Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice
Abstract
1. Introduction
2. Results
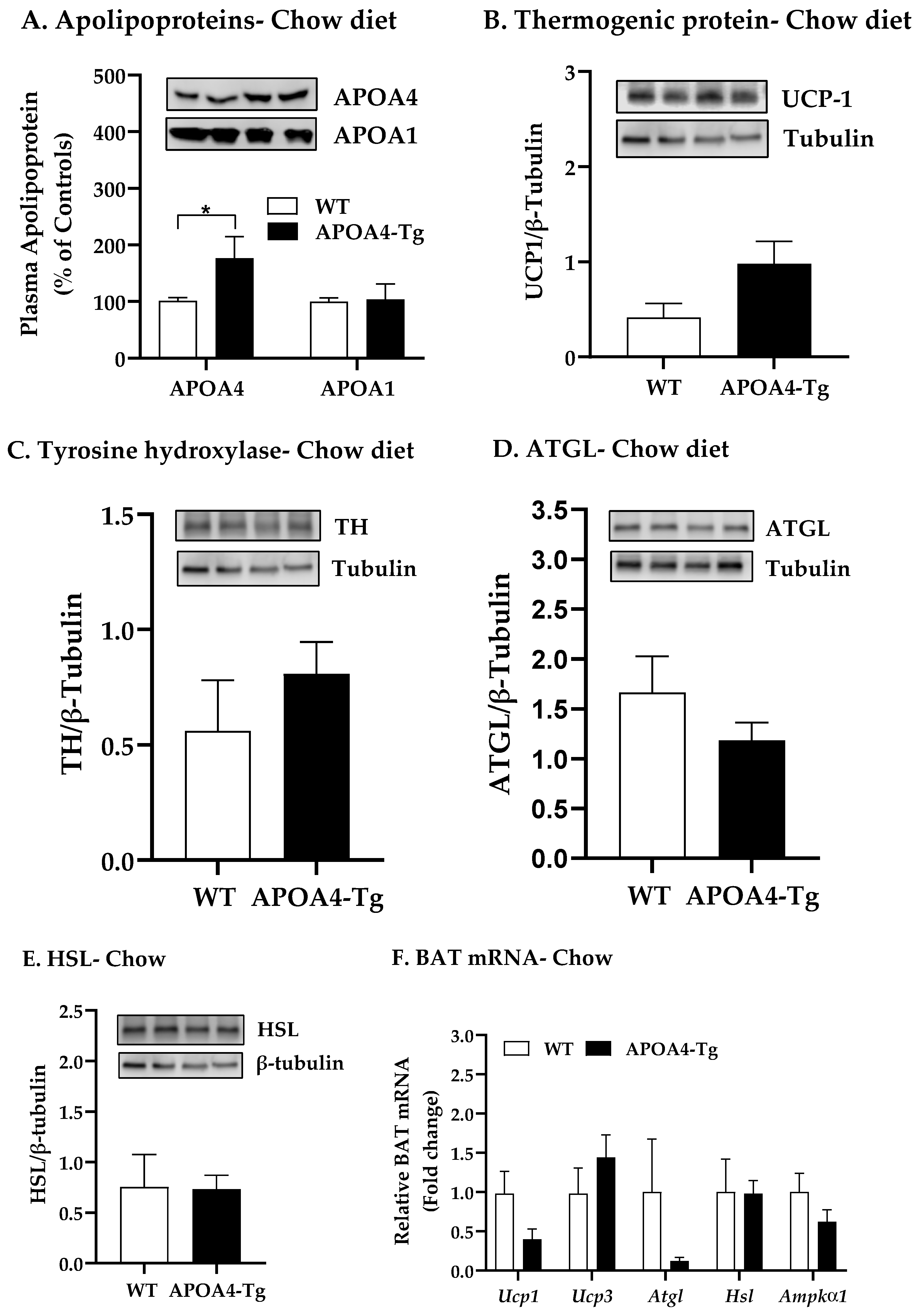
2.1. Body Weight, Fat Mass, Plasma Parameters and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed a Chow Diet
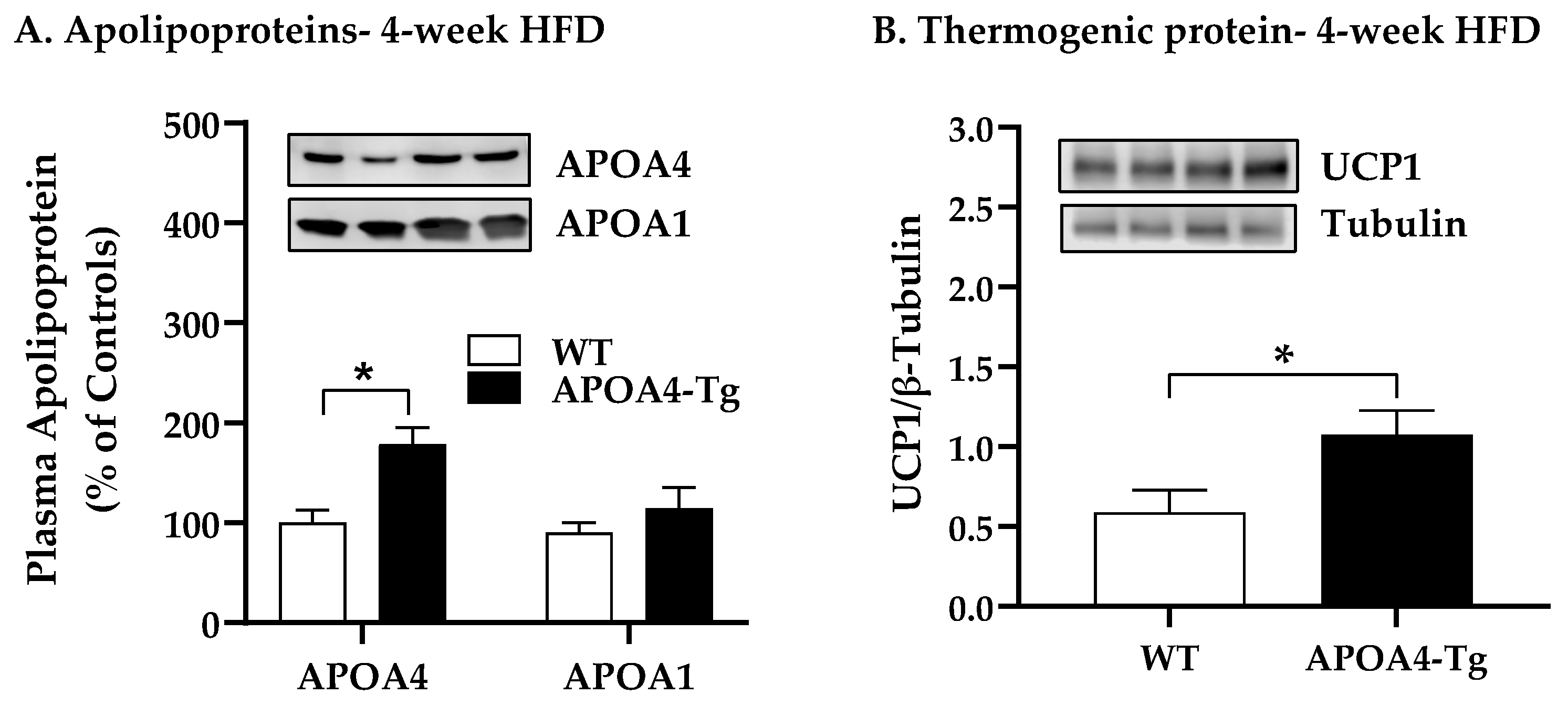
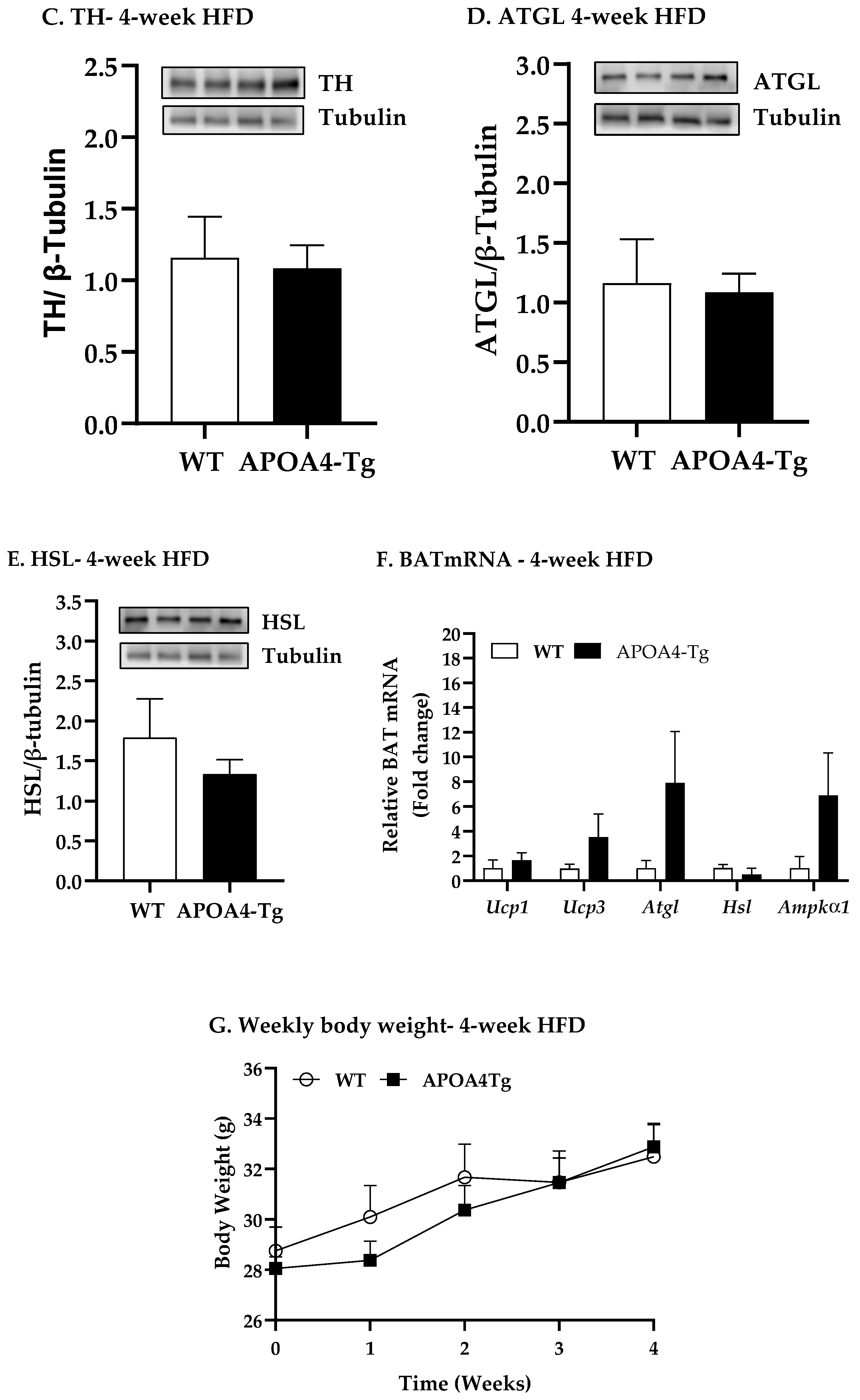
2.2. Body Weight, Fat Mass, Plasma Parameters, and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed an HFD for 4 Weeks
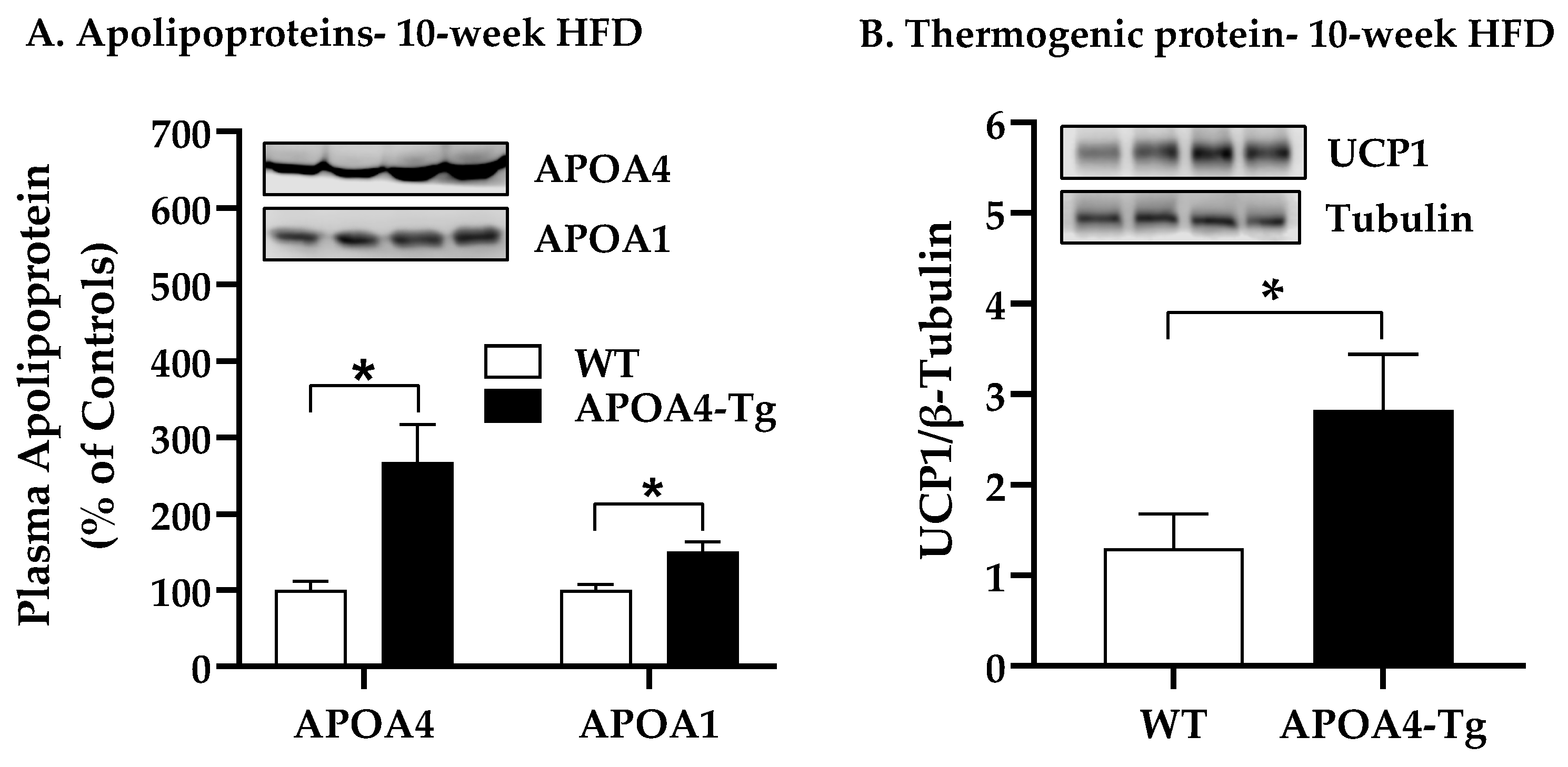
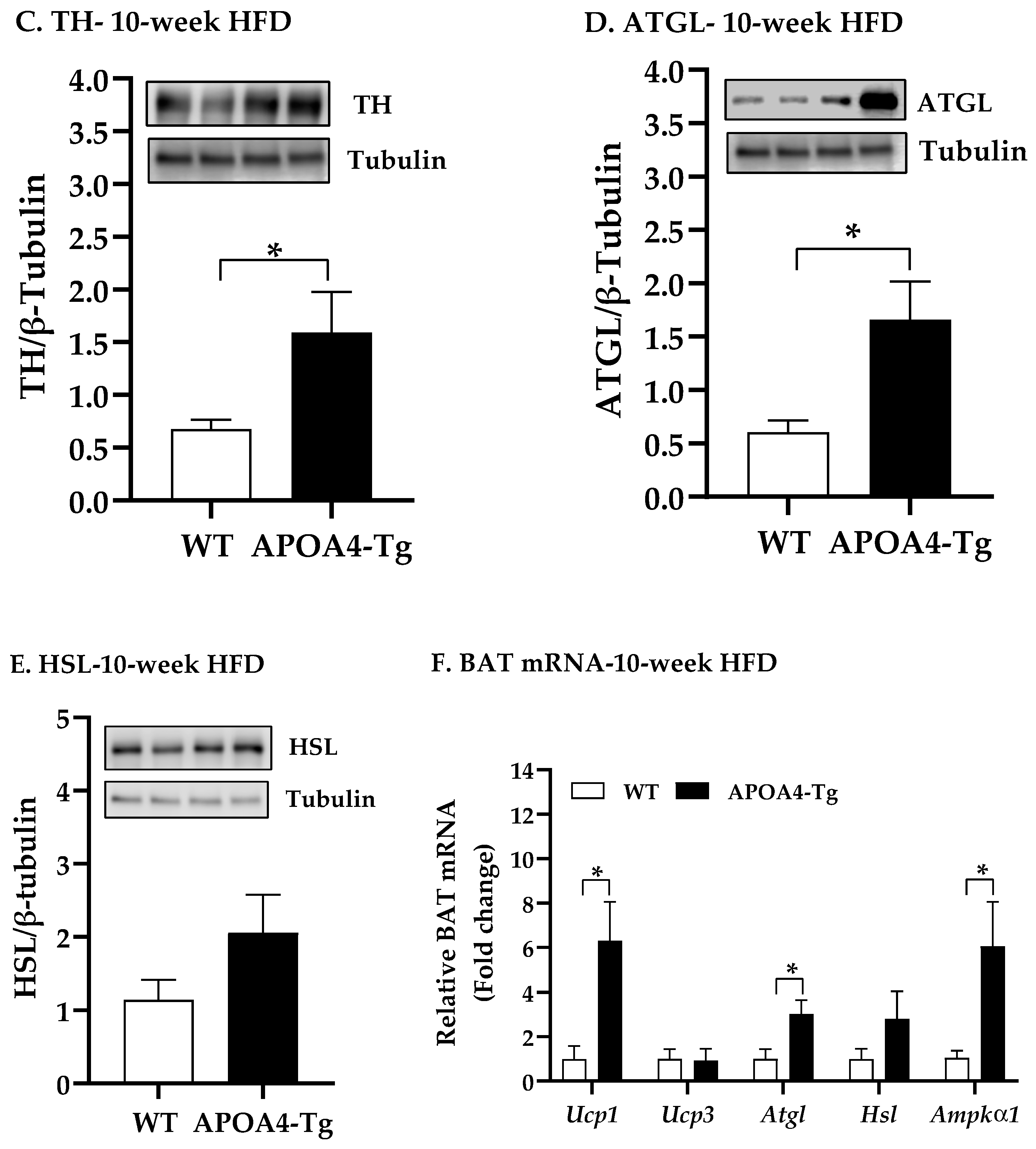
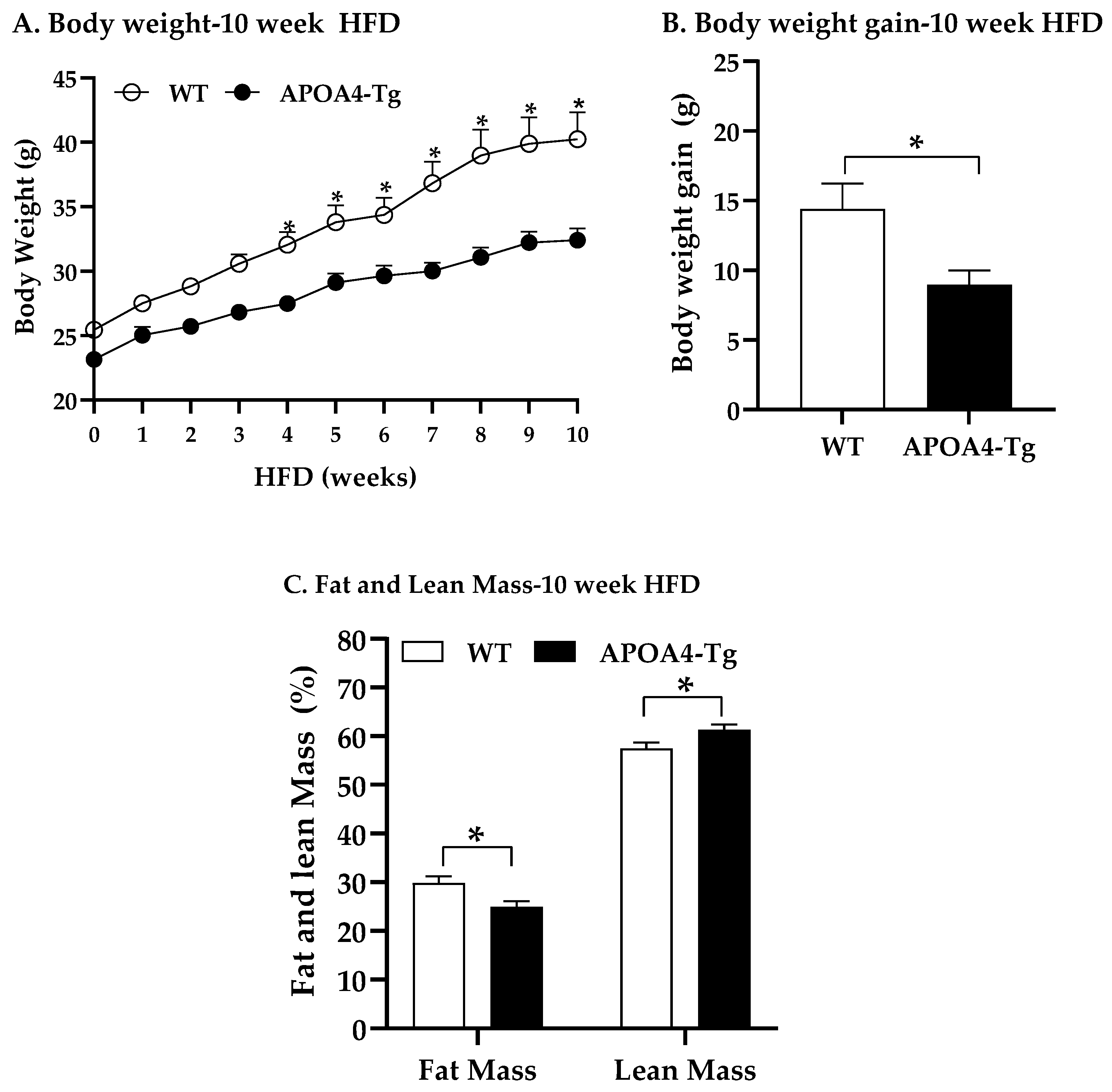
2.3. Body Weight, Fat Mass, Plasma Parameters, and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed an HFD for 10 Weeks
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Body Weight, Fat Mass, Plasma Parameters, and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed a Chow Diet
4.3. Body Weight, Fat Mass, Plasma Parameters and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed an HFD for 4 Weeks
4.4. Body Weight, Fat Mass, Plasma Parameters, and BAT Thermogenic and Lipolytic Proteins in APOA4-Tg Mice Fed an HFD for 10 Weeks
4.5. Thermogenic Protein and Plasma Apolipoprotein Determination
4.6. Plasma Parameters
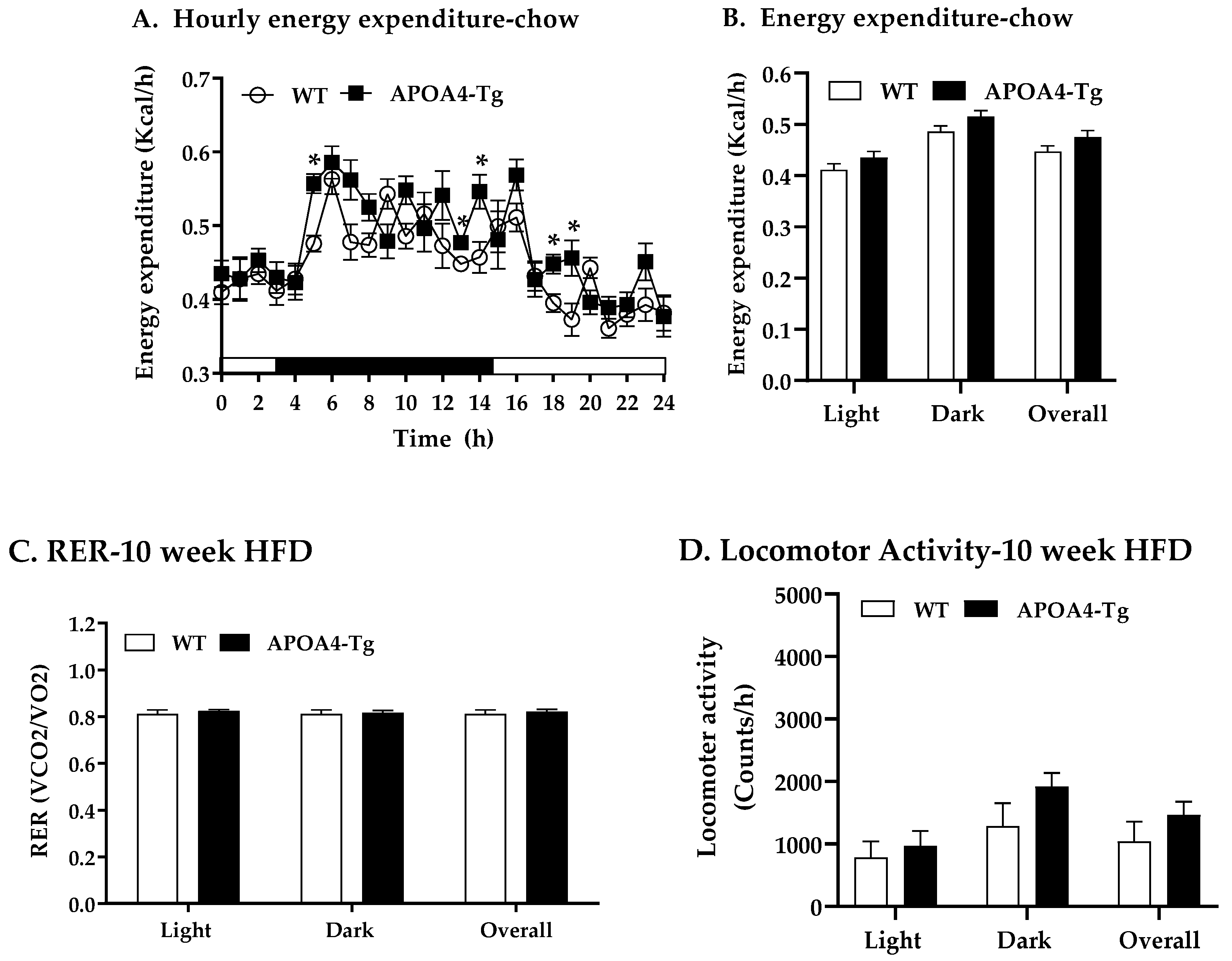
4.7. Energy Expenditure, RER, and Food Intake of Mice Fed a Chow or HFD for 10 Weeks
4.8. Quantitative RT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| APOA4 | Apolipoprotein A4 |
| APOA4-Tg | Transgenic mice with overexpression of mouse APOA4 in the small intestine |
| APOA4-KO | Global knockout of APOA4 |
| ATGL | Adipose triglyceride lipase |
| BAT | Brown adipose tissue |
| EWAT | Epididymal white adipose tissue |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HSL | Hormone sensitive lipase |
| IWAT | Inguinal white adipose tissue |
| LPL | Lipoprotein lipase |
| TG | Triglycerides |
| TH | Tyrosine hydroxylase |
| UCP1 | Uncoupling protein 1 |
| UCP3 | Uncoupling protein 3 |
| VLDL | Very low-density lipoprotein |
| WAT | White adipose tissue |
| WT | Wildtype |
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Callow, A.D. Cardiovascular Disease 2005—The Global Picture. Vascul. Pharmacol. 2006, 45, 302–307. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity Management as a Primary Treatment Goal for Type 2 Diabetes: Time to Reframe the Conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J.; Warwick, B.P. Energy Balance and Brown Fat Activity in Rats Fed Cafeteria Diets or High-Fat, Semisynthetic Diets at Several Levels of Intake. Metabolism 1985, 34, 474–480. [Google Scholar] [CrossRef]
- Levin, B.E.; Triscari, J.; Sullivan, A.C. Altered Sympathetic Activity during Development of Diet-Induced Obesity in Rat. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 1983, 244, R347–R355. [Google Scholar] [CrossRef]
- Vijgen, G.H.E.J.; Bouvy, N.D.; Teule, G.J.J.; Brans, B.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Brown Adipose Tissue in Morbidly Obese Subjects. PLoS ONE 2011, 6, e17247. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Al, E. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a Β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 2011, 17, 200–206. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am. J. Physiol.—Endocrinol. Metab. 2007, 293, 444–452. [Google Scholar] [CrossRef]
- Marette, A.; Bukowiecki, L.J. Noradrenaline Stimulates Glucose Transport in Rat Brown Adipocytes by Activating Thermogenesis: Evidence That Fatty Acid Activation of Mitochondrial Respiration Enhances Glucose Transport. Biochem. J. 1991, 277, 119–124. [Google Scholar] [CrossRef]
- Betz, M.J.; Enerbäck, S. Human Brown Adipose Tissue: What We Have Learned so Far. Diabetes 2015, 64, 2352–2360. [Google Scholar] [CrossRef]
- Blouet, C.; Schwartz, G.J. Duodenal Lipid Sensing Activates Vagal Afferents to Regulate Non-Shivering Brown Fat Thermogenesis in Rats. PLoS ONE 2012, 7, e51898. [Google Scholar] [CrossRef]
- Vijgen, G.H.E.J.; Bouvy, N.D.; Leenen, L.; Rijkers, K.; Cornips, E.; Majoie, M.; Brans, B.; van Marken Lichtenbelt, W.D. Vagus Nerve Stimulation Increases Energy Expenditure: Relation to Brown Adipose Tissue Activity. PLoS ONE 2013, 8, e77221. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and Sensory Innervation of Brown Adipose Tissue. Int. J. Obes. 2010, 34, S36–S42. [Google Scholar] [CrossRef]
- Shi, H.; Song, C.K.; Giordano, A.; Cinti, S.; Bartness, T.J. Sensory or Sympathetic White Adipose Tissue Denervation Differentially Affects Depot Growth and Cellularity. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2005, 288, 1028–1037. [Google Scholar] [CrossRef]
- Molinoff, P.B.; Axelrod, J. biochemistry of Catecholamines. Annu. Rev. Biochem. 1971, 40, 465–500. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-Activated Protein Kinase and Its Downstream Transcriptional Pathways. Cell Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in Adipose Tissue Is Promoted by Adipose Triglyceride Lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-Sensitive Lipase: Control of Intracellular Tri-(Di-)Acylglycerol and Cholesteryl Ester Hydrolysis. J. Lipid Res. 2002, 43, 1585–1594. [Google Scholar] [CrossRef]
- van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.N.; Boon, M.R. Regulation of Brown Fat by AMP-Activated Protein Kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef]
- Bouillaud, F.; Alves-Guerra, M.C.; Ricquier, D. UCPs, at the Interface between Bioenergetics and Metabolism. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 2443–2456. [Google Scholar] [CrossRef]
- Kohan, A.B.; Wang, F.; Lo, C.-M.; Liu, M.; Tso, P. ApoA-IV: Current and Emerging Roles in Intestinal Lipid Metabolism, Glucose Homeostasis, and Satiety. Am. J. Physiol.—Gastrointest. Liver Physiol. 2015, 308, G472–G481. [Google Scholar] [CrossRef]
- Hayashi, H.; Nutting, D.F.; Fujimoto, K.; Cardelli, J.A.; Black, D.; Tso, P. Transport of Lipid and Apolipoproteins A-I and A-IV in Intestinal Lymph of the Rat. J. Lipid Res. 1990, 31, 1613–1625. [Google Scholar] [CrossRef]
- Lo, C.M.; Zhang, D.M.; Pearson, K.; Ma, L.; Sun, W.; Sakai, R.R.; Davidson, W.S.; Liu, M.; Raybould, H.E.; Woods, S.C.; et al. Interaction of Apolipoprotein AIV with Cholecystokinin on the Control of Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1490–R1494. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A Protein Intimately Involved in Metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef]
- Cohen, R.D.; Castellani, L.W.; Qiao, J.H.; Van Lenten, B.J.; Lusis, A.J.; Reue, K. Reduced Aortic Lesions and Elevated High Density Lipoprotein Levels in Transgenic Mice Overexpressing Mouse Apolipoprotein A-IV. J. Clin. Investig. 1997, 99, 1906–1916. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Scheraldi, C.A.; Yacoub, L.K.; Saxena, U.; Bisgaier, C.L. Lipoprotein ApoC-II Activation of Lipoprotein Lipase. Modulation by Apolipoprotein A-IV. J. Biol. Chem. 1990, 265, 4266–4272. [Google Scholar] [CrossRef]
- Zhu, Q.; Weng, J.; Shen, M.; Fish, J.; Shen, Z.; Coschigano, K.T.; Davidson, W.S.; Tso, P.; Shi, H.; Lo, C.C. Apolipoprotein A-IV Enhances Fatty Acid Uptake by Adipose Tissues of Male Mice via Sympathetic Activation. Endocrinology 2020, 161, bqaa042. [Google Scholar] [CrossRef] [PubMed]
- Pence, S.; Zhu, Q.; Binne, E.; Liu, M.; Shi, H.; Lo, C.C. Reduced Diet-Induced Thermogenesis in Apolipoprotein a-IV Deficient Mice. Int. J. Mol. Sci. 2019, 20, 3176. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Rushing, P.A.; D’Alessio, D.; Tso, P. A Controlled High-Fat Diet Induces an Obese Syndrome in Rats. J. Nutr. 2003, 133, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.J.; Painter, R.G. Adaptation of Intestinal Production of Apolipoprotein A-IV during Chronic Feeding of Lipid. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2001, 280, R1155–R1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, L.; Liu, Y.; Woods, S.C.; Seeley, R.J.; D’Alessio, D.; Tso, P. Obesity Induced by a High-Fat Diet Downregulates Apolipoprotein A-IV Gene Expression in Rat Hypothalamus. Am. J. Physiol.—Endocrinol. Metab. 2004, 287, 366–370. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Dantzker, C.; Patton, C.S. Sensitivity of Serum Apolipoprotein A-IV Levels to Changes in Dietary Fat Content. Gastroenterology 1990, 98, 17–24. [Google Scholar] [CrossRef]
- Aalto-Setalälä, K.; Bisgaier, C.L.; Ho, A.; Kieft, K.A.; Traber, M.G.; Kayden, H.J.; Ramakrishnan, R.; Walsh, A.; Essenburg, A.D.; Breslow, J.L. Intestinal Expression of Human Apolipoprotein A-IV in Transgenic Mice Fails to Influence Dietary Lipid Absorption or Feeding Behavior. J. Clin. Investig. 1994, 93, 1776–1786. [Google Scholar] [CrossRef]
- Lo, C.C.; Langhans, W.; Georgievsky, M.; Arnold, M.; Caldwell, J.L.; Cheng, S.; Liu, M.; Woods, S.C.; Tso, P. Apolipoprotein AIV Requires Cholecystokinin and Vagal Nerves to Suppress Food Intake. Endocrinology 2012, 153, 5857–5865. [Google Scholar] [CrossRef]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. 2021, 12, 652444. [Google Scholar] [CrossRef]
- Surwit, R.S.; Wang, S.; Petro, A.E.; Sanchis, D.; Raimbault, S.; Ricquier, D.; Collins, S. Diet-Induced Changes in Uncoupling Proteins in Obesity-Prone and Obesity-Resistant Strains of Mice. Proc. Natl. Acad. Sci. USA 1998, 95, 4061–4065. [Google Scholar] [CrossRef]
- Weng, J.; Lou, D.; Benoit, S.C.; Coschigano, N.; Woods, S.C.; Tso, P.; Lo, C.C. Energy Homeostasis in Apolipoprotein AIV and Cholecystokinin-Deficient Mice. Am J Physiol Regul Integr Comp Physiol 2017, 313, 535–548. [Google Scholar] [CrossRef]
- Pence, S.; Larussa, Z.; Shen, Z.; Liu, M.; Coschigano, K.T.; Shi, H.; Lo, C.C. Central Apolipoprotein A-Iv Stimulates Thermogenesis in Brown Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 1221. [Google Scholar] [CrossRef]
- Shen, L.; Pearson, K.J.; Xiong, Y.; Lo, C.M.; Tso, P.; Woods, S.C.; Davidson, W.S.; Liu, M. Characterization of Apolipoprotein A-IV in Brain Areas Involved in Energy Homeostasis. Physiol. Behav. 2008, 95, 161–167. [Google Scholar] [CrossRef]
- Hoeke, G.; Kooijman, S.; Boon, M.R.; Rensen, P.C.N.; Berbeé, J.F.P. Role of Brown Fat in Lipoprotein Metabolism and Atherosclerosis. Circ. Res. 2016, 118, 173–182. [Google Scholar] [CrossRef]
- Berbeé, J.F.P.; Boon, M.R.; Khedoe, P.P.S.J.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown Fat Activation Reduces Hypercholesterolaemia and Protects from Atherosclerosis Development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Metabolic Consequences of the Presence or Absence of the Thermogenic Capacity of Brown Adipose Tissue in Mice (and Probably in Humans). Int. J. Obes. 2010, 34, S7–S16. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Saraf, M.K.; Annamalai, P.; Yfanti, C.; Chao, T.; Wong, D.; Shinoda, K.; et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016, 23, 1200–1206. [Google Scholar] [CrossRef]
- Camps, L.; Reina, M.; Llobera, M.; Vilaro, S.; Olivecrona, T. Lipoprotein Lipase: Cellular Origin and Functional Distribution. Am. J. Physiol.—Cell Physiol. 1990, 258, 673–681. [Google Scholar] [CrossRef]
- Trayhurn, P.; Duncan, J.S.; Rayner, D.V. Acute Cold-Induced Suppression of Ob (Obese) Gene Expression in White Adipose Tissue of Mice: Mediation by the Sympathetic System. Biochem. J. 1995, 311, 729–733. [Google Scholar] [CrossRef]
- Carneheim, C.; Negergaard, J.; Cannon, B. Cold-Induced β-Adrenergic Recruitment of Lipoprotein Lipase in Brown Fat Is Due to Increased Transcription. Am. J. Physiol.—Endocrinol. Metab. 1988, 254, E155–E161. [Google Scholar] [CrossRef]
- Gomez, P.; Perez-Martinez, P.; Marin, C.; Camargo, A.; Yubero-Serrano, E.M.; Garcia-Rios, A.; Rodriguez, F.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. APOA1 and APOA4 Gene Polymorphisms Influence the Effects of Dietary Fat on LDL Particle Size and Oxidation in Healthy Young Adults. J. Nutr. 2010, 140, 773–778. [Google Scholar] [CrossRef]
- Kohan, A.B.; Wang, F.; Li, X.; Bradshaw, S.; Yang, Q.; Caldwell, J.L.; Bullock, T.M.; Tso, P. Apolipoprotein A-IV Regulates Chylomicron Metabolism-Mechanism and Function. AJP Gastrointest. Liver Physiol. 2012, 302, G628–G636. [Google Scholar] [CrossRef]
- Weinstock, P.H.; Bisgaier, C.L.; Hayek, T.; Aalto-Setala, K.; Sehayek, E.; Wu, L.; Sheiffele, P.; Merkel, M.; Essenburg, A.D.; Breslow, J.L. Decreased HDL Cholesterol Levels but Normal Lipid Absorption, Growth, and Feeding Behavior in Apolipoprotein A-IV Knockout Mice. J. Lipid Res. 1997, 38, 1782–1794. [Google Scholar] [CrossRef]
- Simon, T.; Cook, V.R.; Rao, A.; Weinberg, R.B. Impact of Murine Intestinal Apolipoprotein A-IV Expression on Regional Lipid Absorption, Gene Expression, and Growth. J. Lipid Res. 2011, 52, 1984–1994. [Google Scholar] [CrossRef]
- Maffei, Á.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin Levels in Human and Rodent: Measurement of Plasma Leptin and Ob RNA in Obese and Weight-Reduced Subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Ghibaudi, L.; Cook, J.; Farley, C.; Van Heek, M.; Hwa, J.J. Fat Intake Affects Adiposity, Comorbidity Factors, and Energy Metabolism of Sprague-Dawley Rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Z.; Zhang, Y.; Yang, L.; Pan, Y.; Wang, Z.; Feng, G.S.; Chen, Y. Apolipoprotein A-I Possesses an Anti-Obesity Effect Associated with Increase of Energy Expenditure and up-Regulation of UCP1 in Brown Fat. J. Cell. Mol. Med. 2011, 15, 763–772. [Google Scholar] [CrossRef]
- Peng, J.; Li, X. ping Apolipoprotein A-IV: A Potential Therapeutic Target for Atherosclerosis. Prostaglandins Other Lipid Mediat. 2018, 139, 87–92. [Google Scholar] [CrossRef]
- Rohatgi, A. Reverse Cholesterol Transport and Atherosclerosis: Heterogeneous Observations by Vascular Bed and Plaque Architecture. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2–4. [Google Scholar] [CrossRef]
- Yoshimichi, G.; Lo, C.C.; Tamashiro, K.L.K.; Ma, L.; Lee, D.M.; Begg, D.P.; Liu, M.; Sakai, R.R.; Woods, S.C.; Yoshimatsu, H.; et al. Effect of Peripheral Administration of Cholecystokinin on Food Intake in Apolipoprotein AIV Knockout Mice. AJP Gastrointest. Liver Physiol. 2012, 302, G1336–G1342. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Ravussin, E.; Speakman, J.R.; Galgani, J.E. Progress and Challenges in Analyzing Rodent Energy Expenditure. Nat. Methods 2019, 16, 797–799. [Google Scholar] [CrossRef]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.S.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V.; Galgani, J.E.; Hambly, C.; et al. A Guide to Analysis of Mouse Energy Metabolism. Nat. Methods 2012, 9, 57–63. [Google Scholar] [CrossRef]

| Parameters | WT Mice | APOA4-Tg Mice |
|---|---|---|
| Body weight (g) | 26.4 ± 0.37 | 26.1 ± 0.55 |
| Daily caloric intake (Kcal) | 12.7 ± 2.63 | 12.5 ± 3.60 |
| Fat mass/body weight (%) | 10.6 ± 0.77 | 11.3 ± 0.63 |
| Lean mass/body weight (%) | 77.1 ± 1.65 | 76.4 ± 0.69 |
| BAT (g) | 0.15 ± 0.01 | 0.12 ± 0.02 |
| EWAT (g) | 0.34 ± 0.01 | 0.29 ± 0.03 |
| IWAT (g) | 0.21 ± 0.01 | 0.19 ± 0.02 |
| Leptin (ng/mL) | 1.85 ± 0.60 | 1.93 ± 0.76 |
| Triglycerides (mg/dl) | 50.0 ± 4.01 | 29.9 ± 10.25 * |
| Cholesterol (mg/dl) | 80.8 ± 5.05 | 74.2 ± 3.21 |
| Parameters | WT Mice | APOA4-Tg Mice |
|---|---|---|
| Body weight (g) | 32.5 ± 1.32 | 32.88 ± 0.88 |
| Daily caloric intake (Kcal) | 8.9 ± 0.50 | 9.9 ± 0.42 |
| Fat mass/body weight (%) | 21.3 ± 0.81 | 23.3 ± 1.41 |
| Lean mass/body weight (%) | 66.8 ± 0.52 | 64.6 ± 1.56 |
| BAT (g) | 0.20 ± 0.02 | 0.31 ± 0.03 |
| EWAT (g) | 1.25 ± 0.28 | 1.15 ± 0.21 |
| IWAT (g) | 0.80 ± 0.17 | 1.02 ± 0.12 |
| Leptin (ng/mL) | 14.6 ± 1.28 | 17.3 ± 2.85 |
| Triglycerides (mg/dl) | 60.6 ± 3.1 | 43.1 ± 6.51 * |
| Cholesterol (mg/dl) | 143.8 ± 12.7 | 145.6 ± 11.0 |
| Parameters | WT Mice | APOA4-Tg Mice |
|---|---|---|
| Body weight (g) | 40.6 ± 1.88 | 32.4 ± 0.91 * |
| Daily caloric intake (Kcal) | 11.6 ± 0.70 | 11.4 ± 0.78 |
| BAT (g) | 0.53 ± 0.07 | 0.32 ± 0.05 * |
| EWAT (g) | 2.47 ± 0.28 | 1.33 ± 0.24 * |
| IWAT (g) | 1.39 ± 0.18 | 1.14 ± 0.15 |
| Leptin (ng/mL) | 25.5 ± 1.77 | 16.7 ± 1.10 * |
| Triglycerides (mg/dl) | 66.9 ± 8.71 | 45.5 ± 4.30 * |
| Cholesterol (mg/dl) | 159.3 ± 16.60 | 138.6 ± 14.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaRussa, Z.; Kuo, H.-C.N.; West, K.; Shen, Z.; Wisniewski, K.; Tso, P.; Coschigano, K.T.; Lo, C.C. Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 4231. https://doi.org/10.3390/ijms24044231
LaRussa Z, Kuo H-CN, West K, Shen Z, Wisniewski K, Tso P, Coschigano KT, Lo CC. Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice. International Journal of Molecular Sciences. 2023; 24(4):4231. https://doi.org/10.3390/ijms24044231
Chicago/Turabian StyleLaRussa, Zachary, Hsuan-Chih N. Kuo, Kathryn West, Zhijun Shen, Kevin Wisniewski, Patrick Tso, Karen T. Coschigano, and Chunmin C. Lo. 2023. "Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice" International Journal of Molecular Sciences 24, no. 4: 4231. https://doi.org/10.3390/ijms24044231
APA StyleLaRussa, Z., Kuo, H.-C. N., West, K., Shen, Z., Wisniewski, K., Tso, P., Coschigano, K. T., & Lo, C. C. (2023). Increased BAT Thermogenesis in Male Mouse Apolipoprotein A4 Transgenic Mice. International Journal of Molecular Sciences, 24(4), 4231. https://doi.org/10.3390/ijms24044231








