The Dual Function of RhoGDI2 in Immunity and Cancer
Abstract
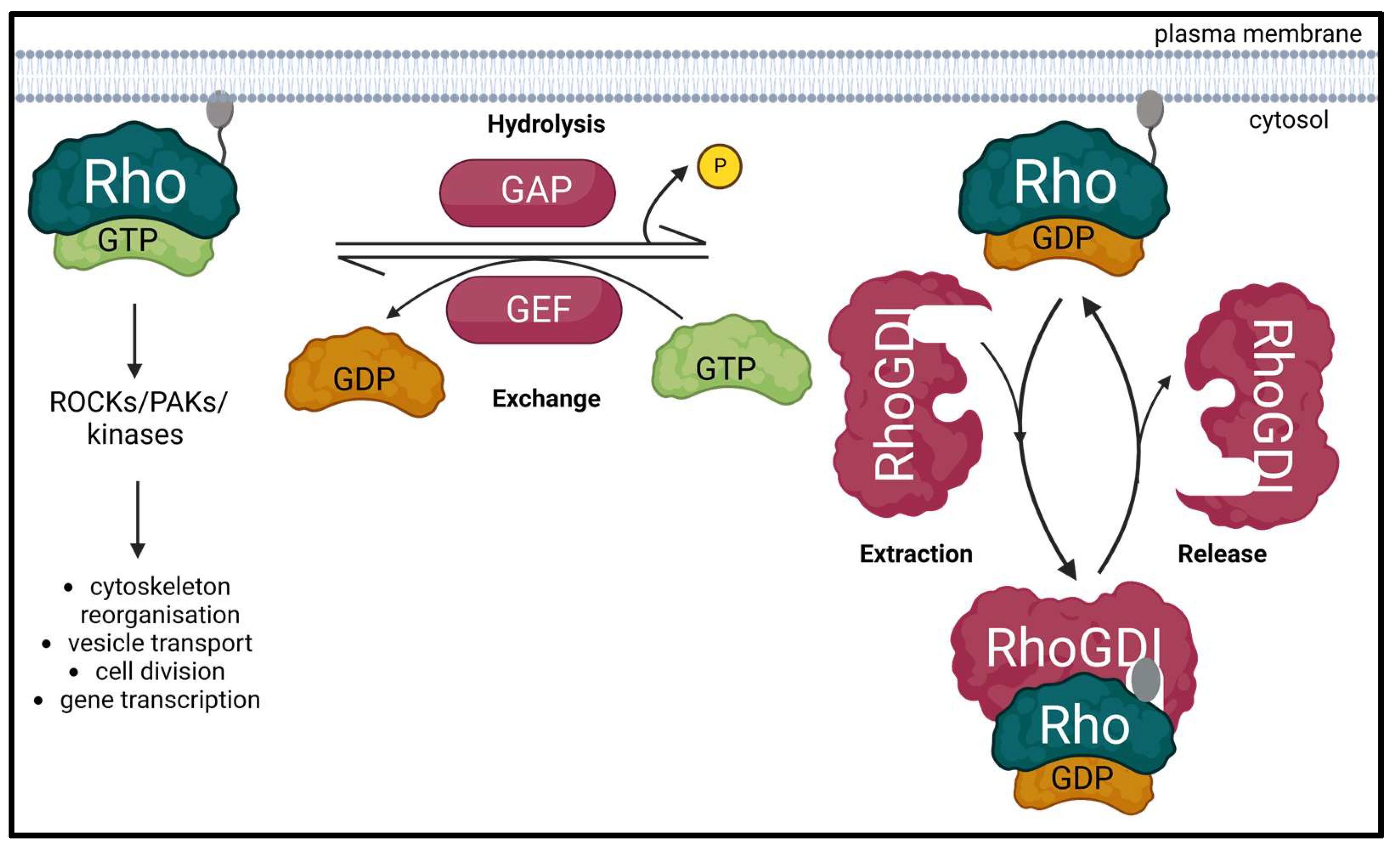
:1. Introduction
2. RhoGDI1 and RhoGDI2: Similarities and Differences
3. Regulatory Functions of RhoGDI2
3.1. Actin Cytoskeletal Organization
3.2. Immune Response
3.2.1. Innate Immune Response
3.2.2. Adaptive Immune Response
3.2.3. Phosphorylated RhoGDI2
3.3. Apoptosis
3.4. HIV-1 Replication
3.5. Vascular Remodeling
4. Regulatory Functions of RhoGDI2 in Cancer
4.1. Ovarian Cancer
4.2. Breast Cancer
4.3. Bladder Cancer
4.4. Osteosarcoma
4.5. Leukemias
4.6. Hodgkin’s Lymphoma
4.7. Pancreatic Cancer
4.8. Colorectal Cancer
4.9. Hepatocellular Carcinoma
4.10. Gastric Cancer
4.11. Melanoma
4.12. Lung Cancer
5. Discussion and Future Directives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.M.; Ridley, A.J. Rho GTPases in cancer cell biology. FEBS Lett. 2008, 582, 2093–2101. [Google Scholar] [CrossRef] [Green Version]
- Rodenburg, W.S.; van Buul, J.D. Rho GTPase signalling networks in cancer cell transendothelial migration. Vasc. Biol. 2021, 3, R77–R95. [Google Scholar] [CrossRef]
- Magalhaes, Y.T.; Farias, J.O.; Silva, L.E.; Forti, F.L. GTPases, genome, actin: A hidden story in DNA damage response and repair mechanisms. DNA Repair 2021, 100, 103070. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in cancer: Friend or foe? Oncogene 2019, 38, 7447–7456. [Google Scholar] [CrossRef]
- Kreider-Letterman, G.; Carr, N.M.; Garcia-Mata, R. Fixing the GAP: The role of RhoGAPs in cancer. Eur. J. Cell Biol. 2022, 101, 151209. [Google Scholar] [CrossRef]
- Maldonado, M.D.M.; Medina, J.I.; Velazquez, L.; Dharmawardhane, S. Targeting Rac and Cdc42 GEFs in Metastatic Cancer. Front. Cell Dev. Biol. 2020, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Kim, J.-T.; Baek, K.E.; Kim, B.-Y.; Lee, H.G. Regulation of Rho GTPases by RhoGDIs in Human Cancers. Cells 2019, 8, 1037. [Google Scholar] [CrossRef] [Green Version]
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase signaling in cancer progression and dissemination. Physiol. Rev. 2022, 102, 455–510. [Google Scholar] [CrossRef]
- Kowluru, A. Roles of GTP and Rho GTPases in pancreatic islet beta cell function and dysfunction. Small GTPases 2020, 12, 323–335. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Kaibuchi, K.; Hori, Y.; Fujioka, H.; Araki, S.; Ueda, T.; Kikuchi, A.; Takai, Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 1990, 5, 1321–1328. [Google Scholar]
- Kowluru, A.; Gleason, N.F. Underappreciated roles for Rho GDP dissociation inhibitors (RhoGDIs) in cell function: Lessons learned from the pancreatic islet β-cell. Biochem. Pharmacol. 2021, 197, 114886. [Google Scholar] [CrossRef]
- Lelias, J.M.; Adra, C.N.; Wulf, G.M.; Guillemot, J.C.; Khagad, M.; Caput, D.; Lim, B. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 1479–1483. [Google Scholar] [CrossRef] [Green Version]
- Scherle, P.; Behrens, T.; Staudt, L.M. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 7568–7572. [Google Scholar] [CrossRef] [Green Version]
- De León-Bautista, M.P.; Cardenas-Aguayo, M.D.C.; Casique-Aguirre, D.; Almaraz-Salinas, M.; Parraguirre-Martinez, S.; Olivo-Diaz, A.; Thompson-Bonilla, M.D.R.; Vargas, M. Immunological and Functional Characterization of RhoGDI3 and Its Molecular Targets RhoG and RhoB in Human Pancreatic Cancerous and Normal Cells. PLoS ONE 2016, 11, e0166370. [Google Scholar] [CrossRef] [Green Version]
- Leffers, H.; Nielsen, M.S.; Andersen, A.H.; Honoré, B.; Madsen, P.; Vandekerckhove, J.; Celis, J.E. Identification of Two Human Rho GDP Dissociation Inhibitor Proteins Whose Overexpression Leads to Disruption of the Actin Cytoskeleton. Exp. Cell Res. 1993, 209, 165–174. [Google Scholar] [CrossRef]
- Garcia-Mata, R.; Boulter, E.; Burridge, K. The ‘invisible hand’: Regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 2011, 12, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Keep, N.; Barnes, M.; Barsukov, I.; Badii, R.; Lian, L.-Y.; Segal, A.W.; Moody, P.C.; Roberts, G.C. A modulator of rho family G proteins, rhoGDI, binds these G proteins via an immunoglobulin-like domain and a flexible N-terminal arm. Structure 1997, 5, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Mosaddeghzadeh, N.; Jasemi, N.S.K.; Majolée, J.; Zhang, S.-C.; Hordijk, P.L.; Dvorsky, R.; Ahmadian, M.R. Electrostatic Forces Mediate the Specificity of RHO GTPase-GDI Interactions. Int. J. Mol. Sci. 2021, 22, 12493. [Google Scholar] [CrossRef]
- Kardol-Hoefnagel, T.; van Logtestijn, S.A.; Otten, H.G. A Review on the Function and Regulation of ARHGDIB/RhoGDI2 Expression Including the Hypothetical Role of ARHGDIB/RhoGDI2 Autoantibodies in Kidney Transplantation. Transplant. Direct 2020, 6, e548. [Google Scholar] [CrossRef]
- Choi, E.-K.; Kim, J.-G.; Kim, H.-J.; Cho, J.-Y.; Jeong, H.; Park, Y.; Islam, R.; Cap, C.K.; Park, J.-B. Regulation of RhoA GTPase and novel target proteins for ROCK. Small GTPases 2017, 11, 95–102. [Google Scholar] [CrossRef]
- Majolée, J.; Podieh, F.; Hordijk, P.L.; Kovačević, I. The interplay of Rac1 activity, ubiquitination and GDI binding and its consequences for endothelial cell spreading. PLoS ONE 2021, 16, e0254386. [Google Scholar] [CrossRef]
- Chinchole, A.N.; Lone, K.A.; Tyagi, S. MLL regulates actin cytoskeleton and cell migration by stabilizing Rho GTPases via the transcription of RhoGDI1. J. Cell Sci. 2022, 135, jcs260042. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, S.; Ba, X.; Xu, T.; Wang, X.; Zeng, X. RhoGDI2 positively regulates the Rho GTPases activation in response to the β2 outside-in signaling in T cells adhesion and migration on ICAM-1. J. Leukoc. Biol. 2019, 106, 431–446. [Google Scholar] [CrossRef]
- Nagar, H.; Kim, S.; Lee, I.; Choi, S.-J.; Piao, S.; Jeon, B.H.; Shong, M.; Kim, C.-S. CRIF1 deficiency suppresses endothelial cell migration via upregulation of RhoGDI2. PLoS ONE 2021, 16, e0256646. [Google Scholar] [CrossRef]
- Yin, L.; Schwartzberg, P.; Scharton-Kerstenj, T.M.; Staudt, L.; Lenardo, M. Immune responses in mice deficient in Ly-GDI, a lymphoid-specific regulator of Rho GTPases. Mol. Immunol. 1997, 34, 481–491. [Google Scholar] [CrossRef]
- Zhu, G.-F.; Xu, Y.-W.; Li, J.; Niu, H.-L.; Ma, W.-X.; Xu, J.; Zhou, P.-R.; Liu, X.; Ye, D.-L.; Liu, X.-R.; et al. Mir20a/106a-WTX axis regulates RhoGDIa/CDC42 signaling and colon cancer progression. Nat. Commun. 2019, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Zhang, X.; Zeng, Y.; Liang, L.; Ding, Y. Overexpression of RhoGDI2 Correlates with Tumor Progression and Poor Prognosis in Colorectal Carcinoma. Ann. Surg. Oncol. 2011, 19, 145–153. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.-T.; Lee, S.-J.; Hwang, Y.S.; Park, S.Y.; Kim, B.-Y.; Yoo, J.; Hong, K.S.; Min, J.-K.; Lee, C.-H.; et al. Protein phosphatase 1B dephosphorylates Rho guanine nucleotide dissociation inhibitor 1 and suppresses cancer cell migration and invasion. Cancer Lett. 2018, 417, 141–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B. D4-GDI, a Rho GTPase Regulator, Promotes Breast Cancer Cell Invasiveness. Cancer Res 2006, 66, 5592–5598. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bi, X.; Huang, X.; Wang, B.; Guo, Q.; Wu, Z. Systematic investigation of biomarker-like role of ARHGDIB in breast cancer. Cancer Biomark. 2020, 28, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zou, H.; Zhan, S.; Cao, K. Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncol. Rep. 2007, 17, 1383–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, M.M.; Williams, M.M.; Hicks, D.J.; Jones, B.; Sanchez, V.; Young, C.D.; Sarbassov, D.D.; Muller, W.J.; Brantley-Sieders, D.; Cook, R.S. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017, 19, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Li, Q.; Huang, L.Y.; Li, D.W.; Wang, Y.W.; Li, X.X.; Cai, S.J. Loss of ARHGDIA expression is associated with poor prognosis in HCC and promotes invasion and metastasis of HCC cells. Int. J. Oncol. 2014, 45, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Yi, J.; Lizhi, L.; Qiucheng, C. Rho GDP Dissociation Inhibitor Beta Promotes Cell Proliferation and Invasion by Modulating the AKT Pathway in Hepatocellular Carcinoma. DNA Cell Biol. 2014, 33, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, J.; Zhao, Y.; Liu, X.; Chen, L.; Xia, Y.; Wang, Y.; Chen, S.; Sun, S.; Shi, B.; et al. KDM6A-ARHGDIB axis blocks metastasis of bladder cancer by inhibiting Rac1. Mol. Cancer 2021, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sottnik, J.L.; Dancik, G.M.; Sahu, D.; Hansel, D.E.; Theodorescu, D.; Schwartz, M.A. An Osteopontin/CD44 Axis in RhoGDI2-Mediated Metastasis Suppression. Cancer Cell 2016, 30, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Stevens, E.V.; Banet, N.; Onesto, C.; Plachco, A.; Alan, J.K.; Nikolaishvili-Feinberg, N.; Midkiff, B.R.; Kuan, P.F.; Liu, J.; Miller, C.R.; et al. RhoGDI2 antagonizes ovarian carcinoma growth, invasion and metastasis. Small GTPases 2011, 2, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Wang, J. Adenosine Inhibits Ovarian Cancer Growth Through Regulating RhoGDI2 Protein Expression. Drug Des. Dev. Ther. 2019, 13, 3837–3844. [Google Scholar] [CrossRef] [Green Version]
- Küppers, R.; Klein, U.; Schwering, I.; Distler, V.; Bräuninger, A.; Cattoretti, G.; Tu, Y.; Stolovitzky, G.A.; Califano, A.; Hansmann, M.-L.; et al. Identification of Hodgkin and Reed-Sternberg cell-specific genes by gene expression profiling. J. Clin. Investig. 2003, 111, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Xu, G.; Sotnikova, A.; Szczepanowski, M.; Giefing, M.; Krause, K.; Krams, M.; Siebert, R.; Jin, J.; Klapper, W. Loss of expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor, in Hodgkin lymphoma. Br. J. Haematol. 2007, 139, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ren, M.; Li, Y.; Liu, Y.; Chen, C.; Su, J.; Su, B.; Xia, H.; Liu, F.; Jiang, H.; et al. Knockdown of RhoGDI2 represses human gastric cancer cell proliferation, invasion and drug resistance via the Rac1/Pak1/LIMK1 pathway. Cancer Lett. 2020, 492, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Ryu, K.-J.; Kim, M.; Kim, T.; Kim, S.-H.; Han, H.; Kim, H.; Hong, K.-S.; Song, C.Y.; Choi, Y.; et al. RhoGDI2-Mediated Rac1 Recruitment to Filamin A Enhances Rac1 Activity and Promotes Invasive Abilities of Gastric Cancer Cells. Cancers 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Hu, Y.; Qin, G.; Gu, W.; Zhu, X.; He, S.; Zhou, J.; Li, D. Depletion of RhoGDI2 expression inhibits the ability of invasion and migration in pancreatic carcinoma. Int. J. Mol. Med. 2014, 34, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Ding, G.; Zhou, L.; Shen, T.; Xu, X.; Zhao, T.; Jia, S.; Cao, L. Interferon Gamma Inhibits CXCL8-Induced Proliferation and Migration of Pancreatic Cancer BxPC-3 Cell Line via a RhoGDI2/Rac1/NF-κB Signaling Pathway. J. Interferon Cytokine Res. 2018, 38, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Hu, Y.; Zhu, D.; Yao, J.; Zhou, J.; Zhang, Y.; He, Z.; Zhang, L.; Zhang, Z.; Yang, J.; et al. RhoGDI2 induced malignant phenotypes of pancreatic cancer cells via regulating Snail expression. Genes Genom. 2022, 44, 561–569. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Guan, L.; Xu, S.; Yuan, Y.; Lv, D.; He, X.; Zhan, J.; Kong, Y.; Guo, J.; et al. Unconventional myosin VIIA promotes melanoma progression. J. Cell Sci. 2018, 131, jcs209924. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Wu, B.; Jiang, H.; Li, H.; Zhang, Y.; Peng, Y.; He, P. Mechanisms of RhoGDI2 Mediated Lung Cancer Epithelial-Mesenchymal Transition Suppression. Cell. Physiol. Biochem. 2014, 34, 2007–2016. [Google Scholar] [CrossRef]
- Niu, H.; Wu, B.; Peng, Y.; Jiang, H.; Zhang, Y.; Wang, J.; Zhang, Y.; He, P. RNA interference-mediated knockdown of RhoGDI2 induces the migration and invasion of human lung cancer A549 cells via activating the PI3K/Akt pathway. Tumor Biol. 2014, 36, 409–419. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Dong, R.; Lin, G.; Zhang, N.; Wang, J.; Lin, N.; Gu, Y.; Ding, L.; Ying, M.; et al. Hypoxia-Induced WSB1 Promotes the Metastatic Potential of Osteosarcoma Cells. Cancer Res 2015, 75, 4839–4851. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Jin, Z.; Yan, F.; You, J.; Xie, J.; Chen, B.; Cheng, G.; Zhu, H.; He, Q.; Hu, Y.; et al. Discovery of 5,6-Bis(4-methoxy-3-methylphenyl)pyridin-2-amine as a WSB1 Degrader to Inhibit Cancer Cell Metastasis. J. Med. Chem. 2021, 64, 8621–8643. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Kondoh, K.; Fukushima, S.; Hashiguchi, A.; Du, W.; Hayashi, M.; Fujimoto, J.-I.; Hata, J.-I.; Yamada, T. Mutated D4-guanine diphosphate–dissociation inhibitor is found in human leukemic cells and promotes leukemic cell invasion. Exp. Hematol. 2008, 36, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, J.; Zheng, H.; Wang, L. Rho GDP-Dissociation Inhibitor 2 Inhibits C-X-C Chemokine Receptor Type 4-Mediated Acute Lymphoblastic Leukemia Cell Migration. Front. Oncol. 2020, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, A.M.B.A.; Ahmed, S.B.M.; Darling, N.J.; Harris, M.; Mott, H.R.; Owen, D. A Complete Survey of RhoGDI Targets Reveals Novel Interactions with Atypical Small GTPases. Biochemistry 2021, 60, 1533–1551. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Tidow, N.; Grothues, D.; Welte, K. Differential expression and regulation of GTPases (RhoA and Rac2) and GDIs (LyGDI and RhoGDI) in neutrophils from patients with severe congenital neutropenia. Blood 2000, 95, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Scheffzek, K.; Stephan, I.; Jensen, O.N.; Illenberger, D.; Gierschik, P. The Rac-RhoGDI complex and the structural basis for the regulation of Rho proteins by RhoGDI. Nat. Struct. Biol. 2000, 7, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Platko, J.V.; Leonard, A.D.; Adra, C.N.; Shaw, R.J.; Cerione, A.R.; Lim, B. A single residue can modify target-binding affinity and activity of the functional domain of the Rho-subfamily GDP dissociation inhibitors. Proc. Natl. Acad. Sci. USA 1995, 92, 2974–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togawa, A.; Miyoshi, J.; Ishizaki, H.; Tanaka, M.; Takakura, A.; Nishioka, H.; Yoshida, H.; Doi, T.; Mizoguchi, A.; Matsuura, N.; et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene 1999, 18, 5373–5380. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, H.; Togawa, A.; Tanaka-Okamoto, M.; Hori, K.; Nishimura, M.; Hamaguchi, A.; Imai, T.; Takai, Y.; Miyoshi, J. Defective Chemokine-Directed Lymphocyte Migration and Development in the Absence of Rho Guanosine Diphosphate-Dissociation Inhibitors α and β. J. Immunol. 2006, 177, 8512–8521. [Google Scholar] [CrossRef] [Green Version]
- Bagci, H.; Sriskandarajah, N.; Robert, A.; Boulais, J.; Elkholi, I.E.; Tran, V.; Lin, Z.-Y.; Thibault, M.-P.; Dubé, N.; Faubert, D.; et al. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat. Cell Biol. 2019, 22, 120–134, Correction in Nat. Cell Biol. 2020, 22, 353. [Google Scholar] [CrossRef]
- Liu, S.; Cui, H.; Li, Q.; Zhang, L.; Na, Q.; Liu, C. RhoGDI2 Is Expressed in Human Trophoblasts and Involved in Their Migration by Inhibiting the Activation of RAC11. Biol. Reprod. 2014, 90, 88. [Google Scholar] [CrossRef] [PubMed]
- Institute for Quality and Efficiency in Health Care (IQWiG). The Innate and Adaptive Immune Systems. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279396/ (accessed on 5 December 2022).
- Mehta, P.; Wavreille, A.-S.; Justiniano, S.; Marsh, R.L.; Yu, J.; Burry, R.W.; Jarjoura, D.; Eubank, T.; Caligiuri, M.A.; Butchar, J.P.; et al. LyGDI, a Novel SHIP-Interacting Protein, Is a Negative Regulator of FcγR-Mediated Phagocytosis. PLoS ONE 2011, 6, e21175. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Chavrier, P.; Caron, E. Actin dynamics during phagocytosis. Semin. Immunol. 2001, 13, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Vitali, R.; Palone, F.; Armuzzi, A.; Fulci, V.; Negroni, A.; Carissimi, C.; Cucchiara, S.; Stronati, L. Proteomic Analysis Identifies Three Reliable Biomarkers of Intestinal Inflammation in the Stools of Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 17, 92–102. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Do, D.N.; Nguyen, T.T.P.; Nguyen, T.L.; Nguyen-Thanh, T.; Nguyen, H.T. Immune–related biomarkers shared by inflammatory bowel disease and liver cancer. PLoS ONE 2022, 17, e0267358. [Google Scholar] [CrossRef]
- Wu, J.; Cao, J.; Fan, Y.; Li, C.; Hu, X. Comprehensive analysis of miRNA–mRNA regulatory network and potential drugs in chronic chagasic cardiomyopathy across human and mouse. BMC Med. Genom. 2021, 14, 283. [Google Scholar] [CrossRef]
- Tabellini, G.; Baronio, M.; Patrizi, O.; Benevenuto, A.; Gazzurelli, L.; Plebani, A.; Parolini, S.; Lougaris, V. The RAC2-PI3K axis regulates human NK cell maturation and function. Clin. Immunol. 2019, 208, 108257. [Google Scholar] [CrossRef]
- Hojjatipour, T.; Aslani, S.; Salimifard, S.; Mikaeili, H.; Hemmatzadeh, M.; Navashenaq, J.G.; Parvin, E.A.; Jadidi-Niaragh, F.; Mohammadi, H. NK cells—Dr. Jekyll and Mr. Hyde in autoimmune rheumatic diseases. Int. Immunopharmacol. 2022, 107, 108682. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, X.; Mao, X.; Wen, D.; Zhang, H.; Wang, J. Inflammatory and immune-related factor Caspase 1 contributes to the development of oral lichen planus. Arch. Oral Biol. 2021, 131, 105244. [Google Scholar] [CrossRef]
- Kamburova, E.G.; Gruijters, M.L.; Kardol-Hoefnagel, T.; Wisse, B.W.; Joosten, I.; Allebes, W.A.; van der Meer, A.; Hilbrands, L.B.; Baas, M.C.; Spierings, E.; et al. Antibodies against ARHGDIB are associated with long-term kidney graft loss. Am. J. Transplant. 2019, 19, 3335–3344. [Google Scholar] [CrossRef] [Green Version]
- Senev, A.; Otten, H.G.; Kamburova, E.G.; Callemeyn, J.; Lerut, E.; Van Sandt, V.; Kuypers, D.; Emonds, M.-P.; Naesens, M. Antibodies against ARHGDIB and ARHGDIB Gene Expression Associate with Kidney Allograft Outcome. Transplantation 2020, 104, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.; Sablik, K.A.; Litjens, N.H.; Otten, H.G.; de Weerd, A.E. ARHGDIB and AT1R autoantibodies are differentially related to the development and presence of chronic antibody-mediated rejection and fibrosis in kidney allografts. Hum. Immunol. 2021, 82, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kardol-Hoefnagel, T.; Michielsen, L.A.; Ehlers, A.M.; van Zuilen, A.D.; Luijk, B.; Otten, H.G. Complement component C3 and C5b-9 deposition on hypoxia reperfused endothelial cells by non-HLA antibodies against RhoGDI2: A player involved in graft failure? Hla 2022, 101, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.-P.; Chang, T.-C.; Boretto, J.; Azuma, T.; Chavrier, P. Differential properties of D4/LyGDI versus RhoGDI: Phosphorylation and rho GTPase selectivity. FEBS Lett. 1998, 422, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Groysman, M.; Hornstein, I.; Alcover, A.; Katzav, S. Vav1 and Ly-GDI Two Regulators of Rho GTPases, Function Cooperatively as Signal Transducers in T Cell Antigen Receptor-induced Pathways. J. Biol. Chem. 2002, 277, 50121–50130. [Google Scholar] [CrossRef] [Green Version]
- Ngo, A.T.P.; Thierheimer, M.L.D.; Babur, Ö.; Rocheleau, A.D.; Huang, T.; Pang, J.; Rigg, R.A.; Mitrugno, A.; Theodorescu, D.; Burchard, J.; et al. Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor Ly-GDI in platelet function: A spatial systems approach. Am. J. Physiol. Physiol. 2017, 312, C527–C536. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Chuang, T.-H.; Cunningham, A.; Turi, T.G.; Hanke, J.H.; Bokoch, G.M.; Danley, D.E. D4-GDI, a Substrate of CPP32, Is Proteolyzed during Fas-induced Apoptosis. J. Biol. Chem. 1996, 271, 11209–11213. [Google Scholar] [CrossRef] [Green Version]
- Krieser, R.J.; Eastman, A. Cleavage and nuclear translocation of the caspase 3 substrate Rho GDP-dissociation inhibitor, D4-GDI, during apoptosis. Cell Death Differ. 1999, 6, 412–419. [Google Scholar] [CrossRef]
- Choi, M.-R.; Groot, M.; Drexler, H.C.A. Functional implications of caspase-mediated RhoGDI2 processing during apoptosis of HL60 and K562 leukemia cells. Apoptosis 2007, 12, 2025–2035. [Google Scholar] [CrossRef]
- Kettritz, R.; Xu, Y.X.; Faass, B.; Klein, J.B.; Müller, E.C.; Otto, A.; Busjahn, A.; Luft, F.C.; Haller, H. TNF-alpha-mediated neutrophil apoptosis involves Ly-GDI, a Rho GTPase regulator. J. Leukoc. Biol. 2000, 68, 277–283. [Google Scholar] [CrossRef]
- Tavares, L.A.; Januário, Y.C.; Dasilva, L.L.P. HIV-1 Hijacking of Host ATPases and GTPases That Control Protein Trafficking. Front. Cell Dev. Biol. 2021, 9, 622610. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Urano, E.; Miyauchi, K.; Ichikawa, R.; Hamatake, M.; Misawa, N.; Sato, K.; Ebina, H.; Koyanagi, Y.; Komano, J. The Hematopoietic Cell-Specific Rho GTPase Inhibitor ARHGDIB/D4GDI Limits HIV Type 1 Replication. AIDS Res. Hum. Retrovir. 2012, 28, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anokhin, B.; Spearman, P. Viral and Host Factors Regulating HIV-1 Envelope Protein Trafficking and Particle Incorporation. Viruses 2022, 14, 1729. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Anversa, P.; Armstrong, P.W.; Brilla, C.G.; Burnett, J.C.; Cruickshank, J.M.; Devereux, R.B.; Giles, T.D.; Korsgaard, N.; Leier, C.V.; et al. Remodeling and reparation of the cardiovascular system. J. Am. Coll. Cardiol. 1992, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Kloc, M.; Ghobrial, R.M. Chronic allograft rejection: A significant hurdle to transplant success. Burn. Trauma 2014, 2, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Dai, F.; Qi, Y.; Guan, W.; Meng, G.; Liu, Z.; Zhang, T.; Yao, W. RhoGDI stability is regulated by SUMOylation and ubiquitination via the AT1 receptor and participates in Ang II-induced smooth muscle proliferation and vascular remodeling. Atherosclerosis 2019, 288, 124–136. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Dai, F.; Qi, Y.; Yang, L.; Liu, Z.; Deng, L.; Yao, W.; Qi, Y.; Deng, W.Y.L. ROCK inhibitors alleviate myofibroblast transdifferentiation and vascular remodeling via decreasing TGFβ1-mediated RhoGDI expression. Gen. Physiol. Biophys. 2019, 38, 271–280. [Google Scholar] [CrossRef]
- Tang, L.; Feng, P.; Qi, Y.; Huang, L.; Liang, X.; Chen, X. TGFβ1 induces myofibroblast transdifferentiation via increasing Smad-mediated RhoGDI-RhoGTPase signaling. Gen. Physiol. Biophys. 2022, 41, 511–521. [Google Scholar] [CrossRef]
- Nagy, Á.; Munkácsy, G.; Győrffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef]
- Huang, H.; Jin, H.; Zhao, H.; Wang, J.; Li, X.; Yan, H.; Wang, S.; Guo, X.; Xue, L.; Li, J.; et al. RhoGDIβ promotes Sp1/MMP-2 expression and bladder cancer invasion through perturbing miR-200c-targeted JNK2 protein translation. Mol. Oncol. 2017, 11, 1579–1594. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Jin, H.; Xu, J.; Gu, J.; Li, X.; Xie, Q.; Huang, H.; Li, J.; Tian, Z.; Jiang, G.; et al. XIAP overexpression promotes bladder cancer invasion in vitro and lung metastasis in vivo via enhancing nucleolin-mediated Rho-GDIβ mRNA stability. Int. J. Cancer 2017, 142, 2040–2055. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hua, X.; Jin, H.; Zhu, J.; Li, Y.; Li, J.; Huang, C. NFκB2 p52 stabilizesrhogdiβmRNA by inhibiting AUF1 protein degradation via a miR-145/Sp1/USP8-dependent axis. Mol. Carcinog. 2019, 58, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devel, L.; Guedeney, N.; Bregant, S.; Chowdhury, A.; Jean, M.; Legembre, P. Role of metalloproteases in the CD95 signaling pathways. Front. Immunol. 2022, 13, 1074099. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Li, Y.; Mao, X.; Tao, W.; Tang, Y.; Lin, Y.; Guo, Q.; Duan, J.; Lin, N. Therapeutic effects of Euphorbia Pekinensis and Glycyrrhiza glabra on Hepatocellular Carcinoma Ascites Partially Via Regulating the Frk-Arhgdib-Inpp5d-Avpr2-Aqp4 Signal Axis. Sci. Rep. 2017, 7, 41925. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.K.; Xuan, Y.L.; He, X.F. FHL1 Overexpression as a Inhibitor of Lung Cancer Cell Invasion via Increasing RhoGDIß mRNA Expression. Cell J. Yakhteh 2022, 24, 239–244. [Google Scholar] [CrossRef]
- Dang, H.; Schiebel, E. Emerging roles of centrosome cohesion. Open Biol. 2022, 12, 220229. [Google Scholar] [CrossRef]
- Ge, R.; Cao, M.; Chen, M.; Liu, M.; Xie, S. Cytoskeletal networks in primary cilia: Current knowledge and perspectives. J. Cell. Physiol. 2022, 237, 3975–3983. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I. Role of Polo-like Kinases Plk1 and Plk4 in the Initiation of Centriole Duplication—Impact on Cancer. Cells 2022, 11, 786. [Google Scholar] [CrossRef]
- Porter, A.P.; Reed, H.; White, G.R.M.; Ogg, E.-L.; Whalley, H.J.; Malliri, A. The RAC1 activator Tiam1 regulates centriole duplication through controlling PLK4 levels. J. Cell Sci. 2021, 134, jcs252502. [Google Scholar] [CrossRef]
- Epting, D.; Slanchev, K.; Boehlke, C.; Hoff, S.; Loges, N.T.; Yasunaga, T.; Indorf, L.; Nestel, S.; Lienkamp, S.S.; Omran, H.; et al. The Rac1 regulator ELMO controls basal body migration and docking in multiciliated cells through interaction with Ezrin. Development 2015, 142, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streets, A.J.; Prosseda, P.P.; Ong, A.C. Polycystin-1 regulates ARHGAP35-dependent centrosomal RhoA activation and ROCK signaling. JCI Insight 2020, 5, e135385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-S.; Maeda, M.; Okamoto, M.; Fujii, M.; Fukutomi, R.; Hori, M.; Tatsuka, M.; Ota, T. Centrosomal localization of RhoGDIβ and its relevance to mitotic processes in cancer cells. Int. J. Oncol. 2012, 42, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, N.; Togari, H.; Minagi, K.; Iwaoka, Y.; Tai, A.; Nakaoji, K.; Hamada, K.; Tatsuka, M. 2-O-Octadecylascorbic acid represses RhoGDIβ expression and ameliorates DNA damage-induced abnormal spindle orientations. J. Cell. Biochem. 2021, 122, 739–751. [Google Scholar] [CrossRef] [PubMed]





| Cancer | RhoGDI | Regulation | Reference(s) |
|---|---|---|---|
| Colorectal cancer | RhoGDI1 | Up | [27] |
| RhoGDI2 | Up | [28] | |
| Breast cancer | RhoGDI1 | Up | [29] |
| RhoGDI2 | Up | [30,31] | |
| Biphasic | [32,33] | ||
| Hepatocellular carcinoma | RhoGDI1 | Down | [34] |
| RhoGDI2 | Up | [35] | |
| Bladder cancer | RhoGDI1 | Down | [36] |
| RhoGDI2 | Down | [37] | |
| Ovarian cancer | RhoGDI2 | Down | [38,39] |
| Hodgkin’s lymphoma | RhoGDI2 | Down | [40,41] |
| Gastric cancer | RhoGDI2 | Up | [42,43] |
| Pancreatic cancer | RhoGDI2 | Up | [44,45,46] |
| Melanoma | RhoGDI2 | Up | [47] |
| Lung cancer | RhoGDI2 | Down | [48,49] |
| Osteosarcoma | RhoGDI2 | Down | [50,51] |
| Leukemias | RhoGDI2 | Down | [52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, M.; Colige, A.; Deroanne, C.F. The Dual Function of RhoGDI2 in Immunity and Cancer. Int. J. Mol. Sci. 2023, 24, 4015. https://doi.org/10.3390/ijms24044015
Tripathi M, Colige A, Deroanne CF. The Dual Function of RhoGDI2 in Immunity and Cancer. International Journal of Molecular Sciences. 2023; 24(4):4015. https://doi.org/10.3390/ijms24044015
Chicago/Turabian StyleTripathi, Mudrika, Alain Colige, and Christophe F. Deroanne. 2023. "The Dual Function of RhoGDI2 in Immunity and Cancer" International Journal of Molecular Sciences 24, no. 4: 4015. https://doi.org/10.3390/ijms24044015







