Biochemical Characterization and Functional Analysis of Glucose Regulated Protein 78 from the Silkworm Bombyx mori
Abstract
:1. Introduction
2. Results
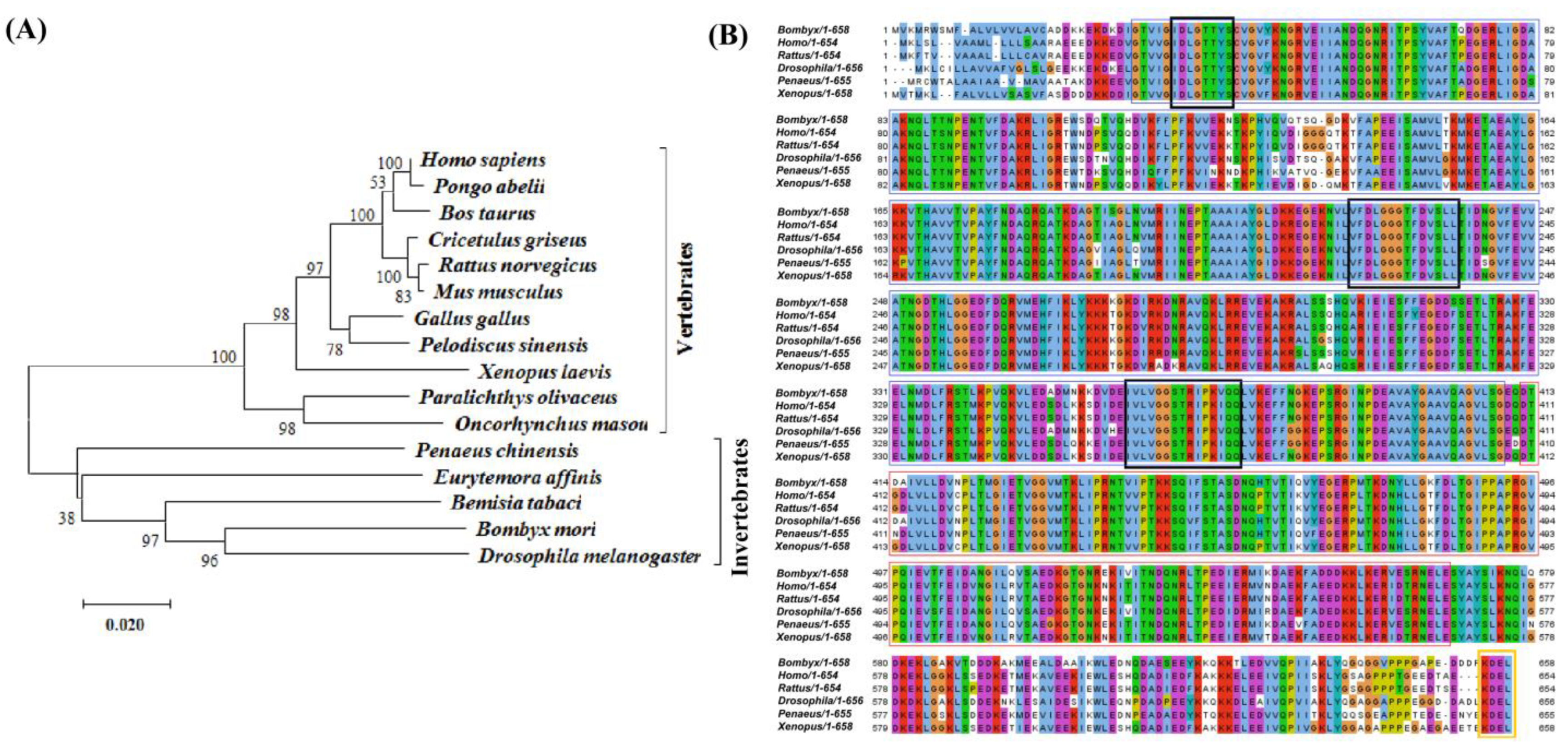
2.1. Cloning and Characterization Analysis of BmGRP78 from Bombyx mori
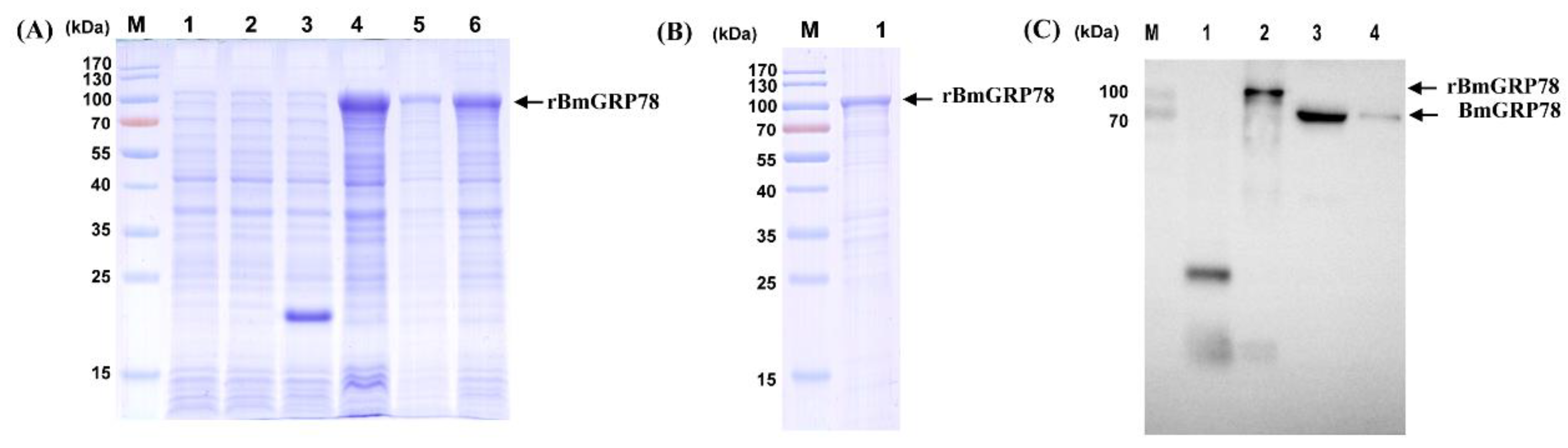
2.2. Expression and Production of Recombinant BmGRP78 (rBmGRP78) and Preparation of Antiserum
2.3. Analysis Expression Patterns of BmGRP78 in Developmental Stages and Larval Tissues
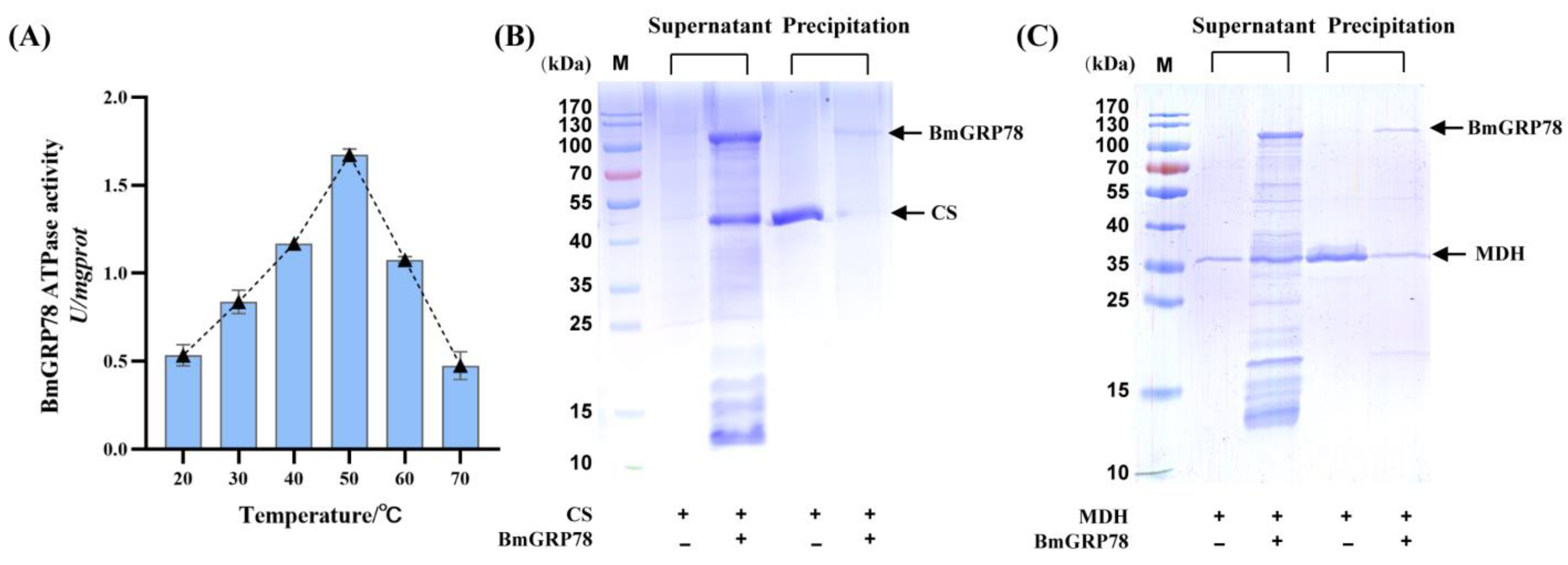
2.4. The ATPase Activity of rBmGRP78
2.5. rBmGRP78 Inhibits Thermal Aggregation of Citrate Synthase (CS) and Malate Dehydrogenase (MDH)
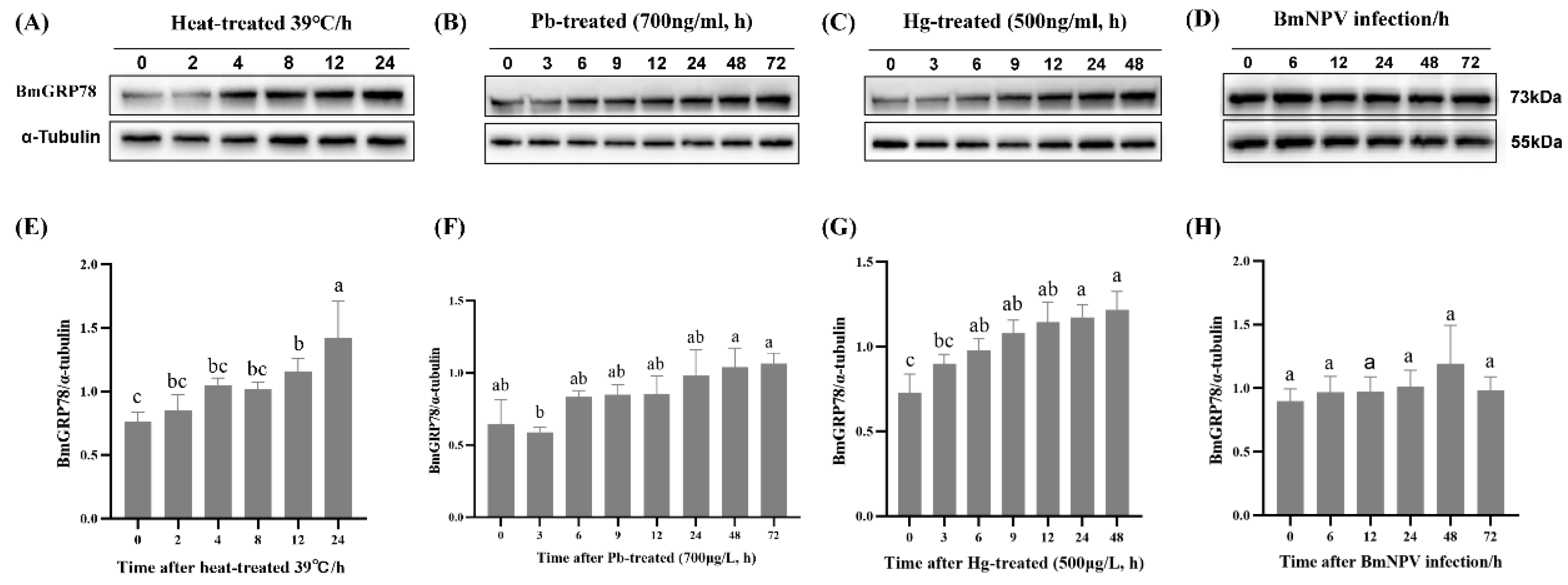
2.6. BmGRP78 Expression in Response to Abiotic and Biotic Stresses in BmN Cells
2.7. Subcellular Localization of BmGRP78 under Different Stress-Induced Conditions in BmN Cells
3. Discussion
4. Materials and Methods
4.1. Biological Materials
4.2. Cloning and Sequence Analysis of BmGRP78
4.3. Bio-Informatic Analysis
4.4. Prokaryotic Expression, Purification, and Antiserum Preparation of Recombinant BmGRP78 (rBmGRP78)
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.6. SDS-PAGE and Western Blotting
4.7. The ATPase Activity Assay
4.8. Thermal Aggregation Assays
4.9. Abiotic and Biotic Treatment
4.10. Subcellular Localization by Confocal Microscopy
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patra, A.; Adhikary, A.; Ghosh, S. The unfolded protein response (UPR) pathway: The unsung hero in breast cancer management. Apoptosis 2022. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.L.; Marzano, N.R.; van Oijen, A.M.; Ecroyd, H. Using Single-Molecule Approaches to Understand the Molecular Mechanisms of Heat-Shock Protein Chaperone Function. J. Mol. Biol. 2018, 430, 4525–4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, T.; Cohen, E. Organismal Protein Homeostasis Mechanisms. Genetics 2020, 215, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Xia, S.; Duan, W.; Liu, W.; Zhang, X.; Wang, Q. GRP78 in lung cancer. J. Transl. Med. 2021, 19, 118. [Google Scholar] [CrossRef]
- Carlos, A.J.; Ha, D.P.; Yeh, D.W.; Van Krieken, R.; Tseng, C.C.; Zhang, P.; Gill, P.; Machida, K.; Lee, A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021, 296, 100759. [Google Scholar] [CrossRef]
- Reid, S.P.; Shurtleff, A.C.; Costantino, J.A.; Tritsch, S.R.; Retterer, C.; Spurgers, K.B.; Bavari, S. HSPA5 is an essential host factor for Ebola virus infection. Antivir. Res. 2014, 109, 171–174. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Ha, D.P.; Zhao, H.; Carlos, A.J.; Wei, S.; Pun, T.K.; Wu, K.; Zandi, E.; Kelly, K.; Lee, A.S. Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-β signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E4245–E4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Kim, H.J.; Kim, H.J.; Kim, D.H.; Han, J.H.; Byeon, H.K.; Lee, K.; Kim, C.H. HSPA5 negatively regulates lysosomal activity through ubiquitination of MUL1 in head and neck cancer. Autophagy 2018, 14, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albakova, Z.; Mangasarova, Y. The HSP Immune Network in Cancer. Front. Immunol. 2021, 12, 796493. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, X.; Meng, Y.; Ma, J.; Zhang, Q.; Shao, G.; Wang, L.; Cheng, X.; Hong, X.; Wang, Y.; et al. A Novel Mechanism of Endoplasmic Reticulum Stress- and c-Myc-Degradation-Mediated Therapeutic Benefits of Antineurokinin-1 Receptor Drugs in Colorectal Cancer. Adv. Sci. 2021, 8, e2101936. [Google Scholar] [CrossRef]
- Cappellozza, S.; Casartelli, M.; Sandrelli, F.; Saviane, A.; Tettamanti, G. Silkworm and Silk: Traditional and Innovative Applications. Insects 2022, 13, 1016. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A Promising Model Organism in Life Science. J. Insect Sci. 2017, 17, 97. [Google Scholar]
- Li, F.; Li, M.; Wang, H.; Mao, T.; Chen, J.; Lu, Z.; Qu, J.; Fang, Y.; Li, B. Effects of phoxim pesticide on the immune system of silkworm midgut. Pestic. Biochem. Physiol. 2020, 164, 58–64. [Google Scholar] [CrossRef]
- Jiang, L. Insights Into the Antiviral Pathways of the Silkworm Bombyx mori. Front. Immunol. 2021, 12, 639092. [Google Scholar] [CrossRef]
- Imai, S.; Kusakabe, T.; Xu, J.; Li, Z.; Shirai, S.; Mon, H.; Morokuma, D.; Lee, J.M. Roles of silkworm endoplasmic reticulum chaperones in the secretion of recombinant proteins expressed by baculovirus system. Mol. Cell. Biochem. 2015, 409, 255–262. [Google Scholar] [CrossRef]
- Lv, M.; Cai, Y.; Hou, W.; Peng, K.; Xu, K.; Lu, C.; Yu, W.; Zhang, W.; Liu, L. The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. Inflamm. Res. 2022, 71, 461–472. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Cell-PLoc: A package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Ogata, T.; Yamazoe, K.; Tanaka, Y.; Inoue, Y.H. Heat shock cognate 70 genes contribute to Drosophila spermatocyte growth progression possibly through the insulin signaling pathway. Dev. Growth Differ. 2021, 63, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Wang, X.; Mao, H.; Wan, Y.; Liu, Y.; Zhang, T.; Hu, C. A mechanism underlies fish GRP78 protection against Pb2+ toxicity. Fish Shellfish Immunol. 2017, 66, 185–188. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020, 80, 554–562. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jaattela, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Rao, P.; Gajbhiye, R.; Kulkarni, V.; Parte, P. Glucose Regulated Protein 78 Phosphorylation in Sperm Undergoes Dynamic Changes during Maturation. PLoS ONE 2015, 10, e0141858. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Jung, E.J.; Hwang, J.M.; Bae, J.W.; Kwon, W.S. GRP78 plays a key role in sperm function via the PI3K/PDK1/AKT pathway. Reprod. Toxicol. 2022, 113, 103–109. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Liu, Q.; Hendrickson, W.A. Conformational equilibria in allosteric control of Hsp70 chaperones. Mol. Cell 2021, 81, 3919–3933.e7. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef]
- Clerico, E.M.; Tilitsky, J.M.; Meng, W.; Gierasch, L.M. How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J. Mol. Biol. 2015, 427, 1575–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, A.L. Chaperone-mediated protein folding. Physiol. Rev. 1999, 79, 425–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K. Signalling pathways in the unfolded protein response: Development from yeast to mammals. J. Biochem. 2009, 146, 743–750. [Google Scholar] [CrossRef]
- Shi-Chen Ou, D.; Lee, S.B.; Chu, C.S.; Chang, L.H.; Chung, B.C.; Juan, L.J. Transcriptional activation of endoplasmic reticulum chaperone GRP78 by HCMV IE1-72 protein. Cell Res. 2011, 21, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sevier, C.S. Formation and Reversibility of BiP Protein Cysteine Oxidation Facilitate Cell Survival during and post Oxidative Stress. J. Biol. Chem. 2016, 291, 7541–7557. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhang, Q.; Sun, X.; Zeh, H.J., 3rd; Lotze, M.T.; Kang, R.; Tang, D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Lei, J.; Zhu, Y.; Chen, X.; Mao, F.; Miao, M.; Quan, Y.; Yu, W. Role of the Bombyx mori nucleopolyhedrovirus LEF3 acetylation on viral replication. Microb. Pathog. 2021, 158, 105109. [Google Scholar] [CrossRef]
- Li, S.; Fei, J.; Cheng, D.; Jin, Y.; Zhang, W.; Zhang, Y.; Lv, Z. Bioinformatics, Tissue Distribution, and Subcellular Localization Analyses of Fk506 Binding Protein 12b from Silkworms. Arch. Insect Biochem. Physiol. 2016, 91, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, S.; Mo, Y.; Zhu, Y.; Chen, X.; Miao, M.; Quan, Y.; Yu, W. Acetylation of fructose-bisphosphate aldolase-mediated glycolysis is essential for Bombyx mori nucleopolyhedrovirus infection. Microb. Pathog. 2022, 170, 105695. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, P.; Yu, H.; Li, W.; Chai, W.; Liang, Z. Based on the whole genome clarified the evolution and expression process of fatty acid desaturase genes in three soybeans. Int. J. Biol. Macromol. 2021, 182, 1966–1980. [Google Scholar] [CrossRef]
- Kiefer, F.; Arnold, K.; Kunzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009, 37, D387–D392. [Google Scholar] [CrossRef] [Green Version]
- Diao, X.; Ye, F.; Zhang, M.; Ren, X.; Tian, X.; Lu, J.; Sun, X.; Hou, Z.; Chen, X.; Li, F.; et al. Identification of oleoylethanolamide as an endogenous ligand for HIF-3α. Nat. Commun. 2022, 13, 2529. [Google Scholar] [CrossRef]
- Xue, S.; Mao, F.; Hu, D.; Yan, H.; Lei, J.; Obeng, E.; Zhou, Y.; Quan, Y.; Yu, W. Acetylation of BmAtg8 inhibits starvation-induced autophagy initiation. Mol. Cell. Biochem. 2019, 457, 73–81. [Google Scholar] [CrossRef]
- You, Z.; Ye, X.; Ye, L.; Qian, Q.; Wu, M.; Song, J.; Che, J.; Zhong, B. Extraordinary Mechanical Properties of Composite Silk Through Hereditable Transgenic Silkworm Expressing Recombinant Major Ampullate Spidroin. Sci. Rep. 2018, 8, 15956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, L.; Shan, Z.; Huang, H.; Chen, H.; Ding, X.; Guo, J.; Liu, L. Transcriptome sequencing analysis of novel sRNAs of Kineococcus radiotolerans in response to ionizing radiation. Microbiol. Res. 2016, 192, 122–129. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, K.; Li, J.; Zeng, C.; Huang, Q.; Nie, Y.; Zhu, J.; Gan, M. Microcalorimetric investigation of the ATPase activity and the refolding activity of GroEL system. J. Therm. Anal. Calorim. 2018, 135, 2411–2418. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Li, D.; Liu, Y.; Sheng, Q.; Lv, Z.; Yu, W.; Nie, Z. BmHSP20.8 is Localized in the Mitochondria and has a Molecular Chaperone Function In Vitro. J. Insect Sci. 2015, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Ungelenk, S.; Moayed, F.; Ho, C.T.; Grousl, T.; Scharf, A.; Mashaghi, A.; Tans, S.; Mayer, M.P.; Mogk, A.; Bukau, B. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 2016, 7, 13673. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Wang, H.; Lu, M.; Ni, X.; Bahri, N.; Zhu, S.; Chen, L.; Wu, Y.; Qiu, J.; Fletcher, J.A.; et al. AXL Inactivation Inhibits Mesothelioma Growth and Migration via Regulation of p53 Expression. Cancers 2020, 12, 2757. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, X.; Xu, X.; Jiang, T.; Zhou, H.; Sheng, Q.; Nie, Z. Cloning, expression profiling, and acetylation identification of alpha-tubulin N-acetyltransferase 1 from Bombyx mori. Arch. Insect Biochem. Physiol. 2018, 98, e21463. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Jin, Y.; Liu, S.Q.; Xu, S.L.; Huang, Y.X.; Xu, Y.S.; Shi, L.G.; Wang, H.B. Genome-wide identification and comparative analysis of lipocalin families in Lepidoptera with an emphasis on Bombyx mori. Insect Sci. 2022, 30, 15–30. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequence (5′-3′) | Application | Length of Product (bp) | |
|---|---|---|---|---|
| BmGRP78 | Forward | ATGGTCAAGATGCG | ||
| Reverse | CAACTCGTCCTTGAAG | |||
| Forward | CCCAAGCTTGCCACCACCACCACCACCACATGGTCAAGATGCG | PCR | 2012 | |
| Reverse | CCGCTCGAGCAACTCGTCCTTGAAG | |||
| Forward | AAGGACATCGGCACAGTAATCG | qRT-PCR | 141 | |
| Reverse | ATCTTGAGTGAAGGCCACGTAT | |||
| RP49 | Forward | TGCTCCCAAATGGATTCCGTAAG | qRT-PCR | 131 |
| Reverse | CACGATCAGCTTCCGCTTCTTC | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Ren, L.; Wang, Y.; Wen, H.; Ji, Y.; Li, C.; Yi, Y.; Jiang, C.; Sheng, Q.; Nie, Z.; et al. Biochemical Characterization and Functional Analysis of Glucose Regulated Protein 78 from the Silkworm Bombyx mori. Int. J. Mol. Sci. 2023, 24, 3964. https://doi.org/10.3390/ijms24043964
Xiao Y, Ren L, Wang Y, Wen H, Ji Y, Li C, Yi Y, Jiang C, Sheng Q, Nie Z, et al. Biochemical Characterization and Functional Analysis of Glucose Regulated Protein 78 from the Silkworm Bombyx mori. International Journal of Molecular Sciences. 2023; 24(4):3964. https://doi.org/10.3390/ijms24043964
Chicago/Turabian StyleXiao, Yao, Lujie Ren, Yanan Wang, Huanhuan Wen, Yongqiang Ji, Chenshou Li, Yangqing Yi, Caiying Jiang, Qing Sheng, Zuoming Nie, and et al. 2023. "Biochemical Characterization and Functional Analysis of Glucose Regulated Protein 78 from the Silkworm Bombyx mori" International Journal of Molecular Sciences 24, no. 4: 3964. https://doi.org/10.3390/ijms24043964





