Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction
Abstract
:1. Introduction
2. Biogenesis and Features of Circular RNAs
3. Cyclization Form of Circular RNAs
3.1. Lariat-Driven Cyclization
3.2. Intron-Pairing-Driven Cyclization
3.3. RNA-Binding-Protein (RBP)-Driven Cyclization
4. Biological Functions of CircRNAs
4.1. CircRNAs as miRNA Sponges
4.2. CircRNAs as Protein Sponges
4.3. CircRNAs Directly Translate Proteins
4.4. CircRNAs Directly Affect Gene Expression
4.5. CircRNAs Regulate mRNA Stability
4.6. CircRNAs Regulate DNA Methylation
4.7. CircRNAs Act as a Retrotransposon to Mediate Pseudo-Genetic Production
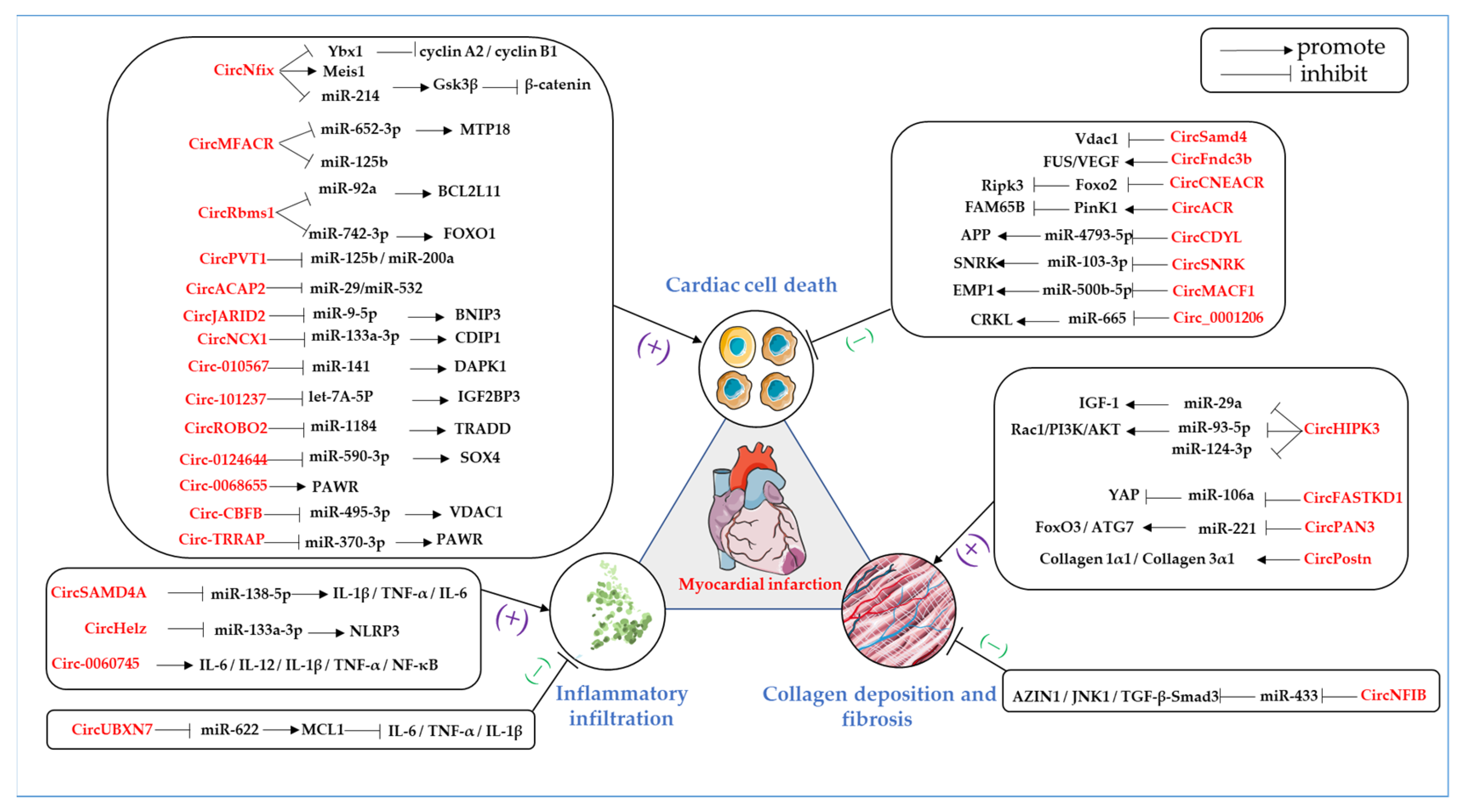
5. Conclusions on the Specific Functions of Circular RNAs in Myocardial Infarction
5.1. Role of Circular RNAs in Cardiac Cell Death and MI
5.1.1. CircRNAs Promote Cardiac Cell Death in MI
- CircNfix
- CircACAP2
- CircMFACR
- CircPVT1
- CircNCX1
- CircJARID2
- CircROBO2
- CircRbms1
- CircCBFB
- CircTRRAP
5.1.2. CircRNAs Suppress Cardiac Cell Death in MI
- CircCNEACR
- CircSamd4
- CircACR
- CircCDYL
- CircFndc3b
- CircSNRK
- CircMACF1
- Circ_0001206
5.2. The Role of Circular RNAs in Inflammatory Infiltration and MI
5.2.1. CircRNAs Promote Inflammatory Infiltration in MI
- CircSAMD4A
- CircHelz
- Circ_0060745
5.2.2. CircRNAs Inhibit Inflammatory Infiltration in MI
5.3. The Roles of Circular RNAs in Collagen Deposition and Fibrosis after MI
5.3.1. CircRNAs Promote Collagen Deposition and Fibrosis in the Healing Stage of MI
- CircFASTKD1
- CircPAN3
- CircHIPK3
- CircPostn
5.3.2. CircRNAs Suppress Collagen Deposition and Fibrosis in the Healing Stage of MI
6. Circular RNAs as Potential Diagnostic Markers of Myocardial Infarction
7. Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wereski, R.; Kimenai, D.M.; Bularga, A.; Taggart, C.; Lowe, D.J.; Mills, N.L.; Chapman, A.R. Risk factors for type 1 and type 2 myocardial infarction. Eur. Heart J. 2022, 43, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D.; et al. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidlöf, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulluck, H.; Hammond-Haley, M.; Fontana, M.; Knight, D.S.; Sirker, A.; Herrey, A.S.; Manisty, C.; Kellman, P.; Moon, J.C.; Hausenloy, D.J. Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J. Cardiovasc. Magn. Reson. 2017, 19, 57. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.V.; Toor, I.; Shah, A.S.; Carruthers, K.; Vesey, A.T.; Alam, S.R.; Sills, A.; Hoo, T.Y.; Melville, A.J.; Langlands, S.P.; et al. Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J. Am. Heart Assoc. 2015, 4, e001956. [Google Scholar] [CrossRef] [Green Version]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.T.; Coca-Prados, M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef]
- Arnberg, A.C.; Van Ommen, G.J.; Grivell, L.A.; Van Bruggen, E.F.; Borst, P. Some yeast mitochondrial RNAs are circular. Cell 1980, 19, 313–319. [Google Scholar] [CrossRef]
- Cocquerelle, C.; Daubersies, P.; Majérus, M.A.; Kerckaert, J.P.; Bailleul, B. Splicing with inverted order of exons occurs proximal to large introns. EMBO J. 1992, 11, 1095–1098. [Google Scholar] [CrossRef]
- Zaphiropoulos, P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: Correlation with exon skipping. Proc. Natl. Acad. Sci. USA 1996, 93, 6536–6541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capel, B.; Swain, A.; Nicolis, S.; Hacker, A.; Walter, M.; Koopman, P.; Goodfellow, P.; Lovell-Badge, R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993, 73, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Danan, M.; Schwartz, S.; Edelheit, S.; Sorek, R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012, 40, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Haddad, G.; Lorenzen, J.M. Biogenesis and Function of Circular RNAs in Health and in Disease. Front. Pharmacol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, C.Y.; Zhang, Y.H.; Wang, Y.H.; Zhou, L.Y.; Li, X.M.; Wang, K.; Chen, X.Z.; Wang, T.; Ju, J.; et al. The circRNA CNEACR regulates necroptosis of cardiomyocytes through Foxa2 suppression. Cell Death Differ. 2022, 29, 527–539. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, S.; Wei, G.; Sun, Y.; Li, C.; Si, X.; Chen, Y.; Tang, Z.; Li, X.; Chen, Y.; et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol. Ther. 2022, 30, 3477–3498. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Dempsey, R.J.; Vemuganti, R. Role of circular RNAs in brain development and CNS diseases. Prog Neurobiol. 2020, 186, 101746. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; An, Y.; Wang, J.; Gao, Y. The Particular Expression Profiles of Circular RNA in Peripheral Blood of Myocardial Infarction Patients by RNA Sequencing. Front. Cardiovasc. Med. 2022, 9, 810257. [Google Scholar] [CrossRef] [PubMed]
- Caba, L.; Florea, L.; Gug, C.; Dimitriu, D.C.; Gorduza, E.V. Circular RNA-Is the Circle Perfect? Biomolecules 2021, 11, 1755. [Google Scholar] [CrossRef]
- Eger, N.; Schoppe, L.; Schuster, S.; Laufs, U.; Boeckel, J.N. Circular RNA Splicing. Adv. Exp. Med. Biol. 2018, 1087, 41–52. [Google Scholar] [CrossRef]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altesha, M.A.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell. Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Ulshöfer, C.J.; Pfafenrot, C.; Bindereif, A.; Schneider, T. Methods to study circRNA-protein interactions. Methods 2021, 196, 36–46. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Ho-Xuan, H.; Glažar, P.; Latini, C.; Heizler, K.; Haase, J.; Hett, R.; Anders, M.; Weichmann, F.; Bruckmann, A.; Van den Berg, D.; et al. Comprehensive analysis of translation from overexpressed circular RNAs reveals pervasive translation from linear transcripts. Nucleic Acids Res. 2020, 48, 10368–10382. [Google Scholar] [CrossRef] [PubMed]
- Welden, J.R.; Stamm, S. Pre-mRNA structures forming circular RNAs. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194410. [Google Scholar] [CrossRef] [PubMed]
- Kos, A.; Dijkema, R.; Arnberg, A.C.; van der Meide, P.H.; Schellekens, H. The hepatitis delta (delta) virus possesses a circular RNA. Nature 1986, 323, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Di Timoteo, G.; Dattilo, D.; Centron-Broco, A.; Colantoni, A.; Guarnacci, M.; Rossi, F.; Incarnato, D.; Oliviero, S.; Fatica, A.; Morlando, M.; et al. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020, 31, 107641. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e29. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Cortés-López, M.; Miura, P. Emerging Functions of Circular RNAs. Yale J. Biol. Med. 2016, 89, 527–537. [Google Scholar]
- Sinha, T.; Panigrahi, C.; Das, D.; Chandra Panda, A. Circular RNA translation, a path to hidden proteome. Wiley Interdiscip. Rev. RNA 2022, 13, e1685. [Google Scholar] [CrossRef]
- Rossi, J.J. A novel nuclear miRNA mediated modulation of a non-coding antisense RNA and its cognate sense coding mRNA. EMBO J. 2011, 30, 4340–4341. [Google Scholar] [CrossRef]
- Ng, W.L.; Marinov, G.K.; Liau, E.S.; Lam, Y.L.; Lim, Y.Y.; Ea, C.K. Inducible RasGEF1B circular RNA is a positive regulator of ICAM-1 in the TLR4/LPS pathway. RNA Biol. 2016, 13, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.H.; Zhang, R.C.; Liu, C.Y.; Dong, Y.H.; Wang, M.; et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Furber, K.L.; Ji, S. Pseudogenes regulate parental gene expression via ceRNA network. J. Cell Mol. Med. 2017, 21, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R. Circular RNA and Alzheimer’s Disease. Adv. Exp. Med. Biol. 2018, 1087, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef]
- Cui, X.; Dong, Y.; Li, M.; Wang, X.; Jiang, M.; Yang, W.; Liu, G.; Sun, S.; Xu, W. A circular RNA from NFIX facilitates oxidative stress-induced H9c2 cells apoptosis. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 715–722. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Li, Q.; Liu, W.; Song, Q.; Jiang, H. CircRNA ACAP2 induces myocardial apoptosis after myocardial infarction by sponging miR-29. Minerva Med. 2022, 113, 128–134. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Zhang, J.; Wang, J.; He, J.; Zhang, Z.; Liu, F. CircRNA ACAP2 Is Overexpressed in Myocardial Infarction and Promotes the Maturation of miR-532 to Induce the Apoptosis of Cardiomyocyte. J. Cardiovasc. Pharmacol. 2021, 78, 247–252. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Deng, W.; Jiang, M. CircRNA MFACR Is Upregulated in Myocardial Infarction and Downregulates miR-125b to Promote Cardiomyocyte Apoptosis Induced by Hypoxia. J. Cardiovasc. Pharmacol. 2021, 78, 802–808. [Google Scholar] [CrossRef]
- Wang, K.; Gan, T.Y.; Li, N.; Liu, C.Y.; Zhou, L.Y.; Gao, J.N.; Chen, C.; Yan, K.W.; Ponnusamy, M.; Zhang, Y.H.; et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Ling, G.X.; Lei, B.F.; Feng, X.; Xie, X.Y.; Fang, C.; Li, Y.G.; Cai, X.W.; Zheng, B.S. Circular RNA PVT1 silencing prevents ischemia-reperfusion injury in rat by targeting microRNA-125b and microRNA-200a. J. Mol. Cell. Cardiol. 2021, 159, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, B.; Wang, Y.; Zhu, H.; Zhang, P.; Jiang, P.; Yang, X.; Sun, J.; Hong, L.; Shao, L. CircJARID2 Regulates Hypoxia-Induced Injury in H9c2 Cells by Affecting miR-9-5p-Mediated BNIP3. J. Cardiovasc. Pharmacol. 2021, 78, e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.P.; Zhang, N.J.; Wang, H.J.; Hu, S.G.; Geng, X. Knockdown of circROBO2 attenuates acute myocardial infarction through regulating the miR-1184/TRADD axis. Mol. Med. 2021, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, Y.; Jiang, Y.; Tan, M.; Liu, C. Circular RNA Rbms1 inhibited the development of myocardial ischemia reperfusion injury by regulating miR-92a/BCL2L11 signaling pathway. Bioengineered 2022, 13, 3082–3092. [Google Scholar] [CrossRef]
- Liu, B.; Guo, K. CircRbms1 knockdown alleviates hypoxia-induced cardiomyocyte injury via regulating the miR-742-3p/FOXO1 axis. Cell. Mol. Biol. Lett. 2022, 27, 31. [Google Scholar] [CrossRef]
- Chen, Y.E.; Yang, H.; Pang, H.B.; Shang, F.Q. Circ-CBFB exacerbates hypoxia/reoxygenation-triggered cardiomyocyte injury via regulating miR-495-3p in a VDAC1-dependent manner. J. Biochem. Mol. Toxicol. 2022, 36, e23189. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, J.; Chen, H.; He, R.; Wu, H. CircTRRAP Knockdown Has Cardioprotective Function in Cardiomyocytes via the Signal Regulation of miR-370-3p/PAWR Axis. Cardiovasc. Ther. 2022, 2022, 7125602. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, W.; Pan, W.; Wang, Z. CircRNA 010567 plays a significant role in myocardial infarction via the regulation of the miRNA-141/DAPK1 axis. J. Thorac. Dis. 2021, 13, 2447–2459. [Google Scholar] [CrossRef]
- Gan, J.; Yuan, J.; Liu, Y.; Lu, Z.; Xue, Y.; Shi, L.; Zeng, H. Circular RNA_101237 mediates anoxia/reoxygenation injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int. J. Mol. Med. 2020, 45, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Pan, W.; Chen, H.; Du, Y.; Jiang, P.; Zeng, D.; Wu, J.; Peng, K. Circ_0124644 Serves as a ceRNA for miR-590-3p to Promote Hypoxia-Induced Cardiomyocytes Injury via Regulating SOX4. Front. Genet. 2021, 12, 667724. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Zheng, M.; Wang, L.; Wei, M.; Yin, Y.; Ma, F.; Li, X.; Zhang, H.; Liu, G. Circ_0068655 Promotes Cardiomyocyte Apoptosis via miR-498/PAWR Axis. Tissue Eng. Regen. Med. 2020, 17, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ma, R.; Cao, J.; Du, X.; Cai, X.; Fan, Y. CircSAMD4A aggravates H/R-induced cardiomyocyte apoptosis and inflammatory response by sponging miR-138-5p. J. Cell. Mol. Med. 2022, 26, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Pang, P.; Li, X.; Yu, S.; Wang, X.; Liu, K.; Ju, J.; Wu, H.; Gao, Y.; Liu, Q.; et al. CircHelz activates NLRP3 inflammasome to promote myocardial injury by sponging miR-133a-3p in mouse ischemic heart. J. Mol. Cell. Cardiol. 2021, 158, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Qian, G.; Wu, H.; Pan, H.; Xie, S.; Sun, Z.; Shao, P.; Tang, G.; Hu, H.; Zhang, S. Knockdown of circ_0060745 alleviates acute myocardial infarction by suppressing NF-κB activation. J. Cell. Mol. Med. 2020, 24, 12401–12410. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Q.; Hu, X.M.; Zhang, Q.; Yang, L.; Lv, X.Z.; Chen, S.; Wu, P.; Duan, D.W.; Lang, Y.H.; Ning, M.; et al. Downregulation of circFASTKD1 ameliorates myocardial infarction by promoting angiogenesis. Aging 2020, 13, 3588–3604. [Google Scholar] [CrossRef]
- Li, F.; Long, T.Y.; Bi, S.S.; Sheikh, S.A.; Zhang, C.L. circPAN3 exerts a profibrotic role via sponging miR-221 through FoxO3/ATG7-activated autophagy in a rat model of myocardial infarction. Life Sci. 2020, 257, 118015. [Google Scholar] [CrossRef]
- Bai, M.; Pan, C.L.; Jiang, G.X.; Zhang, Y.M.; Zhang, Z. CircHIPK3 aggravates myocardial ischemia-reperfusion injury by binding to miRNA-124-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10107–10114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7954657. [Google Scholar] [CrossRef]
- Long, N.; Chu, L.; Jia, J.; Peng, S.; Gao, Y.; Yang, H.; Yang, Y.; Zhao, Y.; Liu, J. CircPOSTN/miR-361-5p/TPX2 axis regulates cell growth, apoptosis and aerobic glycolysis in glioma cells. Cancer Cell Int. 2020, 20, 374. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, M.Y.; Wu, Y.B.; Cui, H.M.; Wei, S.X.; Liu, B.; Wang, R. Circular RNA POSTN Promotes Myocardial Infarction-Induced Myocardial Injury and Cardiac Remodeling by Regulating miR-96-5p/BNIP3 Axis. Front. Cell Dev. Biol. 2020, 8, 618574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Z.; Cheng, Q.; Wang, Z.; Lv, X.; Wang, Z.; Li, N. Circular RNA (circRNA) CDYL Induces Myocardial Regeneration by ceRNA After Myocardial Infarction. Med. Sci. Monit. 2020, 26, e923188. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhao, P.; Sun, L.; Lu, Y.; Zhu, W.; Zhang, J.; Xiang, C.; Mao, Y.; Chen, Q.; Zhang, F. Overexpression of circRNA SNRK targets miR-103-3p to reduce apoptosis and promote cardiac repair through GSK3β/β-catenin pathway in rats with myocardial infarction. Cell Death Discov. 2021, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, G.; Peng, J.; Ren, L.; Lei, L.; Ye, H.; Wang, Z.; Zhao, S. CircMACF1 Attenuates Acute Myocardial Infarction Through miR-500b-5p-EMP1 Axis. J. Cardiovasc. Transl. Res. 2021, 14, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, L.; Wang, Y.; Gao, X.; Tang, H.; Ge, J. Circ_0001206 regulates miR-665/CRKL axis to alleviate hypoxia/reoxygenation-induced cardiomyocyte injury in myocardial infarction. ESC Heart Fail. 2022, 9, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, Z.; Chen, X.; Lu, G.; Zhu, X.; Xu, G. CircUBXN7 mitigates H/R-induced cell apoptosis and inflammatory response through the miR-622-MCL1 axis. Am. J. Transl. Res. 2021, 13, 8711–8727. [Google Scholar] [PubMed]
- Zhu, Y.; Pan, W.; Yang, T.; Meng, X.; Jiang, Z.; Tao, L.; Wang, L. Upregulation of Circular RNA CircNFIB Attenuates Cardiac Fibrosis by Sponging miR-433. Front. Genet. 2019, 10, 564. [Google Scholar] [CrossRef] [Green Version]
- Tondera, D.; Czauderna, F.; Paulick, K.; Schwarzer, R.; Kaufmann, J.; Santel, A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J. Cell Sci. 2005, 118, 3049–3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Liao, J.; Liang, J.; Chen, X.P.; Zhang, B.; Chu, L. Circular RNA HIPK3: A Key Circular RNA in a Variety of Human Cancers. Front. Oncol. 2020, 10, 773. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Yang, J.; Li, Y.; Peng, W.; Wu, M.; Yu, C.; Fang, M. Silencing CircHIPK3 Sponges miR-93-5p to Inhibit the Activation of Rac1/PI3K/AKT Pathway and Improves Myocardial Infarction-Induced Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 645378. [Google Scholar] [CrossRef]
- Kommineni, N.; Saka, R.; Khan, W.; Domb, A.J. Non-polymer drug-eluting coronary stents. Drug Deliv. Transl. Res. 2018, 8, 903–917. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Thygesen, K.; Alpert, J.S.; Jaffe, A.S. Clinical implications of the Third Universal Definition of Myocardial Infarction. Heart 2014, 100, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Labugger, R.; Organ, L.; Collier, C.; Atar, D.; Van Eyk, J.E. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000, 102, 1221–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervaiz, S.; Waskiewicz, D.; Anderson, F.P.; Lawson, C.J.; Lohmann, T.P.; Feng, Y.J.; Contois, J.H.; Wu, A.H. Comparative analysis of cardiac troponin I and creatine ki-nase-MB as markers of acute myocardial infarction. Clin. Cardiol. 1997, 20, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Giugliano, R.P.; Braunwald, E. Updates on Acute Coronary Syndrome: A Review. JAMA Cardiol. 2016, 1, 718–730. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lichtenauer, M.; Wernly, B.; Paar, V.; Pistulli, R.; Rohm, I.; Jung, C.; Figulla, H.R.; Yilmaz, A.; Cadamuro, J.; et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2017, 47, 638–648. [Google Scholar] [CrossRef]
- Jahani, S.; Nazeri, E.; Majidzadeh-A, K.; Jahani, M.; Esmaeili, R. Circular RNA; a new biomarker for breast cancer: A systematic review. J. Cell. Physiol. 2020, 235, 5501–5510. [Google Scholar] [CrossRef]
- Koh, W.; Pan, W.; Gawad, C.; Fan, H.C.; Kerchner, G.A.; Wyss-Coray, T.; Blumenfeld, Y.J.; El-Sayed, Y.Y.; Quake, S.R. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7361–7366. [Google Scholar] [CrossRef] [Green Version]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef]
- Marinescu, M.C.; Lazar, A.L.; Marta, M.M.; Cozma, A.; Catana, C.S. Non-Coding RNAs: Prevention, Diagnosis, and Treatment in Myocardial Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 2728. [Google Scholar] [CrossRef]
- Lin, F.; Yang, Y.; Guo, Q.; Xie, M.; Sun, S.; Wang, X.; Li, D.; Zhang, G.; Li, M.; Wang, J.; et al. Analysis of the Molecular Mechanism of Acute Coronary Syndrome Based on circRNA-miRNA Network Regulation. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 1584052. [Google Scholar] [CrossRef] [PubMed]
- Lavenniah, A.; Luu, T.D.A.; Li, Y.P.; Lim, T.B.; Jiang, J.; Ackers-Johnson, M.; Foo, R.S. Engineered Circular RNA Sponges Act as miRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol. Ther. 2020, 28, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular functions and specific roles of circRNAs in the cardiovascular system. Non-Coding RNA Res. 2018, 3, 75–98. [Google Scholar] [CrossRef]
- Bazan, H.A.; Hatfield, S.A.; Brug, A.; Brooks, A.J.; Lightell, D.J., Jr.; Woods, T.C. Carotid Plaque Rupture Is Accompanied by an Increase in the Ratio of Serum circR-284 to miR-221 Levels. Circ. Cardiovasc. Genet. 2017, 10, e001720. [Google Scholar] [CrossRef] [Green Version]

| Name | Expression | Function | Ref. |
|---|---|---|---|
| CircNfix | Upregulated Downregulated | Pro-apoptotic; inhibits angiogenesis | [45,46] |
| CircACAP2 | Upregulated | Pro-apoptotic | [47,48] |
| CircMFACR | Upregulated | Pro-apoptotic | [49,50] |
| CircPVT1 | Upregulated | Pro-apoptotic | [51] |
| CircNCX1 | Upregulated | Pro-apoptotic | [52] |
| CircJARID2 | Upregulated | Pro-apoptotic | [53] |
| CircROBO2 | Upregulated | Pro-apoptotic | [54] |
| CircRbms1 | Upregulated | Pro-apoptotic | [55,56] |
| Circ-CBFB | Upregulated | Pro-apoptotic | [57] |
| Circ-TRRAP | Upregulated | Pro-apoptotic | [58] |
| Circ-010567 | Upregulated | Pro-apoptotic | [59] |
| Circ-101237 | Upregulated | Pro-apoptotic; promotes autophagy | [60] |
| Circ-0124644 | Upregulated | Pro-apoptotic | [61] |
| Circ-0068655 | Upregulated | Pro-apoptotic | [62] |
| CircSAMD4A | Upregulated | Pro-inflammatory infiltration; pro-apoptotic | [63] |
| CircHelz | Upregulated | Pro-inflammatory infiltration | [64] |
| Circ_0060745 | Upregulated | Pro-inflammatory infiltration; pro-apoptotic | [65] |
| CircFASTKD1 | Upregulated | Inhibits angiogenesis | [66] |
| CircPAN3 | Upregulated | Promotes autophagy; promotes fibrosis | [67] |
| CircHIPK3 | Upregulated | Promotes collagen deposition; promotes fibrosis | [68,69] |
| CircPOSTN | Upregulated | Promotes collagen deposition; promotes fibrosis | [70,71] |
| CircCNEACR | Downregulated | Inhibits programmed necrosis | [20] |
| CircSamd4 | Downregulated | Inhibits apoptosis; inhibits fibrosis | [21] |
| CircACR | Downregulated | Inhibits autophagy | [42] |
| CircCDYL | Downregulated | Inducing myocardial regeneration | [72] |
| CircFndc3b | Downregulated | Inhibits apoptosis; promotes angiogenesis | [41] |
| CircSNRK | Downregulated | Inhibits apoptosis; promotes angiogenesis | [73] |
| CircMACF1 | Downregulated | Inhibits apoptosis | [74] |
| Circ_0001206 | Downregulated | Inhibits apoptosis | [75] |
| CircUBXN7 | Downregulated | Inhibits apoptosis; inhibits inflammatory infiltration | [76] |
| CircNFIB | Downregulated | Inhibits fibrosis | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, Y.; Wang, S.; Wu, X.; Cao, J.; Sun, T. Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4233. https://doi.org/10.3390/ijms24044233
Li J, Han Y, Wang S, Wu X, Cao J, Sun T. Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(4):4233. https://doi.org/10.3390/ijms24044233
Chicago/Turabian StyleLi, Jialei, Yu Han, Shuang Wang, Xiaolei Wu, Jimin Cao, and Teng Sun. 2023. "Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction" International Journal of Molecular Sciences 24, no. 4: 4233. https://doi.org/10.3390/ijms24044233





