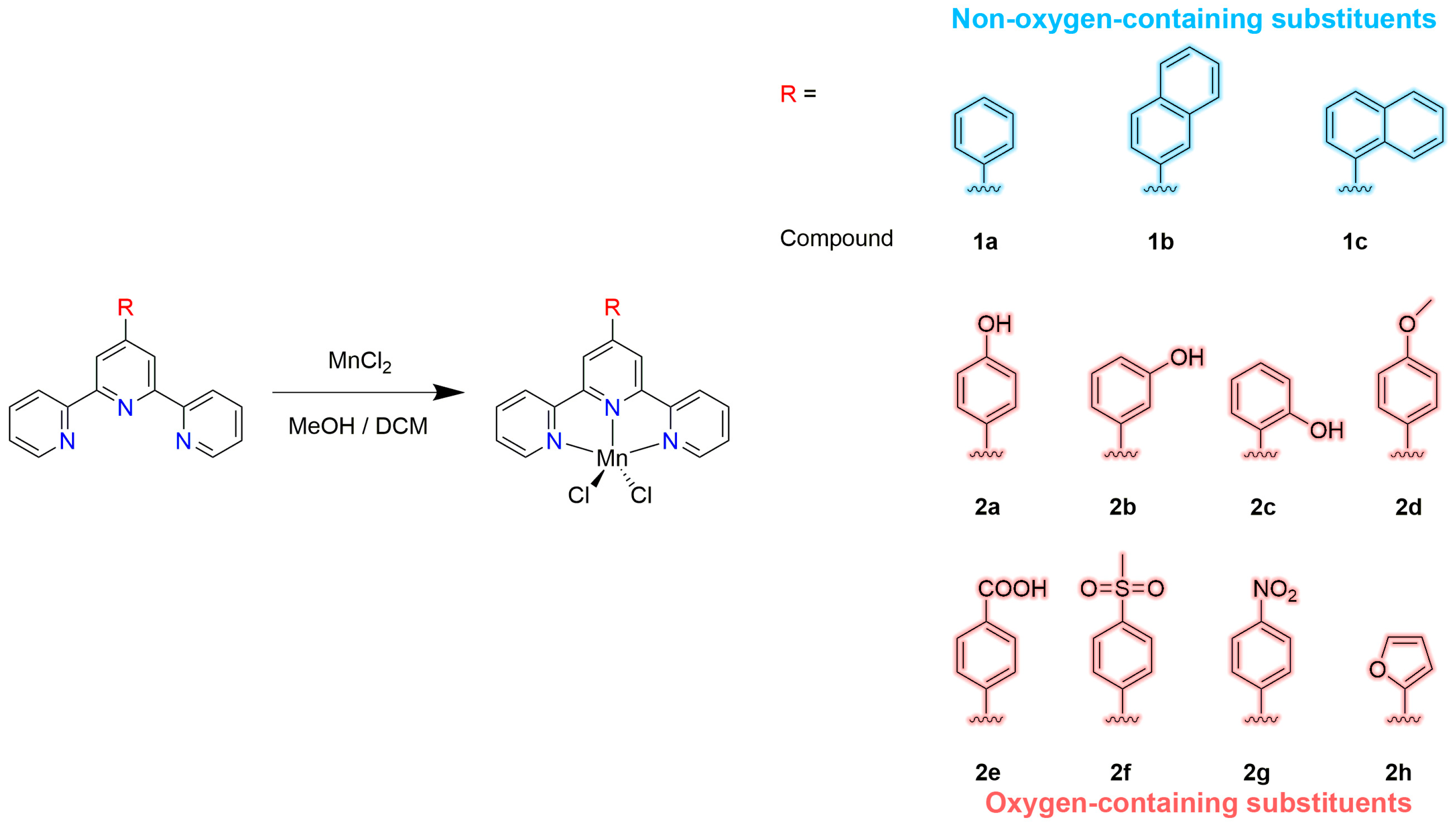
2.1. Synthesis and Characterization
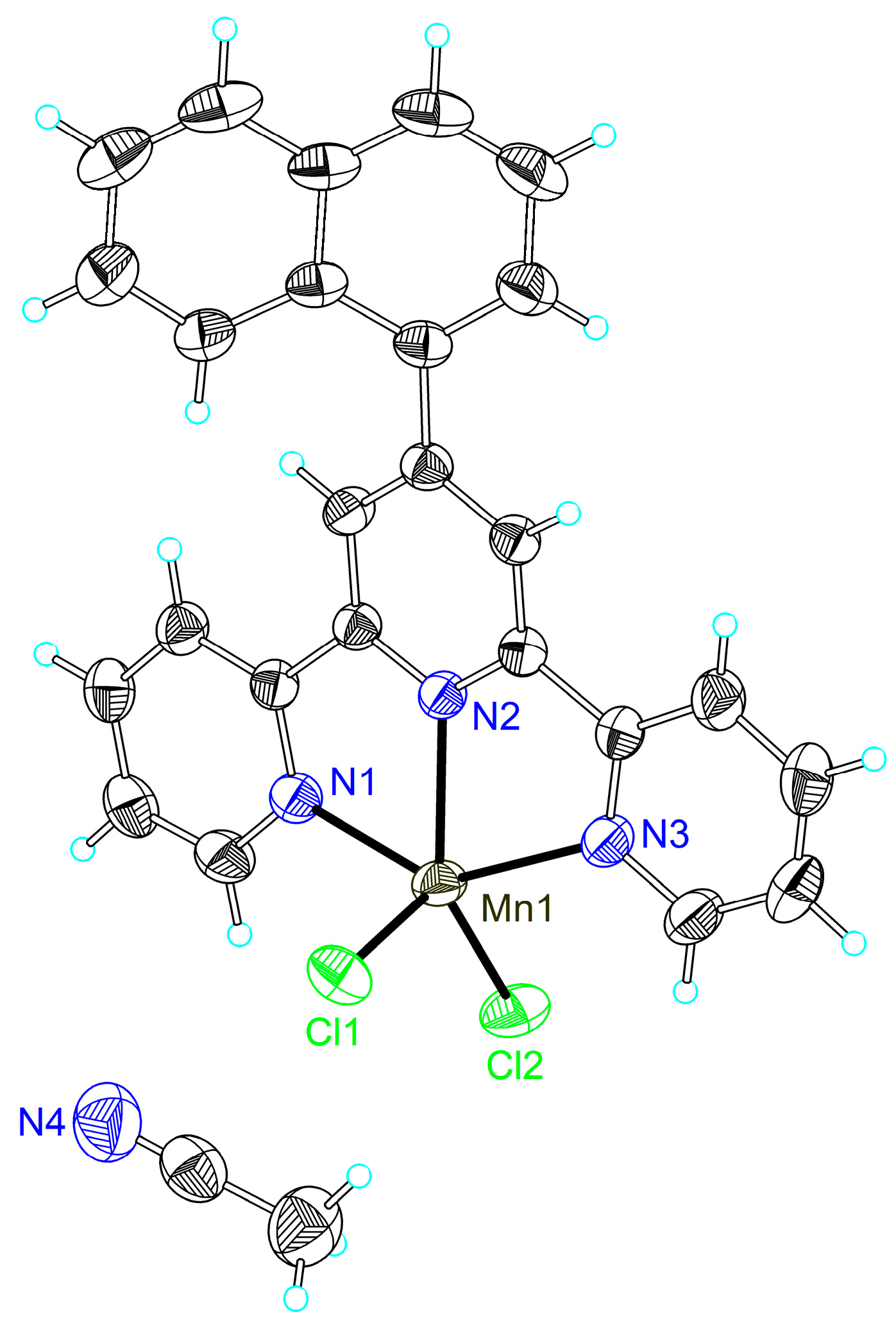
2.2. Single Crystal X-ray Crystallography
2.3. Antiproliferative Properties against Tumor Cells
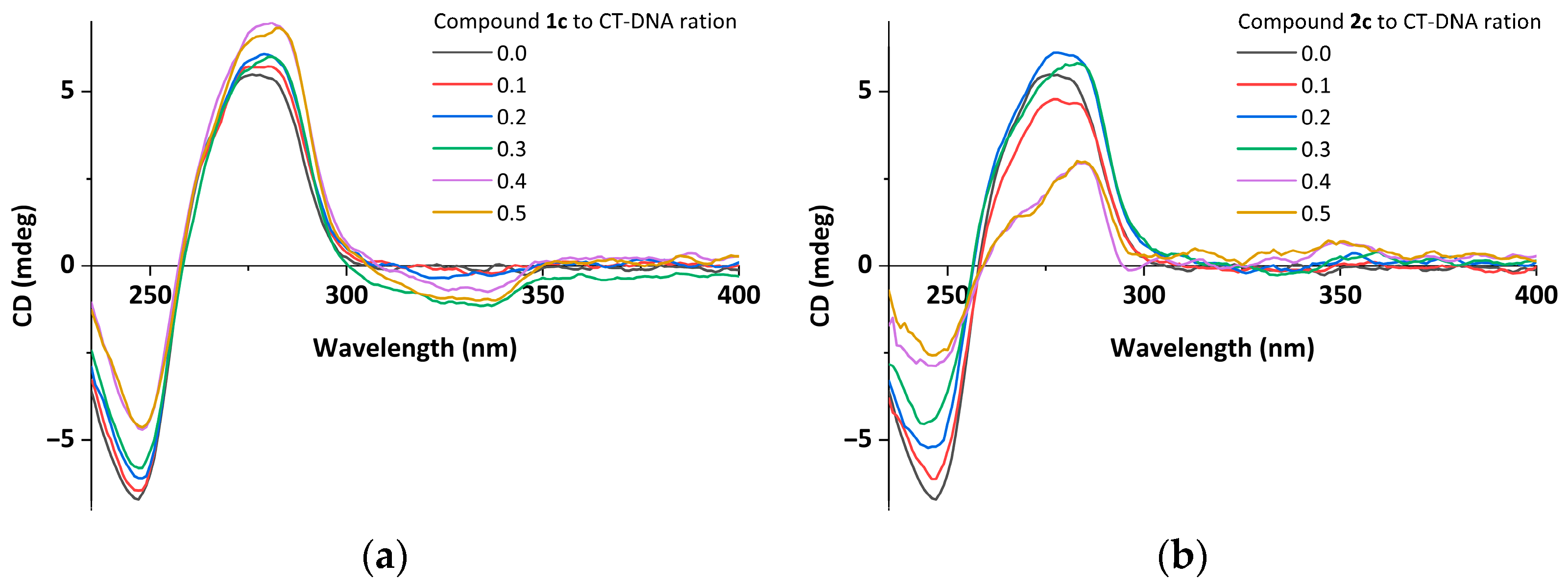
2.4. Circular Dichroism Spectroscopic Studies
2.5. Molecular Docking Studies
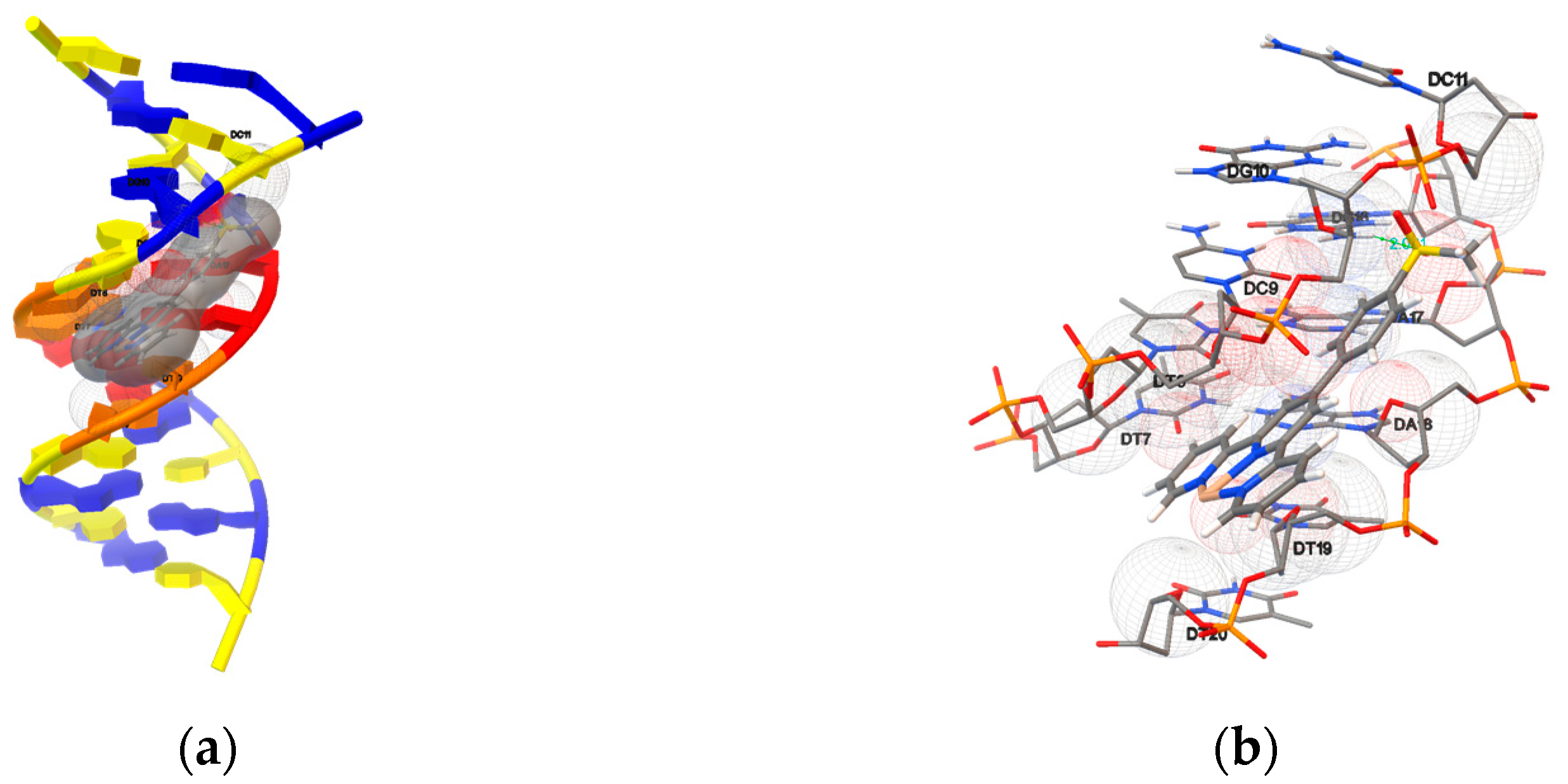
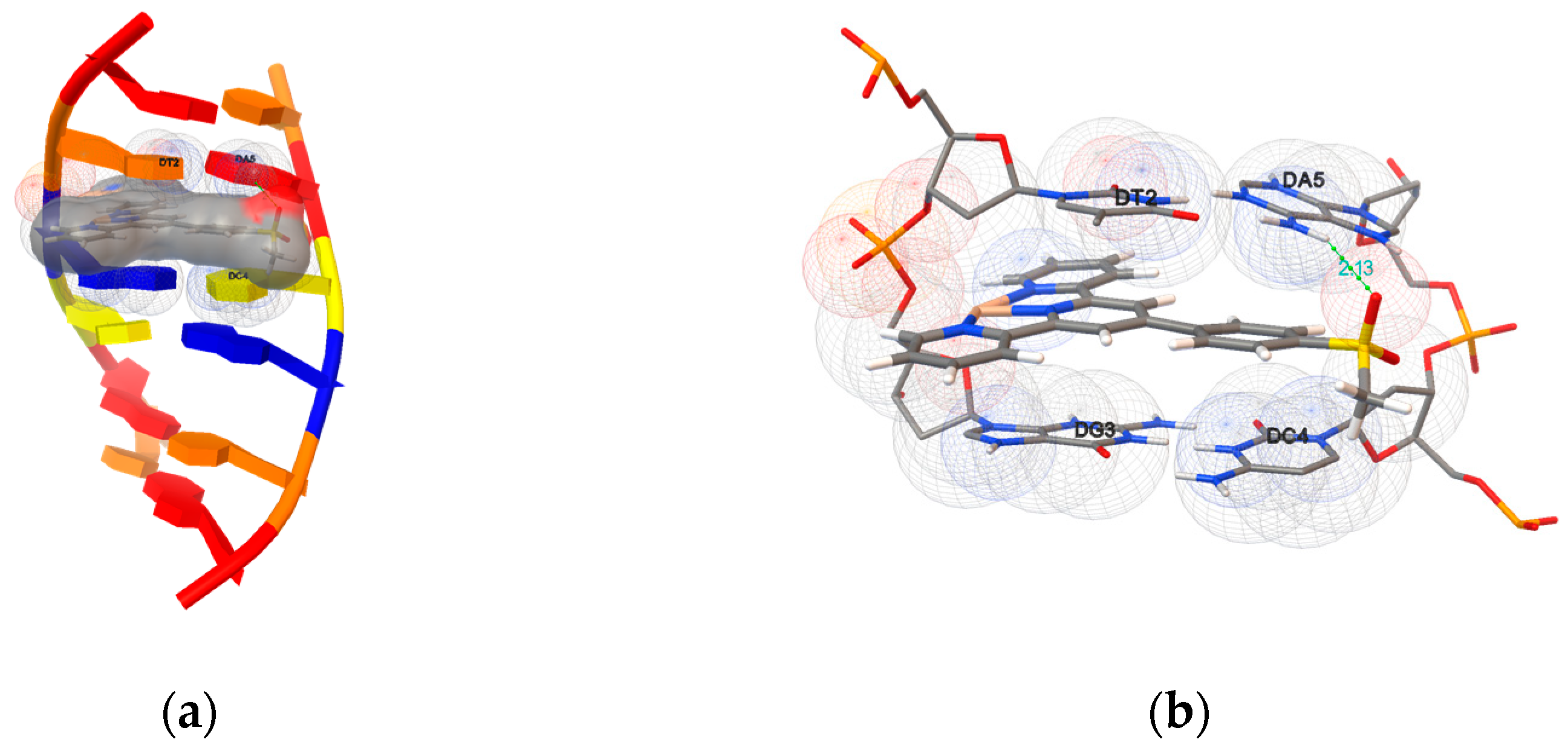
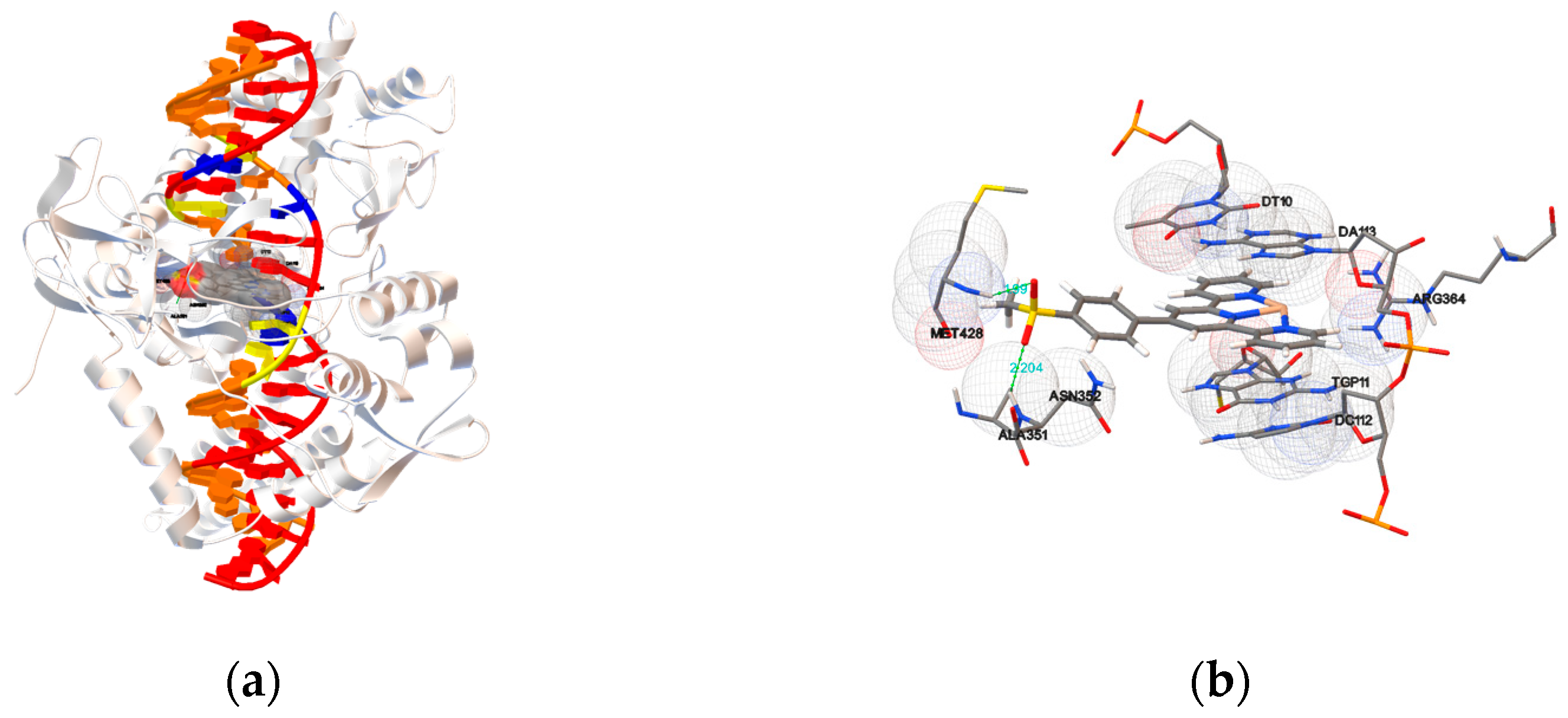
2.5.1. Molecular Docking with DNA
2.5.2. Molecular Docking with Topoisomerase I
3. Methods and Materials
3.1. Chemicals and Reagents
3.2. Instruments and Apparatus
3.3. Synthesis of the Compounds
3.4. Crystallography
3.5. Antiproliferative Activity against Tumor Cells
3.6. Circular Dichroism (CD) Spectropolarimetry
3.7. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Rajput, A.; Osmani, R.A.M.; Singh, E.; Banerjee, R. Cancer: A sui generis threat and its global impact. In Biosensor Based Advanced Cancer Diagnostics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–25. [Google Scholar]
- Musiol, R.; Malecki, P.; Pacholczyk, M.; Mularski, J. Terpyridines as promising antitumor agents: An overview of their discovery and development. Expert Opin. Drug Discov. 2022, 17, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Muller, C.M.; Babak, M.V.; Kubanik, M.; Hanif, M.; Jamieson, S.M.; Hartinger, C.G.; Wright, L.J. Pt (II) pyridinium amidate (PYA) complexes: Preparation and in vitro anticancer activity studies. Inorg. Chim. Acta 2016, 450, 124–130. [Google Scholar] [CrossRef]
- Musumeci, D.; Platella, C.; Riccardi, C.; Merlino, A.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. A first-in-class and a fished out anticancer platinum compound: Cis-[PtCl 2 (NH 3) 2] and cis-[PtI 2 (NH 3) 2] compared for their reactivity towards DNA model systems. Dalton Trans. 2016, 45, 8587–8600. [Google Scholar]
- Zhang, J.; Ye, Z.-w.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar]
- Medrano, M.Á.; Álvarez-Valdés, A.; Perles, J.; Lloret-Fillol, J.; Muñoz-Galván, S.; Carnero, A.; Navarro-Ranninger, C.; Quiroga, A.G. Oxidation of anticancer Pt (II) complexes with monodentate phosphane ligands: Towards stable but active Pt (IV) prodrugs. Chem. Commun. 2013, 49, 4806–4808. [Google Scholar] [CrossRef]
- Infante, I.; Azpiroz, J.M.; Blanco, N.G.; Ruggiero, E.; Ugalde, J.M.; Mareque-Rivas, J.C.; Salassa, L. Quantum dot photoactivation of Pt (IV) anticancer agents: Evidence of an electron transfer mechanism driven by electronic coupling. J. Phys. Chem. C 2014, 118, 8712–8721. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, J.; Xu, Y.; Hou, S.; Sun, L.; Ye, Q.; Lou, L. Design, synthesis and anticancer activity of diam (m) ine platinum (II) complexes bearing a small-molecular cell apoptosis inducer dichloroacetate. J. Inorg. Biochem. 2015, 146, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Urig, S.; Hartmann, M.; Helmke, B.M.; Koncarevic, S.; Allenberger, B.; Kienhoefer, C.; Neher, M.; Steiner, H.-H.; Unterberg, A.J.F.R.B. Medicine, Antiglioma activity of 2, 2′:6′, 2″-terpyridineplatinum (II) complexes in a rat model—Effects on cellular redox metabolism. Free Radic. Biol. Med. 2006, 40, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Darabi, F.; Hadadzadeh, H.; Simpson, J.; Shahpiri, A. A water-soluble Pd (II) complex with a terpyridine ligand: Experimental and molecular modeling studies of the interaction with DNA and BSA; and in vitro cytotoxicity investigations against five human cancer cell lines. New J. Chem. 2016, 40, 9081–9097. [Google Scholar] [CrossRef]
- Mahendiran, D.; Kumar, R.S.; Viswanathan, V.; Velmurugan, D.; Rahiman, A.K. Targeting of DNA molecules, BSA/c-Met tyrosine kinase receptors and anti-proliferative activity of bis (terpyridine) copper (II) complexes. Dalton Trans. 2016, 45, 7794–7814. [Google Scholar] [CrossRef] [PubMed]
- Lazić, D.; Arsenijević, A.; Puchta, R.; Bugarčić, Ž.D.; Rilak, A. DNA binding properties, histidine interaction and cytotoxicity studies of water soluble ruthenium (II) terpyridine complexes. Dalton Trans. 2016, 45, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.M.; Rilak, A.; Bratsos, I.; Klisurić, O.; Vraneš, M.; Gligorijević, N.; Radulović, S.; Bugarčić, Ž.D. New 4′-(4-chlorophenyl)-2,2′:6′,2″-terpyridine ruthenium (II) complexes: Synthesis, characterization, interaction with DNA/BSA and cytotoxicity studies. J. Inorg. Biochem. 2017, 169, 1–12. [Google Scholar] [CrossRef]
- Yu, C.; Chan, K.H.-Y.; Wong, K.M.-C.; Yam, V.W.-W. Nucleic acid-induced self-assembly of a platinum (II) terpyridyl complex: Detection of G-quadruplex formation and nuclease activity. Chem. Commun. 2009, 3756–3758. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, W.; Liang, B.; Pombeiro, A.J. Synthesis, characterization, photoluminescent and thermal properties of zinc (II) 4′-phenyl-terpyridine compounds. New J. Chem. 2013, 37, 1529–1537. [Google Scholar] [CrossRef]
- Becker, K.; Herold-Mende, C.; Park, J.J.; Lowe, G.; Schirmer, R.H. Human thioredoxin reductase is efficiently inhibited by (2,2′:6′,2″-terpyridine) platinum (II) complexes. Possible implications for a novel antitumor strategy. J. Med. Chem. 2001, 44, 2784–2792. [Google Scholar] [CrossRef]
- Lowe, G.; Droz, A.S.; Vilaivan, T.; Weaver, G.W.; Park, J.J.; Pratt, J.M.; Tweedale, L.; Kelland, L.R. Cytotoxicity of 2,2′:6′,2″-Terpyridineplatinum (II) Complexes against Human Ovarian Carcinoma. J. Med. Chem. 1999, 42, 3167–3174. [Google Scholar] [CrossRef]
- Lowe, G.; Droz, A.S.; Vilaivan, T.; Weaver, G.W.; Tweedale, L.; Pratt, J.M.; Rock, P.; Yardley, V.; Croft, S.L. Cytotoxicity of (2,2′:6′,2″-Terpyridine) platinum (II) Complexes to Leishmania d onovani, Trypanosoma c ruzi, and Trypanosoma b rucei. J. Med. Chem. 1999, 42, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bonse, S.; Richards, J.M.; Ross, S.A.; Lowe, G.; Krauth-Siegel, R.L. (2,2′:6′,2″-Terpyridine) platinum (II) Complexes Are Irreversible Inhibitors of Trypanosoma c ruzi Trypanothione Reductase But Not of Human Glutathione Reductase. J. Med. Chem. 2000, 43, 4812–4821. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, W.D.; Wakelin, L.P.; Roos, I.A.; Leopold, V.A. Activity of platinum (II) intercalating agents against murine leukemia L1210. J. Med. Chem. 1985, 28, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Bhattacharyya, D.; Dasgupta, D. Structural basis of DNA recognition by anticancer antibiotics, chromomycin A3, and mithramycin: Roles of minor groove width and ligand flexibility. Biopolym. Orig. Res. Biomol. 2000, 56, 85–95. [Google Scholar] [CrossRef]
- Bugreev, D.; Buneva, V.; Sinitsyna, O.; Nevinsky, G. The Mechanism of the Supercoiled DNA Recognition by the Eukaryotic Type I Topoisomerases: I. The Enzyme Interaction with Nonspecific Oligonucleotides. Russ. J. Bioorg. Chem. 2003, 29, 143–153. [Google Scholar] [CrossRef]
- Jacquemard, U.; Routier, S.; Dias, N.; Lansiaux, A.; Goossens, J.-F.; Bailly, C.; Mérour, J.-Y. Synthesis of 2, 5-and 3, 5-diphenylpyridine derivatives for DNA recognition and cytotoxicity. Eur. J. Med. Chem. 2005, 40, 1087–1095. [Google Scholar] [CrossRef]
- Zimbron, J.M.; Sardo, A.; Heinisch, T.; Wohlschlager, T.; Gradinaru, J.; Massa, C.; Schirmer, T.; Creus, M.; Ward, T.R. Chemo-Genetic Optimization of DNA Recognition by Metallodrugs using a Presenter-Protein Strategy. Chem.—Eur. J. 2010, 16, 12883–12889. [Google Scholar] [CrossRef]
- Lin, C.; Mathad, R.I.; Zhang, Z.; Sidell, N.; Yang, D. Solution structure of a 2: 1 complex of anticancer drug XR5944 with TFF1 estrogen response element: Insights into DNA recognition by a bis-intercalator. Nucleic Acids Res. 2014, 42, 6012–6024. [Google Scholar] [CrossRef]
- Wong, K.M.-C.; Yam, V.W.-W. Luminescence platinum (II) terpyridyl complexes—From fundamental studies to sensory functions. Coord. Chem. Rev. 2007, 251, 2477–2488. [Google Scholar] [CrossRef]
- Wong, K.M.-C.; Yam, V.W.-W. Self-assembly of luminescent alkynylplatinum (II) terpyridyl complexes: Modulation of photophysical properties through aggregation behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, J.; Xue, X.; Huang, L.; Ding, X.; Nong, D.; Chen, H.; Pan, L.; Ma, Z. Synthesis, characterization, photoluminescence, anti-tumor activity, DFT calculations and molecular docking with proteins of zinc (II) halogen substituted terpyridine compounds. Dalton Trans. 2019, 48, 10488–10504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, P.; Liang, B.; Huang, L.; Zhou, Y.; Ma, Z. Effects of counterions of colorful sandwich-type zinc (II) 4′-phenyl-terpyridine compounds on photoluminescent and thermal properties. J. Mol. Struct. 2017, 1146, 504–511. [Google Scholar] [CrossRef]
- Xue, X.; Wang, Q.; Mai, F.; Liang, X.; Huang, Y.; Li, J.; Zhou, Y.; Yang, D.; Ma, Z. Study on the Photoluminescent and Thermal Properties of Zinc Complexes with a N6O4 Macrocyclic Ligand. Molecules 2018, 23, 1735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.-S.; Bu, X.-H.; Yang, M. Synthesis, crystal structure, cytotoxic activity and DNA-binding properties of the copper (II) and zinc (II) complexes with 1-[3-(2-pyridyl) pyrazol-1-ylmethyl] naphthalene. J. Inorg. Biochem. 2005, 99, 1119–1125. [Google Scholar] [CrossRef]
- Sheng, X.; Guo, X.; Lu, X.-M.; Lu, G.-Y.; Shao, Y.; Liu, F.; Xu, Q. DNA binding, cleavage, and cytotoxic activity of the preorganized dinuclear zinc (II) complex of triazacyclononane derivatives. Bioconjugate Chem. 2008, 19, 490–498. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, Y.; Li, Q.; da Silva, M.F.C.G.; da Silva, J.J.F.; Pombeiro, A.J. Synthesis, characterization, solid-state photo-luminescence and anti-tumor activity of zinc (II) 4′-phenyl-terpyridine compounds. J. Inorg. Biochem. 2010, 104, 704–711. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, L.; Zhang, Y.; Xue, X.; Zhou, Y.; Ma, Z. Synthesis, characterization and photoluminescence of substituted terpyridine compounds and their molecular docking studies with bovine hemoglobin. J. Photochem. Photobiol. A Chem. 2018, 358, 17–25. [Google Scholar] [CrossRef]
- Huang, L.; Liu, R.; Li, J.; Liang, X.; Lan, Q.; Shi, X.; Pan, L.; Chen, H.; Ma, Z. Synthesis, characterization, anti-tumor activity, photo-luminescence and BHb/HHb/Hsp90 molecular docking of zinc (II) hydroxyl-terpyridine complexes. J. Inorg. Biochem. 2019, 201, 110790. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Jiang, J.; Liang, X.; Huang, L.; Huang, G.; Chen, H.; Pan, L.; Ma, Z. Zinc (II) Terpyridine Complexes: Substituent Effect on Photoluminescence, Antiproliferative Activity, and DNA Interaction. Molecules 2019, 24, 4519. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Liu, C.; Liu, R.; Liang, X.; Zhou, Y.; Pan, L.; Chen, H.; Ma, Z. Study on the substitution effects of zinc benzoate terpyridine complexes on photoluminescence, antiproliferative potential and DNA binding properties. JBIC J. Biol. Inorg. Chem. 2020, 25, 311–324. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Jiang, J.; Liang, X.; Huang, G.; Yang, D.; Chen, H.; Pan, L.; Ma, Z. Synthesis, characterization, photoluminescence, antiproliferative activity, and DNA interaction of cadmium (II) substituted 4′-phenyl-terpyridine compounds. J. Inorg. Biochem. 2020, 210, 111165. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yan, H.; Jiang, J.; Li, J.; Liang, X.; Yang, D.; Pan, L.; Xie, T.; Ma, Z. Synthesis, Characterization, Photoluminescence, Molecular Docking and Bioactivity of Zinc (II) Compounds Based on Different Substituents. Molecules 2020, 25, 3459. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xing, Y.; Yang, M.; Hu, M.; Liu, B.; da Silva, M.F.C.G.; Pombeiro, A.J. The double-helicate terpyridine silver (I) compound [Ag2L2](SO3CF3) 2 (L= 4′-phenyl-terpyridine) as a building block for di-and mononuclear complexes. Inorg. Chim. Acta 2009, 362, 2921–2926. [Google Scholar] [CrossRef]
- Li, Z.; Yan, H.; Liu, K.; Huang, X.; Niu, M. Syntheses, structures, DNA/BSA binding and cytotoxic activity studies of chiral alcohol-amine Schiff base manganese (II/III) complexes. J. Mol. Struct. 2019, 1195, 470–478. [Google Scholar] [CrossRef]
- Reddy, A.S.; Mao, J.; Krishna, L.S.; Badavath, V.N.; Maji, S. Synthesis, spectral investigation, molecular docking and biological evaluation of Cu (II), Ni (II) and Mn (II) complexes of (E)-2-((2-butyl-4-chloro-1H-imidazol-5-yl) methylene)-N-methylhydrazinecarbothioamide (C10H16N5ClS) and its DFT studies. J. Mol. Struct. 2019, 1196, 338–347. [Google Scholar] [CrossRef]
- Khalaf-Alla, P.A. Antioxidant, Antimicrobial and Antitumor Studies of Transition Metal Complexes Derived from N-(2-Aminoethyl)-1, 3-Propanediamine with DFT Calculations and Molecular Docking Investigation. Appl. Organomet. Chem. 2020, 34, e5628. [Google Scholar] [CrossRef]
- Calladine, C.R.; Drew, H. Understanding DNA: The Molecule and How It Works; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Valente, A.; Podolski-Renić, A.; Poetsch, I.; Filipović, N.; López, Ó.; Turel, I.; Heffeter, P. Metal-and metalloid-based compounds to target and reverse cancer multidrug resistance. Drug Resist. Updates 2021, 58, 100778. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Wang, L.-D.; Zheng, K.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Synthesis and crystal structure of a new copper (II) complex with N, N′-(4, 4′-bithiazole-2, 2′-diyl) diacetimidamide as ligand: Molecular docking, DNA-binding and cytotoxicity activity studies. J. Mol. Struct. 2013, 1037, 15–22. [Google Scholar] [CrossRef]
- Maity, B.; Roy, M.; Banik, B.; Majumdar, R.; Dighe, R.R.; Chakravarty, A.R. Ferrocene-promoted photoactivated DNA cleavage and anticancer activity of terpyridyl copper (II) phenanthroline complexes. Organometallics 2010, 29, 3632–3641. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Hernández-Gil, J.; Martínez-Ruíz, A.; Castiñeiras, A.; Liu-González, M.; Pallardó, F.V.; Borrás, J.; Piña, G.A. DNA binding, nuclease activity, DNA photocleavage and cytotoxic properties of Cu (II) complexes of N-substituted sulfonamides. J. Inorg. Biochem. 2013, 121, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Du, K.-J.; Wang, J.-Q.; Liang, J.-W.; Kou, J.-F.; Hou, X.-J.; Ji, L.-N.; Chao, H. Synthesis, crystal structure, DNA interaction and anticancer activity of tridentate copper (II) complexes. J. Inorg. Biochem. 2013, 119, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Tan, K.W.; Ng, S.W. Topoisomerase I inhibition and DNA cleavage by zinc, copper, and nickel derivatives of 2-[2-bromoethyliminomethyl]-4-[ethoxymethyl] phenol complexes exhibiting anti-proliferation and anti-metastasis activity. J. Inorg. Biochem. 2016, 159, 14–21. [Google Scholar] [CrossRef]
- Neves, A.P.; Pereira, M.X.; Peterson, E.J.; Kipping, R.; Vargas, M.D.; Silva-Jr, F.P.; Carneiro, J.W.M.; Farrell, N.P. Exploring the DNA binding/cleavage, cellular accumulation and topoisomerase inhibition of 2-hydroxy-3-(aminomethyl)-1, 4-naphthoquinone Mannich bases and their platinum (II) complexes. J. Inorg. Biochem. 2013, 119, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Ko, T.-P.; Su, W.-C.; Su, T.-L.; Wang, A.H.-J. Terpyridine–platinum (II) complexes are effective inhibitors of mammalian topoisomerases and human thioredoxin reductase 1. J. Inorg. Biochem. 2009, 103, 1082–1092. [Google Scholar] [CrossRef]
- Kwon, H.-B.; Park, C.; Jeon, K.-H.; Lee, E.; Park, S.-E.; Jun, K.-Y.; Kadayat, T.M.; Thapa, P.; Karki, R.; Na, Y. A series of novel terpyridine-skeleton molecule derivants inhibit tumor growth and metastasis by targeting topoisomerases. J. Med. Chem. 2015, 58, 1100–1122. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, B.; Da Silva, M.F.C.G.; Silva, J.; Mendo, A.S.; Baptista, P.V.; Fernandes, A.R.; Pombeiro, A.J. Synthesis, characterization, thermal properties and antiproliferative potential of copper (II) 4′-phenyl-terpyridine compounds. Dalton Trans. 2016, 45, 5339–5355. [Google Scholar] [CrossRef]
- Son, J.-K.; Zhao, L.-X.; Basnet, A.; Thapa, P.; Karki, R.; Na, Y.; Jahng, Y.; Jeong, T.C.; Jeong, B.-S.; Lee, C.-S. Synthesis of 2, 6-diaryl-substituted pyridines and their antitumor activities. Eur. J. Med. Chem. 2008, 43, 675–682. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zhao, H.; Tan, L. Ruthenium (II) polypyridyl complexes with 1, 8-naphthalimide group as DNA binder, photonuclease, and dual inhibitors of topoisomerases I and IIα. J. Inorg. Biochem. 2016, 163, 88–94. [Google Scholar] [CrossRef]
- Long, X.-J.; Dai, J.-W.; Wu, J.-Z. Synthesis and supramolecular networks of mono-and dinuclear manganese chloride complexes with 2-(2, 2′: 6′, 2 ”-terpyridin-4′-yl) phenol. J. Coord. Chem. 2012, 65, 316–324. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Wang, Z.; Liu, R.; Luo, B.; Yang, D.; Chen, H.; Pan, L.; Ma, Z. Copper chloride complexes with substituted 4′-phenyl-terpyridine ligands: Synthesis, characterization, antiproliferative activities and DNA interactions. Dalton Trans. 2021, 50, 8243–8257. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tagore, R.; Das, S.; Incarvito, C.; Faller, J.; Crabtree, R.H.; Brudvig, G.W. General synthesis of di-μ-oxo dimanganese complexes as functional models for the oxygen evolving complex of photosystem II. Inorg. Chem. 2005, 44, 7661–7670. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Nordén, B.; Kurucsev, T. Analysing DNA complexes by circular and linear dichroism. J. Mol. Recognit. 1994, 7, 141–155. [Google Scholar] [CrossRef]
- Garbett, N.C.; Ragazzon, P.A.; Chaires, J.B. Circular dichroism to determine binding mode and affinity of ligand–DNA interactions. Nat. Protoc. 2007, 2, 3166–3172. [Google Scholar] [CrossRef]
- Gray, D.M.; Ratliff, R.L.; Vaughan, M.R. [19] Circular dichroism spectroscopy of DNA. Methods Enzymol. 1992, 211, 389–406. [Google Scholar]
- Kashanian, S.; Askari, S.; Ahmadi, F.; Omidfar, K.; Ghobadi, S.; Tarighat, F.A. In vitro study of DNA interaction with clodinafop-propargyl herbicide. DNA Cell Biol. 2008, 27, 581–586. [Google Scholar] [CrossRef]
- Dehkordi, M.N.; Bordbar, A.-K.; Lincoln, P.; Mirkhani, V. Spectroscopic study on the interaction of ct-DNA with manganese Salen complex containing triphenyl phosphonium groups. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 50–54. [Google Scholar] [CrossRef]
- Rohs, R.; Bloch, I.; Sklenar, H.; Shakked, Z. Molecular flexibility in ab initio drug docking to DNA: Binding-site and binding-mode transitions in all-atom Monte Carlo simulations. Nucleic Acids Res. 2005, 33, 7048–7057. [Google Scholar] [CrossRef]
- Tabassum, S.; Zaki, M.; Afzal, M.; Arjmand, F. Synthesis and characterization of Cu (II)-based anticancer chemotherapeutic agent targeting topoisomerase Iα: In vitro DNA binding, pBR322 cleavage, molecular docking studies and cytotoxicity against human cancer cell lines. Eur. J. Med. Chem. 2014, 74, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Agilent, C.P. CrysAlis PRO; Agilent Technologies: Yarnton, England, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS Program for Empirical Absorption Correction for Area Detector Data; University of Göttingen: Göttingen, Germany.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Putz, H.; Brandenburg, K. Diamond-Crystal and Molecular Structure Visualization; Crystal Impact-GbR, Kreuzherrenstr: Bonn, Germany, 2018. [Google Scholar]
- Xiao, X.; Antony, S.; Pommier, Y.; Cushman, M. On the Binding of Indeno [1, 2-c] isoquinolines in the DNA− Topoisomerase I Cleavage Complex. J. Med. Chem. 2005, 48, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W. PyMOL Molecular Viewer; DeLano Scientific LLC: Palo Alto, CA, USA, 2008. [Google Scholar]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.—Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]

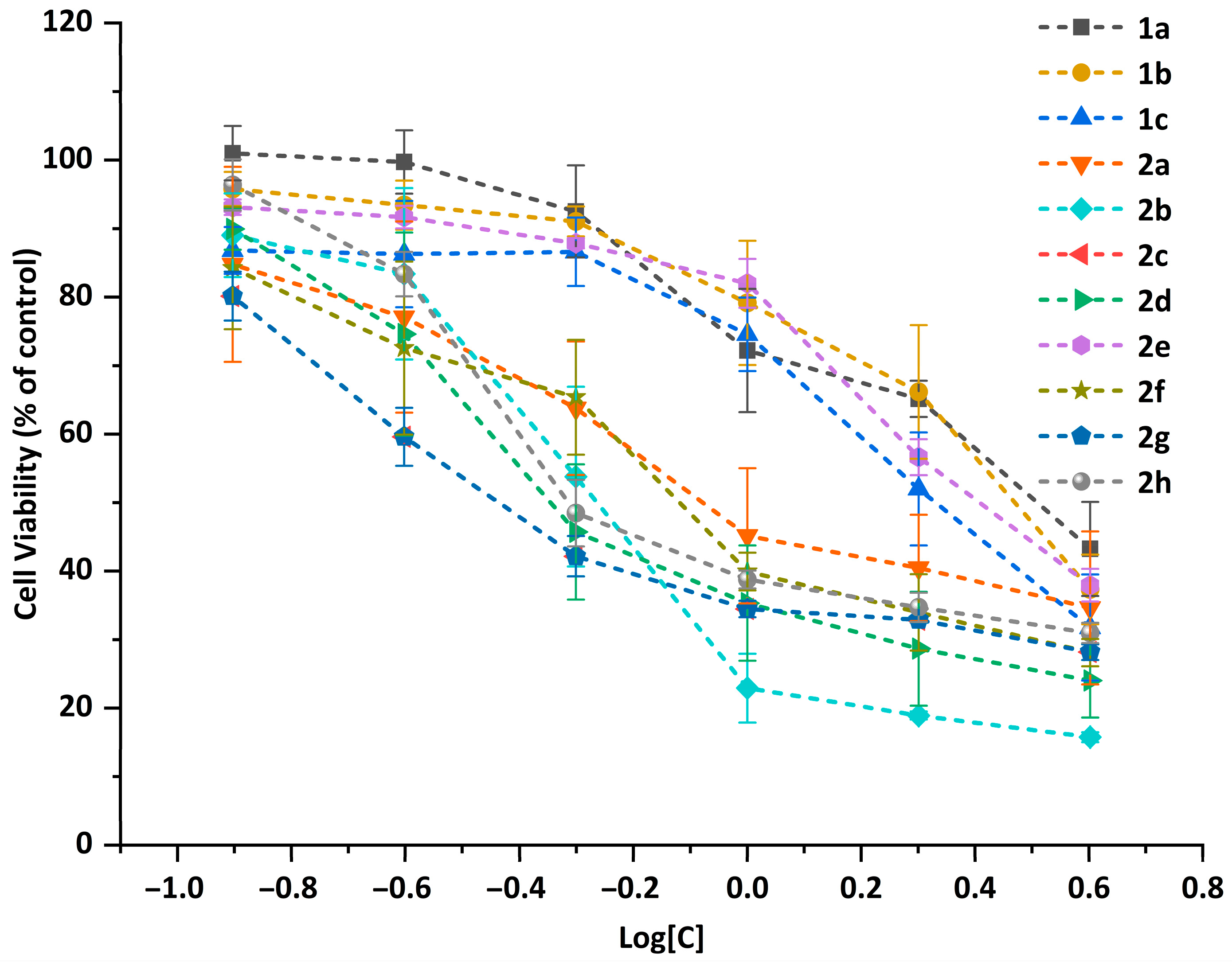
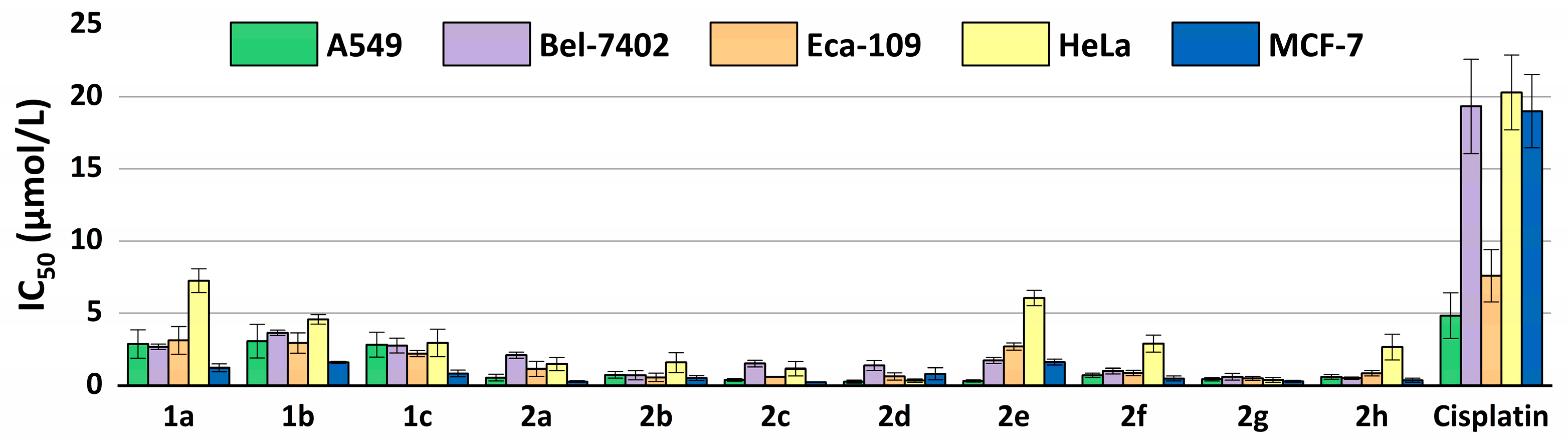
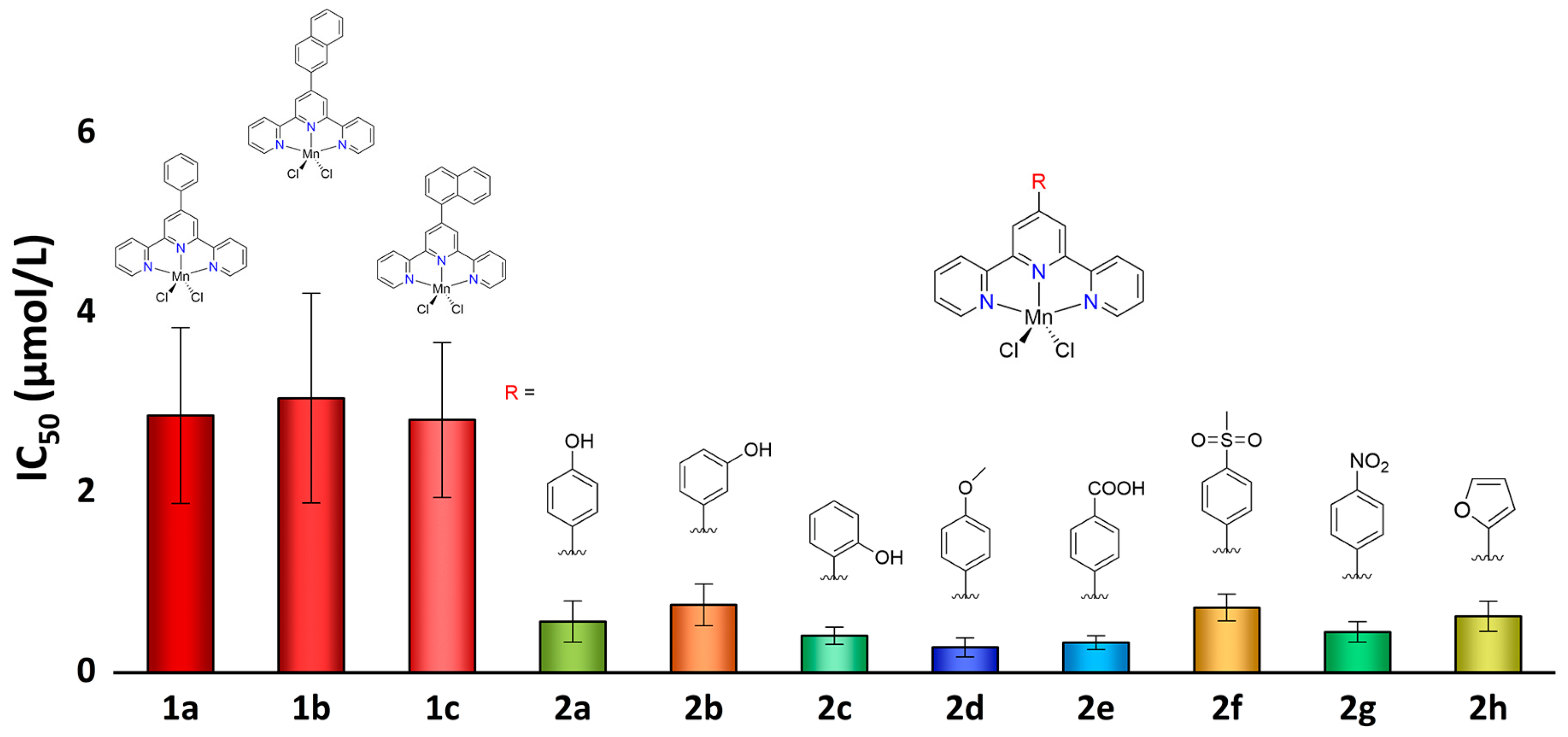

| Compounds | A549 | Bel-7402 | Eca-109 | HeLa | MCF-7 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μmol/L) | 95% Confidence Intervals (μmol/L) | IC50 (μmol/L) | 95% Confidence Intervals (μmol/L) | IC50 (μmol/L) | 95% Confidence Intervals (μmol/L) | IC50 (μmol/L) | 95% Confidence Intervals (μmol/L) | IC50 (μmol/L) | 95% Confidence Intervals (μmol/L) | |
| 1a | 2.858 | 2.142–3.813 | 2.667 | 0.455–0.718 | 3.115 | 2.575–3.769 | 7.249 | 6.118–8.589 | 1.229 | 0.980–1.540 |
| 1b | 3.052 | 1.628–5.724 | 3.631 | 0.434–0.650 | 2.927 | 2.222–3.857 | 4.566 | 3.857–5.406 | 1.594 | 1.244–2.042 |
| 1c | 2.811 | 2.034–3.883 | 2.751 | 0.308–0.450 | 2.196 | 1.818–2.652 | 2.935 | 1.765–4.883 | 0.850 | 0.671–1.077 |
| 2a | 0.567 | 0.398–0.808 | 2.088 | 1.329–1.590 | 1.153 | 0.714–1.863 | 1.486 | 1.086–2.031 | 0.284 | 0.227–0.355 |
| 2b | 0.754 | 0.519–1.096 | 0.730 | 0.616–0.796 | 0.579 | 0.486–0.688 | 1.583 | 1.085–2.310 | 0.525 | 0.399–0.691 |
| 2c | 0.409 | 0.242–0.692 | 1.510 | 1.043–1.227 | 0.614 | 0.488–0.774 | 1.160 | 0.969–1.389 | 0.249 | 0.207–0.301 |
| 2d | 0.281 | 0.217–0.364 | 1.382 | 0.304–0.471 | 0.645 | 0.502–0.829 | 0.356 | 0.280–0.452 | 0.819 | 0.583–1.151 |
| 2e | 0.334 | 0.287–0.390 | 1.720 | 0.656–0.876 | 2.690 | 2.452–2.950 | 6.047 | 5.304–6.894 | 1.610 | 1.240–2.090 |
| 2f | 0.723 | 0.538–0.970 | 1.011 | 0.441–0.605 | 0.886 | 0.717–1.094 | 2.889 | 1.815–4.600 | 0.501 | 0.360–0.698 |
| 2g | 0.453 | 0.313–0.655 | 0.626 | 0.616–0.796 | 0.514 | 0.414–0.638 | 0.412 | 0.266–0.639 | 0.304 | 0.249–0.372 |
| 2h | 0.626 | 0.455–0.862 | 0.523 | 1.043–1.227 | 0.868 | 0.677–1.111 | 2.648 | 1.683–4.167 | 0.379 | 0.317–0.454 |
| Cisplatin | 4.832 | 3.501–6.669 | 19.34 | 16.27–22.98 | 7.594 | 5.879–9.809 | 20.30 | 17.87–23.06 | 19.00 | 16.19–22.30 |
| Compounds | Affinity (kcal/mol) | ||
|---|---|---|---|
| B-DNA (1BNA) | Oligonucleotide (4JD8) | DNA-Topo I Complex (1SC7) | |
| 1a | −9.11 | −9.32 | −9.97 |
| 1b | −10.34 | −9.61 | −10.23 |
| 1c | −10.27 | −9.93 | −10.89 |
| 2a | −9.07 | −9.28 | −9.58 |
| 2b | −9.21 | −8.78 | −10.26 |
| 2c | −9.29 | −8.97 | −10.39 |
| 2d | −9.16 | −8.41 | −9.97 |
| 2e | −8.55 | −8.07 | −9.75 |
| 2f | −10.86 | −10.23 | −12.34 |
| 2g | −9.38 | −9.20 | −10.53 |
| 2h | −8.67 | −8.11 | −9.06 |
| Compounds | Receptor | Bonds Formed | Bond Distance (Å) | Bond Energy (kcal/mol) |
|---|---|---|---|---|
| 2a | B-DNA (1BNA) | O–H…O (DA18) | 2.105 | −4.619 |
| Oligonucleotide (4JD8) | O–H…O (DT2) | 1.993 | −0.824 | |
| DNA-Topo I complex (1SC7) | O–H…O (ASN722) | 2.133 | −3.223 | |
| 2b | B-DNA (1BNA) | O–H…O (DT19) | 2.175 | −4.074 |
| Oligonucleotide (4JD8) | O–H…O (DT2) | 2.073 | −4.052 | |
| DNA-Topo I complex (1SC7) | O–H…O (ASN722) | 2.081 | −0.401 | |
| DNA-Topo I complex (1SC7) | O…H–N (ASN722) | 1.956 | −3.016 | |
| 2c | B-DNA (1BNA) | O–H…O (DT7) | 1.804 | −1.485 |
| Oligonucleotide (4JD8) | O–H…O (DG3) | 2.231 | −5.235 | |
| DNA-Topo I complex (1SC7) | O–H…O (TGP11) | 2.134 | −6.073 | |
| 2f | B-DNA (1BNA) | O…H–N(DG16) | 2.061 | −5.108 |
| Oligonucleotide (4JD8) | O…H–N(DA5) | 2.130 | −4.107 | |
| DNA-Topo I complex (1SC7) | O…H–N (MET428) | 1.990 | −4.015 | |
| DNA-Topo I complex (1SC7) | O…H–N (ALA351) | 2.204 | −1.600 | |
| 2g | B-DNA (1BNA) | O…H–N (DG14) | 2.079 | −1.999 |
| Oligonucleotide (4JD8) | O…H–N(DA5) | 2.201 | −2.900 | |
| DNA-Topo I complex (1SC7) | O…H–N (LYS425) | 1.877 | −4.568 |
| Compound | 1a | 1c | 2a | 2b | 2c | 2f | 2g |
|---|---|---|---|---|---|---|---|
| Empirical formula | C21H15Cl2MnN3 | C27H20Cl2MnN4 | C21H15Cl2MnN3O | C21H15Cl2MnN3O | C21H19Cl2MnN3O | C22H17Cl2MnN3O2S | C21H14N4O2Cl2Mn |
| Formula weight | 435.20 | 526.31 | 451.2 | 451.2 | 455.23 | 513.29 | 480.2 |
| Temperature | 123(2) K | 293(2) K | 293(2) K | 293(2) K | 293(2) K | 293(2) K | 150(2) K |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Monoclinic | Triclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P-1 | P21/n | P21/n | P-1 | P21/n | C2/c |
| a (Å) | 12.1515(5) | 8.6474(6) | 12.0365(11) | 12.2520(15) | 8.9693(18) | 10.2754(15) | 11.1314(16) |
| b (Å) | 9.7548(4) | 11.0270(7) | 9.8576(10) | 9.7098(10) | 10.584(2) | 18.416(2) | 22.013(3) |
| c (Å) | 16.6109(6) | 13.3718(10) | 17.1166(17) | 17.083(2) | 12.780(3) | 12.7472(17) | 8.6607(11) |
| α (°) | 90.00 | 85.629(3) | 90 | 90 | 103.63(3) | 90 | 90 |
| β (°) | 106.581(2) | 74.006(3) | 107.487(11) | 107.349(14) | 108.46(3) | 110.752(16) | 110.471(8) |
| γ (°) | 90.00 | 87.326(3) | 90 | 90 | 96.63(3) | 90 | 90 |
| Volume (Å3) | 1887.10(13) | 1221.70(15) | 1937.1(3) | 1939.8(4) | 1094.4(4) | 2255.7(5) | 1988.1(5) |
| Z | 4 | 2 | 4 | 4 | 2 | 4 | 4 |
| Calculated density (Mg/m3) | 1.532 | 1.431 | 1.547 | 1.545 | 1.382 | 1.511 | 1.604 |
| Absorption coefficient (mm−1) | 0.993 | 0.782 | 0.974 | 0.973 | 0.863 | 0.939 | 0.96 |
| F(000) | 884 | 538 | 916 | 916 | 466 | 1044 | 972 |
| Crystal size (mm−1) | 0.41 × 0.33 × 0.29 | 0.40 × 0.36 × 0.36 | 0.48 × 0.33 × 0.31 | 0.48 × 0.46 × 0.26 | 0.41 × 0.23 × 0.16 | 0.45 × 0.40 × 0.38 | 0.26 × 0.22 × 0.15 |
| θmax, θmin (°) | 30.69, 1.85 | 26.45, 2.36 | 29.40, 3.24 | 29.47, 3.21 | 29.27, 2.84 | 29.54, 2.80 | 30.07, 2.75 |
| Index range h | –17 → 17 | –10 → 10 | –16 → 14 | –15 → 14 | –8 → 12 | –13 → 13 | –11 → 15 |
| K | –13 → 13 | –13 → 13 | –13 → 12 | –13 → 10 | –14 → 13 | –25 → 17 | –27 → 30 |
| L | –23 → 23 | –16 → 16 | –23 → 22 | –16 → 23 | –17 → 16 | –16 → 17 | –11 → 12 |
| Reflections collected /unique | 36,786/5834 [R(int) = 0.0378] | 41,099/5022 [R(int) = 0.0555] | 10,821/4560 [R(int) = 0.0214] | 11,912/4640 [R(int) = 0.0228] | 9493/5049 [R(int) = 0.0164] | 12,816/5327 [R(int) = 0.0248] | 10,177/2910 [R(int) = 0.0558] |
| Data/restraints/parameters | 5834/0/244 | 5022/0/376 | 4560/0/254 | 4640/0/253 | 5049/0/287 | 5327/0/280 | 2910/0/177 |
| GOF on F2 | 1.034 | 1.003 | 1.031 | 1.002 | 1.034 | 1.030 | 1.031 |
| Final R indices [I>2σ(I)] | R1 = 0.0248 wR2 = 0.0683 | R1 = 0.0304 wR2 = 0.0804 | R1 = 0.0314 wR2 = 0.0777 | R1 = 0.0320 wR2 = 0.0801 | R1 = 0.0368 wR2 = 0.0949 | R1 = 0.0364 wR2 = 0.0957 | R1 = 0.0492 wR2 = 0.1123 |
| R indices (all data) | R1 = 0.0283 wR2 = 0.0704 | R1 = 0.0424 wR2 = 0.0899 | R1 = 0.0423 wR2 = 0.0866 | R1 = 0.0457 wR2 = 0.0910 | R1 = 0.0476 wR2 = 0.1046 | R1 = 0.0540 wR2 = 0.1078 | R1 = 0.0862 wR2 = 0.1296 |
| Largest diff. peak and hole (e Å–3) | 0.41 and −0.25 | 0.19 and −0.32 | 0.26 and −0.27 | 0.20 and −0.29 | 0.38 and −0.41 | 0.79 and −0.34 | 0.93 and −0.64 |
| CCDC number | 2062271 | 2062273 | 2062274 | 2062275 | 2062276 | 2062277 | 2062272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, M.; Jiang, J.; Huang, J.; Chen, H.; Pan, L.; Nesterov, D.S.; Ma, Z.; Pombeiro, A.J.L. A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification. Int. J. Mol. Sci. 2023, 24, 3903. https://doi.org/10.3390/ijms24043903
Li J, Chen M, Jiang J, Huang J, Chen H, Pan L, Nesterov DS, Ma Z, Pombeiro AJL. A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification. International Journal of Molecular Sciences. 2023; 24(4):3903. https://doi.org/10.3390/ijms24043903
Chicago/Turabian StyleLi, Jiahe, Min Chen, Jinzhang Jiang, Jieyou Huang, Hailan Chen, Lixia Pan, Dmytro S. Nesterov, Zhen Ma, and Armando J. L. Pombeiro. 2023. "A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification" International Journal of Molecular Sciences 24, no. 4: 3903. https://doi.org/10.3390/ijms24043903
APA StyleLi, J., Chen, M., Jiang, J., Huang, J., Chen, H., Pan, L., Nesterov, D. S., Ma, Z., & Pombeiro, A. J. L. (2023). A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification. International Journal of Molecular Sciences, 24(4), 3903. https://doi.org/10.3390/ijms24043903







