Response to Abemaciclib and Immunotherapy Rechallenge with Nivolumab and Ipilimumab in a Heavily Pretreated TMB-H Metastatic Squamous Cell Lung Cancer with CDKN2A Mutation, PIK3CA Amplification and TPS 80%: A Case Report
Abstract
1. Introduction
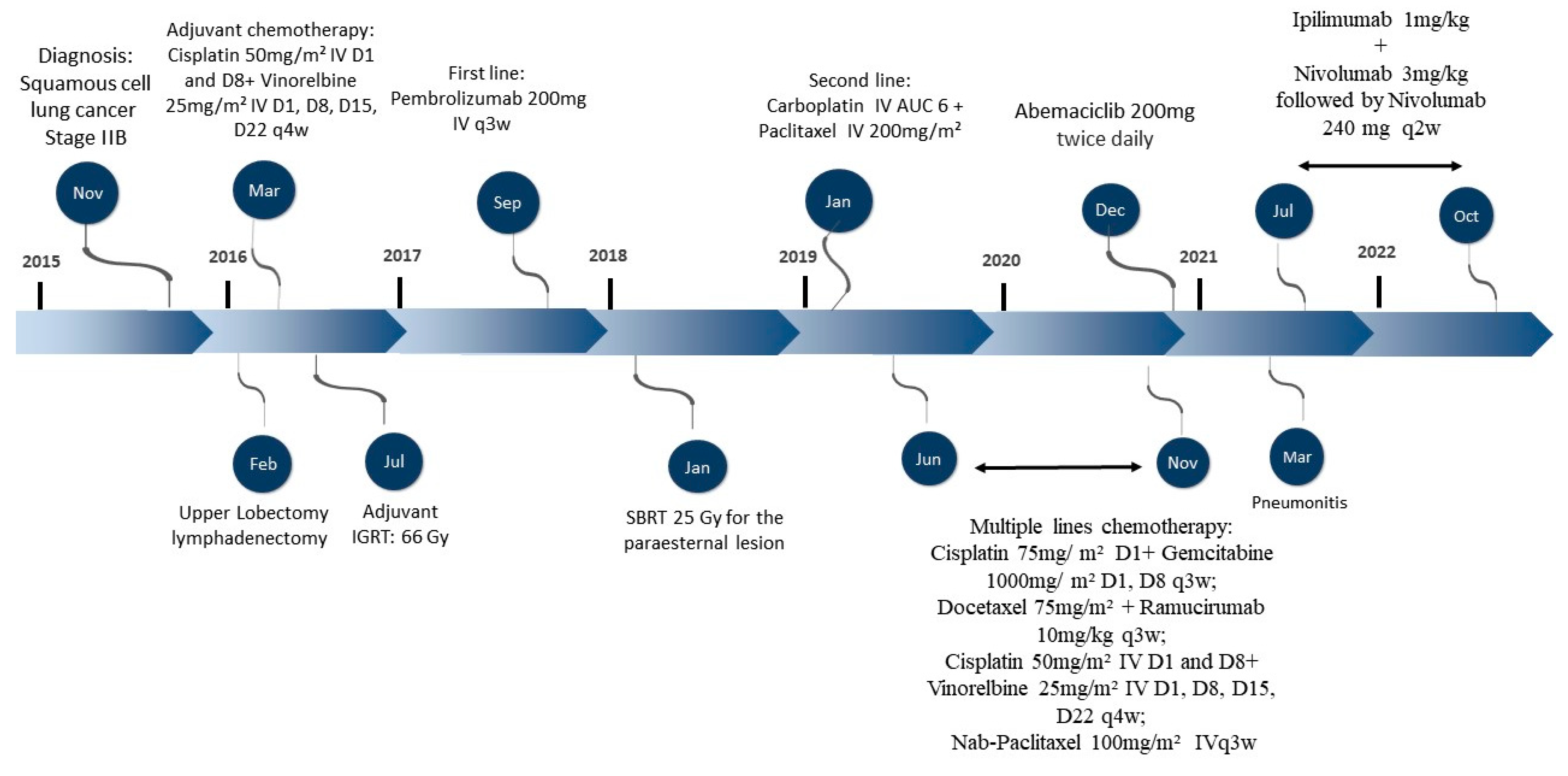
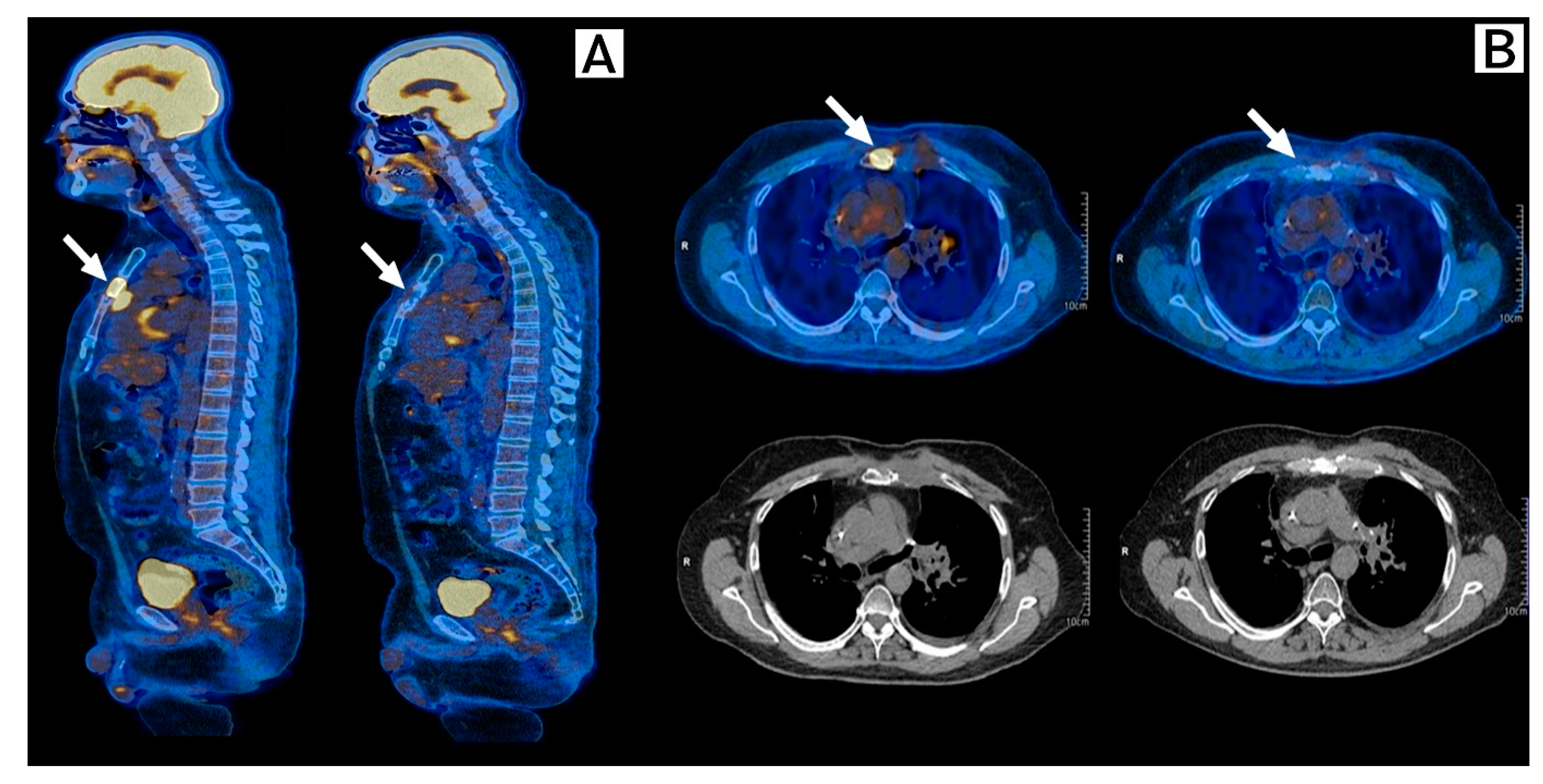
2. Case Description
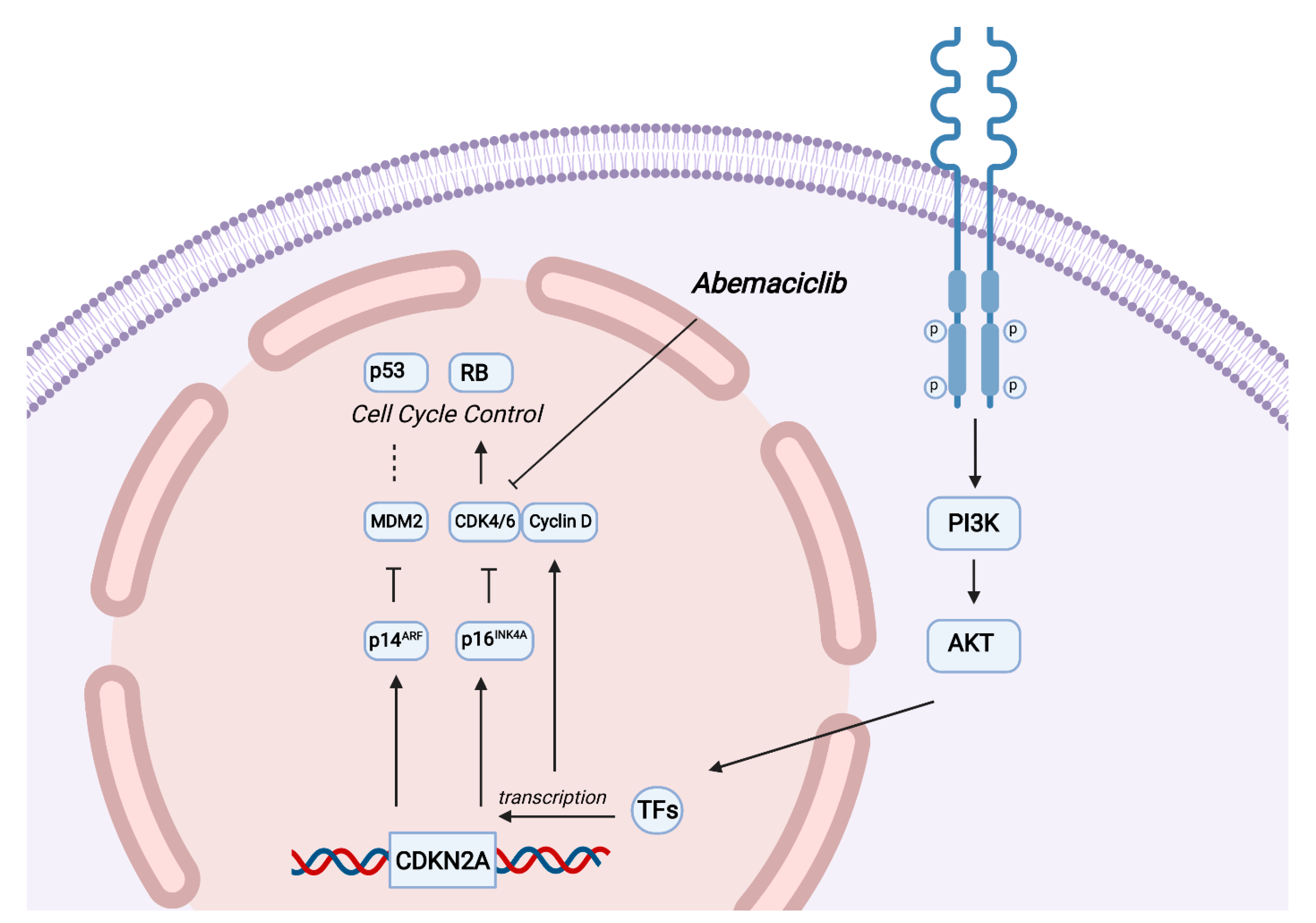
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Thomas, A.; Liu, S.V.; Subramaniam, D.S.; Giaccone, G. Refining the Treatment of NSCLC According to Histological and Molecular Subtypes. Nat. Rev. Clin. Oncol. 2015, 12, 511–526. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Voet, D.; Lawrence, M.S.; Voet, D.; Jing, R.; Cibulskis, K.; Sivachenko, A.; Stojanov, P.; McKenna, A.; Lander, E.S.; et al. Comprehensive Genomic Characterization of Squamous Cell Lung Cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Jeong, E.-H.; Lee, T.-G.; Ko, Y.J.; Kim, S.Y.; Kim, H.-R.; Kim, H.; Kim, C.H. Anti-Tumor Effect of CDK Inhibitors on CDKN2A-Defective Squamous Cell Lung Cancer Cells. Cell. Oncol. 2018, 41, 663–675. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, L.; Costa, M.A.S.M.; Fournier, M.V.; de Oliveira Romano, S.; do Nascimento, C.M.; Barbosa, A.S.; dos Santos Moreira, A.; Scherrer, L.R.; Ornellas, M.H.F.; Alves, G. Mutation Profiling in the PIK3CA, TP53, and CDKN2A Genes in Circulating Free DNA and Impalpable Breast Lesions. Ann. Diagn. Pathol. 2019, 39, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt Pathway in Cell Cycle Progression, Apoptosis, and Neoplastic Transformation: A Target for Cancer Chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef]
- Goldman, J.W.; Gandhi, L.; Patnaik, A.; Rosen, L.S.; Hilton, J.F.; Papadopoulos, K.P.; Tolaney, S.M.; Beeram, M.; Rasco, D.W.; Myrand, S.P.; et al. Clinical Activity of LY2835219, a Novel Cell Cycle Inhibitor Selective for CDK4 and CDK6, in Patients with Non-Small Cell Lung Cancer. J. Clin. Oncol. 2014, 32, 8026. [Google Scholar] [CrossRef]
- Gopalan, P.K.; Villegas, A.G.; Cao, C.; Pinder-Schenck, M.; Chiappori, A.; Hou, W.; Zajac-Kaye, M.; Ivey, A.M.; Kaye, F.J. CDK4/6 Inhibition Stabilizes Disease in Patients with P16-Null Non-Small Cell Lung Cancer and Is Synergistic with MTOR Inhibition. Oncotarget 2018, 9, 37352–37366. [Google Scholar] [CrossRef]
- Goldman, J.W.; Mazieres, J.; Barlesi, F.; Koczywas, M.; Dragnev, K.H.; Göksel, T.; Cortot, A.B.; Girard, N.; Wesseler, C.; Bischoff, H.; et al. A Randomized Phase 3 Study of Abemaciclib versus Erlotinib in Previously Treated Patients with Stage IV NSCLC with KRAS Mutation: JUNIPER. J. Clin. Oncol. 2018, 36, 9025. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Bondarenko, I.; Ciuleanu, T.-E.; Bryl, M.; Fülöp, A.; Vicente, D.; Bischoff, H.; Hurt, K.; Lu, Y.; Estrem, S.; et al. A Randomized Phase 2 Study of Abemaciclib versus Docetaxel in Patients with Stage IV Squamous Non-Small Cell Lung Cancer (SqNSCLC) Previously Treated with Platinum-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 9059. [Google Scholar] [CrossRef]
- Kim, E.S.; Kelly, K.; Paz-Ares, L.G.; Garrido, P.; Jalal, S.; Mahadevan, D.; Gutierrez, M.; Provencio, M.; Schaefer, E.; Shaheen, M.; et al. Abemaciclib in Combination with Single-Agent Options in Patients with Stage IV Non–Small Cell Lung Cancer: A Phase Ib Study. Clin. Cancer Res. 2018, 24, 5543–5551. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Redman, M.W.; Albain, K.S.; McGary, E.C.; Rafique, N.M.; Petro, D.; Waqar, S.N.; Minichiello, K.; Miao, J.; Papadimitrakopoulou, V.A.; et al. SWOG S1400C (NCT02154490)—A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alteration–Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J. Thorac. Oncol. 2019, 14, 1853–1859. [Google Scholar] [CrossRef]
- Ahn, E.R.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Dib, E.G.; Haggstrom, D.E.; Alguire, K.B.; Calfa, C.J.; Cannon, T.L.; Crilley, P.A.; et al. Palbociclib in Patients With Non-Small-Cell Lung Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2020, 4, 757–766. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.H.; Nally, K.; Ronan, K.; Clarke, G.; Amu, S.; Almeida, A.S.; Flavin, R.; Finn, S. Cancer Immunotherapy with Immune Checkpoint Inhibitors-Biomarkers of Response and Toxicity; Current Limitations and Future Promise. Diagnostics 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Wurtz, A.; Goldberg, S.B.; Rimm, D.; Schalper, K.; Kaech, S.; Kavathas, P.; Chiang, A.; Lilenbaum, R.; Zelterman, D.; et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 831–839. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Casaclang Verzosa, G.; Pezeshki, A.; et al. Naturally Occurring P16 Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Cilluffo, D.; Barra, V.; Di Leonardo, A. P14ARF: The Absence That Makes the Difference. Genes 2020, 11, 824. [Google Scholar] [CrossRef]
- Clark, A.S.; Karasic, T.B.; DeMichele, A.; Vaughn, D.J.; O’Hara, M.; Perini, R.; Zhang, P.; Lal, P.; Feldman, M.; Gallagher, M.; et al. Palbociclib (PD0332991)—A Selective and Potent Cyclin-Dependent Kinase Inhibitor: A Review of Pharmacodynamics and Clinical Development. JAMA Oncol. 2016, 2, 253–260. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the P53-MDM2 Pathway for Neuroblastoma Therapy: Rays of Hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling Mechanisms of P53-Mediated Tumour Suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, F.; He, H.; Chen, Y.; Lin, H.; Chen, P.; Chen, X.; Liu, S. TP53 and CDKN2A Mutations in Patients with Early-Stage Lung Squamous Cell Carcinoma: An Analysis of the Correlations and Prognostic Outcomes Wang et Al. Analysis of TP53 and CDKN2A Mutations in LUSC. Ann. Transl. Med. 2021, 9, 1330. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Huober, J.; García-Sáenz, J.A.; Masuda, N.; Sohn, J.H.; Andre, V.A.M.; Barriga, S.; Cox, J.; Goetz, M. Management of Abemaciclib-Associated Adverse Events in Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Safety Analysis of MONARCH 2 and MONARCH 3. Oncologist 2021, 26, e53–e65. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Leonardelli, S.; Sucker, A.; Schadendorf, D.; Griewank, K.G.; Paschen, A. Tumor CDKN2A-Associated JAK2 Loss and Susceptibility to Immunotherapy Resistance. J. Natl. Cancer Inst. 2018, 110, 677–681. [Google Scholar] [CrossRef]
- Lim, S.M.; Hong, M.H.; Kim, H.R. Immunotherapy for Non-Small Cell Lung Cancer: Current Landscape and Future Perspectives. Immune Netw. 2020, 20, e10. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Gibney, G.T.; rey Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol. 2016, 16, e542–e551. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Anna Giacomo, M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- ODonnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Ziemer, M. Rechallenge with Checkpoint Inhibitors in Metastatic Melanoma. JDDG J. Ger. Soc. Dermatol. 2020, 18, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Uson Junior, P.L.S.; Nagalo, B.M.; Ahn, D.H.; Bekaii-Saab, T.; Borad, M.J. Combination immunotherapy for hepatocellular carcinoma: Where are we currently? Sem. Liver Dis. 2021, 41, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Frisone, D.; Friedlaender, A.; Addeo, A.; Tsantoulis, P. The Landscape of Immunotherapy Resistance in NSCLC. Front. Oncol. 2022, 12, 817548. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Therapy | Phase | N | Molecular Analysis | Response to CDKi |
|---|---|---|---|---|---|
| Goldman et al., 2014 [8] | Abemaciclib | I | 49 | KRAS | DCR: 54% for KRAS mut |
| Gopalan et al., 2018 [9] | Palbociclib | II | 16 | p16 null | SD: 50% lasting 4–10 m |
| Goldman et al., 2018 [10] | Abemaciclib vs. Erlotinib | III | 453 | KRAS mut. (Codons 12 and 13) | mOS: 7.4 m vs. 7.8 m mPFS: 3.6 m vs. 1.8 m DCR: 54% vs. 31% ORR: 8.9% vs. 2.7% |
| Scagliotti et al., 2018 [11] | Abemaciclib vs. Docetaxel | II | 159 | NMA | mOS: 7 m vs. 12.4 m mPFS: 2.5 m vs. 4.2 m DCR: 50% vs. 64% ORR: 2.8% vs. 4.2% |
| Kim et al., 2018 [12] | Abemaciclib plus Pemetrexed, Gemcitabine or Ramucirumab | Ib | 86 | NMA | DCR: 52% for pemetrexed group DCR: 25% for gemcitabine group DCR: 54% for Ramucirumab group |
| Edelman et al., 2019 [13] | Palbociclib | II | 32 | CDK4, CCND1, CCND2, CCND3 amplifications | mOS: 7.1 m mPFS: 1.7 m ORR: 6% SD: 38% |
| Ahn et al., 2020 [14] | Palbociclib | II (TAPUR Studt) | 29 | CDKN2A alterations/No Rb mutations | mOS: 21.6 w mPFS: 8.1 w DCR: 31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias e Silva, D.; Borba, G.B.; Beal, J.R.; Botrus, G.; Osawa, A.; Araújo, S.E.A.; Moura, F.; Guendelmann, R.A.K.; Uson Junior, P.L.S. Response to Abemaciclib and Immunotherapy Rechallenge with Nivolumab and Ipilimumab in a Heavily Pretreated TMB-H Metastatic Squamous Cell Lung Cancer with CDKN2A Mutation, PIK3CA Amplification and TPS 80%: A Case Report. Int. J. Mol. Sci. 2023, 24, 4209. https://doi.org/10.3390/ijms24044209
Dias e Silva D, Borba GB, Beal JR, Botrus G, Osawa A, Araújo SEA, Moura F, Guendelmann RAK, Uson Junior PLS. Response to Abemaciclib and Immunotherapy Rechallenge with Nivolumab and Ipilimumab in a Heavily Pretreated TMB-H Metastatic Squamous Cell Lung Cancer with CDKN2A Mutation, PIK3CA Amplification and TPS 80%: A Case Report. International Journal of Molecular Sciences. 2023; 24(4):4209. https://doi.org/10.3390/ijms24044209
Chicago/Turabian StyleDias e Silva, Douglas, Guilherme Bes Borba, Juliana Rodrigues Beal, Gehan Botrus, Akemi Osawa, Sérgio Eduardo Alonso Araújo, Fernando Moura, Rafael Aliosha Kaliks Guendelmann, and Pedro Luiz Serrano Uson Junior. 2023. "Response to Abemaciclib and Immunotherapy Rechallenge with Nivolumab and Ipilimumab in a Heavily Pretreated TMB-H Metastatic Squamous Cell Lung Cancer with CDKN2A Mutation, PIK3CA Amplification and TPS 80%: A Case Report" International Journal of Molecular Sciences 24, no. 4: 4209. https://doi.org/10.3390/ijms24044209
APA StyleDias e Silva, D., Borba, G. B., Beal, J. R., Botrus, G., Osawa, A., Araújo, S. E. A., Moura, F., Guendelmann, R. A. K., & Uson Junior, P. L. S. (2023). Response to Abemaciclib and Immunotherapy Rechallenge with Nivolumab and Ipilimumab in a Heavily Pretreated TMB-H Metastatic Squamous Cell Lung Cancer with CDKN2A Mutation, PIK3CA Amplification and TPS 80%: A Case Report. International Journal of Molecular Sciences, 24(4), 4209. https://doi.org/10.3390/ijms24044209






