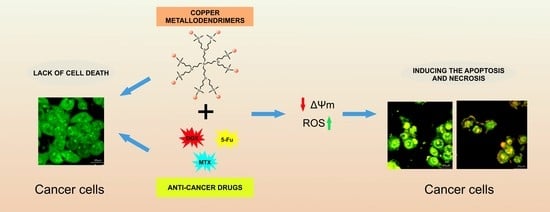
Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models
Abstract
:1. Introduction
2. Results
2.1. Zeta Potential and Size
2.2. Cytotoxicity
2.3. Generation of Intracellular Reactive Oxygen Species (ROS)
2.4. Mitochondrial Membrane Potential
2.5. Confocal Microscopy Imaging and Flow Cytometry Analysis of Cell Cycle and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Metallodendrimers
4.2. Drugs
4.3. Zeta Size
4.4. Zeta Potential
4.5. Cells
4.6. Cytotoxicity
4.7. ROS Level
4.8. Mitochondrial Membrane Potential
4.9. Annexin V/Propidium Iodide Double-Staining Assay
4.10. Orange Acridine/Ethidium Bromide Fluorescent Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Ma, L.; Merlin, D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin. Drug Deliv. 2016, 14, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Hołota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; del Olmo, N.S.; García-Gallego, S.; Ortega, P.; de la Mata, F.J.; Ionov, M.; Bryszewska, M. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Young, S.W.S.; Stenzel, M.; Jia-Lin, Y. Nanoparticle-siRNA: A potential cancer therapy? Crit. Rev. Oncol. Hematol. 2016, 98, 159–169. [Google Scholar] [CrossRef]
- Das, S.K.; Khatri, S.; Das, N.G. Effect of methotrexate conjugated PAMAM dendrimers on the viability of MES-SA uterine cancer cells. J. Pharm. Bioallied Sci. 2014, 6, 297–302. [Google Scholar] [CrossRef]
- Jin, Y.; Ren, X.; Wang, W.; Ke, L.; Ning, E.; Du, L.; Bradshaw, J. A 5-fluorouracil-loaded pH-responsive dendrimer nanocarrier for tumor targeting. Int. J. Pharm. 2011, 420, 378–384. [Google Scholar] [CrossRef]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef]
- Zhang, N.; Li, S.; Hua, H.; Liu, D.; Song, L.; Sun, P.; Huang, W.; Tang, Y.; Zhao, Y. Low density lipoprotein receptor targeted doxorubicin/DNA-Gold Nanorods as a chemo- and thermo-dual therapy for prostate cancer. Int. J. Pharm. 2016, 513, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Choi, S.K. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int. J. Mol. Sci. 2015, 16, 1772–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, A.; Burden, A. Noninvasive monitoring for methotrexate hepatotoxicity. Br. J. Dermatol. 2005, 152, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, V.; Saide, A.; Mitidieri, E.; di Villa, B.R.D.E.; Sorrentino, R.; Russo, G.; Russo, A. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-β-synthase in colon cancer cells lacking p53. Oncotarget 2016, 7, 50333–50348. [Google Scholar] [CrossRef] [Green Version]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Michlewska, S.; Maroto, M.; Hołota, M.; Kubczak, M.; del Olmo, N.S.; Ortega, P.; Shcharbin, D.; de la Mata, F.J.; Bryszewska, M.; Ionov, M. Combined therapy of ruthenium dendrimers and anti-cancer drugs against human leukemic cells. Dalton Trans. 2021, 50, 9500–9511. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Shcharbin, D.; Shcharbina, N.; Milowska, K.; de la Mata, F.J.; Muñoz-Fernandez, M.A.; Mignani, S.; Gomez-Ramirez, R.; Majoral, J.-P.; Bryszewska, M. Interference of cationic polymeric nanoparticles with clinical chemistry tests—Clinical relevance. Int. J. Pharm. 2014, 473, 599–606. [Google Scholar] [CrossRef]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Maly, M.; Ramirez, R.G.; de la Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef]
- Carloni, R.; del Olmo, N.S.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules 2019, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- Sanz Del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; Garcia-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu(ii)-metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, N.S.; Holota, M.; Michlewska, S.; Gómez, R.; Ortega, P.; Ionov, M.; de la Mata, F.J.; Bryszewska, M. Copper (II) metallodendrimers combined with pro-apoptotic sirnas as a promising strategy against breast cancer cells. Pharmaceutics 2020, 12, 727. [Google Scholar] [CrossRef]
- Canonico, B.; Carloni, R.; del Olmo, N.S.; Papa, S.; Nasoni, M.G.; Fattori, A.; Cangiotti, M.; de la Mata, F.J.; Ottaviani, M.F.; García-Gallego, S. Fine-Tuning the Interaction and Therapeutic Effect of Cu(II) Carbosilane Metallodendrimers in Cancer Cells: An In Vitro Electron Paramagnetic Resonance Study. Mol. Pharm. 2020, 17, 2691–2702. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Apelgot, S.; Coppey, J.; Grisvard, J.; Guillé, E.; Sissoëff, I. Distribution of copper-64 in control mice and in mice bearing ascitic Krebs tumor cells. Cancer Res 1981, 41, 1502–1507. [Google Scholar]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Element Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Zowczak, M.; Iskra, M.; Torlinski, L.; Cofta, S. Analysis of Serum Copper and Zinc Concentrations in Cancer Patients. Biol. Trace Element Res. 2001, 82, 1–8. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper complexes in cancer therapy. In Metallo-Drugs: Development and Action of Anticancer Agents; Walter de Gruyter GmbH: Berlin, Germany, 2018; Volume 18, pp. 469–506. [Google Scholar]
- Michlewska, S.; Kubczak, M.; Maroto-Díaz, M.; del Olmo, N.S.; Ortega, P.; Shcharbin, D.; Ramirez, R.G.; de la Mata, F.J.; Ionov, M.; Bryszewska, M. Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug. Biomolecules 2019, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Del Olmo, N.S.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; De La Mata, F.J.; et al. Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells. Int. J. Mol. Sci. 2020, 21, 4119. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Dzmitruk, V.; Rogalska, A.; Denel, M.; Gapinska, M.; et al. Ruthenium dendrimers against acute promyelocytic leukemia: In vitro studies on HL-60 cells. Futur. Med. Chem. 2019, 11, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.; Rao, T.; He, X.; Xu, T. Pharmaceutical applications of dendrimers: Promising nanocarriers for drug delivery. Front. Biosci. 2008, 13, 1447–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Ramirez, R.G.; de la Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef]
- Pandi, P.; Jain, A.; Kommineni, N.; Ionov, M.; Bryszewska, M.; Khan, W. Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int. J. Pharm. 2018, 550, 240–250. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; Cresteil, T.; Majoral, J.-P. First-in-Class Combination Therapy of a Copper(II) Metallo-Phosphorus Dendrimer with Cytotoxic Agents. Oncology 2018, 94, 324–328. [Google Scholar] [CrossRef]
- Szwed, A.; Miłowska, K.; Michlewska, S.; Moreno, S.; Shcharbin, D.; Gomez-Ramirez, R.; de la Mata, F.J.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro. Biomolecules 2020, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Benhar, M.; Engelberg, D.; Levitzki, A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Lucken-Ardjomande, S.; Martinou, J.-C. Regulation of Bcl-2 proteins and of the permeability of the outer mitochondrial membrane. Comptes Rendus Biol. 2005, 328, 616–631. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Gajek, A.; Ksiezak, M.; Dzialak, P.; Klajnert-Maculewicz, B. Molecular Mechanisms of Antitumor Activity of PAMAM Dendrimer Conjugates with Anticancer Drugs and a Monoclonal Antibody. Polymers 2019, 11, 1422. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.-P.; Cheng, G.; Zheng, S.-Y. Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Sun, Y.; Kang, C.; Sun, H.; Wei, W. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J. Drug Target. 2016, 25, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sanz del Olmo, N.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; de la Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef] [PubMed]








| Compound | Generation/Number of Surface Groups | Molecular Weight (g/mol) |
|---|---|---|
| CCD-Cl-0 | 0/1 | 414.93 |
| CCD-Cl-1 | 1/4 | 1627.68 |
| CCD-Cl-2 | 2/8 | 3696.01 |
| CCD-NO-0 | 0/1 | 468.04 |
| CCD-NO-1 | 1/4 | 1840.10 |
| CCD-NO-2 | 2/8 | 3992.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołota, M.; Michlewska, S.; Garcia-Gallego, S.; del Olmo, N.S.; Ortega, P.; Bryszewska, M.; de la Mata, F.J.; Ionov, M. Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models. Int. J. Mol. Sci. 2023, 24, 4076. https://doi.org/10.3390/ijms24044076
Hołota M, Michlewska S, Garcia-Gallego S, del Olmo NS, Ortega P, Bryszewska M, de la Mata FJ, Ionov M. Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models. International Journal of Molecular Sciences. 2023; 24(4):4076. https://doi.org/10.3390/ijms24044076
Chicago/Turabian StyleHołota, Marcin, Sylwia Michlewska, Sandra Garcia-Gallego, Natalia Sanz del Olmo, Paula Ortega, Maria Bryszewska, Francisco Javier de la Mata, and Maksim Ionov. 2023. "Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models" International Journal of Molecular Sciences 24, no. 4: 4076. https://doi.org/10.3390/ijms24044076
APA StyleHołota, M., Michlewska, S., Garcia-Gallego, S., del Olmo, N. S., Ortega, P., Bryszewska, M., de la Mata, F. J., & Ionov, M. (2023). Combination of Copper Metallodendrimers with Conventional Antitumor Drugs to Combat Cancer in In Vitro Models. International Journal of Molecular Sciences, 24(4), 4076. https://doi.org/10.3390/ijms24044076