Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting
Abstract
1. Introduction
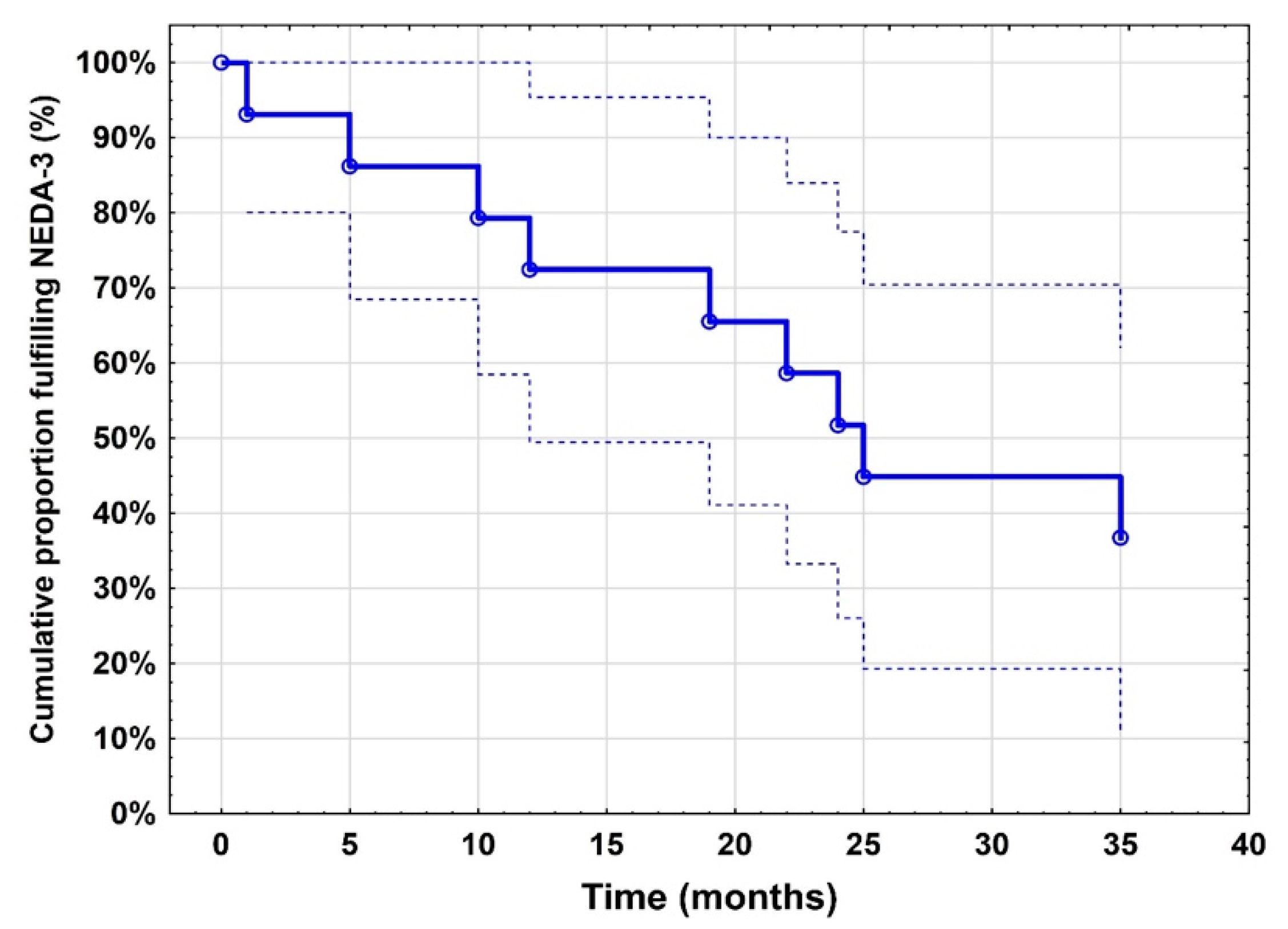
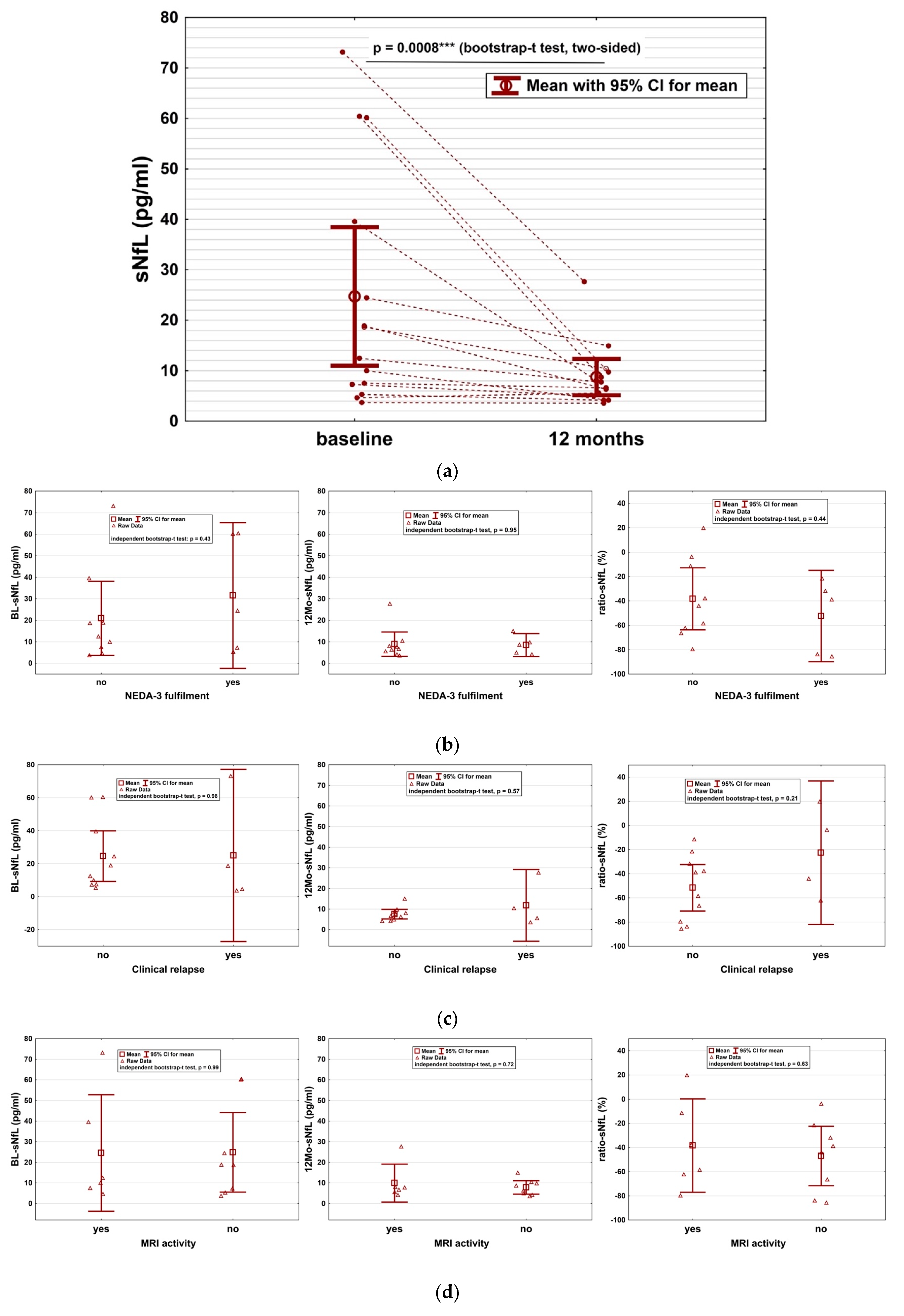
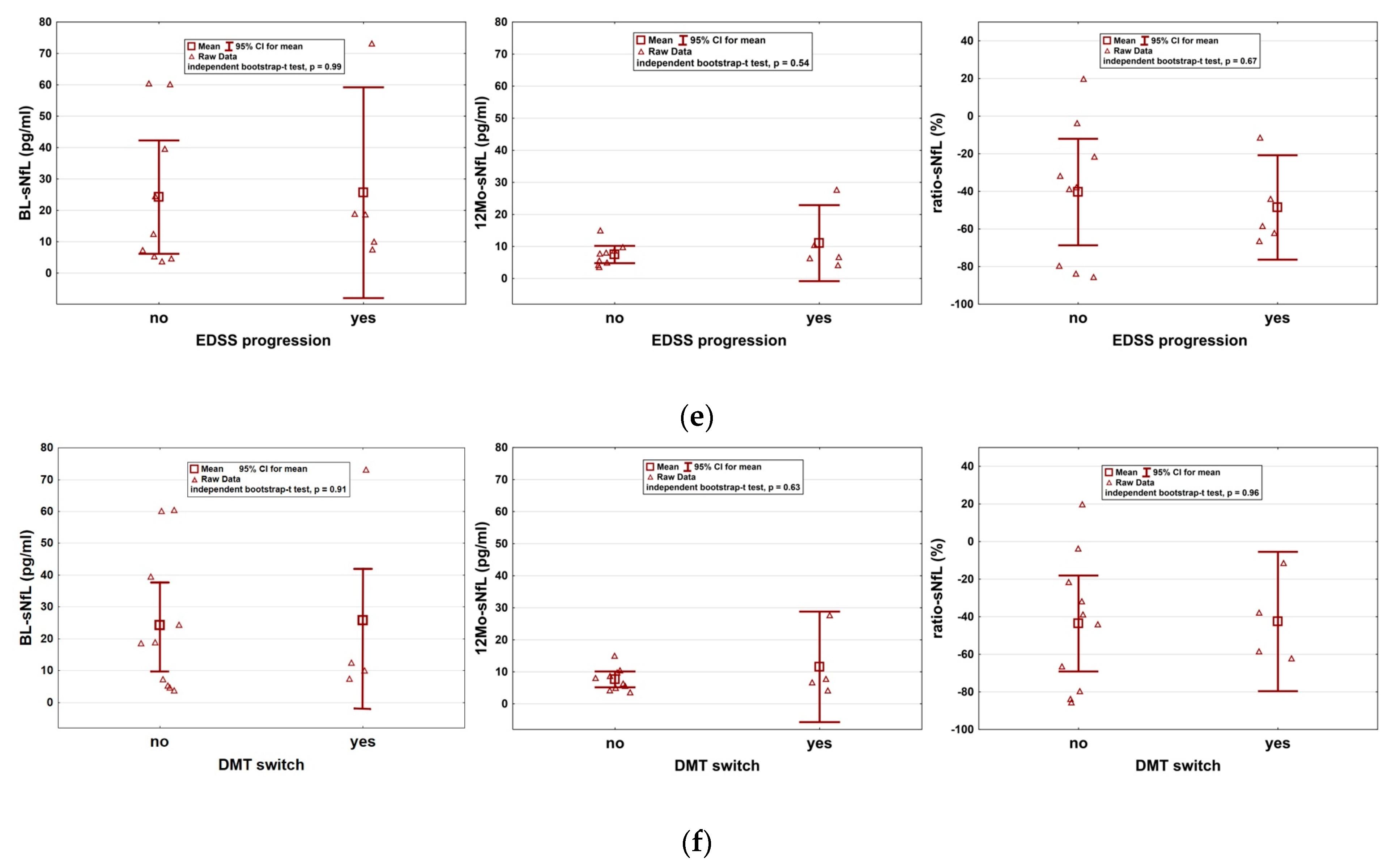
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Lunemann, J.D.; Ruck, T.; Muraro, P.A.; Bar-Or, A.; Wiendl, H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Hamlett, A.; Viglietta, V.; Greenberg, S.; CLARITY Study Group. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol. 2011, 10, 329–337. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Thebault, S.; Abdoli, M.; Fereshtehnejad, S.M.; Tessier, D.; Tabard-Cossa, V.; Freedman, M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020, 10, 10381. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Bittner, S.; Oh, J.; Havrdova, E.K.; Tintore, M.; Zipp, F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain 2021, 144, 2954–2963. [Google Scholar] [CrossRef]
- Paolicelli, D.; Ruggieri, M.; Manni, A.; Gargano, C.D.; Carleo, G.; Palazzo, C.; Iaffaldano, A.; Bollo, L.; Guerra, T.; Saracino, A.; et al. Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sci. 2022, 12, 1595. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Herrod, S.S.; Schmierer, K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult. Scler. Relat. Disord. 2019, 30, 176–186. [Google Scholar] [CrossRef]
- Moser, T.; Akgun, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Marta, M.; Pryce, G.; Giovannoni, G.; Schmierer, K. Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis. EBioMedicine 2017, 16, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Hoepner, L.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Cladribine Alters Immune Cell Surface Molecules for Adhesion and Costimulation: Further Insights to the Mode of Action in Multiple Sclerosis. Cells 2021, 10, 3116. [Google Scholar] [CrossRef] [PubMed]
- Leist, T.P.; Weissert, R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Stelmasiak, Z.; Grieb, P. Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult. Scler. Relat. Disord. 2019, 27, 117–120. [Google Scholar] [CrossRef]
- Håkansson, I.; Tisell, A.; Cassel, P.; Blennow, K.; Zetterberg, H.; Lundberg, P.; Dahle, C.; Vrethem, M.; Ernerudh, J. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J. Neuroinflammation 2018, 15, 209. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007-15. [Google Scholar] [CrossRef]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237. [Google Scholar] [CrossRef]
- Kuhle, J.; Daizadeh, N.; Benkert, P.; Maceski, A.; Barro, C.; Michalak, Z.; Sormani, M.P.; Godin, J.; Shankara, S.; Samad, T.A.; et al. Sustained reduction of serum neurofilament light chain over 7 years by alemtuzumab in early relapsing-remitting MS. Mult. Scler. 2022, 28, 573–582. [Google Scholar] [CrossRef]
- Mariottini, A.; Marchi, L.; Innocenti, C.; Di Cristinzi, M.; Pasca, M.; Filippini, S.; Barilaro, A.; Mechi, C.; Fani, A.; Mazzanti, B.; et al. Intermediate-Intensity Autologous Hematopoietic Stem Cell Transplantation Reduces Serum Neurofilament Light Chains and Brain Atrophy in Aggressive Multiple Sclerosis. Front. Neurol. 2022, 13, 820256. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; Leurs, C.E.; van Lierop, Z.Y.; van Kempen, Z.L.; Dekker, I.; Twaalfhoven, H.A.; Moraal, B.; Barkhof, F.; Uitdehaag, B.M.; Killestein, J.; et al. Serum Neurofilament Light Association with Progression in Natalizumab-Treated Patients with Relapsing-Remitting Multiple Sclerosis. Neurology 2021, 97, e1898–e1905. [Google Scholar] [CrossRef] [PubMed]
- Walo-Delgado, P.E.; Sainz de la Maza, S.; Villarrubia, N.; Monreal, E.; Medina, S.; Espiño, M.; Fernández-Velasco, J.I.; Rodríguez-Martín, E.; Roldán, E.; Lourido, D.; et al. Low serum neurofilament light chain values identify optimal responders to dimethyl fumarate in multiple sclerosis treatment. Sci. Rep. 2021, 11, 9299. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; Van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- University of California, San Francisco MS-EPIC Team; Cree, B.A.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Zhu, A.H.; et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar]
- Moser, T.; Ziemssen, T.; Sellner, J. Real-world evidence for cladribine tablets in multiple sclerosis: Further insights into efficacy and safety. Wien Med Wochenschr. 2022, 172, 365–372. [Google Scholar] [CrossRef]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef]
- Leist, T.P.; Comi, G.; Cree, B.A.; Coyle, P.K.; Freedman, M.S.; Hartung, H.P.; Vermersch, P.; Casset-Semanaz, F.; Scaramozza, M. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): A phase 3 randomised trial. Lancet Neurol. 2014, 13, 257–267. [Google Scholar] [CrossRef]
- Maltby, V.E.; Lea, R.A.; Monif, M.; Fabis-Pedrini, M.J.; Buzzard, K.; Kalincik, T.; Kermode, A.G.; Taylor, B.; Hodgkinson, S.; McCombe, P.; et al. Efficacy of Cladribine Tablets as a Treatment for People with Multiple Sclerosis: Protocol for the CLOBAS Study (Cladribine, a Multicenter, Long-term Efficacy and Biomarker Australian Study). JMIR Res. Protoc. 2021, 10, e24969. [Google Scholar] [CrossRef]
- Akgün, K.; Kretschmann, N.; Haase, R.; Proschmann, U.; Kitzler, H.H.; Reichmann, H.; Ziemssen, T. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e555. [Google Scholar] [CrossRef]
- Giovannoni, G.; Turner, B.; Gnanapavan, S.; Offiah, C.; Schmierer, K.; Marta, M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult. Scler. Relat. Disord. 2015, 4, 329–333. [Google Scholar] [CrossRef] [PubMed]
| Age (y), mean (SD) | 35.4 (9.3) |
| Female, No. (%) | 12 (86) |
| No. RRMS at baseline (%) | 14 (100) |
| Median follow-up in months (IQR) | 41.5 (36.0–46.3) |
| Median EDSS (range) at BL | 1.8 (0–3.5) |
| Median EDSS (range) at EOS | 1.3 (0–6.5) |
| Mean disease duration at BL, y (SD) | 7.9 (7.4) |
| Patients with DMTs before CLAD (%) First-line DMTs (%) Second-line DMTs (%) No. DMTs before CLAD, median (min; max) | 12/14 (86) 9/12 (75) 3/12 (25) 1.8 (0; 4) |
| Patients with DMTs after CLAD (%) Anti-CD20 infusion (%) S1P-modulator (%) Months from CLAD to DMT switch, mean (SD) | 4/14 (29) 3 (75) 1 (25) 32 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiberl, M.; Feige, J.; Hilpold, P.; Hitzl, W.; Machegger, L.; Buchmann, A.; Khalil, M.; Trinka, E.; Harrer, A.; Wipfler, P.; et al. Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting. Int. J. Mol. Sci. 2023, 24, 4067. https://doi.org/10.3390/ijms24044067
Seiberl M, Feige J, Hilpold P, Hitzl W, Machegger L, Buchmann A, Khalil M, Trinka E, Harrer A, Wipfler P, et al. Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting. International Journal of Molecular Sciences. 2023; 24(4):4067. https://doi.org/10.3390/ijms24044067
Chicago/Turabian StyleSeiberl, Michael, Julia Feige, Patrick Hilpold, Wolfgang Hitzl, Lukas Machegger, Arabella Buchmann, Michael Khalil, Eugen Trinka, Andrea Harrer, Peter Wipfler, and et al. 2023. "Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting" International Journal of Molecular Sciences 24, no. 4: 4067. https://doi.org/10.3390/ijms24044067
APA StyleSeiberl, M., Feige, J., Hilpold, P., Hitzl, W., Machegger, L., Buchmann, A., Khalil, M., Trinka, E., Harrer, A., Wipfler, P., & Moser, T. (2023). Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting. International Journal of Molecular Sciences, 24(4), 4067. https://doi.org/10.3390/ijms24044067






