Multiple Roles of the Stress Sensor GCN2 in Immune Cells
Abstract
1. Introduction
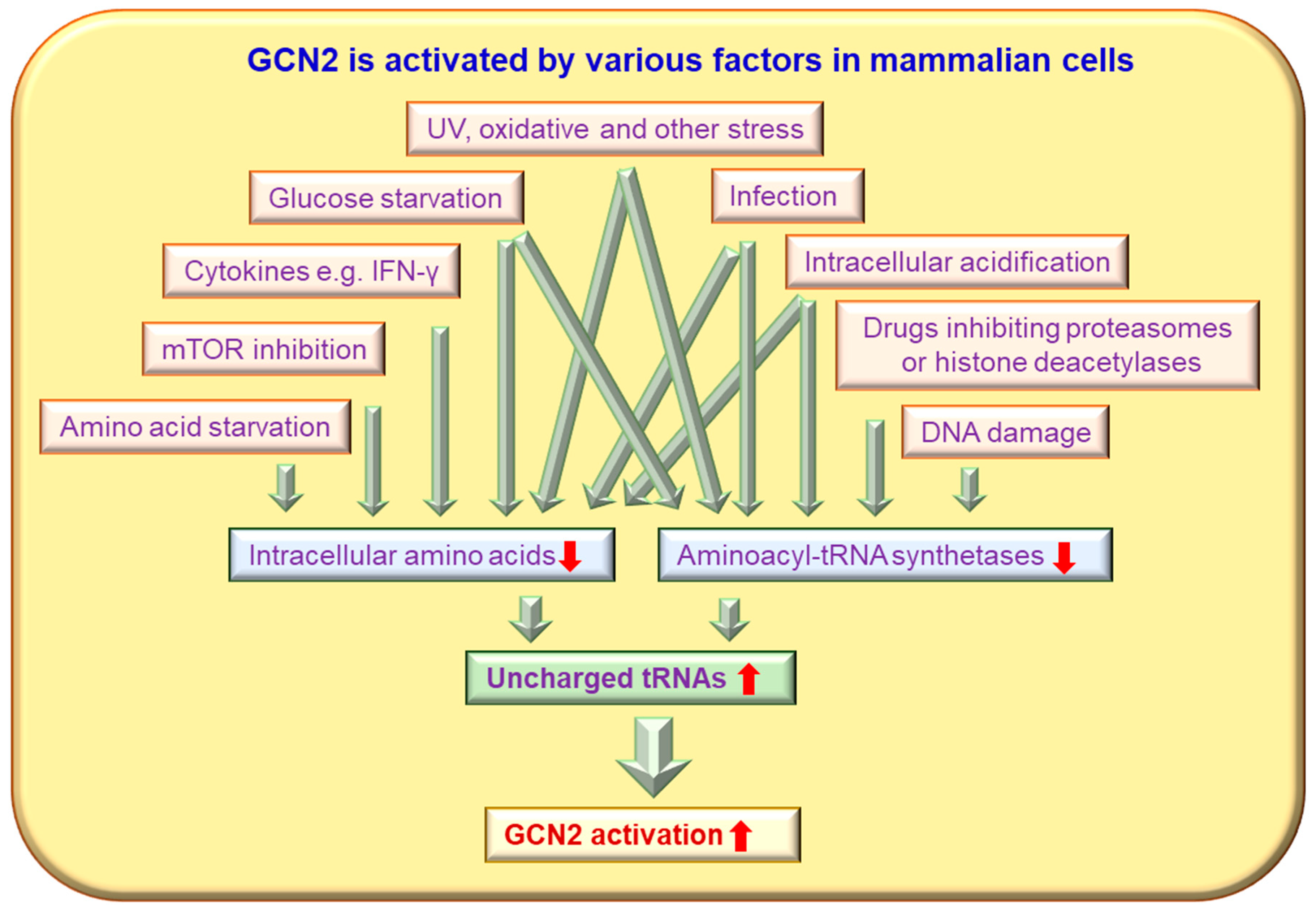
2. Molecular Structure, Expression and Activation of GCN2
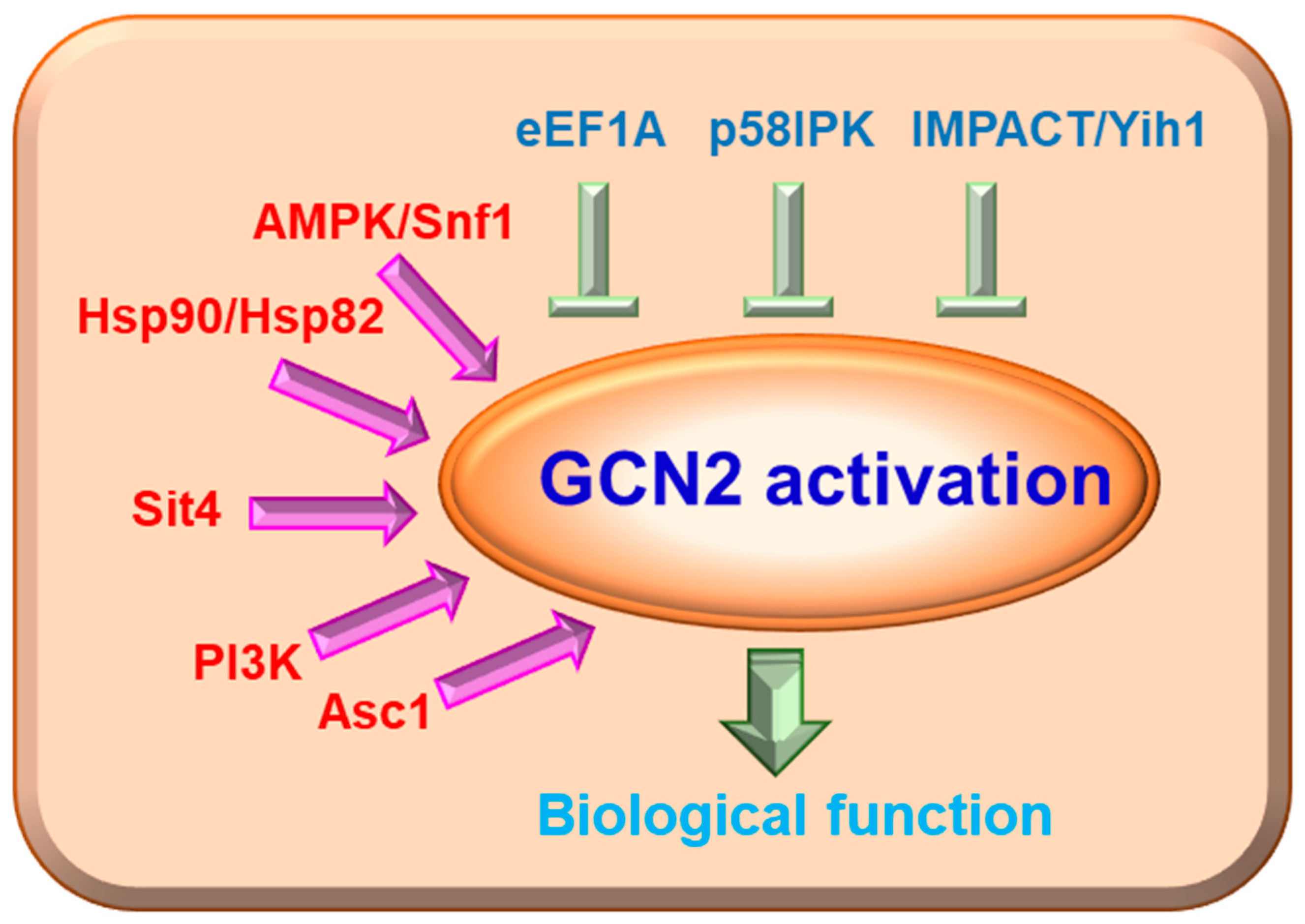
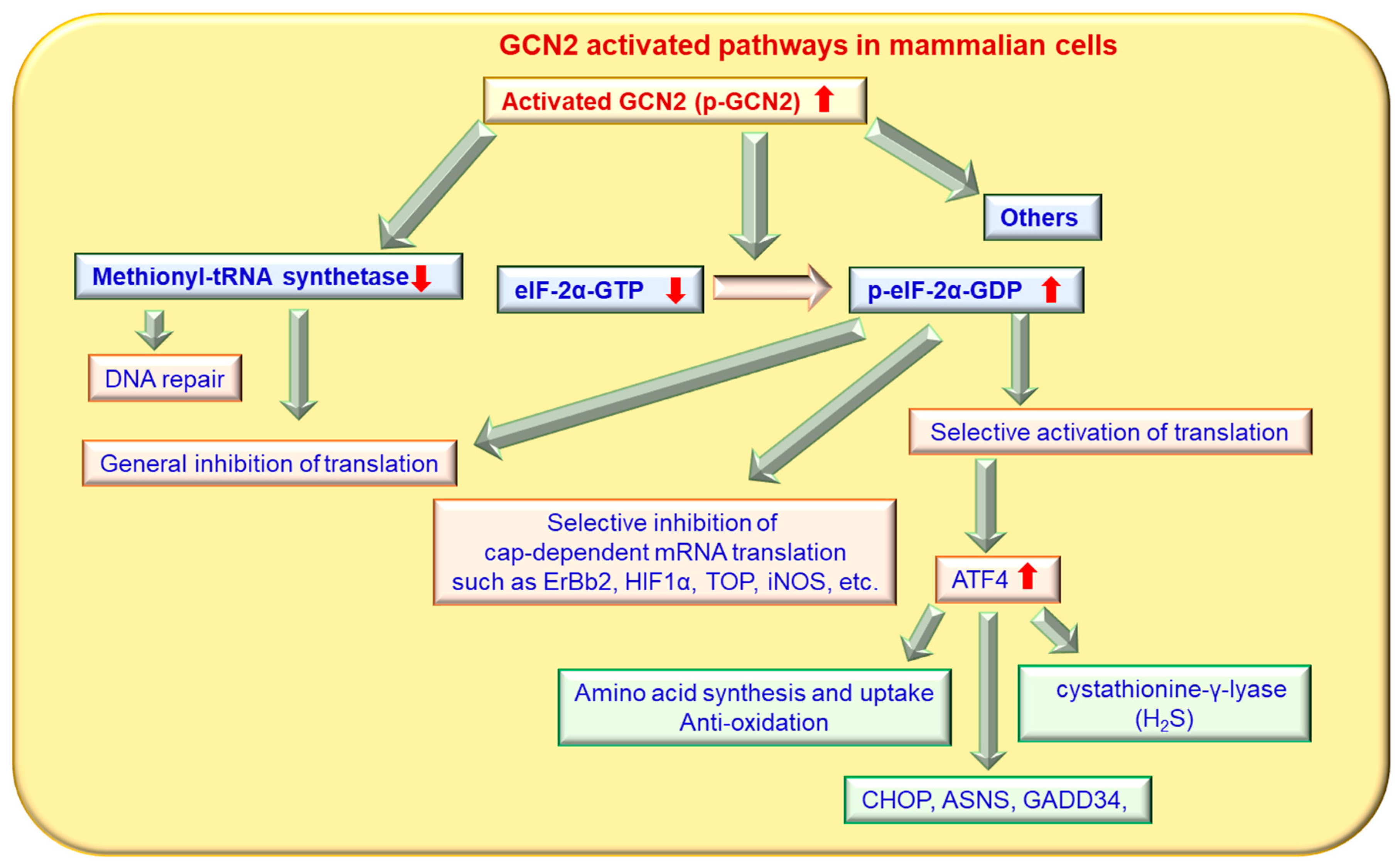
3. GCN2 and Its Associated Signaling Pathways
4. Biological Functions of GCN2
4.1. GCN2 and the Oxidative Stress Response
4.2. GCN2 and Cell Survival, Cell Cycle and Cell Differentiation
4.3. GCN2 and Autophagy
4.4. GCN2, mTOR and Metabolism
5. GCN2 in the Immune System
5.1. GCN2 in Innate Cells
5.2. GCN2 in T Cells
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4E-BP1 | eIF4E-binding protein |
| Aft1 | DNA-binding transcription factor 1 |
| AhR | aryl hydrocarbon receptor |
| AMPK | AMP-activated serine/threonine protein kinase |
| APCs | Antigen presenting cells |
| Asc1 | Activating signal cointegrator–1 |
| ATF4 | Activating transcription factor 4 |
| ATM | Ataxia-telangiectasia, mutated protein kinases |
| ATR | ATM and Rad3-related protein kinases |
| BMECs | Bovine mammary epithelial cells |
| C/EBPβ | CCAAT enhancer-binding protein-beta |
| CHOP | C/EBP homologous protein |
| CNS | Central nervous system |
| CTD | C-terminal ribosome domain |
| EAE | Experimental autoimmune encephalomyelitis |
| eEF1A | Eukaryotic translation elongation factor 1A |
| eIF-2 | Eukaryotic initiation factor 2 |
| eIF2B | Eukaryotic translation initiation factor 2B |
| eIF2G | Eukaryotic translation initiation factor 2 gamma |
| eIF-2α | Eukaryotic translation initiation factor 2 alpha |
| eIF5 | Eukaryotic translation initiation factor 5 |
| ErBb2 | Erb-b2 receptor tyrosine kinase 2 |
| FASN | Fatty acid synthase |
| FDA | Food and Drug Administration |
| Foxo | Forkhead box O class |
| GCN1 | General control nonderepressible 1 |
| GCN2 | General control nonderepressible 2 |
| Gcn4p | General control nondepressible 4 protein |
| GSK-β | Glycogen synthase kinase 3 beta |
| HisRS | Histidyl-tRNA synthetase |
| Hsp82 | Heat shock protein 82 |
| Hsp90 | Heat shock protein 90 |
| IDO | Indolamine 2,3-dioxygenase |
| IMPACT | Impact RWD domain protein |
| ISR | Integrated stress response |
| MDSCs | Myeloid derived suppressor cells |
| mRNA | Messenger RNA |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NEDD4L | NEDD4 like E3 ubiquitin protein ligase |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| ORFs | Open reading frames |
| PERK | Protein kinase RNA-like ER kinase |
| PI3K | Phosphoinositide 3-kinase |
| PKB | Protein kinase B |
| PKR | Double-stranded RNA-dependent protein kinase |
| PP6C | Protein phosphatase 6 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 |
| Rack1 | Receptor for activated C-kinase |
| REDD1 | Regulated in development and DNA damage responses 1 |
| REG1 | Resistance to glucose repression protein 1 |
| ROS | Reactive oxygen species |
| RWD | RING finger and WD repeat containing |
| S6K | S6 kinase |
| Sit4 | Serine/threonine-protein phosphatase PP1-1 |
| SLC7A5 | Solute carrier family 7 member 5 |
| SLE | Systemic lupus erythematosus |
| Snf1 | Sucrose non-fermenting 1 |
| SREBP1C | Sterol regulatory element-binding protein 1C |
| STAT3 | Signal transducer and activator of transcription 3 |
| TIA-1 | T-cell intracellular antigen 1 |
| TIAR | TIA1 cytotoxic granule-associated RNA binding protein like 1 |
| TME | Tumor [141] microenvironment |
| TOP | Translation of 5′-terminal oligopyrimidine tracts |
| TOR2 | Target of rapamycin 2 |
| TRAIL-R2 | TNF-related apoptosis-inducing ligand receptor 2 |
| Treg | Regulatory T cells |
| tRNA | Transfer RNA |
| UPR | Unfolded protein response |
| Yih1 | IMPACT homolog 1 |
References
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Taniuchi, S.; Miyake, M.; Tsugawa, K.; Oyadomari, M.; Oyadomari, S. Integrated stress response of vertebrates is regulated by four eIF2alpha kinases. Sci. Rep. 2016, 6, 32886. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2alpha kinases: Their structures and functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef] [PubMed]
- Grallert, B.; Boye, E. GCN2, an old dog with new tricks. Biochem. Soc. Trans. 2013, 41, 1687–1691. [Google Scholar] [CrossRef]
- Santos-Ribeiro, D.; Lecocq, M.; De Beukelaer, M.; Verleden, S.; Bouzin, C.; Ambroise, J.; Dorfmuller, P.; Yakoub, Y.; Huaux, F.; Quarck, R.; et al. Disruption of GCN2 Pathway Aggravates Vascular and Parenchymal Remodeling During Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Halaby, M.J.; Hezaveh, K.; Lamorte, S.; Ciudad, M.T.; Kloetgen, A.; MacLeod, B.L.; Guo, M.; Chakravarthy, A.; Medina, T.D.S.; Ugel, S.; et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci. Immunol. 2019, 4, eaax8189. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol. Rev. 1988, 52, 248–273. [Google Scholar] [CrossRef]
- Baird, T.D.; Wek, R.C. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 2012, 3, 307–321. [Google Scholar] [CrossRef]
- Valbuena, N.; Rozalen, A.E.; Moreno, S. Fission yeast TORC1 prevents eIF2alpha phosphorylation in response to nitrogen and amino acids via Gcn2 kinase. J. Cell. Sci. 2012, 125, 5955–5959. [Google Scholar] [CrossRef]
- Berlanga, J.J.; Santoyo, J.; De Haro, C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur. J. Biochem. 1999, 265, 754–762. [Google Scholar] [CrossRef]
- Wek, S.A.; Zhu, S.; Wek, R.C. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 1995, 15, 4497–4506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sobolev, A.Y.; Wek, R.C. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J. Biol. Chem. 1996, 271, 24989–24994. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, H.; Garcia-Barrio, M.; Anderson, J.; Hinnebusch, A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 2000, 6, 269–279. [Google Scholar] [CrossRef]
- Yamazaki, H.; Kasai, S.; Mimura, J.; Ye, P.; Inose-Maruyama, A.; Tanji, K.; Wakabayashi, K.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Ribosome binding protein GCN1 regulates the cell cycle and cell proliferation and is essential for the embryonic development of mice. PLoS Genet. 2020, 16, e1008693. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef]
- Lageix, S.; Zhang, J.; Rothenburg, S.; Hinnebusch, A.G. Interaction between the tRNA-binding and C-terminal domains of Yeast Gcn2 regulates kinase activity in vivo. PLoS Genet. 2015, 11, e1004991. [Google Scholar] [CrossRef]
- Fennell, C.; Babbitt, S.; Russo, I.; Wilkes, J.; Ranford-Cartwright, L.; Goldberg, D.E.; Doerig, C. PfeIK1, a eukaryotic initiation factor 2alpha kinase of the human malaria parasite Plasmodium falciparum, regulates stress-response to amino-acid starvation. Malar. J. 2009, 8, 99. [Google Scholar] [CrossRef]
- Konrad, C.; Wek, R.C.; Sullivan, W.J., Jr. GCN2-like eIF2alpha kinase manages the amino acid starvation response in Toxoplasma gondii. Int. J. Parasitol. 2014, 44, 139–146. [Google Scholar] [CrossRef]
- Rai, M.; Xiong, Y.; Singleton, C.K. Disruption of the ifkA and ifkB genes results in altered cell adhesion, morphological defects and a propensity to form pre-stalk O cells during development of Dictyostelium. Differentiation 2006, 74, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Lu, J.; Gadalla, D.S.; Hendrich, O.; Gronke, S.; Partridge, L. The Role of GCN2 Kinase in Mediating the Effects of Amino Acids on Longevity and Feeding Behaviour in Drosophila. Front. Aging 2022, 3, 944466. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Harding, H.P.; Raught, B.; Gingras, A.C.; Berlanga, J.J.; Scheuner, D.; Kaufman, R.J.; Ron, D.; Sonenberg, N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr. Biol. 2002, 12, 1279–1286. [Google Scholar] [CrossRef]
- Peidis, P.; Papadakis, A.I.; Rajesh, K.; Koromilas, A.E. HDAC pharmacological inhibition promotes cell death through the eIF2alpha kinases PKR and GCN2. Aging 2010, 2, 669–677. [Google Scholar] [CrossRef]
- Yang, R.; Wek, S.A.; Wek, R.C. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol. Cell. Biol. 2000, 20, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Brooks, H.L. Phosphorylation of eIF2alpha via the general control kinase, GCN2, modulates the ability of renal medullary cells to survive high urea stress. Am. J. Physiol. Renal Physiol. 2011, 301, F1202–F1207. [Google Scholar] [CrossRef][Green Version]
- Eleftheriadis, T.; Tsogka, K.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Activation of general control nonderepressible 2 kinase protects human glomerular endothelial cells from harmful high-glucose-induced molecular pathways. Int. Urol. Nephrol. 2016, 48, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Castilho, B.A.; Shanmugam, R.; Silva, R.C.; Ramesh, R.; Himme, B.M.; Sattlegger, E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim. Biophys. Acta 2014, 1843, 1948–1968. [Google Scholar] [CrossRef]
- Tang, C.P.; Clark, O.; Ferrarone, J.R.; Campos, C.; Lalani, A.S.; Chodera, J.D.; Intlekofer, A.M.; Elemento, O.; Mellinghoff, I.K. GCN2 kinase activation by ATP-competitive kinase inhibitors. Nat. Chem. Biol. 2022, 18, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Anda, S.; Zach, R.; Grallert, B. Activation of Gcn2 in response to different stresses. PLoS ONE 2017, 12, e0182143. [Google Scholar] [CrossRef] [PubMed]
- Ghavidel, A.; Kislinger, T.; Pogoutse, O.; Sopko, R.; Jurisica, I.; Emili, A. Impaired tRNA nuclear export links DNA damage and cell-cycle checkpoint. Cell 2007, 131, 915–926. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Machida, K.; Mizunuma, M.; Emoto, Y.; Sato, N.; Miyahara, K.; Hirata, D.; Usui, T.; Takahashi, H.; Osada, H.; et al. Identification of Saccharomyces cerevisiae isoleucyl-tRNA synthetase as a target of the G1-specific inhibitor Reveromycin A. J. Biol. Chem. 2002, 277, 28810–28814. [Google Scholar] [CrossRef]
- Habibi, D.; Ogloff, N.; Jalili, R.B.; Yost, A.; Weng, A.P.; Ghahary, A.; Ong, C.J. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Invest. New Drugs 2012, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, A.; Munch, C.; Hanssum, A.; Bertolotti, A. Failure of amino acid homeostasis causes cell death following proteasome inhibition. Mol. Cell 2012, 48, 242–253. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Nomura, W.; Maeta, K.; Kita, K.; Izawa, S.; Inoue, Y. Role of Gcn4 for adaptation to methylglyoxal in Saccharomyces cerevisiae: Methylglyoxal attenuates protein synthesis through phosphorylation of eIF2alpha. Biochem. Biophys. Res. Commun. 2008, 376, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Nomura, W.; Maeta, K.; Kita, K.; Izawa, S.; Inoue, Y. Methylglyoxal activates Gcn2 to phosphorylate eIF2alpha independently of the TOR pathway in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 86, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, V.A.; Hinnebusch, A.G. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 2003, 17, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Obata, T.; Ota, K.; Sasaki, T.; Ito, T. Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2 alpha kinase GCN2. J. Biol. Chem. 2003, 278, 20457–20460. [Google Scholar] [CrossRef]
- Rodland, G.E.; Tvegard, T.; Boye, E.; Grallert, B. Crosstalk between the Tor and Gcn2 pathways in response to different stresses. Cell Cycle 2014, 13, 453–461. [Google Scholar] [CrossRef][Green Version]
- Staschke, K.A.; Dey, S.; Zaborske, J.M.; Palam, L.R.; McClintick, J.N.; Pan, T.; Edenberg, H.J.; Wek, R.C. Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J. Biol. Chem. 2010, 285, 16893–16911. [Google Scholar] [CrossRef]
- Lewerenz, J.; Baxter, P.; Kassubek, R.; Albrecht, P.; Van Liefferinge, J.; Westhoff, M.A.; Halatsch, M.E.; Karpel-Massler, G.; Meakin, P.J.; Hayes, J.D.; et al. Phosphoinositide 3-kinases upregulate system xc(-) via eukaryotic initiation factor 2alpha and activating transcription factor 4—A pathway active in glioblastomas and epilepsy. Antioxid. Redox Signal 2014, 20, 2907–2922. [Google Scholar] [CrossRef]
- Kazemi, S.; Mounir, Z.; Baltzis, D.; Raven, J.F.; Wang, S.; Krishnamoorthy, J.L.; Pluquet, O.; Pelletier, J.; Koromilas, A.E. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol. Biol. Cell 2007, 18, 3635–3644. [Google Scholar] [CrossRef]
- Visweswaraiah, J.; Lageix, S.; Castilho, B.A.; Izotova, L.; Kinzy, T.G.; Hinnebusch, A.G.; Sattlegger, E. Evidence that eukaryotic translation elongation factor 1A (eEF1A) binds the Gcn2 protein C terminus and inhibits Gcn2 activity. J. Biol. Chem. 2011, 286, 36568–36579. [Google Scholar] [CrossRef]
- Sattlegger, E.; Swanson, M.J.; Ashcraft, E.A.; Jennings, J.L.; Fekete, R.A.; Link, A.J.; Hinnebusch, A.G. YIH1 is an actin-binding protein that inhibits protein kinase GCN2 and impairs general amino acid control when overexpressed. J. Biol. Chem. 2004, 279, 29952–29962. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.; Sattlegger, E.; Jiang, H.Y.; Longo, B.M.; Jaqueta, C.B.; Hinnebusch, A.G.; Wek, R.C.; Mello, L.E.; Castilho, B.A. IMPACT, a protein preferentially expressed in the mouse brain, binds GCN1 and inhibits GCN2 activation. J. Biol. Chem. 2005, 280, 28316–28323. [Google Scholar] [CrossRef] [PubMed]
- Roffe, M.; Hajj, G.N.; Azevedo, H.F.; Alves, V.S.; Castilho, B.A. IMPACT is a developmentally regulated protein in neurons that opposes the eukaryotic initiation factor 2alpha kinase GCN2 in the modulation of neurite outgrowth. J. Biol. Chem. 2013, 288, 10860–10869. [Google Scholar] [CrossRef]
- Ferraz, R.C.; Camara, H.; De-Souza, E.A.; Pinto, S.; Pinca, A.P.; Silva, R.C.; Sato, V.N.; Castilho, B.A.; Mori, M.A. IMPACT is a GCN2 inhibitor that limits lifespan in Caenorhabditis elegans. BMC Biol. 2016, 14, 87. [Google Scholar] [CrossRef]
- Roobol, A.; Roobol, J.; Bastide, A.; Knight, J.R.; Willis, A.E.; Smales, C.M. p58IPK is an inhibitor of the eIF2alpha kinase GCN2 and its localization and expression underpin protein synthesis and ER processing capacity. Biochem. J. 2015, 465, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Donze, O.; Picard, D. Hsp90 binds and regulates Gcn2, the ligand-inducible kinase of the alpha subunit of eukaryotic translation initiation factor 2 [corrected]. Mol. Cell. Biol. 1999, 19, 8422–8432. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Cherkasova, V.; Qiu, H.; Hinnebusch, A.G. Snf1 promotes phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 by activating Gcn2 and inhibiting phosphatases Glc7 and Sit4. Mol. Cell. Biol. 2010, 30, 2862–2873. [Google Scholar] [CrossRef]
- Hedbacker, K.; Carlson, M. SNF1/AMPK pathways in yeast. Front. Biosci. 2008, 13, 2408–2420. [Google Scholar] [CrossRef]
- Adams, D.R.; Ron, D.; Kiely, P.A. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun. Signal. 2011, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Tarumoto, Y.; Kanoh, J.; Ishikawa, F. Receptor for activated C-kinase (RACK1) homolog Cpc2 facilitates the general amino acid control response through Gcn2 kinase in fission yeast. J. Biol. Chem. 2013, 288, 19260–19268. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Porter, A.C.; Olsen, D.A.; Cavener, D.R.; Wek, R.C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics 2000, 154, 787–801. [Google Scholar] [CrossRef]
- Santoyo, J.; Alcalde, J.; Mendez, R.; Pulido, D.; de Haro, C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2alpha) kinase from Drosophila melanogaster. Homology To yeast GCN2 protein kinase. J. Biol. Chem. 1997, 272, 12544–12550. [Google Scholar] [CrossRef]
- Merrick, W.C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 1992, 56, 291–315. [Google Scholar] [CrossRef]
- Hershey, J.W. Translational control in mammalian cells. Annu. Rev. Biochem. 1991, 60, 717–755. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Chen, S.; Gilks, N.; Li, W.; Miller, I.J.; Stahl, J.; Anderson, P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 2002, 13, 195–210. [Google Scholar] [CrossRef]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar] [CrossRef]
- Averous, J.; Bruhat, A.; Jousse, C.; Carraro, V.; Thiel, G.; Fafournoux, P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J. Biol. Chem. 2004, 279, 15706. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Yue, W.; Bi, Y.; Shen, X.; Gao, J.; Xu, X.; Lu, Z. GCN2 deficiency protects against high fat diet induced hepatic steatosis and insulin resistance in mice. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 3257–3267. [Google Scholar] [CrossRef]
- Kwon, N.H.; Kang, T.; Lee, J.Y.; Kim, H.H.; Kim, H.R.; Hong, J.; Oh, Y.S.; Han, J.M.; Ku, M.J.; Lee, S.Y.; et al. Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3. Proc. Natl. Acad. Sci. USA 2011, 108, 19635–19640. [Google Scholar] [CrossRef]
- Dokladal, L.; Stumpe, M.; Pillet, B.; Hu, Z.; Garcia Osuna, G.M.; Kressler, D.; Dengjel, J.; De Virgilio, C. Global phosphoproteomics pinpoints uncharted Gcn2-mediated mechanisms of translational control. Mol. Cell 2021, 81, 1879–1889.e1876. [Google Scholar] [CrossRef]
- Sequeira, S.J.; Wen, H.C.; Avivar-Valderas, A.; Farias, E.F.; Aguirre-Ghiso, J.A. Inhibition of eIF2alpha dephosphorylation inhibits ErbB2-induced deregulation of mammary acinar morphogenesis. BMC Cell Biol. 2009, 10, 64. [Google Scholar] [CrossRef]
- Lou, J.J.; Chua, Y.L.; Chew, E.H.; Gao, J.; Bushell, M.; Hagen, T. Inhibition of hypoxia-inducible factor-1alpha (HIF-1alpha) protein synthesis by DNA damage inducing agents. PLoS ONE 2010, 5, e10522. [Google Scholar] [CrossRef]
- Damgaard, C.K.; Lykke-Andersen, J. Translational coregulation of 5’TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011, 25, 2057–2068. [Google Scholar] [CrossRef]
- You, S.; Li, H.; Hu, Z.; Zhang, W. eIF2alpha kinases PERK and GCN2 act on FOXO to potentiate FOXO activity. Genes Cells 2018, 23, 786–793. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Tang, Y.; Wang, Y.; Zhang, M.; Yang, Z.; Nyirimigabo, E.; Wei, B.; Lu, Z.; Ji, G. GCN2 deficiency protects mice from denervation-induced skeletal muscle atrophy via inhibiting FoxO3a nuclear translocation. Protein Cell 2018, 9, 966–970. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-gamma in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef]
- Xia, X.J.; Gao, Y.Y.; Zhang, J.; Wang, L.; Zhao, S.; Che, Y.Y.; Ao, C.J.; Yang, H.J.; Wang, J.Q.; Lei, L.C. Autophagy mediated by arginine depletion activation of the nutrient sensor GCN2 contributes to interferon-gamma-induced malignant transformation of primary bovine mammary epithelial cells. Cell Death Discov. 2016, 2, 15065. [Google Scholar] [CrossRef]
- Ren, W.; Li, Y.; Xia, X.; Guo, W.; Zhai, T.; Jin, Y.; Che, Y.; Gao, H.; Duan, X.; Ma, H.; et al. Arginine inhibits the malignant transformation induced by interferon-gamma through the NF-kappaB-GCN2/eIF2alpha signaling pathway in mammary epithelial cells in vitro and in vivo. Exp. Cell Res. 2018, 368, 236–247. [Google Scholar] [CrossRef]
- Caballero-Molada, M.; Planes, M.D.; Benlloch, H.; Atares, S.; Naranjo, M.A.; Serrano, R. The Gcn2-eIF2alpha pathway connects iron and amino acid homeostasis in Saccharomyces cerevisiae. Biochem. J. 2018, 475, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Yerbes, R.; Mora-Molina, R.; Fernandez-Farran, F.J.; Hiraldo, L.; Lopez-Rivas, A.; Palacios, C. Limiting glutamine utilization activates a GCN2/TRAIL-R2/Caspase-8 apoptotic pathway in glutamine-addicted tumor cells. Cell Death Dis. 2022, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Averous, J.; Lambert-Langlais, S.; Cherasse, Y.; Carraro, V.; Parry, L.; B’Chir, W.; Jousse, C.; Maurin, A.C.; Bruhat, A.; Fafournoux, P. Amino acid deprivation regulates the stress-inducible gene p8 via the GCN2/ATF4 pathway. Biochem. Biophys. Res. Commun. 2011, 413, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kimura, H. A new role of GCN2 in the nucleolus. Biochem. Biophys. Res. Commun. 2017, 485, 484–491. [Google Scholar] [CrossRef]
- Zhang, P.; McGrath, B.C.; Reinert, J.; Olsen, D.S.; Lei, L.; Gill, S.; Wek, S.A.; Vattem, K.M.; Wek, R.C.; Kimball, S.R.; et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002, 22, 6681–6688. [Google Scholar] [CrossRef]
- Anthony, T.G.; McDaniel, B.J.; Byerley, R.L.; McGrath, B.C.; Cavener, D.R.; McNurlan, M.A.; Wek, R.C. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 2004, 279, 36553–36561. [Google Scholar] [CrossRef] [PubMed]
- Murguia, J.R.; Serrano, R. New functions of protein kinase Gcn2 in yeast and mammals. IUBMB Life 2012, 64, 971–974. [Google Scholar] [CrossRef]
- Balasubramanian, M.N.; Butterworth, E.A.; Kilberg, M.S. Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E789–E799. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.S.; Motta, F.L.; Roffe, M.; Delamano, A.; Pesquero, J.B.; Castilho, B.A. GCN2 activation and eIF2alpha phosphorylation in the maturation of mouse oocytes. Biochem. Biophys. Res. Commun. 2009, 378, 41–44. [Google Scholar] [CrossRef]
- Hu, Z.; Xia, B.; Postnikoff, S.D.; Shen, Z.J.; Tomoiaga, A.S.; Harkness, T.A.; Seol, J.H.; Li, W.; Chen, K.; Tyler, J.K. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. Elife 2018, 7, e35551. [Google Scholar] [CrossRef] [PubMed]
- Cordova, R.A.; Misra, J.; Amin, P.H.; Klunk, A.J.; Damayanti, N.P.; Carlson, K.R.; Elmendorf, A.J.; Kim, H.G.; Mirek, E.T.; Elzey, B.D.; et al. GCN2 eIF2 kinase promotes prostate cancer by maintaining amino acid homeostasis. Elife 2022, 11, e81083. [Google Scholar] [CrossRef]
- Missiaen, R.; Anderson, N.M.; Kim, L.C.; Nance, B.; Burrows, M.; Skuli, N.; Carens, M.; Riscal, R.; Steensels, A.; Li, F.; et al. GCN2 inhibition sensitizes arginine-deprived hepatocellular carcinoma cells to senolytic treatment. Cell Metab. 2022, 34, 1151–1167.e1157. [Google Scholar] [CrossRef]
- Li, C.; Wu, B.; Li, Y.; Chen, J.; Ye, Z.; Tian, X.; Wang, J.; Xu, X.; Pan, S.; Zheng, Y.; et al. Amino acid catabolism regulates hematopoietic stem cell proteostasis via a GCN2-eIF2alpha axis. Cell Stem Cell 2022, 29, 1119–1134.e7. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Crespo, M.; Filippidis, G.; Antoniadis, N.; Liakopoulos, V.; Stefanidis, I. The effect of anti-HLA class I antibodies on the immunological properties of human glomerular endothelial cells and their modification by mTOR inhibition or GCN2 kinase activation. Mol. Med. Rep. 2021, 23, 355. [Google Scholar] [CrossRef]
- Arriazu, E.; Perez de Obanos, M.P.; Lopez-Zabalza, M.J.; Herraiz, M.T.; Iraburu, M.J. Amino acid deprivation decreases intracellular levels of reactive oxygen species in hepatic stellate cells. Cell. Physiol. Biochem. 2010, 26, 281–290. [Google Scholar] [CrossRef]
- Miles, R.R.; Amin, P.H.; Diaz, M.B.; Misra, J.; Aukerman, E.; Das, A.; Ghosh, N.; Guith, T.; Knierman, M.D.; Roy, S.; et al. The eIF2 kinase GCN2 directs keratinocyte collective cell migration during wound healing via coordination of reactive oxygen species and amino acids. J. Biol. Chem. 2021, 297, 101257. [Google Scholar] [CrossRef]
- Chaveroux, C.; Lambert-Langlais, S.; Parry, L.; Carraro, V.; Jousse, C.; Maurin, A.C.; Bruhat, A.; Marceau, G.; Sapin, V.; Averous, J.; et al. Identification of GCN2 as new redox regulator for oxidative stress prevention in vivo. Biochem. Biophys. Res. Commun. 2011, 415, 120–124. [Google Scholar] [CrossRef]
- Sahu, D.; Gupta, S.; Hau, A.M.; Nakashima, K.; Leivo, M.Z.; Searles, S.C.; Elson, P.; Bomalaski, J.S.; Casteel, D.E.; Boss, G.R.; et al. Argininosuccinate Synthetase 1 Loss in Invasive Bladder Cancer Regulates Survival through General Control Nonderepressible 2 Kinase-Mediated Eukaryotic Initiation Factor 2alpha Activity and Is Targetable by Pegylated Arginine Deiminase. Am. J. Pathol. 2016, 18, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, R.; Nagy, G.; Dotu, I.; Chuang, J.H.; Ackerman, S.L. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. Elife 2016, 5, e14295. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.; Yuan, J.; Wu, Y.; Shen, X.; Gao, J.; Feng, W.; Lu, Z. GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 2018, 17, 25–34. [Google Scholar] [CrossRef]
- Collier, A.E.; Wek, R.C.; Spandau, D.F. Human Keratinocyte Differentiation Requires Translational Control by the eIF2alpha Kinase GCN2. J. Invest. Dermatol. 2017, 137, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Vasudevan, D.; Kang, K.; Kim, K.; Park, J.E.; Zhang, N.; Zeng, X.; Neubert, T.A.; Marr, M.T., 2nd; Ryoo, H.D. 4E-BP is a target of the GCN2-ATF4 pathway during Drosophila development and aging. J. Cell Biol. 2017, 216, 115–129. [Google Scholar] [CrossRef]
- Zid, B.M.; Rogers, A.N.; Katewa, S.D.; Vargas, M.A.; Kolipinski, M.C.; Lu, T.A.; Benzer, S.; Kapahi, P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 2009, 139, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef]
- Wei, C.; Lin, M.; Jinjun, B.; Su, F.; Dan, C.; Yan, C.; Jie, Y.; Jin, Z.; Zi-Chun, H.; Wu, Y. Involvement of general control nonderepressible kinase 2 in cancer cell apoptosis by posttranslational mechanisms. Mol. Biol. Cell 2015, 26, 1044–1057. [Google Scholar] [CrossRef]
- Krohn, M.; Skjolberg, H.C.; Soltani, H.; Grallert, B.; Boye, E. The G1-S checkpoint in fission yeast is not a general DNA damage checkpoint. J. Cell Sci. 2008, 121, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Berlanga, J.J.; de Haro, C. New roles of the fission yeast eIF2alpha kinases Hri1 and Gcn2 in response to nutritional stress. J. Cell Sci. 2013, 126, 3010–3020. [Google Scholar] [PubMed]
- Hamanaka, R.B.; Bennett, B.S.; Cullinan, S.B.; Diehl, J.A. PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol. Biol. Cell 2005, 16, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.L.; Cerniglia, G.J.; Johannes, G.J.; Ye, J.; Ryeom, S.; Koumenis, C. Translational Upregulation of an Individual p21Cip1 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress. PLoS Genet. 2015, 11, e1005212. [Google Scholar] [CrossRef]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef]
- Fougeray, S.; Mami, I.; Bertho, G.; Beaune, P.; Thervet, E.; Pallet, N. Tryptophan depletion and the kinase GCN2 mediate IFN-gamma-induced autophagy. J. Immunol. 2012, 189, 2954–2964. [Google Scholar] [CrossRef]
- Xia, X.; Che, Y.; Gao, Y.; Zhao, S.; Ao, C.; Yang, H.; Liu, J.; Liu, G.; Han, W.; Wang, Y.; et al. Arginine Supplementation Recovered the IFN-gamma-Mediated Decrease in Milk Protein and Fat Synthesis by Inhibiting the GCN2/eIF2alpha Pathway, Which Induces Autophagy in Primary Bovine Mammary Epithelial Cells. Mol. Cells 2016, 39, 410–417. [Google Scholar]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How autophagy controls the intestinal epithelial barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Ravindran, R.; Loebbermann, J.; Nakaya, H.I.; Khan, N.; Ma, H.; Gama, L.; Machiah, D.K.; Lawson, B.; Hakimpour, P.; Wang, Y.C.; et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 2016, 531, 523–527. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Averous, J.; Lambert-Langlais, S.; Mesclon, F.; Carraro, V.; Parry, L.; Jousse, C.; Bruhat, A.; Maurin, A.C.; Pierre, P.; Proud, C.G.; et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep. 2016, 6, 27698. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Palm, W.; Peng, M.; King, B.; Lindsten, T.; Li, M.O.; Koumenis, C.; Thompson, C.B. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015, 29, 2331–2336. [Google Scholar] [CrossRef]
- Wengrod, J.; Wang, D.; Weiss, S.; Zhong, H.; Osman, I.; Gardner, L.B. Phosphorylation of eIF2alpha triggered by mTORC1 inhibition and PP6C activation is required for autophagy and is aberrant in PP6C-mutated melanoma. Sci. Signal. 2015, 8, ra27. [Google Scholar] [CrossRef]
- Jin, H.O.; Hong, S.E.; Kim, J.Y.; Jang, S.K.; Park, I.C. Amino acid deprivation induces AKT activation by inducing GCN2/ATF4/REDD1 axis. Cell Death Dis. 2021, 12, 1127. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Indoleamine 2,3-dioxygenase depletes tryptophan, activates general control non-derepressible 2 kinase and down-regulates key enzymes involved in fatty acid synthesis in primary human CD4+ T cells. Immunology 2015, 146, 292–300. [Google Scholar] [CrossRef]
- Ye, J.; Mancuso, A.; Tong, X.; Ward, P.S.; Fan, J.; Rabinowitz, J.D.; Thompson, C.B. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2012, 109, 6904–6909. [Google Scholar] [CrossRef]
- Xu, X.; Hu, J.; McGrath, B.C.; Cavener, D.R. GCN2 regulates the CCAAT enhancer binding protein beta and hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1007–E1017. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Clark, N.K.; Sam, J.; Cotham, V.C.; Ueberheide, B.; Marr, M.T., 2nd; Ryoo, H.D. The GCN2-ATF4 Signaling Pathway Induces 4E-BP to Bias Translation and Boost Antimicrobial Peptide Synthesis in Response to Bacterial Infection. Cell Rep. 2017, 21, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Sorbara, M.T.; Vuckovic, D.; Ling, A.; Soares, F.; Carneiro, L.A.; Yang, C.; Emili, A.; Philpott, D.J.; Girardin, S.E. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 2012, 11, 563–575. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Khan, N.; Nakaya, H.I.; Li, S.; Loebbermann, J.; Maddur, M.S.; Park, Y.; Jones, D.P.; Chappert, P.; Davoust, J.; et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 2014, 343, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Mondal, A.; DuHadaway, J.B.; Sutanto-Ward, E.; Laury-Kleintop, L.D.; Thomas, S.; Prendergast, G.C.; Mandik-Nayak, L.; Muller, A.J. IDO1 Signaling through GCN2 in a Subpopulation of Gr-1(+) Cells Shifts the IFNgamma/IL6 Balance to Promote Neovascularization. Cancer Immunol. Res. 2021, 9, 514–528. [Google Scholar] [CrossRef]
- Jackson, J.J.; Shibuya, G.M.; Ravishankar, B.; Adusumilli, L.; Bradford, D.; Brockstedt, D.G.; Bucher, C.; Bui, M.; Cho, C.; Colas, C.; et al. Potent GCN2 Inhibitor Capable of Reversing MDSC-Driven T Cell Suppression Demonstrates In Vivo Efficacy as a Single Agent and in Combination with Anti-Angiogenesis Therapy. J. Med. Chem. 2022, 65, 12895–12924. [Google Scholar] [CrossRef]
- Tomé, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Nutr. Diabetes 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, L.; Bradley, J.; Liu, K.; Bardhan, K.; Ron, D.; Mellor, A.L.; Munn, D.H.; McGaha, T.L. GCN2-dependent metabolic stress is essential for endotoxemic cytokine induction and pathology. Mol. Cell. Biol. 2014, 34, 428–438. [Google Scholar] [CrossRef]
- Arriazu, E.; Ruiz de Galarreta, M.; Lopez-Zabalza, M.J.; Leung, T.M.; Nieto, N.; Iraburu, M.J. GCN2 kinase is a key regulator of fibrogenesis and acute and chronic liver injury induced by carbon tetrachloride in mice. Lab. Investig. 2013, 93, 303–310. [Google Scholar] [CrossRef][Green Version]
- Colonna, L.; Lood, C.; Elkon, K.B. Beyond apoptosis in lupus. Curr. Opin. Rheumatol. 2014, 26, 459–466. [Google Scholar] [CrossRef]
- Ravishankar, B.; Liu, H.; Shinde, R.; Chandler, P.; Baban, B.; Tanaka, M.; Munn, D.H.; Mellor, A.L.; Karlsson, M.C.; McGaha, T.L. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 3909–3914. [Google Scholar] [CrossRef]
- Ravishankar, B.; Liu, H.; Shinde, R.; Chaudhary, K.; Xiao, W.; Bradley, J.; Koritzinsky, M.; Madaio, M.P.; McGaha, T.L. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc. Natl. Acad. Sci. USA 2015, 112, 10774–10779. [Google Scholar] [CrossRef] [PubMed]
- Toboz, P.; Amiri, M.; Tabatabaei, N.; Dufour, C.R.; Kim, S.H.; Fillebeen, C.; Ayemoba, C.E.; Khoutorsky, A.; Nairz, M.; Shao, L.; et al. The amino acid sensor GCN2 controls red blood cell clearance and iron metabolism through regulation of liver macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2121251119. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, F.; Du, L.; Niu, Y.; Yin, H.; Zhou, Z.; Jiang, X.; Jiang, H.; Yuan, F.; Liu, K.; et al. Activation of GCN2 in macrophages promotes white adipose tissue browning and lipolysis under leucine deprivation. FASEB J. 2021, 35, e21652. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Tsogka, K.; Sounidaki, M.; Liakopoulos, V.; Stefanidis, I. Indoleamine 2,3-dioxygenase downregulates T-cell receptor complex zetachain and cMyc, and reduces proliferation, lactate dehydrogenase levels and mitochondrial glutaminase in human Tcells. Mol. Med. Rep. 2016, 13, 925–932. [Google Scholar] [CrossRef][Green Version]
- Eleftheriadis, T.; Pissas, G.; Yiannaki, E.; Markala, D.; Arampatzis, S.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive T-cells. Hum. Immunol. 2013, 74, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Sonner, J.K.; Deumelandt, K.; Ott, M.; Thome, C.M.; Rauschenbach, K.J.; Schulz, S.; Munteanu, B.; Mohapatra, S.; Adam, I.; Hofer, A.C.; et al. The stress kinase GCN2 does not mediate suppression of antitumor T cell responses by tryptophan catabolism in experimental melanomas. Oncoimmunology 2016, 5, e1240858. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.; Ron, D.; Mellor, A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Orsini, H.; Araujo, L.P.; Maricato, J.T.; Guereschi, M.G.; Mariano, M.; Castilho, B.A.; Basso, A.S. GCN2 kinase plays an important role triggering the remission phase of experimental autoimmune encephalomyelitis (EAE) in mice. Brain Behav. Immun. 2014, 37, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Keil, M.; Sonner, J.K.; Lanz, T.V.; Oezen, I.; Bunse, T.; Bittner, S.; Meyer, H.V.; Meuth, S.G.; Wick, W.; Platten, M. General control non-derepressible 2 (GCN2) in T cells controls disease progression of autoimmune neuroinflammation. J. Neuroimmunol. 2016, 297, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, Y.; Zhang, J.; Shi, L.; Lei, T.; Hou, Y.; Lu, Z.; Zhao, Y. The amino acid sensor general control nonderepressible 2 (GCN2) controls TH9 cells and allergic airway inflammation. J. Allergy Clin. Immunol. 2019, 144, 1091–1105. [Google Scholar] [CrossRef]
- Sundrud, M.S.; Koralov, S.B.; Feuerer, M.; Calado, D.P.; Kozhaya, A.E.; Rhule-Smith, A.; Lefebvre, R.E.; Unutmaz, D.; Mazitschek, R.; Waldner, H.; et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 2009, 324, 1334–1338. [Google Scholar] [CrossRef]
- Domblides, C.; Lartigue, L.; Faustin, B. Control of the Antitumor Immune Response by Cancer Metabolism. Cells 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef]
- Carlson, T.J.; Pellerin, A.; Djuretic, I.M.; Trivigno, C.; Koralov, S.B.; Rao, A.; Sundrud, M.S. Halofuginone-induced amino acid starvation regulates Stat3-dependent Th17 effector function and reduces established autoimmune inflammation. J. Immunol. 2014, 192, 2167–2176. [Google Scholar] [CrossRef]
- Rashidi, A.; Miska, J.; Lee-Chang, C.; Kanojia, D.; Panek, W.K.; Lopez-Rosas, A.; Zhang, P.; Han, Y.; Xiao, T.; Pituch, K.C.; et al. GCN2 is essential for CD8(+) T cell survival and function in murine models of malignant glioma. Cancer Immunol. Immunother. 2020, 69, 81–94. [Google Scholar] [CrossRef]
- Van de Velde, L.A.; Guo, X.J.; Barbaric, L.; Smith, A.M.; Oguin, T.H., 3rd; Thomas, P.G.; Murray, P.J. Stress Kinase GCN2 Controls the Proliferative Fitness and Trafficking of Cytotoxic T Cells Independent of Environmental Amino Acid Sensing. Cell Rep. 2016, 17, 2247–2258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Guo, H.; Hou, Y.; Lei, T.; Wei, D.; Zhao, Y. Multiple Roles of the Stress Sensor GCN2 in Immune Cells. Int. J. Mol. Sci. 2023, 24, 4285. https://doi.org/10.3390/ijms24054285
Zhao C, Guo H, Hou Y, Lei T, Wei D, Zhao Y. Multiple Roles of the Stress Sensor GCN2 in Immune Cells. International Journal of Molecular Sciences. 2023; 24(5):4285. https://doi.org/10.3390/ijms24054285
Chicago/Turabian StyleZhao, Chenxu, Han Guo, Yangxiao Hou, Tong Lei, Dong Wei, and Yong Zhao. 2023. "Multiple Roles of the Stress Sensor GCN2 in Immune Cells" International Journal of Molecular Sciences 24, no. 5: 4285. https://doi.org/10.3390/ijms24054285
APA StyleZhao, C., Guo, H., Hou, Y., Lei, T., Wei, D., & Zhao, Y. (2023). Multiple Roles of the Stress Sensor GCN2 in Immune Cells. International Journal of Molecular Sciences, 24(5), 4285. https://doi.org/10.3390/ijms24054285






