The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds
Abstract
1. Introduction
2. Normal Wound Healing
2.1. Coagulation
2.2. Inflammation
2.3. Proliferation
2.4. Remodeling
3. Functional Impairments of Keratinocytes in Chronic Diabetic Wounds
3.1. Increased Oxidative Stress in Keratinocytes
3.2. Abnormal Expression of Matrix Metalloproteinases (MMPs)
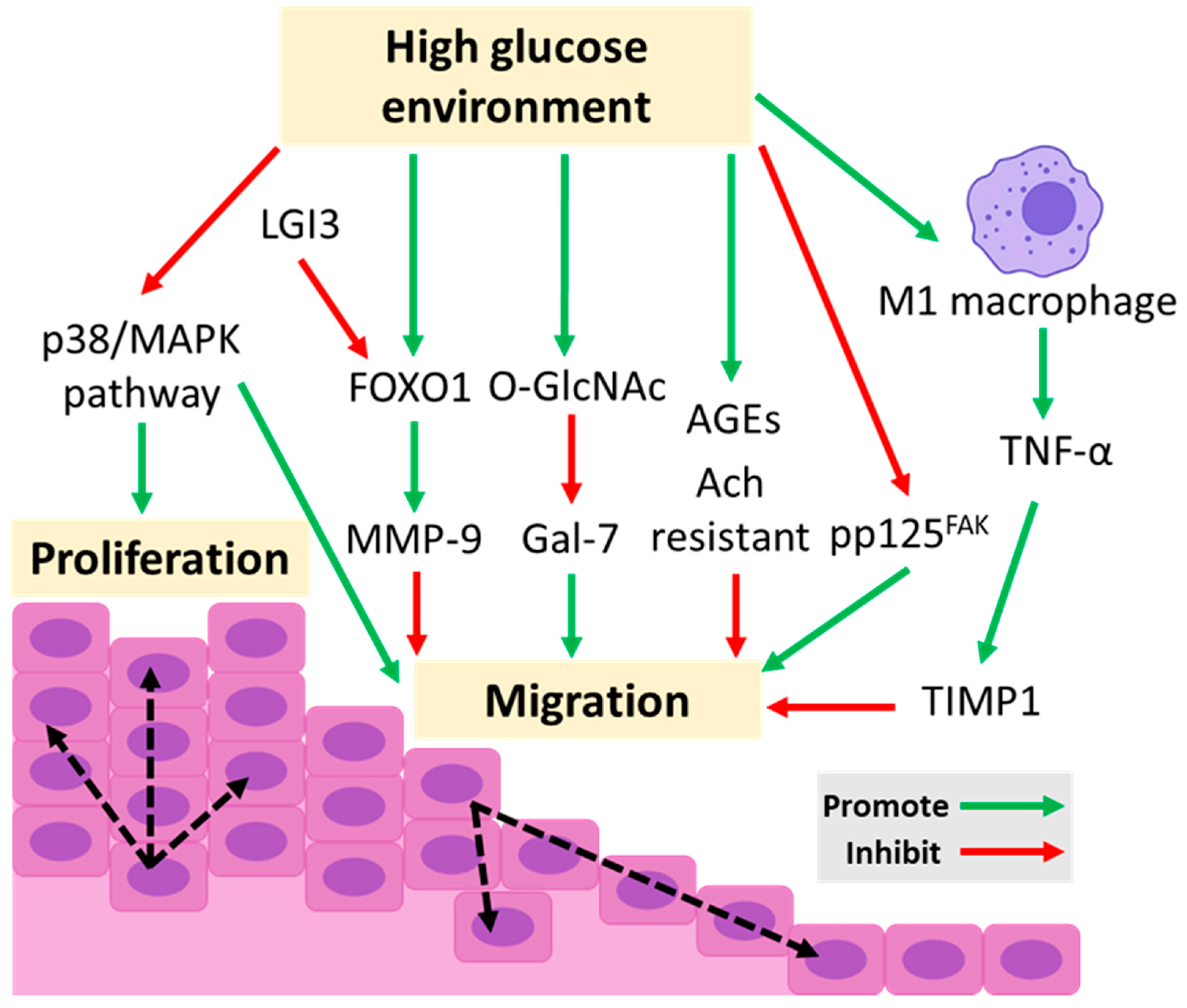
3.3. Impaired Proliferation and Migration of Keratinocyte
3.4. Chronic Inflammation
3.5. Chronic Infection
3.6. Impaired Angiogenesis
4. Novel Therapeutic Strategies for the Treatment of Diabetic Wounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Robbins, J.M.; Strauss, G.; Aron, D.; Long, J.; Kuba, J.; Kaplan, Y. Mortality rates and diabetic foot ulcers: Is it time to communicate mortality risk to patients with diabetic foot ulceration? J. Am. Podiatr. Med. Assoc. 2008, 98, 489–493. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Tomic-Canic, M. Role of keratinocytes in healing of chronic wounds. Surg. Technol. Int. 2008, 17, 105–112. [Google Scholar]
- Wang, Y.; Graves, D.T. Keratinocyte Function in Normal and Diabetic Wounds and Modulation by FOXO1. J. Diabetes Res. 2020, 2020, 3714704. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Xue, M.; Le, N.T.; Jackson, C.J. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin. Ther. Targets 2006, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Maheshwari, A.; Chandra, A. Biomarkers for wound healing and their evaluation. J. Wound Care 2016, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Dovi, J.V.; Szpaderska, A.M.; DiPietro, L.A. Neutrophil function in the healing wound: Adding insult to injury? Thromb. Haemost. 2004, 92, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Moon, M.L.; McNeil, L.K.; Freund, G.G. Macrophages make me sick: How macrophage activation states influence sickness behavior. Psychoneuroendocrinology 2011, 36, 1431–1440. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Burgess, M.; Wicks, K.; Gardasevic, M.; Mace, K.A. Cx3CR1 Expression Identifies Distinct Macrophage Populations That Contribute Differentially to Inflammation and Repair. Immunohorizons 2019, 3, 262–273. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef]
- Pang, J.; Maienschein-Cline, M.; Koh, T.J. Monocyte/Macrophage Heterogeneity during Skin Wound Healing in Mice. J. Immunol. 2022, 209, 1999–2011. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Martins-Green, M. The Yin and Yang of Integrin Function in Re-Epithelialization During Wound Healing. Adv. Wound Care 2013, 2, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Szekeres, C.; Milano, V.; Svenson, K.B.; Nilsen-Hamilton, M.; Kreidberg, J.A.; DiPersio, C.M. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J. Cell Sci. 2009, 122, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Liu, J.; Liu, G.; Zhou, W.; Liu, Y.; Hu, L.; Xiong, L.; Ye, S.; Wu, Y. Icariin promotes wound healing by enhancing the migration and proliferation of keratinocytes via the AKT and ERK signaling pathway. Int. J. Mol. Med. 2018, 42, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating kerationcyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D. Chronic wound fluid--thinking outside the box. Wound Repair Regen. 2011, 19, 287–291. [Google Scholar] [CrossRef]
- Hiraoka, C.; Toki, F.; Shiraishi, K.; Sayama, K.; Nishimura, E.K.; Miura, H.; Higashiyama, S.; Nanba, D. Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J. Dermatol. Sci. 2016, 82, 84–94. [Google Scholar] [CrossRef]
- Sklenářová, R.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. [Google Scholar] [CrossRef]
- Ham, S.A.; Hwang, J.S.; Yoo, T.; Lee, W.J.; Paek, K.S.; Oh, J.W.; Park, C.K.; Kim, J.H.; Do, J.T.; Kim, J.H.; et al. Ligand-activated PPARδ upregulates α-smooth muscle actin expression in human dermal fibroblasts: A potential role for PPARδ in wound healing. J. Dermatol. Sci. 2015, 80, 186–195. [Google Scholar] [CrossRef]
- Shephard, P.; Martin, G.; Smola-Hess, S.; Brunner, G.; Krieg, T.; Smola, H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am. J. Pathol. 2004, 164, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Chen, X.F.; Tang, W.; Lin, W.D.; Liu, Z.Y.; Lu, X.X.; Zhang, B.; Ye, F.; Liu, Z.M.; Zou, J.J.; Liao, W.Q. Receptor for advanced glycation end as drug targets in diabetes-induced skin lesion. Am. J. Transl. Res. 2017, 9, 330–342. [Google Scholar]
- Widlansky, M.E.; Wang, J.; Shenouda, S.M.; Hagen, T.M.; Smith, A.R.; Kizhakekuttu, T.J.; Kluge, M.A.; Weihrauch, D.; Gutterman, D.D.; Vita, J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010, 156, 15–25. [Google Scholar] [CrossRef]
- Siqueira, M.F.; Li, J.; Chehab, L.; Desta, T.; Chino, T.; Krothpali, N.; Behl, Y.; Alikhani, M.; Yang, J.; Braasch, C.; et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia 2010, 53, 378–388. [Google Scholar] [CrossRef]
- Kim, J.H.; Ruegger, P.R.; Lebig, E.G.; VanSchalkwyk, S.; Jeske, D.R.; Hsiao, A.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation. Front. Cell. Infect. Microbiol. 2020, 10, 259. [Google Scholar] [CrossRef]
- Lan, C.C.; Wu, C.S.; Huang, S.M.; Wu, I.H.; Chen, G.S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the Study of Skin Wound Healing. The Role of Nrf2 and NF-κB; Biomedical Papers of the Medical Faculty of Palacky University in Olomouc; Palacky University in Olomouc: Olomouc, Czech Republic, 2017; Volume 161, pp. 1–13. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef]
- Lan, C.C.; Liu, I.H.; Fang, A.H.; Wen, C.H.; Wu, C.S. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br. J. Dermatol. 2008, 159, 1103–1115. [Google Scholar] [CrossRef]
- Lan, C.C.; Wu, C.S.; Kuo, H.Y.; Huang, S.M.; Chen, G.S. Hyperglycaemic conditions hamper keratinocyte locomotion via sequential inhibition of distinct pathways: New insights on poor wound closure in patients with diabetes. Br. J. Dermatol. 2009, 160, 1206–1214. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.S.; Chao, D.; Wu, C.H.; Li, C.C.; Chen, G.S.; Lan, C.C. High-glucose-cultivated peripheral blood mononuclear cells impaired keratinocyte function via reduced IL-22 expression: Implications on impaired diabetic wound healing. Exp. Dermatol. 2015, 24, 639–641. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Song, Z.Q.; Wang, R.X.; Yu, D.M.; Wang, P.H.; Lu, S.L.; Tian, M.; Xie, T.; Huang, F.; Yang, G.Z. [Impact of advanced glycosylation end products-modified human serum albumin on migration of epidermal keratinocytes: An in vitro experiment]. Zhonghua Yi Xue Za Zhi 2008, 88, 2690–2694. [Google Scholar]
- Huang, S.M.; Wu, C.S.; Chiu, M.H.; Yang, H.J.; Chen, G.S.; Lan, C.E. High-glucose environment induced intracellular O-GlcNAc glycosylation and reduced galectin-7 expression in keratinocytes: Implications on impaired diabetic wound healing. J. Dermatol. Sci. 2017, 87, 168–175. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A.; van Koningsveld, R.; Chen, M.; Woodley, D.T. Hypoxia induces epidermal keratinocyte matrix metalloproteinase-9 secretion via the protein kinase C pathway. J. Cell. Physiol. 2008, 214, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T.; Fan, J.; Cheng, C.F.; Li, Y.; Chen, M.; Bu, G.; Li, W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration. J. Cell. Sci. 2009, 122, 1495–1498. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, X.; Xu, X.; Teng, M.; Huang, C.; Zhang, D.; Zhang, Q.; Zhang, J.; Huang, Y. Hypoxia regulates CD9-mediated keratinocyte migration via the P38/MAPK pathway. Sci. Rep. 2014, 4, 6304. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jiang, X.; Ren, X.; Sun, H.; Zhang, D.; Zhang, Q.; Zhang, J.; Huang, Y. The Galvanotactic Migration of Keratinocytes is Enhanced by Hypoxic Preconditioning. Sci. Rep. 2015, 5, 10289. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Gomes, L.R.; Vessoni, A.T.; Menck, C.F.M. Microenvironment and autophagy cross-talk: Implications in cancer therapy. Pharmacol. Res. 2016, 107, 300–307. [Google Scholar] [CrossRef]
- Rybstein, M.D.; Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. The autophagic network and cancer. Nat. Cell Biol. 2018, 20, 243–251. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Matsuyama, A.; Osawa, M.; Hayakawa, T. BNIP3 plays crucial roles in the differentiation and maintenance of epidermal keratinocytes. J. Investig. Dermatol. 2014, 134, 1627–1635. [Google Scholar] [CrossRef]
- Akinduro, O.; Sully, K.; Patel, A.; Robinson, D.J.; Chikh, A.; McPhail, G.; Braun, K.M.; Philpott, M.P.; Harwood, C.A.; Byrne, C.; et al. Constitutive Autophagy and Nucleophagy during Epidermal Differentiation. J. Investig. Dermatol. 2016, 136, 1460–1470. [Google Scholar] [CrossRef]
- Gosselin, K.; Deruy, E.; Martien, S.; Vercamer, C.; Bouali, F.; Dujardin, T.; Slomianny, C.; Houel-Renault, L.; Chelli, F.; De Launoit, Y.; et al. Senescent keratinocytes die by autophagic programmed cell death. Am. J. Pathol. 2009, 174, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Deruy, E.; Nassour, J.; Martin, N.; Vercamer, C.; Malaquin, N.; Bertout, J.; Chelli, F.; Pourtier, A.; Pluquet, O.; Abbadie, C. Level of macroautophagy drives senescent keratinocytes into cell death or neoplastic evasion. Cell Death Dis. 2014, 5, e1577. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.N.; Mowers, E.E.; Drake, L.E.; Collier, C.; Chen, H.; Zamora, M.; Mui, S.; Macleod, K.F. Autophagy Promotes Focal Adhesion Disassembly and Cell Motility of Metastatic Tumor Cells through the Direct Interaction of Paxillin with LC3. Cell Rep. 2016, 15, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jiao, L.; Wang, Y.; Yu, Y.; Ming, L. SIRT1 induces epithelial-mesenchymal transition by promoting autophagic degradation of E-cadherin in melanoma cells. Cell Death Dis. 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Zhang, Q.; Zhang, D.; Xiang, F.; Jia, J.; Wei, P.; Zhang, J.; Hu, J.; Huang, Y. High Glucose Suppresses Keratinocyte Migration Through the Inhibition of p38 MAPK/Autophagy Pathway. Front. Physiol. 2019, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Wu, C.S.; Chiu, M.H.; Wu, C.H.; Chang, Y.T.; Chen, G.S.; Lan, C.E. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019, 96, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.Y.; Kim, I.W.; Yun, H.Y.; Kim, D.S. LGI3 promotes human keratinocyte migration in high-glucose environments by increasing the expression of β-catenin. Pharmazie 2022, 77, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Jeong, H.S.; Kwon, N.S.; Baek, K.J.; Yun, H.Y.; Kim, D.S. LGI3 promotes human keratinocyte differentiation via the Akt pathway. Exp. Dermatol. 2018, 27, 1224–1229. [Google Scholar] [CrossRef]
- Singkhorn, S.; Tantisira, M.H.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother. Res. 2018, 32, 1397–1403. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Park, W.J.; Kim, M.K.; Baek, K.J.; Kwon, N.S.; Yun, H.Y.; Kim, D.S. Leucine-rich glioma inactivated 3 promotes HaCaT keratinocyte migration. Wound Repair Regen. 2013, 21, 634–640. [Google Scholar] [CrossRef]
- Sakai, S.; Endo, Y.; Ozawa, N.; Sugawara, T.; Kusaka, A.; Sayo, T.; Tagami, H.; Inoue, S. Characteristics of the epidermis and stratum corneum of hairless mice with experimentally induced diabetes mellitus. J. Investig. Dermatol. 2003, 120, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Breeden, M.; Hübner, G.; Greenhalgh, D.G.; Longaker, M.T. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J. Investig. Dermatol. 1994, 103, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Galkowska, H.; Olszewsk, W.L.; Wojewodzka, U.; Mijal, J.; Filipiuk, E. Expression of apoptosis- and cell cycle-related proteins in epidermis of venous leg and diabetic foot ulcers. Surgery 2003, 134, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Goren, I.; Linke, A.; Müller, E.; Pfeilschifter, J.; Frank, S. The suppressor of cytokine signaling-3 is upregulated in impaired skin repair: Implications for keratinocyte proliferation. J. Investig. Dermatol. 2006, 126, 477–485. [Google Scholar] [CrossRef]
- Becker, D.L.; Thrasivoulou, C.; Phillips, A.R. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta 2012, 1818, 2068–2075. [Google Scholar] [CrossRef]
- Sutcliffe, J.E.; Chin, K.Y.; Thrasivoulou, C.; Serena, T.E.; O’Neil, S.; Hu, R.; White, A.M.; Madden, L.; Richards, T.; Phillips, A.R.; et al. Abnormal connexin expression in human chronic wounds. Br. J. Dermatol. 2015, 173, 1205–1215. [Google Scholar] [CrossRef]
- Cogliati, B.; Vinken, M.; Silva, T.C.; Araújo, C.M.M.; Aloia, T.P.A.; Chaible, L.M.; Mori, C.M.C.; Dagli, M.L.Z. Connexin 43 deficiency accelerates skin wound healing and extracellular matrix remodeling in mice. J. Dermatol. Sci. 2015, 79, 50–56. [Google Scholar] [CrossRef]
- Hana, A.; Booken, D.; Henrich, C.; Gratchev, A.; Maas-Szabowski, N.; Goerdt, S.; Kurzen, H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007, 80, 2214–2220. [Google Scholar] [CrossRef]
- Kurzen, H.; Wessler, I.; Kirkpatrick, C.J.; Kawashima, K.; Grando, S.A. The non-neuronal cholinergic system of human skin. Horm. Metab. Res. 2007, 39, 125–135. [Google Scholar] [CrossRef]
- Tan, M.W.Y.; Tan, W.R.; Kong, Z.Q.; Toh, J.H.; Wee, W.K.J.; Teo, E.M.L.; Cheng, H.S.; Wang, X.; Tan, N.S. High Glucose Restraint of Acetylcholine-Induced Keratinocyte Epithelial-Mesenchymal Transition Is Mitigated by p38 Inhibition. J. Investig. Dermatol. 2021, 141, 1438–1449.e1439. [Google Scholar] [CrossRef]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Hyperglycemia Induces Toll-Like Receptor Activity Through Increased Oxidative Stress. Metab. Syndr. Relat. Disord. 2016, 14, 239–241. [Google Scholar] [CrossRef]
- Cheng, T.L.; Lai, C.H.; Chen, P.K.; Cho, C.F.; Hsu, Y.Y.; Wang, K.C.; Lin, W.L.; Chang, B.I.; Liu, S.K.; Wu, Y.T.; et al. Thrombomodulin promotes diabetic wound healing by regulating toll-like receptor 4 expression. J. Investig. Dermatol. 2015, 135, 1668–1675. [Google Scholar] [CrossRef]
- Wang, W.; Yan, X.; Lin, Y.; Ge, H.; Tan, Q. Wnt7a promotes wound healing by regulation of angiogenesis and inflammation: Issues on diabetes and obesity. J. Dermatol. Sci. 2018, 91, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Yan, X.; Tan, Q. Wnt7a regulates high autophagic and inflammatory response of epidermis in high-glucose environment. Burns 2020, 46, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, H.; Schmidt, R.; Geisslinger, G.; Pfeilschifter, J.; Frank, S. Wound inflammation in diabetic ob/ob mice: Functional coupling of prostaglandin biosynthesis to cyclooxygenase-1 activity in diabetes-impaired wound healing. Diabetes 2005, 54, 1543–1551. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Petherick, K.J.; Williams, A.C.; Lane, J.D.; Ordóñez-Morán, P.; Huelsken, J.; Collard, T.J.; Smartt, H.J.; Batson, J.; Malik, K.; Paraskeva, C.; et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013, 32, 1903–1916. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A.; Hong, S.J. Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J. Am. Coll. Surg. 2012, 215, 388–399. [Google Scholar] [CrossRef]
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe 2019, 25, 641–655.e5. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Xu, F.; Dong, G.; Li, S.; Tian, C.; Ponugoti, B.; Graves, D.T. Effect of bacteria on the wound healing behavior of oral epithelial cells. PLoS ONE 2014, 9, e89475. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Wu, C.S.; Huang, S.M.; Kuo, H.Y.; Wu, I.H.; Liang, C.W.; Chen, G.S. High-glucose environment reduces human β-defensin-2 expression in human keratinocytes: Implications for poor diabetic wound healing. Br. J. Dermatol. 2012, 166, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Wu, C.S.; Huang, S.M.; Kuo, H.Y.; Wu, I.H.; Wen, C.H.; Chai, C.Y.; Fang, A.H.; Chen, G.S. High-Glucose Environment Inhibits p38MAPK Signaling and Reduces Human β-Defensin-3 Expression [corrected] in Keratinocytes. Mol. Med. 2011, 17, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Donnarumma, G.; Paoletti, I.; Longanesi-Cattani, I.; Bifulco, K.; Tufano, M.A.; Carriero, M.V. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 2009, 30, 267–272. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.H.; Jung, M.; Chung, C.H.; Hasham, R.; Park, C.S.; Choi, E.H. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 2011, 20, 969–974. [Google Scholar] [CrossRef]
- Cruz Díaz, L.A.; Flores Miramontes, M.G.; Chávez Hurtado, P.; Allen, K.; Gonzalez Ávila, M.; Prado Montes de Oca, E. Ascorbic acid, ultraviolet C rays, and glucose but not hyperthermia are elicitors of human β-defensin 1 mRNA in normal keratinocytes. Biomed Res. Int. 2015, 2015, 714580. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Stephens, E.H.; Nguyen, T.C.; Blazejewski, J.G.; Vekilov, D.P.; Connell, J.P.; Itoh, A.; Ingels, N.B., Jr.; Miller, D.C.; Grande-Allen, K.J. Extracellular matrix remodeling in wound healing of critical size defects in the mitral valve leaflet. Heart Vessels 2016, 31, 1186–1195. [Google Scholar] [CrossRef]
- Tsuji, T.; Sun, Y.; Kishimoto, K.; Olson, K.A.; Liu, S.; Hirukawa, S.; Hu, G.F. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005, 65, 1352–1360. [Google Scholar] [CrossRef]
- Takzaree, N.; Hadjiakhondi, A.; Hassanzadeh, G.; Rouini, M.R.; Manayi, A.; Zolbin, M.M. Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of aloe vera gel. Can. J. Physiol. Pharmacol. 2016, 94, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Marin-Luevano, P.; Trujillo, V.; Rodriguez-Carlos, A.; González-Curiel, I.; Enciso-Moreno, J.A.; Hancock, R.E.W.; Rivas-Santiago, B. Induction by innate defence regulator peptide 1018 of pro-angiogenic molecules and endothelial cell migration in a high glucose environment. Peptides 2018, 101, 135–144. [Google Scholar] [CrossRef]
- Lan, C.C.; Huang, S.M.; Wu, C.S.; Wu, C.H.; Chen, G.S. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl. Res. 2016, 169, e101–e103. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Hübner, G.; Breier, G.; Longaker, M.T.; Greenhalgh, D.G.; Werner, S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995, 270, 12607–12613. [Google Scholar] [CrossRef] [PubMed]
- Goren, I.; Müller, E.; Schiefelbein, D.; Gutwein, P.; Seitz, O.; Pfeilschifter, J.; Frank, S. Akt1 controls insulin-driven VEGF biosynthesis from keratinocytes: Implications for normal and diabetes-impaired skin repair in mice. J. Investig. Dermatol. 2009, 129, 752–764. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Deshpande, S.K.; Rastogi, A. Novel topical esmolol hydrochloride improves wound healing in diabetes by inhibiting aldose reductase, generation of advanced glycation end products, and facilitating the migration of fibroblasts. Front. Endocrinol. 2022, 13, 926129. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Combination of epinephrine with esmolol attenuates post-resuscitation myocardial dysfunction in a porcine model of cardiac arrest. PLoS ONE 2013, 8, e82677. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Etich, J.; Bergmeier, V.; Pitzler, L.; Brachvogel, B. Identification of a reference gene for the quantification of mRNA and miRNA expression during skin wound healing. Connect. Tissue Res. 2017, 58, 196–207. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Tang, Y.; Xie, D.; Deng, L.; Chen, M.; Wang, Y. Decreased expression of miR-204-3p in peripheral blood and wound margin tissue associated with the onset and poor wound healing of diabetic foot ulcers. Int. Wound J. 2022, 20, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, e2203308. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Lee, Y.; Ryu, H.A.; Jang, Y.; Lee, K.M.; Choi, Y.; Choi, W.J.; Lee, M.; Park, K.M.; Park, K.D.; et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016, 38, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sinha, U.K.; Gallagher, L.A. Effects of steel scalpel, ultrasonic scalpel, CO2 laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa. Laryngoscope 2003, 113, 228–236. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Han, S.K.; Lee, J.W.; Chang, H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes—A pilot study. Wound Repair Regen. 2012, 20, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, D.; Beatriz, P.M.; Jussara, R.; Fabiana, B. Tissue therapy with autologous dermal and epidermal culture cells for diabetic foot ulcers. Cell Tissue Bank. 2012, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Vivarelli, M.; Desideri, C.M.; Ippolito, G.; Marchionni, N.; Mannucci, E. Autologous skin fibroblast and keratinocyte grafts in the treatment of chronic foot ulcers in aging type 2 diabetic patients. J. Am. Podiatr. Med. Assoc. 2011, 101, 55–58. [Google Scholar] [CrossRef]
- Krasilnikova, O.A.; Baranovskii, D.S.; Lyundup, A.V.; Shegay, P.V.; Kaprin, A.D.; Klabukov, I.D. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev. Rep. 2022, 18, 1974–1985. [Google Scholar] [CrossRef]
- Ansel, J.C.; Tiesman, J.P.; Olerud, J.E.; Krueger, J.G.; Krane, J.F.; Tara, D.C.; Shipley, G.D.; Gilbertson, D.; Usui, M.L.; Hart, C.E. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J. Clin. Investig. 1993, 92, 671–678. [Google Scholar] [CrossRef]
- Haynes, S.L.; Shuttleworth, C.A.; Kielty, C.M. Keratinocytes express fibrillin and assemble microfibrils: Implications for dermal matrix organization. Br. J. Dermatol. 1997, 137, 17–23. [Google Scholar] [CrossRef]
- Beele, H.; Naeyaert, J.M.; Goeteyn, M.; De Mil, M.; Kint, A. Repeated cultured epidermal allografts in the treatment of chronic leg ulcers of various origins. Dermatologica 1991, 183, 31–35. [Google Scholar] [CrossRef]
- Hwang, Y.G.; Lee, J.W.; Park, K.H.; Han, S.H. Allogeneic keratinocyte for intractable chronic diabetic foot ulcers: A prospective observational study. Int. Wound J. 2019, 16, 486–491. [Google Scholar] [CrossRef]
- Verdi, J.; Shirian, S.; Saleh, M.; Khadem Haghighian, H.; Kavianpour, M. Mesenchymal Stem Cells Regenerate Diabetic Foot Ulcers: A Review Article. World J. Plast. Surg. 2022, 11, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Qiao, G.H.; Wang, M.; Yu, L.; Sun, Y.; Shi, H.; Ma, T.L. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front. Cell Dev. Biol. 2022, 10, 812262. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 2022, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.D.; Reinke, P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shan, Y.; Wen, Y.; Sun, J.; Du, H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef] [PubMed]
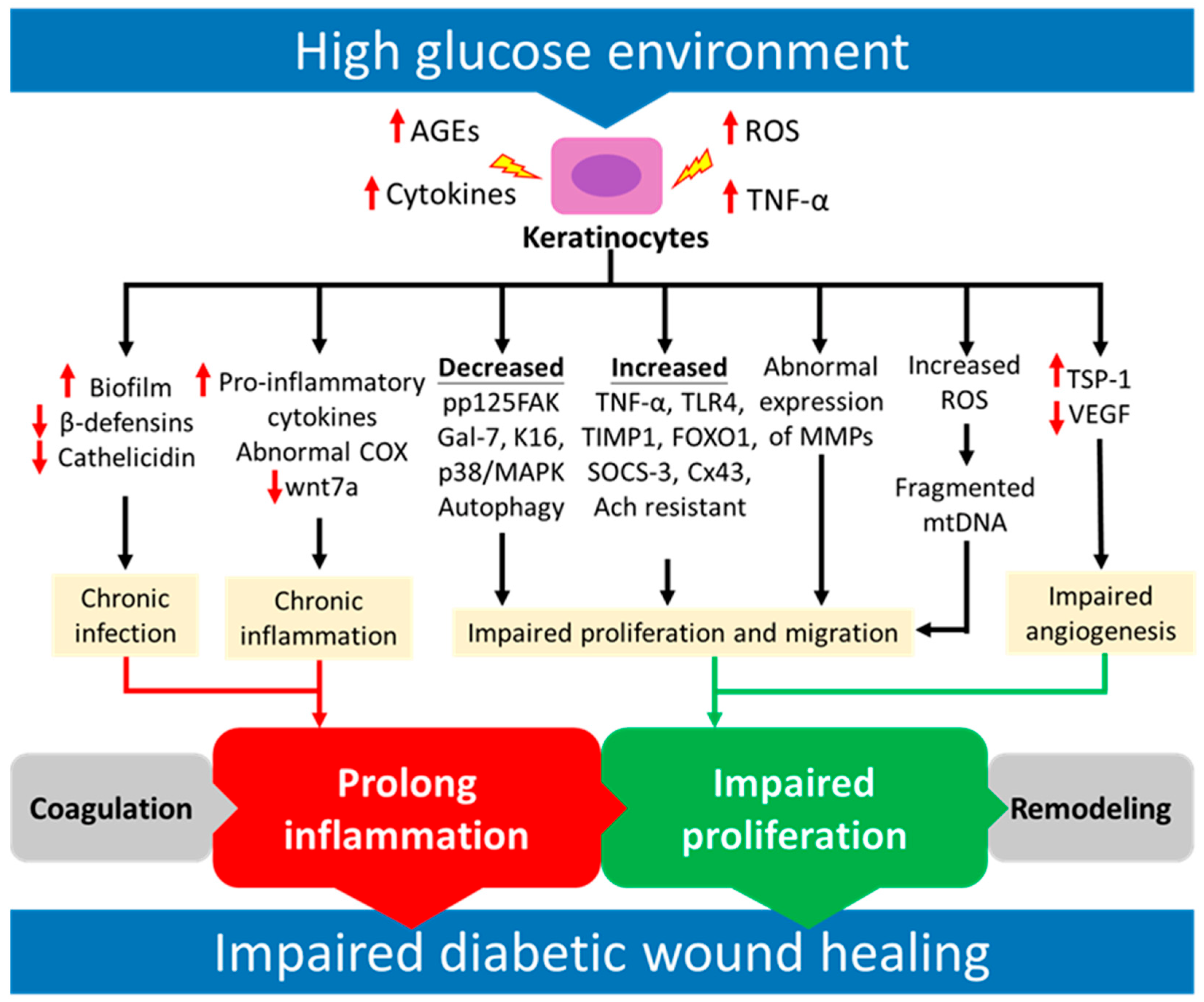
| Dysfunction | Mechanism | Ref. |
|---|---|---|
| Increased oxidative stress and ROS | Impaired antioxidant ability results in mitochondria damage | [52] |
| Abnormal expression of MMPs | Decreased mRNA level and activity of MMP-2 and MMP-9 and increased TIMP-1 | [57] |
| Decreased expression of MMP-1 and α2β1 integrin | [58] | |
| Decreased expression of IL-22 may suppress production of MMP-3 in keratinocytes | [59] | |
| Impaired proliferation and migration of KCs | Decreased expression of phosphorylated p125FAK | [57] |
| Increased AGEs | [61] | |
| Increased O-GlcNAc glycosylation and decreased expression of Gal-7 | [62] | |
| Downregulated the p38/MAPK pathway followed by inactivation of autophagy | [76] | |
| Increased percentage of M1 macrophage infiltration followed by increased secretion of TNF-α, which upregulates TIMP1 expression | [77] | |
| Increased expression of FOXO1 stimulates the expression of MMP-9 | [7] | |
| Impaired expression of K16 | [58] | |
| Increased expression of suppressor of cytokine signaling-3 (SOCS-3) | [85] | |
| Increased expression of Connexin 43 | [86] | |
| Increased keratinocyte resistance to acetylcholine (Ach) | [91] | |
| Chronic inflammation | Increased neutrophil and macrophage infiltration | [49] |
| Increased pro-inflammatory cytokines (IL-1, IL-6, IL-8 and TNF-α) | [93] | |
| Activation of the TNF-α and TLR4 signaling pathway | [95] | |
| Decreased expression of Wnt family member 7A (wnt7a) | [97] | |
| Increased expression of MIP-2 and MCP1 | [92] | |
| Decreased COX-1 expression and increased COX-2 expression | [98] | |
| Chronic infection | Reduced diversity of microbiota and promoted biofilm formation | [48] |
| Bacteria directly influenced keratinocytes (increasing apoptosis, diminishing keratinocyte migration and proliferation) | [103] | |
| Decreasing mRNA and protein levels of human β-defensins-2 (HBD-2) and HBD-3 | [104] | |
| Decreased expression of mRNA and protein of cathelicidin | [107] | |
| Impaired angiogenesis | Impaired the recruitment and migration of endothelial cells and EPCs | [112] |
| Increased the expression of Thrombospondin-1 (TSP-1) | [114] | |
| Decreased the expression of vascular endothelial growth factor (VEGF) | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.-C.; Lan, C.-C.E. The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 4290. https://doi.org/10.3390/ijms24054290
Fang W-C, Lan C-CE. The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. International Journal of Molecular Sciences. 2023; 24(5):4290. https://doi.org/10.3390/ijms24054290
Chicago/Turabian StyleFang, Wei-Cheng, and Cheng-Che E. Lan. 2023. "The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds" International Journal of Molecular Sciences 24, no. 5: 4290. https://doi.org/10.3390/ijms24054290
APA StyleFang, W.-C., & Lan, C.-C. E. (2023). The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. International Journal of Molecular Sciences, 24(5), 4290. https://doi.org/10.3390/ijms24054290




